Abstract
Objective:
Although cerebral ischemia can activate endogenous reparative processes, such as proliferation of endogenous neural stem cells (NSCs) in the subventricular zone (SVZ) and subgranular zone (SGZ), the majority of these new cells die shortly after injury and do not appropriately differentiate into neurons, or migrate and functionally integrate into the brain. The purpose of this study was to examine a novel strategy for treatment of stroke after injury by optimizing the survival of ischemia-induced endogenous NSCs in the SVZ and SGZ.
Methods:
Adult SVZ and SGZ NSCs were grown as neurospheres in culture and treated with a p53 inactivator, pifithrin-α (PFT-α), and an amyloid precursor protein (APP)-lowering drug, posiphen, and effects on neurosphere number, size and neuronal differentiation were evaluated. This combined sequential treatment approach was then evaluated in mice challenged with middle cerebral artery occlusion (MCAo). Locomotor behavior and cognition were evaluated at 4 weeks, and the number of new surviving neurons was quantified in nestin creERT2-YFP mice.
Results:
PFT-α and posiphen enhanced the self-renewal, proliferation rate and neuronal differentiation of adult SVZ and SGZ NSCs in culture. Their sequential combination in mice challenged with MCAo-induced stroke mitigated locomotor and cognitive impairments and increased the survival of SVZ and SGZ NSCs cells. PFT-α and the combined posiphen+PFT-α treatment similarly improved locomotion behavior in stroke challenged mice. Notably, however, the combined treatment provided significantly more potent cognitive function enhancement in stroke mice, as compared with PFT-α single treatment.
Interpretation:
Delayed combined sequential treatment with an inhibitor of p53 dependent apoptosis (PFT-α) and APP synthesis (posiphen) proved able to enhance stroke-induced endogenous neurogenesis and improve the functional recovery in stroke animals. Whereas the combined sequential treatment provided no further improvement in locomotor function, as compared with PFT-α alone treatment, suggesting a potential ceiling in the locomotion behavioral outcome in stroke animals, combined treatment more potently augmented cognitive function recovery after stroke.
Keywords: Stroke, neuroregeneration, p53, APP
Introduction
Stroke is one of the leading causes of death and disability worldwide and places a heavy burden on the economy in our society. Current treatment strategies for stroke primarily focus on reducing the size of ischemic damage and on rescuing dying cells early after occurrence. Treatments, such as the use of thrombolytic agents, are often limited by a narrow therapeutic time window1. However, regenerative processes remain active within the brain from days to weeks after stroke-induced damage occurs and, although these processes are often inefficient, their optimization may provide a second window for treatment2. Prior work by our group3–5 and others6,7 has demonstrated that modulating endogenous neurogenesis represents a promising therapeutic paradigm for the treatment of stroke. We previously demonstrated that administration of pifithrin-α (PFT-α; 2 mg/kg), a synthetic p53 inactivator that blocks p53-dependent apoptosis8, 5 days after stroke is able to enhance the proliferation and migration of neural stem cells (NSCs) and inhibit the apoptosis of newly born cells, resulting in improved behavioral recovery in rodents with focal ischemia in the cerebral cortex induced by 90 min middle cerebral artery occlusion (MCAo)3. In cell culture studies, PFT-α (0.01–0.05 µM) augmented the proliferation and survival of embryonic NSCs, increasing both neurosphere number and diameter3; a finding that later studies have likewise reproduced9.
Embryonic NSCs express relatively high levels of p5310 that appear to be critical for the induction of apoptosis to prune excess neural progenitor cells (NPCs) during neonatal development of the central nervous system11. p53 likewise appears to be important in the self-renewal of adult NSCs12, and studies in rodents suggest that the length of time that neurogenesis can be maintained in the adult is dependent on the capacity of stem cells in neurogenic regions of the brain to proliferate, which is controlled at least in part by p5313. Whether adult neurospheres respond to PFT-α-mediated p53 inactivation in the same manner as do embryonic neurospheres remains unknown, but is of relevance to the endogenous regenerative processes that occur following a stroke in the adult brain.
The microenvironment surrounding NSCs in the adult brain is altered during focal ischemia and hypoxia as occur in stroke, in which levels of key proteins with the capacity to impact NSC proliferation and differentiation, such as amyloid precursor protein (APP), are upregulated14,15 and lead to the potential to impair neurogenesis16–18. APP over expression, together with elevation of its toxic metabolite amyloid-β peptide (Aβ), can be inhibited in neuronal cultures, the brain of animals and in humans by the experimental drugs (-)-phenserine (phenserine) and (+)-phenserine (posiphen)19–21. In the light of phenserine’s ability to inhibit neuronal apoptosis following ischemia/reperfusion injury and reduce stroke volume22, together with posiphen’s ability to enhance the neuronal differentiation and migration of transplanted as well as endogenous NSCs in hippocampus and cortex in mice16,23,24, these agents hold promise to optimize the neuroregenerative processes that occur following a stroke. To enhance both the activation and sequential expansion of NSCs in vivo, in this study we examined the efficacy of a sequential PFT-α and posiphen combination for the treatment of stroke in mice.
Materials and Methods
SVZ and SGZ Neural Stem Cell Culture
Primary stem cell cultures were obtained from C57BL/6 J mice at 4–6 weeks of age. After euthanization, whole brains were immediately harvested and dissected under microscope to obtain the subventricular zone (SVZ) or subgranular zone (SGZ) tissue. After mechanical dissociation with a stab knife, the tissue fragments were processed using trypsin and resuspended as individual cells at a density of 104 cells/cm2 in neurobasal media with growth factors epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) (NBM-GF). Subsequent passaging of cells was performed using Accutase® (Innovative #AT-104, San Diego, CA, USA) every 7 days until the cells established viable lines, and cellular debris was naturally diminished after each passage. At day 4 of each passage, the proliferating spheres were fed with NBM-GF. We used neurospheres at passage P3–P8 in this study.
Neurosphere Proliferation Assay
After dissociation the neurospheres during passaging, individual cells were plated at a density of 104 cells/cm2 in an untreated 96-well plate in 100 µl of NBM-GF with the chosen drug treatment (or vehicle, in the case of control samples). On day 4, 100 µl of NBM-GF with drug treatment (or vehicle) was fed to each well. On day 7, images of each well were taken using a GelCount machine (Oxford Optronix, Abingdon, UK). Though most spheres will be tagged automatically using the GelCount software set to the appropriate user-set parameters, each image was manually reviewed and tags were added/removed manually to ensure proper inclusion of neurospheres and exclusion of non-specific debris. From the GelCount data output, neurosphere count and diameter data was obtained and analyzed statistically.
Neurosphere Differentiation Assay and Sequential Treatment
Briefly, glass coverslips in an untreated 24-well plate were coated with poly-ornithine and laminin. After splitting neurospheres during passaging, individual cells were plated at a density of 104 cells/cm2 in 500 µl of NBM-GF with PFT-α or vehicle. Every other day (i.e. on days 2, 4, etc.), 250 µl of media was removed from each well and 300 µl of fresh NBM-GF (with PFT-α or vehicle) was added. When the attached cells reached approximately 80% confluency (around day 5), all NBM-GF within each well was gently removed and immediately replaced with neurobasal media without growth factors (NBM) along with either posiphen or vehicle. Each well was fed daily, thereafter, by removing 250 µl of the media and adding 300 µl of the media containing the respective drug or vehicle. On day 7 after complete replacement of the NBM-GF, the wells were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature and stored in phosphate-buffered saline at 4oC until ready to stain. The wells were probed with primary antibodies in 1:500 dilution (rabbit anti-microtubule associated protein 2 [MAP2; Millipore AB5622, Burlington, MA, USA]) overnight at 4oC and subsequently with fluorescent secondary antibodies (Alexa Fluor by Thermo Fisher, Waltham, MA, USA). The glass coverslips were then carefully removed from each well and mounted on a microscope slide (neuronal attachment-side facing down) in Mowiol containing 4’,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO, USA). Two to three coverslips were analyzed per condition. Random selections of field in each coverslip were chosen and imaged by Stereo Investigator software (MBF Bioscience, Williston, VT, USA), and quantitative data was obtained by using NIH ImageJ software. A total of 8–10 images at 40× were analyzed for each condition.
