Abstract
Background:
Despite well established empiric dose adjustments for drug and disease-state interactions, the impact of body mass index (BM) on warfarin remains unclear. The objective of this study is to evaluate warfarin requirements in hospitalized patients, stratified by BMI.
Methods:
This retrospective review included two cohorts of patients: cohort A (patients admitted with a therapeutic international normalized ratio (INR)) and cohort B (newly initiated on warfarin during hospitalization). Exclusion criteria included: age under 18 years, pregnancy, INR (goal 2.5–3.5), and warfarin thromboprophylaxis post orthopedic surgery. The primary outcome was mean total weekly dose (TWD) of warfarin based on weight classification: underweight (BMI <18 kg/m2), normal/overweight (BMI 18–29.9 kg/m2), obese (BMI 30–39.9 kg/m2), and morbidly obese (BMI ⩾ 40 kg/m2). Data were extracted from two community hospitals in reverse chronologic order during July 2015–June 2013 until both study institutions evaluated 100 patients per cohort in each BMI classification or until all patients had been evaluated within the prespecified timeframe.
Results:
A total of 585 patients were included in cohort A (26 underweight, 200 normal/overweight, 200 obese, 159 morbidly obese). There was a statistically significant difference in TWD as determined by one-way analysis of variance (p < 0.05). A Tukey post hoc test revealed a statistically significantly higher TWD in morbidly obese (41.5 mg) compared with underweight (25.6 mg, p < 0.05), normal/overweight (28.8 mg, p < 0.05) and obese patients (32.4 mg, p < 0.05). In cohort B, 379 patients were evaluated (9 underweight, 166 normal/overweight, 152 obese, 52 morbidly obese). Overall, 191 patients had a therapeutic INR on discharge (88.9% underweight, 52.4% normal/overweight, 44.1% obese, 55.8% morbidly obese, p = 0.035). Of those, there was a statistically significant difference in TWD (p = 0.021) with a higher TWD in the morbidly obese (41 mg) compared with underweight patients (24.4 mg, p = 0.017).
Conclusions:
Based on the results of this study, morbidly obese patients may require higher TWD to obtain and maintain a therapeutic INR.
Keywords: anticoagulation, atrial fibrillation, obesity, venous thromboembolism, warfarin
Background
Rates of obesity have been increasing worldwide. In 2016, more than 1.9 billion adults (39%) were overweight with over 600 million (13%) being obese.1 Health care practitioners are frequently challenged with drug dosing in obese patients and faced with concerns of under- or overdosing in this patient population. Many clinical trials exclude or have limited obese patients enrolled, making it difficult to determine safe and efficacious dosing regimens.
Warfarin was the only oral anticoagulant on the market in the United States for over 50 years.2 Despite the approval of direct acting oral anticoagulants (DOACs), warfarin is still prescribed for approximately 35% of patients with atrial fibrillation. Even with decades of clinical experience, warfarin therapy poses numerous challenges.2,3 Variations in patient characteristics such as age, race, genetic makeup, and concomitant disease states, as well as drug and dietary interactions, impact warfarin requirements.2,4,5 The usual recommended empiric starting dose for warfarin is 5–10 mg daily. A reduced starting dose, typically less than 5 mg daily, is recommended in elderly patients, patients with impaired nutrition, liver disease, or congestive heart failure, and in patients who are at high risk of bleeding.4
Pharmacogenetic algorithms have been developed to help guide clinicians to initiate a more patient-specific warfarin regimen that incorporates many of the characteristics that warrant a lower initial dose, including race, age, smoking, genotypes, and body surface area (BSA).5,6 In one of the pharmacogenomic models, BSA accounted for 11% of the warfarin dose for each 0.25 m2 increase in BSA. Currently, guidelines recommend against the routine use of pharmacogenetic testing for guiding doses of warfarin.5 Despite BSA being incorporated into pharmacogenetic algorithms, empiric dose adjustments accounting for body weight are not well established for nongenotype-guided warfarin dosing.