Murine Model of Transient Focal Ischemia
Transient MCAo was induced in male 10–12-week-old C57/BL6 mice (Jackson Laboratory, Bar Harbor, Maine, USA) or male NestincreERT2-YFP mice (C57/BL6 congenic background, 10–12-week-old) mice as described in our previous studies5. Briefly, transient MCAo was induced by an intraluminal suture method. Mice were anaesthetized with isoflurane and a midline neck incision was made to expose the left common carotid artery (lCCA). The isolated lCCA was temporarily ligated with a silk suture during the whole period of occlusion. The left external carotid artery (lECA), and the left internal carotid artery (lICA) were also isolated by dissection of fascia. The lICA was clamped with an artery clamp above a loose suture tie around the lICA. The lECA was ligated and cut to make a lECA stump. A nylon filament suture with a silicon coated tip (Doccol Co., Sharon, MA, USA) was inserted into the lECA stump and further pushed along the lumen of the internal carotid artery while the clamp was released, and then the tie around lICA was tightened. After 35 min of occlusion, the nylon suture was gently removed from the left middle cerebral artery (lMCA) to allow reperfusion of the lMCA territory. The lECA stump was permanently ligated, and the temporary ligation of the lCCA and lICA were removed to allow normal blood flow to the brain. During the surgery, mice were placed above a temperature controlled heating blanket to maintain normal body temperature and, after the 35-min occlusion, the skin wound was sutured closed and the mice were placed in a heated animal intensive care unit chamber until recovery. To ensure consistent and successful blockage of the middle cerebral artery, we monitored ischemia in all animals by Laser Doppler flowmetry (PeriFlux System 5000, Perimed, Järfälla-Stockholm, Sweden).
Treatment
On post-stroke day 3, animals were randomly assigned into two equal groups and subjected to locomotion behavioral tests to confirm similar behavioral deficits between groups. PFT-α (2 mg/kg/day) or vehicle (10% dimethyl sulfoxide (DMSO) in saline) was injected daily intra-peritoneally for 7 days starting from post-stroke day 5. This well-tolerated dose and time of PFT-α were selected from our prior study and other subsequent studies using similar treatment paradigms3,25,26. At 24 h after the last injection of PFT-α, animals received 25 mg/kg/day posiphen for 14 consecutive days subcutaneously administered via an Alzet osmotic pump (Durect Corp., Cupertino, CA, USA; Alzet pump #2002). This equates to an equivalent posiphen dose of 120 mg daily in a 60-kg human, following normalization of body surface area between the two species in accord with United States Food and Drug Administration guidelines (US FDA)27. Single doses of up to 160 mg posiphen have been administered to humans21. This treatment window for posiphen was chosen because transient amplification of the NSCs follows the self-renewal of NSCs. Since the in vitro data suggest that PFT-α increases the self-renewal of NSCs and posiphen enhances the size of NSCs neurospheres (a cellular event that is likely due to increased expansion of transient amplifying cells), sequential treatment with posiphen following PFT-α treatment was evaluated in this study. As both SVZ and SGZ NSCs have been shown to be activated from 1–2 weeks after stroke, and because newly born cells formed in the 1st week after MCAo appear to survive for up to 3–4 weeks,28–31 posiphen was administered for 2 weeks following PFT-α treatment. Control mice were implanted with alike Alzet pumps that were filled with physiological saline. PFT-α (>99% chemical purity) and posiphen (>99% chemical and chiral purity, in the form of its water-soluble tartrate salt) were synthesized in accord with our prior studies8,32.
Behavioral Assays
Locomotor function
Animals were placed in an Accuscan activity monitor (Columbus, OH, USA) one day before and 3, 10 and 24 days after MCAo for behavioral evaluation over a 1 h duration, as previously described33. The monitor possesses 16 horizontal and 8 vertical infrared sensors that are spaced 2.5 cm apart. Each animal was placed in a 42 × 42 × 31 cm Plexiglas open box for 60 min. The total horizontal activity (the total number of beam interruptions that occurred for the horizontal sensors) and the total vertical activity (the total number of beam interruptions that occurred for the vertical sensors) were calculated using the automated Versamax software (Accuscan). This behavioral test was automatically monitored by a computer and associated software, and thereby avoided any potential observer bias.
Novel Object Recognition
The novel object recognition (NOR) test was used to evaluate cognitive deficits at post-stroke week 4, as described previously34. On the day before training, mice were allowed to explore the experimental apparatus (a dark gray open field box) for 5 min without the presence of any testing objects. During the training phase, mice were placed in the experimental apparatus for 5 min with two identical objects. After a retention interval of 24 h, mice were placed back into the arena in which one of the familiar objects was replaced by a novel one for the test trial. The kind of object presented during the training as well as its position during the test trial were counterbalanced and randomly permutated. The testing trail was recorded by camera and the time near each of the objects together with the frequency for visiting each of the objects was scored at a later time by an observer blinded to the treatment. A mouse was scored as exploring an object whenever it was within 1 cm from the object and facing it. The new object preference index (PI) was calculated as follows PI = (time near new object−time near familiar object)/(time near new object+time near familiar object). Objects were cleaned with 70% ethanol between each animal to remove any odor cues. Animals that did not explore both objects for more than 30 s over the course of the 5-min test session (i.e. less than 10% of the total test time) were excluded from the analysis.
In vivo SVZ and SGZ NSCs Labeling and Quantification
Male nestin-CreERT2-R26R-YFP mice (8–10 weeks old) were administered tamoxifen (TAM) (dissolved in 10% EtOH/90% sunflower oil) by gavage feeding at a dose of 180 mg/kg daily for 5 consecutive days. This dosing regimen was previously demonstrated to provide maximal recombination with minimal mortality and successfully monitored the activated SVZ NSCs by stroke5,33. For NSC fate mapping, TAM treated mice received MCAo surgery 10 days after the last TAM administration, and mice were perfused at 4 weeks post-stroke to harvest the brain for immunostaining. This time frame was specifically chosen to label NSCs in adult mice before the introduction of MCAo but also allow the clearance of TAM from mice at the time of MCAo to prevent labeling of reactive astrocytes, which also upregulate nestin expression after a stroke.
At 4 weeks after stroke, mice were perfused transcardially with a solution of 4% paraformaldehyde (PFA, pH 7.4) in 0.1 M phosphate buffer (PB, pH 7.4). Brains were removed from the skull, post-fixed in 4% PFA overnight at 4°C, and sequentially transferred to 20% and 30% sucrose in 0.1 M phosphate buffer, pH 7.4 solutions overnight. Brains were frozen on dry ice and sectioned on a cryostat to obtain coronal sections of 25 μm in thickness. These sections were then incubated with blocking buffer for 1 h. A series of primary antibodies were prepared in the blocking buffer and the sections were incubated in the solution overnight. The antibodies used were rabbit anti-GFP (green fluorescent protein) (1:1000; Invitrogen, Carlsbad, CA, USA). After incubation with primary antibody solution, the sections were washed and incubated for 4 h at room temperature in diluted secondary antibody prepared with blocking solution (secondary antibody conjugated with Alexa 488, 1:1000; Life Technologies, Carlsbad, CA, USA). The sections were then washed with Tween tris-buffered saline (TTBS), mounted and coverslipped. Images were acquired using an Olympus microscope (Shinjuku, Tokyo, Japan). Omission of primary or secondary antibodies resulted in no staining and served as negative controls. Group and treatment information were blinded to the image analyzer. Quantification of total yellow fluorescent protein (YFP) cells was performed as described previously5.
Quantification of Brain Atrophy in Stroke Animals
One set of post-stroke 4-week brain sections (25 µm) were mounted on slides. The sections were then stained in 10% Giemsa KH2PO4 buffered solution (pH 4.5) for 30 min at 40°C. After a brief rinse, slides were de-stained, differentiated, and dehydrated in absolute ethanol. Thereafter, the sections were cleared in xylene and then coverslipped. Slides were scanned in a Path Scan Enabler IV slide scanner, and areas of the brain images were quantified using ImageJ software. The calculation formula of atrophy rate is as follows:
∑(area of contralateral side - area of stroke side) / ∑area of contralateral side.
Statistics
Results are expressed by mean ± standard error of the mean (SEM) of the indicated number of experiments. Statistical analysis was performed using Student’s t test, and one- or two-way analysis of variance (ANOVA), as appropriate, with Student–Newman–Keuls post-hoc tests or Bonferroni post-hoc tests for repeated behavioral measurements. A p-value equal to or less than 0.05 was considered as statistically significant.
Results
PFT-α or Posiphen Increases the Self-Renewal and Proliferation Rate of Adult SVZ and SGZ NSCs in Culture
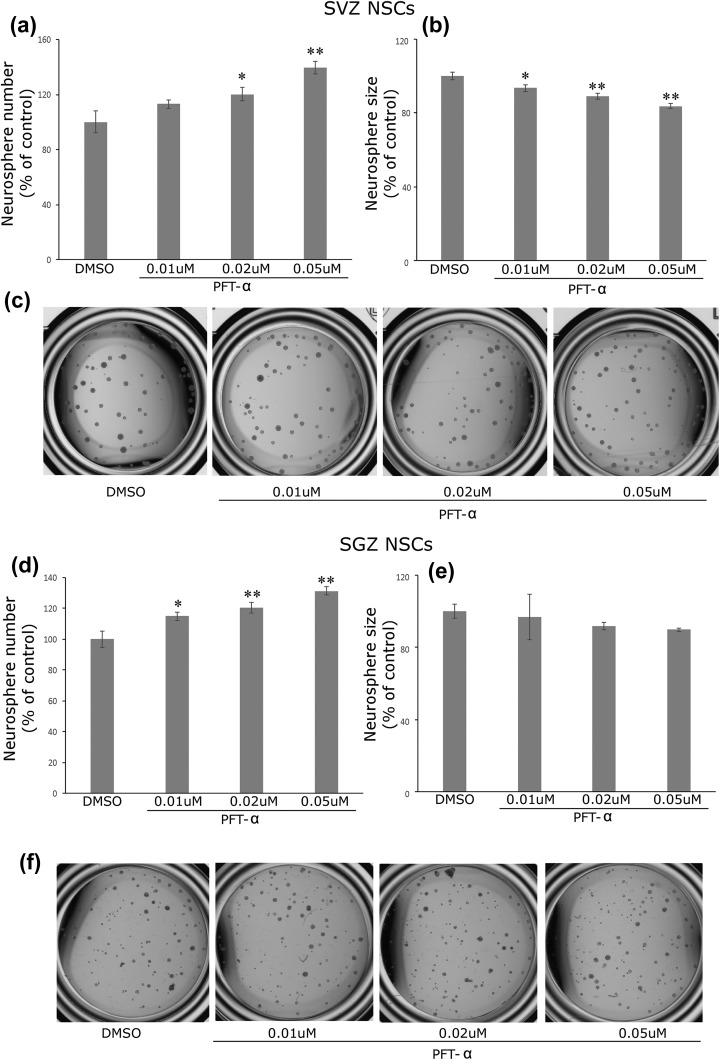
Our previous study demonstrated that PFT-α increases the self-renewal and proliferation of embryonic NSCs in vitro5. In this study, we examined whether PFT-α or posiphen treatment has similar effects on adult SVZ NSCs. SVZ neurosphere cultures were hence established from adult mice (4–5 weeks old) and passaged in the presence of EGF and bFGF. Drug treatment was initiated at the time of passaging and lasted for 7 days until the number and size of neurospheres were analyzed. Our data shows that PFT-α (0.01–0.05 μM) treatment dose-dependently increased the total number of neurospheres formed, suggesting that PFT-α increases the self-renewal property of the SVZ NSCs (p < 0.05, ANOVA, statistical analysis results shown in Figure 1(a), n = 6–8 wells for each condition). This result is consistent with our previously observed effects of PFT-α in embryonic NSC cultures5. Interestingly, in adult SVZ neurosphere cultures, PFT-α decreased the size (i.e. diameter of neurospheres) of SVZ spheres (p < 0.05, ANOVA, statistical analysis results shown in Figure 1(b)); a result that is opposite from the action of PFT-α on embryonic SVZ cultures. This suggests that whereas PFT-α augments the self-renewal of SVZ NSCs it decreases the proliferation of its progeny in adult SVZ NSCs. In accord with this, PFT-α had similar effects on adult hippocampal SGZ NSC neurosphere number (p < 0.05, ANOVA, Figure 1(d)) but did not alter neurosphere size in SGZ cultures (Figure 1(e)). This suggests that p53 may play a similar role in the regulation of self-renewal in both SVZ and SGZ NSCs, but might play differential roles regarding the expansion of these two population of NSCs in adulthood.
Figure 1.
PFT-α increases the number of adult SVZ neurospheres (a) and decreases the size of individual neurosphere (b). (c) representative image of neurospheres under different treatment condition. (d) PFT-α increases the number of SGZ neurospheres (d). There is no difference in the neurosphere size for the concentration of PFT-α tested. Mean+ SEM. *, p < 0.05 and **, p < 0.01 compared with control, ANOVA. N = 6–8 wells each condition. ANOVA: analysis of variance; DMSO: dimethyl sulfoxide; NSC: neural stem cell; PFT-α: pifithrin-α; SEM: standard error of the mean; SGZ: subgranular zone; SVZ: subventricular zone.
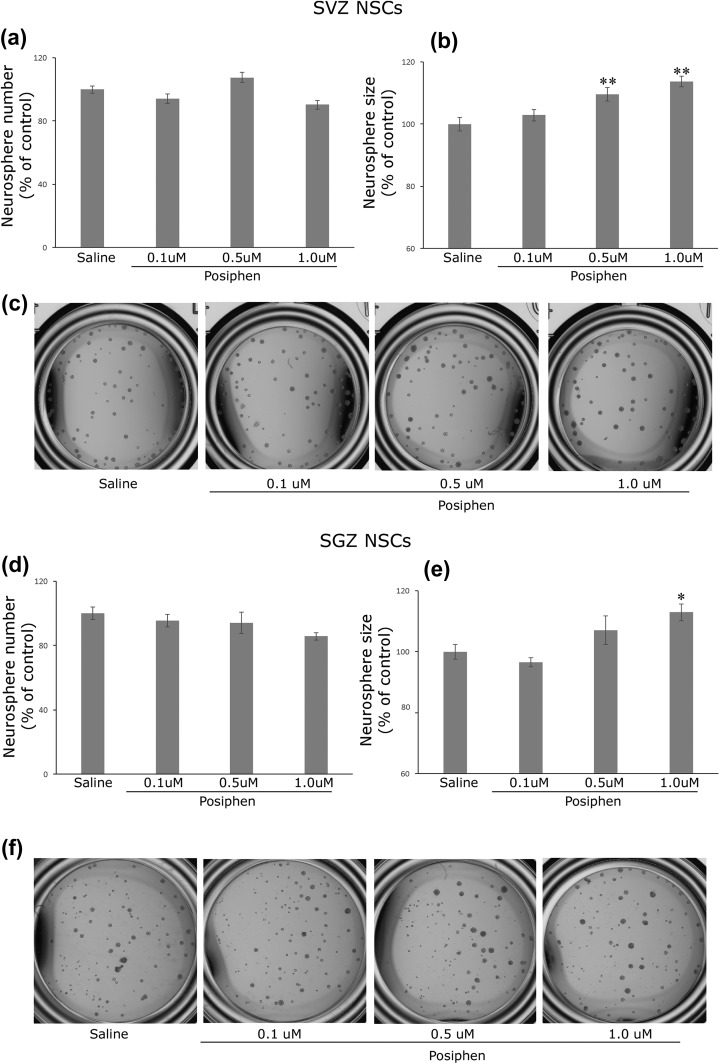
Posiphen (0.1–1.0 μM) treatment induced no difference in SVZ neural sphere number (Figure 2(a)) but increased the average size of individual neurospheres (p < 0.05, ANOVA, Figure 2(b)), a result that is consistent with our previous finding in embryonic SVZ NSCs cultures23. This suggests that posiphen does not affect the self-renewal (stemness) of the NSCs but increases the proliferation rate of progeny cells (the activated NSCs and transient proliferating cells). Consistently, posiphen demonstrated similar effects on adult hippocampal SGZ NSCs (Figure 2(d) and Figure 2(e) (p < 0.05, ANOVA)), suggesting that posiphen enhances the proliferation rate of each neurosphere colony without changing the number of cells that maintained the stemness in both adult SVZ and SGZ NSCs.
Figure 2.
Posiphen has no effect on the number of adult SVZ neurospheres (a) or SGZ neurospheres (d) and increases the size of individual neurosphere originated from SVZ (b) and SGZ (e). (c) and (f) representative image of SVZ or SGZ neurospheres under different treatment condition. (d) PFT-α increases the number of SGZ neurospheres (d). There is no difference in the neurosphere size for the concentration of PFT-α tested. Mean + SEM. *, p < 0.05 and **, p < 0.01 compared with control, ANOVA. N = 6–8 wells each condition. ANOVA: analysis of variance; NSC: neural stem cell; PFT-α: pifithrin-α; SEM: standard error of the mean; SGZ: subgranular zone; SVZ: subventricular zone.