With the exception of pharmacogenetic models, few studies have been conducted to establish if weight or body mass index (BMI) has an effect on warfarin requirements. Based on limited information available, some data have demonstrated that obesity is associated with higher warfarin requirements to achieve a therapeutic INR due to a higher volume of distribution related to fat solubility and increased clearance.7–9
As the rates of obesity continue to increase, health care practitioners are frequently presented with the clinical question of what warfarin dose is most appropriate for their patients with varying weight extremes. The objective of this study is to compare the total weekly dose (TWD) of warfarin in patients admitted with therapeutic INRs and patients newly initiated on warfarin in the hospital, stratified by BMI.
Methods
A retrospective chart review was conducted at two community hospitals and evaluated two cohorts of patients. Cohort A consisted of hospitalized patients admitted with a therapeutic INR and cohort B included patients newly initiated on warfarin during the index hospitalization. Data were extracted from the institutions’ electronic medical record systems. Prior to data collection, this study was approved by the Institutional Review Board at both study institutions (Missouri Baptist Medical Center #1061 and SSM Health St Mary’s Hospital #15-11-0767). Informed consent was waived due to the retrospective nature of the study: research involves no more than minimal risk to the subjects, waiver or alteration will not adversely affect the rights and welfare of the subjects, research could not practicably be carried out without the waiver or alteration, and whenever appropriate, the subjects will be provided with additional pertinent information after participation [United States Department of Human Services 45CFR46.116(d)(1-4)].
All patients admitted with a therapeutic INR within 48 h of admission and a goal INR of 2.0–3.0 based on the American College of Chest Physician guideline recommendations were eligible for inclusion in cohort A.4 Inclusion criteria for cohort B included patients newly initiated on warfarin with an INR goal of 2.0–3.0 who received at least 4 days of warfarin therapy during the index hospitalization.4 Patients were consecutively evaluated in reverse chronologic order starting on 1 July 2015 through 1 July 2013 until 100 patients at each institution were included in each bodyweight classification for both cohorts [underweight (BMI < 18 kg/m2), normal/overweight (BMI < 18–29.9 kg/m2), obese (BMI < 30–39.9 kg/m2), and morbidly obese (BMI ⩾ 40 kg/m2)]. Patients were excluded if they were under 18 years of age, pregnant, had a mechanical mitral valve, an INR goal of 2.5–3.5, or received warfarin for deep vein thrombosis prophylaxis following orthopedic surgery.
Study outcomes
The primary outcome for both cohorts was the mean TWD required to obtain a therapeutic INR, stratified by body weight classifications (underweight, normal/overweight, obese, and morbidly obese). In cohort B, patients must have a therapeutic INR upon discharge to be included in the analysis for the primary outcome. The TWD was calculated based on the patients’ weekly warfarin requirements prior to admission as reported by the patient or obtained from pharmacy refill histories during the home medication reconciliation process (cohort A) or the average daily warfarin dose extrapolated to equate average TWD (cohort B). A subgroup analysis accounting for key drug interactions and smoking status was also conducted.10
Statistical analysis
Descriptive statistics were used to describe baseline characteristics. Data were stratified according to BMI into four bodyweight classifications: underweight, normal/overweight, obese, and morbidly obese. Differences among the mean TWD of the groups was assessed using one-way analysis of variance (ANOVA). A Tukey post hoc test was utilized to determine which BMI classifications had a statistically significant difference in TWD of warfarin. An analysis of covariance (ANCOVA) with a post hoc Bonferroni test was conducted to control for differences in baseline demographics. A bivariate analysis was used to assess whether there was an association between BMI and the TWD of warfarin. A linear regression analysis and linear fit model were used to evaluate the association between BMI and TWD of warfarin. All analyses were performed using IBM SPSS Statistics Version 24. A p value less than 0.05 was considered to indicate statistical significance.
Results
A total of 585 patients were included in cohort A (26 underweight, 200 normal/overweight, 200 obese, and 159 morbidly obese) and 379 patients were included in cohort B (9 underweight patients, 166 normal/overweight patients, 152 obese patients, and 52 morbidly obese patients). Approximately 50% of the normal/overweight, obese, and morbidly obese patients were men, with a lower representation in the underweight cohort (15.4%–22.2%). In both cohorts A and B, the mean age decreased as the BMI increased (Table 1), with the morbidly obese being statistically younger compared with the other weight classifications (65.6 and 56.3 respectively) (p < 0.05). In patients admitted with a therapeutic INR (cohort A), the most common reason for anticoagulation was atrial fibrillation (57.7%–72%), while the most common reason for initiating warfarin in hospitalized patients was for the treatment of a venous thromboembolism (36.5%–47%).
Table 1.
Baseline demographics.
| Characteristic, n (%) | Therapeutic upon admission |
Newly initiated |
||||||
|---|---|---|---|---|---|---|---|---|
| Underweight (n = 26) |
Normal/overweight (n = 200) |
Obese (n = 200) |
Morbidly obese (n = 159) |
Underweight (n = 9) |
Normal/overweight (n = 166) |
Obese (n = 152) |
Morbidly obese (n = 52) |
|
| Age, mean* | 76.7 | 77.8 | 71.4 | 65.6 | 75.5 | 70.3 | 66.6 | 56.3 |
| Sex, male$ | 4 (15.4) | 98 (49) | 98 (49) | 76 (47.8) | 2 (22.2) | 87 (52.4) | 73 (48) | 24 (46.2) |
| Weight, mean (kg)* | 44.9 | 73.4 | 98.7 | 137 | 63.1 | 108 | 136.5 | 184.5 |
| BMI, mean (kg/m2)* | 16.3 | 25.3 | 34.1 | 48.2 | 16.5 | 25.1 | 33.5 | 46.7 |
| Race | ||||||||
| Caucasian | 24 (92.3) | 152 (76) | 129 (64.5) | 111 (69.8) | 7 (77.8) | 117 (70.5) | 110 (72.4) | 35 (67.3) |
| Black | 2 (7.7) | 44 (22) | 64 (32) | 47 (29.6) | 2 (22.2) | 45 (27.1) | 39 (25.7) | 15 (28.8) |
| Other | 0 | 4 (2) | 7 (3.5) | 1 (0.6) | 0 | 4 (2.4) | 3 (2.7) | 2 (3.8) |
| Current smoker | 0 | 21 (10.5) | 17 (8.5) | 17 (10.7) | 3 (33.3) | 19 (11.4) | 18 (11.8) | 8 (15.4) |
| Interacting medication | 5 (19.2) | 33 (16.5) | 38 (19) | 17 (10.7) | 3 (33.3) | 69 (41.6) | 79 (52) | 28 (53.8) |
| Amiodarone | 4 (15.4) | 25 (12.5) | 33 (16.5) | 12 (7.5) | 2 (22.2) | 59 (35.5) | 72 (47.4) | 22 (42.3) |
| Carbamazepine | 0 | 1 (0.5) | 1 (0.5) | 0 | 0 | 0 | 0 | 2 (3.8) |
| Fluconazole‡ | 0 | 3 (1.5) | 0 | 2 (1.3) | 1 (11.1) | 6 (3.6) | 2 (1.3) | 6 (11.5) |
| Metronidazole | 1 (3.8) | 7 (3.5) | 4 (2) | 2 (1.3) | 0 | 12 (7.2) | 9 (5.9) | 5 (9.6) |
| Phenobarbital | 0 | 1 (0.5) | 1 (0.5) | 0 | 0 | 1 (0.6) | 0 | 0 |
| Sulfamethoxazole/trimethoprim | 0 | 2 (1) | 2 (1) | 2 (1.3) | 0 | 4 (2.4) | 2 (1.3) | 1 (1.9) |
| Indication | ||||||||
| Atrial fibrillation | 15 (57.7) | 138 (69) | 144 (72) | 96 (60.4) | 3 (33.3) | 25 (15.1) | 26 (17.1) | 16 (30.8) |
| VTE treatment | 9 (34.6) | 42 (21) | 53 (26.5) | 55 (34.6) | 4 (44.4) | 78 (47) | 56 (36.8) | 19 (36.5) |
| Valve repair or replacement | 1 (3.8) | 30 (15) | 23 (11.5) | 10 (6.3) | 1 (11.1) | 42 (25.3) | 61 (40.1) | 15 (28.8) |
| Other | 1 (3.8) | 4 (2) | 6 (3) | 4 (2.5) | 0 | 1 (.6) | 4 (2.6) | 3 (5.8) |
p Value < 0.05 in both cohorts.
p Value < 0.05 in cohort A.
p Value < 0.05 in cohort B.