PFT-α or Posiphen Increases MAP2 Positive Cells in a NSC Differentiation Assay in Culture
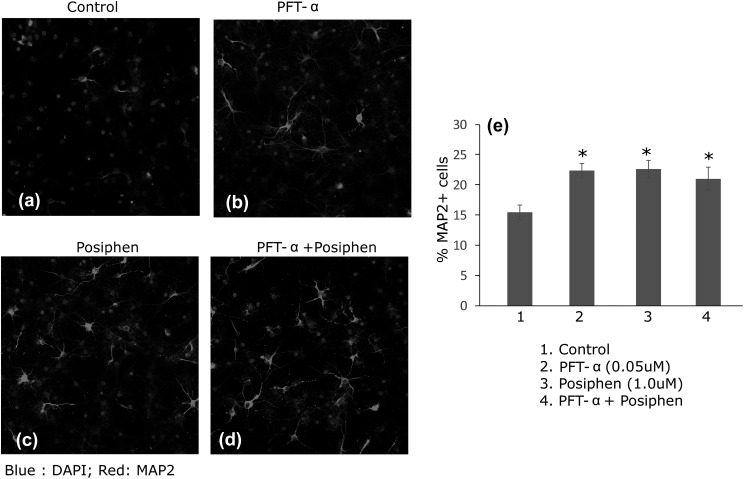
To evaluate the effects of combined sequential treatment of PFT-α and posiphen on the proliferation and differentiation/survival of NSCs, we utilized the sequential proliferation/differentiation protocol, as previously described35. This proliferation/differentiation protocol utilizes an attached monolayer of NSCs to allow the evaluation of sequential proliferation and differentiation/survival of NSCs, and it additionally enables the sequential treatment of NSCs with different agents. Specifically, NSCs were treated with either PFT-α (DIV 1–5), posiphen (DIV 6–12) or combined treatment (PFT-α DIV 1–5 followed by posiphen (DIV 6–12). Our data (Figure 3) showed that either PFT-α (Figure 3(b)) or posiphen (Figure 3(c)) treatment alone increased the total percentage of MAP2 positive cells per image field (Figure 3(e), p < 0.05, ANOVA). Combined treatment (Figure 3(d)) was not able to further increase the percentage of MAP2-positive cells derived from adult SVZ NSCs (Figure 3(e), p = 0.579 PFT-α versus combined and p = 0.784 posiphen versus combined, ANOVA).
Figure 3.
Compared with control (saline) (a), PFT-α (b) or posiphen (c) increases the total number of MAP2+ neuronal cells following NSC differentiation. Sequential and combined PFT-α and posiphen treatment (d) does not further enhance the effect of PFT-α or posiphen single treatment. Quantification of the percentage of MAP2+ cells is shown in panel (e). Mean+SEM. *, p < 0.05 compared with control, ANOVA. No significant difference among groups 2, 3 and 4. ANOVA: analysis of variance; DAPI: 4’,6-diamidino-2-phenylindole; MAP2: microtubule associated protein 2; NSC: neural stem cell; PFT-α: pifithrin-α; SEM: standard error of the mean.
Combined PFT-α and Posiphen Treatment Increases Surviving SVZ and SGZ NSCs Cells in Stroke Mice and Decreases the Atrophy Rate at 4 Weeks After Stroke
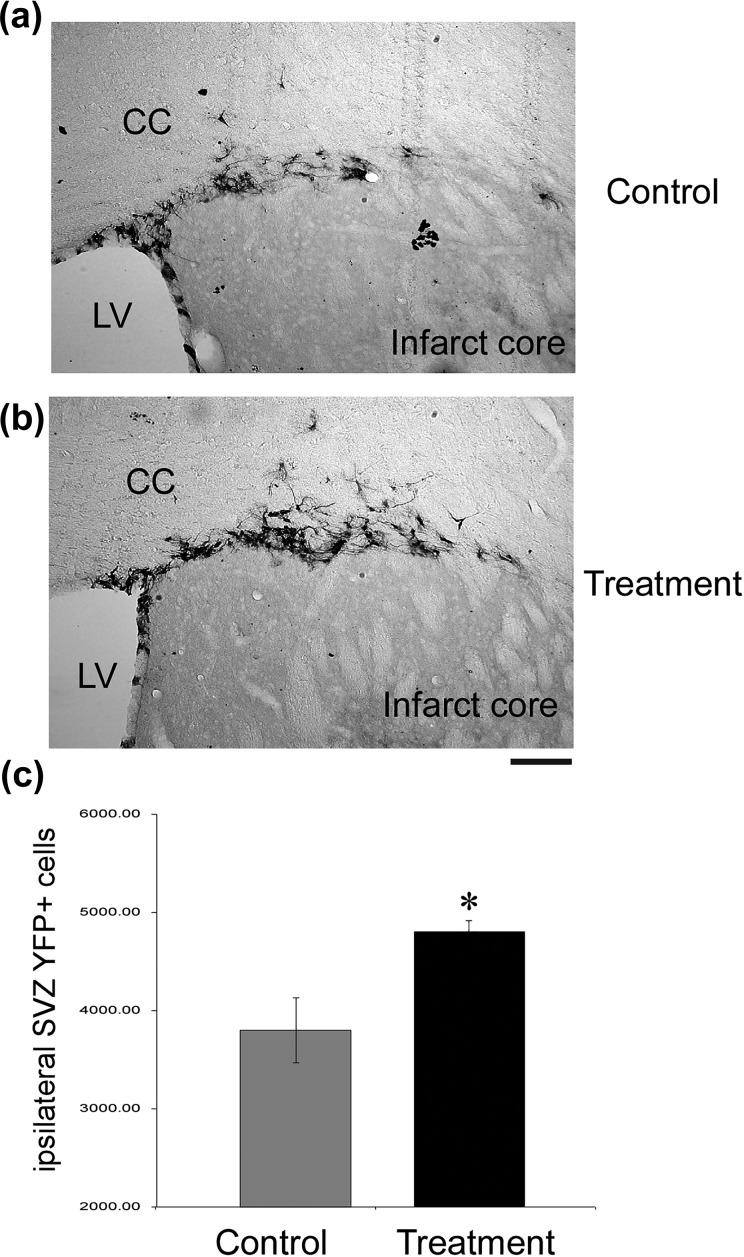
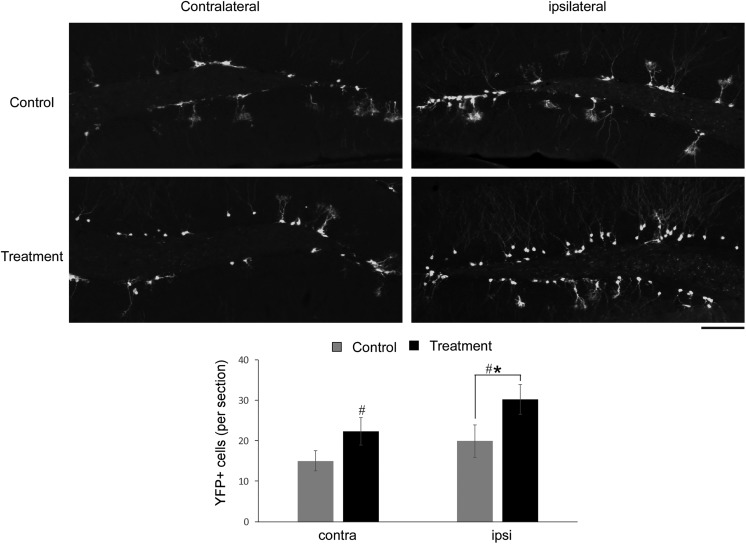
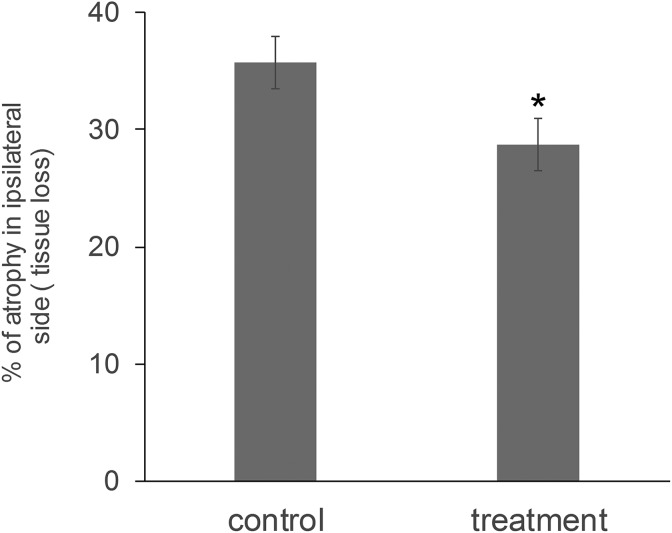
To examine the effect of combined PFT- α and posiphen treatment on SVZ and SGZ NSCs within the brain of stroke animals, we utilized nestin creERT2-YFP mice as they can be used to label, track, and phenotype stem cells and their progeny in the adult SVZ and SGZ36 (see Figure 4 for experimental timeline). The use of these mice allowed the specific labeling of NSCs within their brain 10 days prior to the induction of stroke and the analyses of the number of NSCs and their progeny (YFP+ cells) within the area adjacent to the striatum infarct core and the hippocampus at 4 weeks post-stroke in vehicle as well as PFT-α and posiphen combined treatment groups. We and others have shown that administration of TAM 10 days before MCAo provides specific labeling of SVZ and SGZ NSCs5,31,37. As demonstrated in Figure 5, the total number of YFP+ cells within the area adjoining the SVZ, adjacent to the ischemic striatal area, proved to be elevated in the combined PFT-α and posiphen treated stroke mouse group, as compared with the vehicle-treated control group (Figure 5, p < 0.05, Student’s t test, n = 7). In contrast, the contralateral side had no or minimal elevation or migration of SVZ YFP+ cells, similar to previous reports (data not shown)5,31,37. For the hippocampal NSCs, at 4 weeks post-stroke we observed an increased number of YFP+ cells on the ipsilateral side of the dentate gyrus in the combined PFT-α and posiphen treatment group as compared with the vehicle group (Figure 6, two-way ANOVA, treatment versus vehicle: F1, 29 = 6.445, p = 0.017, post-hoc analysis p = 0.045 for ipsilateral side, n = 7 for each group); suggesting an enhanced proliferation or survival of newly generated cells within the hippocampus consequent to combined PFT-α and posiphen treatment. Because enhanced neurogenesis after stroke has been reported previously to lead to decreased atrophy rate in the ipsilateral brain26,36,37, we also examined the degree of atrophy in vehicle or combined treatment post-stroke brain at 4 weeks. Our results demonstrate (Figure 7) that, consistent with our previous studies, combined sequential treatment in post-stroke animals, indeed, led to decreased atrophy in the stroke hemisphere; potentially due to the enhanced neurogenesis. Although magnetic resonance imaging scanning was not carried out to determine the extent of the acute infarct in this study, due to the fact that behavioral screening demonstrated that the two groups had similar behavioral deficits at post-stroke day 3 and the treatment was not initiated until post-stroke day 5 when acute neuronal apoptosis is no longer active3, the difference in brain atrophy evaluated at 4 weeks post-stroke is not likely due to differences in initial infarct area but, rather, derives from altered neurogenesis or neurorepair.