VTE, venous thromboembolism.
In cohort A, there was a statistically significant difference in TWD between groups as determined by one-way ANOVA (p < 0.05). A Tukey post hoc test revealed a statistically significantly higher TWD in the morbidly obese (41.5 mg) compared with underweight patients (25.6 mg, p < 0.05), normal/overweight patients (28.8 mg, p < 0.05) and obese patients (32.4 mg, p < 0.05) (Table 2). There were no statistically significant differences between the other groups. When adjusting for age, a statistically significant difference was still observed between obese patients compared with underweight, normal/overweight, and obese patients (p < 0.05) (Table 2). There was a weak, but statistically significant positive correlation between TWD of warfarin and BMI (R = 0.279, R2 = 0.078, p < 0.05), suggesting that BMI accounts for approximately 7.8% of the TWD of warfarin. For each one-unit increase in BMI, the TWD of warfarin increased by 0.46 mg. Using the linear fit model (Figure 1), the average TWD of warfarin can be estimated using the formula: 17.58 + 0.46 × BMI. Based on this analysis, as a patient’s BMI increased, higher warfarin requirements were needed to maintain a therapeutic INR.
Table 2.
Weekly warfarin requirements stratified by body mass index.
| Statistical analysis | Therapeutic upon admission |
Newly initiated, therapeutic at discharge |
||||||
|---|---|---|---|---|---|---|---|---|
| Underweight (n = 26) |
Normal/overweight (n = 200) |
Obese (n = 200) |
Morbidly obese (n = 159) |
Underweight (n = 8) |
Normal/overweight (n = 87) |
Obese (n = 67) |
Morbidly obese (n = 29) |
|
| Minimum (mg) | 6 | 3.5 | 6 | 7 | 5.25 | 15.75 | 17.5 | 15.4 |
| Maximum | 59 | 105 | 160 | 140 | 44.2 | 84.58 | 74.38 | 80.5 |
| Mean* | 25.6 | 28.8 | 32.4 | 41.5 | 24.4 | 34.89 | 36.49 | 41.01 |
| Mean adjusted for age$ | 27.6 | 31.3 | 32 | 38.4 | 26.5 | 35.7 | 36 | 39.1 |
| Median$ | 15.75 | 28 | 29 | 35.5 | 24.34 | 32.85 | 33 | 37.69 |
| Mode | 14 | 35 | 35 | 35 | – | 28 | 28 | 46.2 |
| Standard deviation | 18.63 | 15.47 | 18.57 | 18.61 | 13.52 | 13 | 13 | 18.13 |
| Variance | 347 | 239 | 344.7 | 346.3 | 182.67 | 168.93 | 169.05 | 328.59 |
p Value < 0.05 in both cohorts.
p Value < 0.05 in cohort A.
Figure 1.
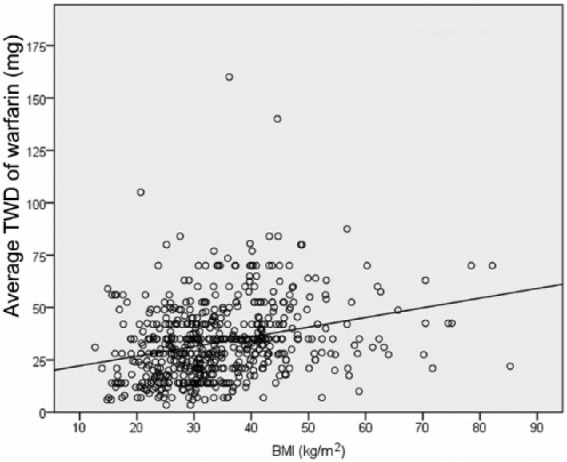
Linear fit of total weekly dose (TWD) by body mass index (BMI) in cohort A (patients admitted with a therapeutic INR).
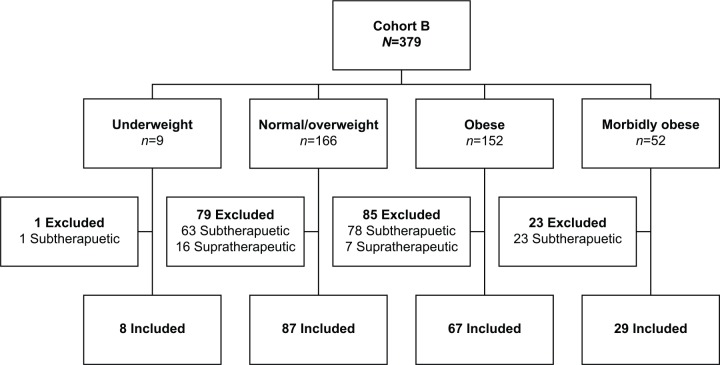
In cohort B, only 191 patients had a therapeutic INR on discharge (88.9% underweight, 52.4% normal/overweight, 44.1% obese, 55.8% morbidly obese, p = 0.035) (Figure 2). Of those discharged with a therapeutic INR, there was a statistically significant difference in TWD between groups as determined by one-way ANOVA (p = 0.021). A Tukey post hoc test revealed a statistically significantly higher TWD in the morbidly obese (41 mg) compared with underweight patients (24.4 mg, p = 0.017). There were no statistically significant differences between the other groups or between time to therapeutic INR (underweight 5.75 days, normal/overweight 5.29 days, obese 5.24 days, morbidly obese 5.24 days; p = 0.939). However, when adjusting for age, there was a numerically, but not statistically significant difference in the TWD among BMI classifications (Table 2) (p = 0.154). Of patients discharged with a therapeutic INR, the morbidly obese had a longer length of stay (LOS) (15.5 days) compared with normal/overweight patients (10.7 days; p = 0.019) and obese patients (10.6 days; p = 0.022) but not underweight patients (12.5 days; p = 0.755). This could possibly reflect a sicker patient population and does not necessarily indicate that patients resided in the hospital solely to attain a therapeutic INR.
Figure 2.
Patient inclusion in cohort B for primary endpoint.
There were no statistically significant differences between the number of patients with key interacting medications among BMI classifications in either cohort (p > 0.05) (Table 1). In cohort B, the only statistically significant difference noted in drug interaction was a higher number of morbidly obese and underweight patients concomitantly taking fluconazole. A subanalysis of cohort A found that patients taking amiodarone required approximately 30%–40% less warfarin than those not taking amiodarone (Table 3) (p = 0.011). Due to the small sample size, inconclusive results were found for patients receiving other drugs that commonly impact warfarin requirements.
Table 3.
Cohort A exploratory subgroup analysis of mean warfarin requirements.
| Underweight (n = 26) |
Normal/overweight (n = 200) |
Obese (n = 200) |
Morbidly obese (n = 159) |
|
|---|---|---|---|---|
| Overall mean | 25.6 mg | 28.8 mg | 32.4 mg | 41.5 mg |
| Sex | ||||
| Male | n = 4 | n = 98 | n = 98 | n = 76 |
| 26.5 mg | 30.2 mg | 35.9 mg | 44.9 mg | |
| Female | n = 22 | n = 102 | n = 102 | n = 83 |
| 25.4 mg | 27.3 mg | 29 mg | 38.4 mg | |
| Race | ||||
| White | n = 24 | n = 152 | n = 129 | n = 111 |
| 25.6 mg | 27.6 mg | 32.5 mg | 39 | |
| Black | n = 2 | n = 44 | n = 64 | n = 47 |
| 24.5 mg | 32.8 mg | 33.6 mg | 47.2 mg | |
| Other | – | n = 4 | n = 7 | n = 1 |
| – | 29.1 mg | 18 mg | 52.5 mg | |
| Tobacco status | ||||
| Smoker | – | n = 21 | n = 17 | n = 17 |
| – | 39.6 mg | 39 mg | 41.3 mg | |
| Nonsmoker | n = 26 | n = 179 | n = 183 | n = 142 |
| 25.6 mg | 27.5 mg | 31.8 mg | 41.5 mg | |
| Interacting medications | ||||
| Amiodarone | ||||
| Yes | n = 4 | n = 25 | n = 33 | n = 12 |
| 20.5 mg | 17.6 mg | 22.3 mg | 30.3 mg | |
| No | n = 22 | n = 175 | n = 167 | n = 147 |
| 26.5 mg | 30.4 mg | 34.4 mg | 42.4 mg | |
| Fluconazole | ||||
| Yes | – | n = 3 | – | n = 2 |
| – | 28 mg | – | 38.5 mg | |
| No | n = 26 | n = 197 | n = 200 | n = 157 |
| 25.6 mg | 28.8 mg | 32.4 mg | 41.5 mg | |
| Metronidazole | ||||
| Yes | n = 1 | n = 7 | n = 4 | n = 2 |
| 49 mg | 27.9 mg | 21.8 mg | 38.5 mg | |
| No | n = 25 | n = 193 | n = 196 | n = 157 |
| 24.6 mg | 28.8 mg | 32.6 mg | 41.5 mg | |
| Sulfamethoxazole/trimethoprim | ||||
| Yes | – | n = 2 | n = 2 | n = 2 |