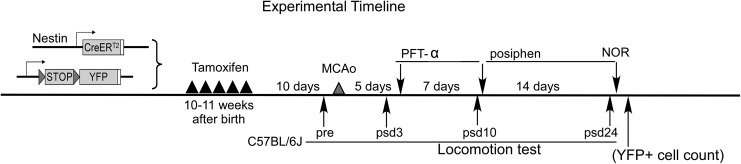
Figure 4.
Experimental timeline. To label endogenous NSCs, nestin creERT2-YFP mice received five TAM treatments to label SVZ and SGZ NSCs 10 days before stroke surgery. For the behavioral test, C57bl/6j mice were used without TAM treatment. All animals were subjected to pre and post-stroke day 3 locomotion tests to ensure that two equal groups of animals were randomly assigned to either control group or treatment group. At post-stroke day 5 treatment group received PFT-α treatment for 7 days and posiphen treatment for 14 days. Control animals receive 10% DMSO in saline for 7 days and saline only for 14 days. Animals were subjected to locomotion tests and NOR tests on the indicated days. Brains were harvested at 4 weeks post-stroke to quantify YFP+ cells. DMSO: dimethyl sulfoxide; MCAo: middle cerebral artery occlusion; NOR: novel object recognition; NSC: neural stem cell; PFT-α: pifithrin-α; SGZ: subgranular zone; SVZ: subventricular zone; TAM: tamoxifen; YFP: yellow fluorescent protein.
Figure 5.
Post-stroke combined treatment with PFT-α and posiphen increased the total migrating number of YFP+ cells adjacent to the infarct core at 4 weeks following MCAo, as compared with the vehicle-treated control group. The total YFP+ cells were calculated using multiple sections following stereological principles described in the Methods Section. Mean+SEM. * indicates p < 0.05, Student’s t test; n = 7 per group. Scale bar = 100 µm. MCAo: middle cerebral artery occlusion; PFT-α: pifithrin-α; SEM: standard error of the mean; YFP: yellow fluorescent protein.
Figure 6.
Post-stroke combined treatment with PFT-α and posiphen increased the total number of YFP+ cells within the ipsilateral side of the hippocampus at 4 weeks following MCAo, as compared with the vehicle-treated control group. Total YFP+ cells were calculated using 3–5 sections per animal and are shown as average total cells per section. Mean+SEM. #indicates p < 0.05, control versus treatment (combined PFT-α and posiphen), two-way ANOVA. *indicates p < 0.05, Student–Newman–Keuls post-hoc analysis; n = 7 per group. ANOVA: analysis of variance; MCAo: middle cerebral artery occlusion; PFT-α: pifithrin-α; SEM: standard error of the mean; YFP: yellow fluorescent protein.
Figure 7.
Post-stroke combined treatment with PFT-α and posiphen resulted in decreased brain atrophy in the stroke cerebral hemisphere at 4 weeks. Mean+SEM. * indicates p < 0.05, Student’s t test; n = 12–13 per group. PFT-α: pifithrin-α; SEM: standard error of the mean.
Delayed PFT-α and Posiphen Combined Treatment Increased the Locomotor Activity in Post-Stroke Mice
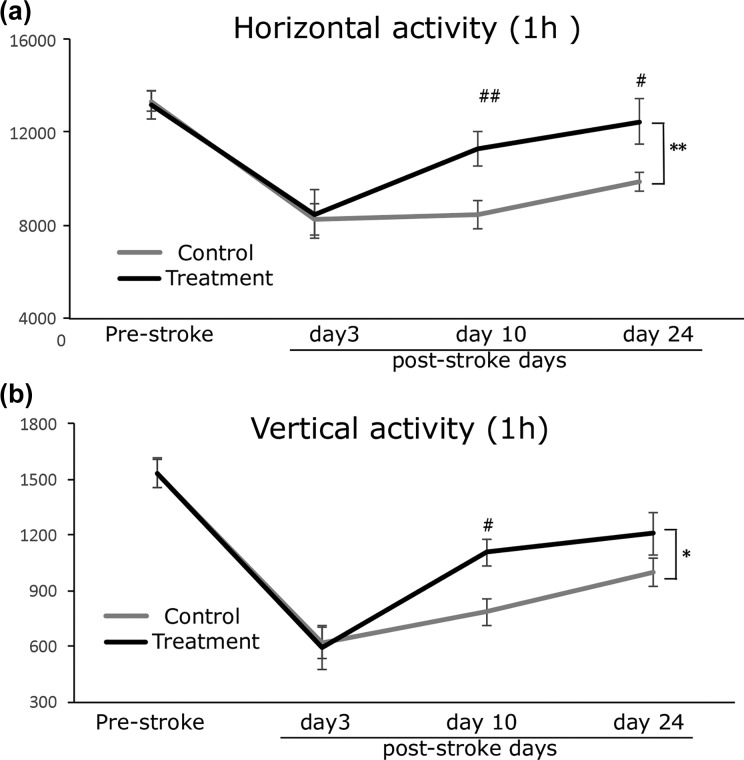
As PFT-α increased the self-renewal of NSCs and posiphen increased the proliferation and expansion of clonal NSCs in our cellular studies, we hypothesized that sequential and combined treatment with PFT-α and posiphen might lead to sustained enhancement of activation and proliferation of NSCs in vivo, which, in turn, might have a positive impact on post-stroke functional recovery. To test this hypothesis, we treated post-stroke animals with PFT-α for 7 days at 2 mg/kg/day, a dose that proved effective in rats in our previous studies23, starting from post-stroke day 5. Then, after 7 days of PFT-α treatment, animals received 14 days of posiphen treatment at 25 mg/kg/day (see Figure 4 for experimental timeline). This posiphen dose is equivalent to a 120 mg daily in a 60-kg human, following normalization of body surface area between mice and humans in accord with US FDA guidelines27, and proved well tolerated in humans when split across 24 h21 (as was achieved in the present study by administration via osmotic pump). Our data showed that the two experimental groups (control and treatment (PFT-α+posiphen) group) had no difference in their baseline locomotion activity, as demonstrated by the same horizontal and vertical activity scores in the pre-stroke evaluation test. At 3 days after stroke, both groups showed a similar significant decrease in both horizontal and vertical activity scores, which reflected the influence of MCAo in their locomotion function (Figure 8(a) and (b)). This acute post-stroke day 3 locomotion activity evaluation showed no difference between the two groups of mice, confirming the occurrence of similar stroke defects between the two groups before treatment initiation. After treatment, mice that received post-stroke PFT-α and posiphen treatment (n = 17) showed enhanced recovery in motor function compared with vehicle-treated mice (n = 17), demonstrated by significant increases in both their horizontal activity and vertical activity scores (HACTV; Figure 8(a), two-way ANOVA for repeated measurements, treatment versus vehicle: F1,135 = 7.547, p = 0.007), vertical activity (VACT; Figure 8(b), treatment versus vehicle: F1, 135 = 4.088, p = 0.045). Post-hoc pair wise comparison revealed that combined PFT-α and posiphen treatment administered post-stroke demonstrated a significant enhancement in both horizontal activity and VACT on post-stroke day 10, (Figure 8, Bonferri post-hoc analysis, ANOVA, p = 0.007 of horizontal activity and p = 0.013 for VACT). The effects of this combined treatment plateaued at 24 days after stroke, providing scores that remained significantly improved compared with vehicle-treated mice in horizontal activity (p = 0.012) but no longer in VACT (p = 0.106); an effect that we have observed in a previous study using a sonic hedgehog agonist, suggesting that spontaneous recovery in VACT might reach a ceiling during later time points after stroke. To determine whether combined sequential treatment of PFT-α and posiphen is more potent than PFT-α treatment alone in relation to locomotion behavioral improvement, we compared the percentage of improvement in both HACTV and VACT in this study to results published in our previous study using PFT-α only treatment3. As shown in Table 1, combined PFT-α and posiphen treatment does not lead to significant further improvement of the locomotor behavioral function as compared with PFT-α treatment alone.