| – | 31.8 mg | 37 mg | 57.8 mg | |
| No | n = 26 | n = 198 | n = 198 | n = 157 |
| 25.6 mg | 28.7 mg | 32.3 mg | 41.3 mg | |
Discussion
The results of this study are consistent with the limited, but recently published literature supporting higher warfarin requirements to obtain and maintain a therapeutic INR in morbidly obese patients.7–9,11–13 Routledge and colleagues conducted a multiple regression analysis in the 1970s. The investigators reported that patient weight is related to warfarin requirements and accounts for approximately 3.77% of the variation in dose.9 A decade later, Kirking and colleagues were unable to establish a correlation between mean maintenance dose and lean body weight (r = 0.17).12 Unfortunately, patient weight extremes were not reported. In 1971–1975 approximately 14.5% of adults had a BMI of at least 30 kg/m2. The prevalence in America nearly doubled by 2000, with approximately 30.4% with a BMI of at least 30 kg/m2.13 Given the increasing rates of obesity, it is unlikely that a substantial number of obese and morbidly obese patients were included in earlier studies.
Another retrospective study, including 831 outpatients on stable warfarin regimens for at least 3 days, found a strong correlation between warfarin dose and BMI. For each one point increase in BMI (kg/m2), the weekly dose of warfarin increased by 0.69 mg (average TWD of warfarin = 12.34 + 0.69 × BMI) (p < 0.001).14 Based on these results, a patient with a BMI of 30 kg/m2 would require approximately 33 mg of warfarin per week. Similar results were observed in the current study. The equation yielded from the linear fit model in cohort A estimates 31 mg of warfarin per week for a patient with the same BMI. Alternatively, Kabegambe and colleagues found a trend of higher warfarin dosing requirements with increasing BMIs, however this was not found to be statistically significant.9
The results are hampered by a number of limitations, such as the retrospective study design and small sample size. Patients were only evaluated at two hospitals during a prespecified timeframe, yielding less than the desired 100 patients per arm per institution. In cohort B, only patients who were discharged with a therapeutic INR were included in the analysis; this yielded an even smaller sample size. The small sample size could explain the lack of difference in average TWD between weight classifications once adjusted for age. Despite the lack of statistical significance, a numerical difference was noted and the average TWD between cohort A and B remained similar: underweight (27.6 mg and 26.6 mg), normal/overweight (31.3 mg and 35.7 mg), obese (32 mg and 36 mg), and morbidly obese (38.4 mg and 39.1 mg).
The retrospective nature of the study depended on the quality of the home medication reconciliations performed on admission to accurately reflect the warfarin dose the patient was receiving as an outpatient and to reliably capture drug interactions. Additionally, disease states such as liver dysfunction and heart failure were not captured in cohort A or B, and only a single therapeutic INR within 48 h of admission was required for study inclusion in cohort A. The time in therapeutic range was not calculated for the duration of the hospital stay and would have provided more insight into the stability of the warfarin regimens the patients were receiving. Therefore, patients could have been deemed as having a stable regimen without knowing or evaluating additional INRs. Based on the retrospective nature of the study, the investigators were also unable to determine when warfarin therapy was initiated in patients in cohort A.