Figure 8.
Post-stroke combined PFT-α and posiphen treatment enhanced functional recovery in locomotor function in stroke mice (a). Total horizontal activity and (b) total vertical activity over a 60-min duration measured by automatic open field locomotion chambers at different time points in vehicle or treatment groups. Mean+SEM. *or ** p < 0.05 or p < 0.01, two-way ANOVA vehicle versus treatment. # or ##, p < 0.05 or p < 0.01, post-hoc Bonferroni test vehicle versus treatment for each time point (n = 17 for each group). ANOVA: analysis of variance; PFT-α: pifithrin-α; SEM: standard error of the mean.
Table 1.
Comparison of the single and combined treatment.
| PFT-α only (a) | PFT-α+posiphen (b) | *p-value | |||
|---|---|---|---|---|---|
| % of vehicle | SEM | % of vehicle | SEM | (a) versus (b) | |
| HACTV | 119.4 | 5.4 | 126.5 | 9.8 | 0.54 |
| VACT | 118.3 | 8 | 120.7 | 11.6 | 0.86 |
HACTV: horizontal activity, total number of beam interruptions that occurred in the horizontal sensor during a given sample period; PFT-α: pifithrin-α; VACT: vertical activity, total number of beam interruptions that occurred in the vertical sensor during a given sample period; SEM: standard error of the mean.
Data presented as % of vehicle group at 4 weeks post-stroke.
*Student’s t test.
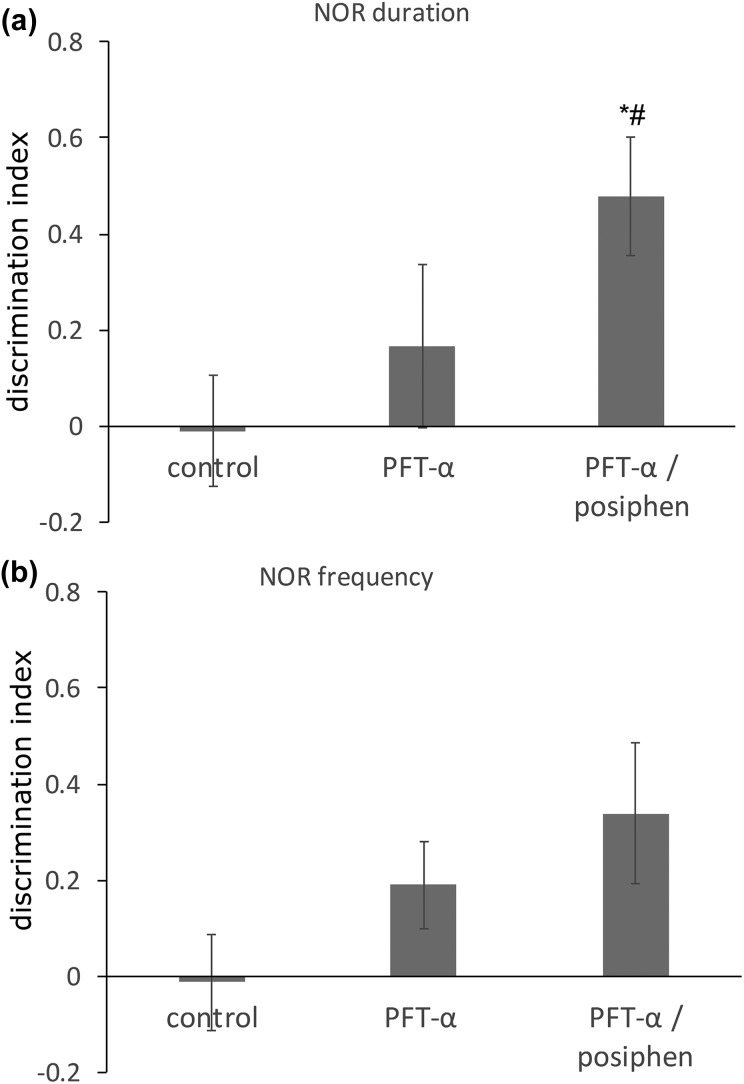
In the light of our in vitro neurosphere analysis demonstrating a PFT-α and posiphen-induced enhancement of self-renewal and proliferation in hippocampal SGZ NSCs cells in addition to SVZ NSCs, we evaluated cognitive function in post-stroke mice from our study. Specifically, stroke-induced functional deficits in learning and memory were evaluated in vehicle, PFT-α only and combined treatment groups at 4 weeks post-MCAo by NOR assay. Previous studies have shown that stroke induces deficits in NOR tasks in mice38,39. In accord with this, as demonstrated in Figure 9(a) and (b), mice administered vehicle lacked the ability to discriminate between the novel versus original objects at 4 weeks after stroke, whereas those that received the combined PFT-α and posiphen treatment showed a higher preference to the novel object, in relation to both the frequency of visits to and time spent at the novel object (Figure 9(a) and 9(b), p < 0.05 and p = 0.06, ANOVA, n = 9 for each group), as compared with vehicle-treated mice. In addition, the combined treatment group showed a significantly higher discrimination index when compared with the PFT-α only group in relation to time spent at the novel object (Figure 9(a), p < 0.05). Together, these data indicate that combined PFT-α and posiphen treatment enhanced cognitive function in stroke mice and proved to be more potent than PFT-α treatment alone in this paradigm.
Figure 9.
Post-stroke combined PFT-α and posiphen treatment enhanced cognitive function in stroke mice. The discrimination index for (a) NOR time spent and (b) NOR frequency of visits showed that the combined treatment group possessed improved cognitive function in this paradigm, as compared with either the control group or PFT-α only group. Mean+SEM *p < 0.05 control versus combined treatment group, # < 0.05 PFT-α only group versus combined treatment group. (n = 7–9 each group). NOR: novel object recognition; PFT-α: pifithrin-α; SEM: standard error of the mean.
Discussion
The effective time-dependent treatment window (4.5 h duration) of tissue plasminogen activator (tPA), the only US FDA-approved stroke therapy, limits its application to a relatively small percentage of patients, thereby rendering the development of a successful delayed treatment for stroke an urgent need in the light of the 6.2 million deaths that occur worldwide annually. Previous work by our group3–5 and others1 has demonstrated that optimizing the endogenous neurogenesis process that is triggered following a stroke represents a promising novel therapeutic strategy.
Adult hippocampal neurogenesis is believed to perform a key role in memory function and mood, with the development and integration of adult-born neurons following a progression of morphological and physiological events that proceed over several weeks duration40–43. The conventional view is that adult neurogenesis recapitulates the processes involved in neuronal development during embryogenesis.44 During development, the central nervous system undergoes massive waves of neuronal expansion accompanied by neuronal death that are necessary for cellular selection and specification; as only a very small fraction of newly formed neurons receive the required trophic support and are selected for survival in an activity-dependent manner45. Developmental apoptosis is hence fundamental to the creation of the correct neuronal connections and, in large part, is underpinned by p53 and its downstream signaling cascade, that provide a crucial convergent checkpoint to selectively eradicate newborn neurons that are not selected for differentiation and integration into the preexisting neuronal network. p53 is hence highly expressed in proliferating and early differentiating NPCs within both the embryonic and adult rodent brain12,46. Indeed, within the adult SVZ, p53 deletion increases the proliferation and survival of NSCs, and within the embryonic olfactory bulb an absence of p53 is associated with an elevation in the proliferation rate and neuronal differentiation of neurospheres12,45,46.
Adult hippocampal neurogenesis has been observed across vertebrate species47, with its basal rate generating a significant proportion of adult-generated functional neurons48,49. This rate is elevated by voluntary exercise, an enriched environment, as well pathological conditions such as ischemia and seizures41,50,51. In pathological conditions, such as ischemia and hypoxia, the microenvironment of the brain is very different from the healthy state, in which levels of key proteins with the capacity to impact NSC proliferation and differentiation are upregulated and can lead to compromised neurogenesis, as would be predicted under conditions that upregulate p53. We previously demonstrated that the administration of PFT-α (2 mg/kg) starting at 5 days after stroke is able to enhance the proliferation and migration of NSCs, and inhibit the apoptosis of newly born cells, resulting in improved behavioral recovery3. Our current studies both validate and extend this past research defining the role of p53 inactivation on NSCs in both the SVZ and SGZ niches.
A notable protein that is dramatically upregulated by ischemia, hypoxia and neuronal injury is APP14,15,52,53 that is best known as the parent molecule of the hallmark Alzheimer’s disease (AD) neurotoxin, amyloid-β peptide (Aβ) that accumulates within the brains of patients with AD and related disorders54. Although much has been elucidated regarding the proteolytic processing of APP in the generation of Aβ, the function of APP has yet to be fully clarified. APP is an integral membrane protein that is expressed in cells from many tissues, but is found in particularly high levels within neurons concentrated at synapses. A role for APP has been linked to synapse formation, neural plasticity, iron transport, and the differentiation of NSCs to generate cells of either neuronal or glial lineage16,54–56. APP is considered an acute response protein, because of rapid changes in its expression following neuronal stress, whether induced by neuronal silencing, oxidative stress, inflammation or elevated iron levels57.
The biological relevance of such APP upregulation remains to be fully elucidated. Acutely, elevated APP levels may be detrimental to the ischemic brain, as APP overexpressing animals have larger infarct areas than their wild-type littermates58,59, and the anti-apoptotic, APP lowering drug (-)-phenserine reduces stroke volume and improves behavioral outcome measures22. More chronically, abnormally high levels of APP brain expression can impair neurogenesis60; leading to eventual glial, rather than neuronal cell differentiation of NSCs16,54–56. Additionally, neurotoxic Aβ peptide deriving from APP amyloidogenic processing has been found to accumulate in vulnerable neurons in the post-ischemic hippocampus61, in accord with the finding that patients with stroke and cerebral infarction are at high risk for AD62,63.
The experimental AD drugs posiphen as well as (-)-phenserine interact via the 5’-untranslated region of APP mRNA to inhibit ribosomal access and block APP translation19,20,57. In neuronal cultures and the brain of wild-type and AD transgenic mice, Posiphen and (-)-phenserine have been demonstrated to lower APP and Aβ levels16,20 in a dose-dependent fashion and thus represent interesting candidate drugs to reduce APP, as well as toxic products deriving from it, in stroke. Posiphen has proven to be well tolerated orally in phase I clinical trials in healthy human volunteers, allowing acute dose escalation to 160 mg and demonstrating a no observed adverse effect level of 80 mg21. In a proof-of-mechanism study in mild cognitive impairment subjects, a 4×60 mg daily (i.e. a total of 120 mg) posiphen dose lowered cerebrospinal fluid levels of APP by up to 59.9% without toxicity and, additionally, significantly lowered cerebrospinal fluid levels of tau and inflammatory markers21. In light of previous studies suggesting that elevated levels of p53 and APP occur in and are detrimental to ischemic brain, may impact NSC survival and induce the differentiation of endogenous NPCs away from a neuronal phenotype, we combined approaches to lower p53 activation and APP levels in stoke as a therapeutic strategy.
Our study demonstrated enhanced functional recovery in stroke animals treated with sequential PFT-α and posiphen. Specifically, delayed treatment of PFT-α followed by posiphen significantly improved locomotion function as well as cognitive function in memory and learning at 1 month after stroke (Figures 8 and 9). In this study, we first examined the effects of PFT-α and posiphen on neurosphere formation from adult mouse SVZ and SGZ. There are multiple cell types that reside in adult neurogenic niches. For example, self-renewing NPCs in the SVZ are glial fibrillary acidic protein and nestin positive, slowly dividing astrocytes, which are also called type B cells. Type B cells proliferate and produce transit-amplifying progenitor cells, which are also called type C cells. Type C cells proliferate rapidly and differentiate into neuroblasts (type A cells). Type A neuroblasts then migrate into target regions and terminally differentiate into various cell types47. Our data suggest that in adult SVZ neurosphere cultures, PFT-α treatment increases the total number of neurosphere formations; however, it also decreases the size of the neurosphere. This suggests that PFT-α may maintain the self-renewal property of adult SVZ NSCs (type B cells) at the expense of NSC expansion (type C and type A cells). Interestingly, posiphen treatment proved able to increase the size of neurospheres without changing the total number of neurospheres, suggesting that APP might be involved in the regulation of expansion of type B and type A cells. Stroke has been shown to be able to activate type B cells, which is followed by the expansion of type C and type A cells. Given the temporal sequence of the cellular events, we therefore evaluated whether sequential and combined treatment with PFT-α and the APP inhibitor posiphen could increase MAP2+ neuronal cells following differentiation in vitro and the number of newly generated NSCs cells within the SVZ and SGZ in vivo. Our results showed that both PFT-α and posiphen treatment increased the percentage of MAP2+ neuronal cells following differentiation in vitro and there appeared to be a ceiling effect for the improved neuronal differentiation in vitro (Figure 3). Furthermore, our in vivo results confirmed that combined sequential treatment, indeed, increased the total number of YFP+ cells (NSCs and their progeny) at 4 weeks after stroke, within both the striatum adjacent to the SVZ and SGZ of the hippocampus (Figures 6 and 7). We also compared the efficacy of combined sequential treatment to PFT-α only treatment, comparison of both locomotion function and cognitive function showed that combined treatment more potently enhanced cognitive function but had similar actions on locomotion function, as compared with single PFT-α treatment. This suggests that PFT-α treatment alone may have reached a ceiling in relation to locomotion functional improvement. However, additional posiphen treatment can, importantly, further enhance cognitive functional recovery in stroke animals.
Notable in our in vivo study, the posiphen dose used (25 mg/kg daily) is of clinical relevance; equating to an equivalent posiphen dose of 120 mg daily in a 60-kg human (following normalization of body surface area between mice and humans, in accord with US FDA guidelines58,64. Moreover, human brain posiphen levels were predicted to be in the order of 3.5 μM following a well-tolerated 60 mg dose,21 that is in line with concentrations of posiphen used in our cellular studies. Whereas PFT-α and p53 inactivating agents on the same backbone8,64,65 have yet to be approved for clinical use, agents that mitigate neuronal apoptosis and impact p53 and/or its downstream targets, potentially (-)-phenserine and exendin-4,22,66–68 may provide such actions and, thereby support the clinical translation of a combined sequential inhibition of p53 and APP approach to augment and optimize neurogenesis following neuronal injury in humans. In addition, although extensive manipulation of stem cells has the conceivable risk of leading to tumor formation in mice, particularly if undertaken chronically, we did not observe any tumor formation in combination treatment mice over the duration of our study. Furthermore, the fact that there appears to be a ceiling effect of neurosphere proliferation/differentiation in vitro and the potential ceiling effect that was evident for locomotion functional recovery in vivo, suggest that dual therapy is safe in mice; particularly in the light of our short-term, transient treatment schedule. With the more potent efficacy in cognitive function recovery demonstrated by the combined treatment, further work is warranted to safely maximize endogenous neurogenesis as a new and much needed treatment strategy for stroke, especially in relation to optimizing cognition.