Drug interactions, such as amiodarone, should still be taken into account as patients concomitantly taking warfarin required lower doses than those without the drug interaction in patients in the same BMI class.3 There was not a sufficient number of patients in each body weight classification to draw conclusions for other drug interactions and conflicting results are reported for patients receiving sulfamethizole/trimethoprim.10 Similarly, it is difficult to extrapolate differences in race and the impact on requirements in patients of varying weight classifications as over 70% of the patients included in the analysis were white.
Conclusion
Data from this evaluation suggest that BMI, especially 40 kg/m2 and over, in addition to other traditional factors affecting warfarin requirements, should be taken into account when determining warfarin doses.
Acknowledgments
The authors presented the preliminary results of this analysis as a poster presentation at the American College of Clinical Pharmacy Virtual Poster Symposium, 17 May 2017 and the American College of Clinical Pharmacy Annual Meeting, 7 October 2017.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Katie B. Tellor  https://orcid.org/0000-0003-0980-9888
https://orcid.org/0000-0003-0980-9888
Contributor Information
Katie B. Tellor, Department of Pharmacy Practice, St Louis College of Pharmacy, 4588 Parkview Place, St Louis, MO 63110, USA.
Steffany N. Nguyen, Memorial Hermann, Texas Medical Center, Houston, TX, USA
Amanda C. Bultas, Barnes Jewish Hospital, St Louis, MO, USA
Anastasia L. Armbruster, Department of Pharmacy Practice, St Louis College of Pharmacy, St Louis, MO, USA
Nicholas A. Greenwald, St Louis College of Pharmacy, St Louis, MO, USA
Abigail M. Yancey, Department of Pharmacy Practice, St Louis College of Pharmacy, St Louis, MO, USA
References
- 1. Obesity and Overweight. World Health Organization, http://www.who.int/mediacentre/factsheets/fs311/en/ (2017, accessed 5 December 2017).
- 2. Bristol-Meyers Squibb. Coumadin® [package insert]. Bristol-Myers Squibb Company: Princeton, New Jersey, 2017. [Google Scholar]
- 3. Marzec JN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 2017; 69: 2475–2484. [DOI] [PubMed] [Google Scholar]
- 4. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 14: e44S–88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holbrook A, Schulman S, Witt DM, et al. Evidence–based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e152S–e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther 2008; 84: 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wallace JL, Reaves AB, Tolley EA, et al. Comparison of initial warfarin response in obese patients versus non-obese patients. J Thromb Thrombolysis 2013; 36: 96–101. [DOI] [PubMed] [Google Scholar]
- 8. Patel JP, Roberts LN, Arya R. Anticoagulating obese patients in the modern era. Br J Haematol 2011; 155: 137–149. [DOI] [PubMed] [Google Scholar]
- 9. Kabagambe EK, Beasley TM, Limdi NA. Vitamin K intake, body mass index and warfarin maintenance dose. Cardiology 2013; 126: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005; 165: 1095–1106. [DOI] [PubMed] [Google Scholar]
- 11. Routledge PA, Chapman PH, Davies DM, et al. Factors affecting warfarin requirements: a prospective population study. Eur J Clin Pharmacol 1979; 15: 319–322. [DOI] [PubMed] [Google Scholar]
- 12. Kirking DM, Cohen IA, Shue ME, et al. Relationship of age, weight and body surface area to warfarin maintenance dose requirements. J Clin Hosp Pharm 1985; 10: 101–105. [DOI] [PubMed] [Google Scholar]
- 13. Wilborn C, Beckham J, Campbell B, et al. Obesity: prevalence, theories, medical consequences, management, and research directions. J Int Soc Sports Nutr 2005; 2: 4–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mueller JA, Patel T, Halawa A, et al. Warfarin dosing and body mass index. Ann Pharmacother 2014; 48: 584–588. [DOI] [PubMed] [Google Scholar]