Authors’ Note
Paul Kim and Austin Barnett contributed equally to this manuscript.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human. All animal protocols were conducted under National Institutes Health (NIH) Guidelines using the NIH handbook Animals in Research and were approved by the Institutional Animal Care and Use Committee (Case Western Reserve University).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: Nigel H. Greig is an inventor on the original patent relating to posiphen, but has assigned all rights to the National Institutes of Health and US Government. All other authors indicate no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the NIH Grant (R01NS091213), and by the Intramural Research Program of the National Institute on Aging, NIH, USA. Flavia Turcato was supported by a scholarship from National Council for Scientific and Technological Development (CNPq), Brazil.
References
- 1. Luo Y. Cell-based therapy for stroke. J Neural Transm (Vienna). 2011;118(1):61–74. [DOI] [PubMed] [Google Scholar]
- 2. Zhang R, Zhang Z, Chopp M. Function of neural stem cells in ischemic brain repair processes. J Cereb Blood Flow Metab. 2016;36(12):2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, Wang Y. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol. 2009;65(5):520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo Y, Shen H, Liu HS, Yu SJ, Reiner DJ, Harvey BK, Hoffer BJ, Yang Y, Wang Y. CART peptide induces neuroregeneration in stroke rats. J Cereb Blood Flow Metab. 2013;33(2):300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jin Y, Barnett A, Zhang Y, Yu X, Luo Y. Poststroke sonic hedgehog agonist treatment improves functional recovery by enhancing neurogenesis and angiogenesis. Stroke. 2017;48(6):1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50(5):602–611. [DOI] [PubMed] [Google Scholar]
- 7. Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA, 2002;99(18):11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu X, Yu QS, Cutler RG, Culmsee CW, Holloway HW, Lahiri DK, Mattson MP, Greig NH. Novel p53 inactivators with neuroprotective action: syntheses and pharmacological evaluation of 2-imino-2,3,4,5,6,7-hexahydrobenzothiazole and 2-imino-2,3,4,5,6,7-hexahydrobenzoxazole derivatives. J Med Chem. 2002;45(23):5090–5097. [DOI] [PubMed] [Google Scholar]
- 9. Lei XH, Zhao D, Li YL, Li XF, Sun X, Du WZ, Sun Y, Hao ZF, Xin SY, Liu C, Zhang ZR, Jiang CL. Pifithrin-alpha enhances the survival of transplanted neural stem cells in stroke rats by inhibiting p53 nuclear translocation. CNS Neurosci Ther. 2013;19(2):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hede SM, Nazarenko I, Nistér M, Lindström MS. Novel perspectives on p53 function in neural stem cells and brain tumors. J Oncol. 2011;2011:852970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7(2):165–171. [DOI] [PubMed] [Google Scholar]
- 12. Meletis K, Wirta V, Hede SM, Nistér M, Lundeberg J, Frisén J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133(2):363–369. [DOI] [PubMed] [Google Scholar]
- 13. Medrano S, Burns-Cusato M, Atienza MB, Rahimi D, Scrable H. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiol Aging. 2009;30(3):483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stephenson DT, Rash K, Clemens JA. Amyloid precursor protein accumulates in regions of neurodegeneration following focal cerebral ischemia in the rat. Brain Res. 1992;593(1):128–135. [DOI] [PubMed] [Google Scholar]
- 15. Banati RB, Gehrmann J, Wiessner C, Hossmann KA, Kreutzberg GW. Glial expression of the beta-amyloid precursor protein (APP) in global ischemia. J Cereb Blood Flow Metab. 1995;15(4):647–654. [DOI] [PubMed] [Google Scholar]
- 16. Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, Sugaya K. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc Natl Acad Sci USA. 2007;104(30):12506–12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sugaya K, Kwak YD, Ohmitsu O, Marutle A, Greig NH, Choumrina E. Practical issues in stem cell therapy for Alzheimer’s disease. Curr Alzheimer Res. 2007;4(4):370–377. [DOI] [PubMed] [Google Scholar]
- 18. Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Med. 2002;1(2):125–135. [DOI] [PubMed] [Google Scholar]
- 19. Shaw KT, Utsuki T, Rogers J, Yu QS, Sambamurti K, Brossi A, Ge YW, Lahiri DK, Greig NH. Phenserine regulates translation of beta -amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development. Proc Natl Acad Sci USA. 2001;98(13):7605–7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lahiri DK, Chen D, Maloney B, Holloway HW, Yu QS, Utsuki T, Giordano T, Sambamurti K, Greig NH. The experimental Alzheimer’s disease drug posiphen [(+)-phenserine] lowers amyloid-beta peptide levels in cell culture and mice. J Pharmacol Exp Ther. 2007;320(1):386–396. [DOI] [PubMed] [Google Scholar]
- 21. Maccecchini ML, Chang MY, Pan C, John V, Zetterberg H, Greig NH. Posiphen as a candidate drug to lower CSF amyloid precursor protein, amyloid-beta peptide and tau levels: target engagement, tolerability and pharmacokinetics in humans. J Neurol Neurosurg Psychiatry. 2012;83(9):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang CF, Lai JH, Wu JC, Greig NH, Becker RE, Luo Y, Chen YH, Kang SJ, Chiang YH, Chen KY. (-)-Phenserine inhibits neuronal apoptosis following ischemia/reperfusion injury. Brain Res. 2017;1677:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lilja AM, Luo Y, Yu QS, Röjdner J, Li Y, Marini AM, Marutle A, Nordberg A, Greig NH. Neurotrophic and neuroprotective actions of (-)- and (+)-phenserine, candidate drugs for Alzheimer’s disease. PLoS One. 2013;8(1):e54887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lilja AM, Röjdner J, Mustafiz T, Thomé CM, Storelli E, Gonzalez D, Unger-Lithner C, Greig NH, Nordberg A, Marutle A. Age-dependent neuroplasticity mechanisms in Alzheimer Tg2576 mice following modulation of brain amyloid-beta levels. PLoS One. 2013;8(3):e58752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He X, Lu Y, Lin X, Jiang L, Tang Y, Tang G, Chen X, Zhang Z, Wang Y, Yang GY. Optical inhibition of striatal neurons promotes focal neurogenesis and neurobehavioral recovery in mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2017;37(3):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pu H, Jiang X, Hu X1, Xia J, Hong D, Zhang W, Gao Y, Chen J, Shi Y. Delayed docosahexaenoic acid treatment combined with dietary supplementation of omega-3 fatty acids promotes long-term neurovascular restoration after ischemic stroke. Transl Stroke Res. 2016;7(6):521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. US Department of Health and Human Services, F.a.D.A., Center for Drug Evaluation and Research. Guidance for industry, estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers, 2005.
- 28. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. [DOI] [PubMed] [Google Scholar]
- 29. Palma-Tortosa S, García-Culebras A, Moraga A, Hurtado O, Perez-Ruiz A, Durán-Laforet V, Parra J, Cuartero MI, Pradillo JM, Moro MA, Lizasoain I. Specific Features of SVZ Neurogenesis After Cortical Ischemia: a Longitudinal Study. Sci Rep. 2017;7(1):16343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dempsey RJ, Sailor KA, Bowen KK, Türeyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87(3):586–597. [DOI] [PubMed] [Google Scholar]
- 31. Zhang RL, Chopp M, Roberts C, Liu X, Wei M, Nejad-Davarani SP, Wang X, Zhang ZG. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS One. 2014;9(12):e113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu QS, Pei XF, Holloway HW, Greig NH, Brossi A. Total syntheses and anticholinesterase activities of (3aS)-N(8)-norphysostigmine, (3aS)-N(8)-norphenserine, their antipodal isomers, and other N(8)-substituted analogues. J Med Chem. 1997;40(18):2895–2901. [DOI] [PubMed] [Google Scholar]
- 33. Jin Y, Raviv N, Barnett A, Bambakidis NC, Filichia E, Luo Y. The shh signaling pathway is upregulated in multiple cell types in cortical ischemia and influences the outcome of stroke in an animal model. PLoS One. 2015;10(4):e0124657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gabbita SP, Srivastava MK, Eslami P, Johnson MF, Kobritz NK, Tweedie D, Greig NH, Zemlan FP, Sharma SP, Harris-White ME. Early intervention with a small molecule inhibitor for tumor necrosis factor-alpha prevents cognitive deficits in a triple transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2012;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker TL, Kempermann G. Kempermann, One mouse, two cultures: isolation and culture of adult neural stem cells from the two neurogenic zones of individual mice. J Vis Exp. 2014;(84):e51225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, Yang GY, Chen Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke. 2008;39(4):1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loris ZB, Pieper AA, Dietrich WD. Dietrich, The neuroprotective compound P7C3-A20 promotes neurogenesis and improves cognitive function after ischemic stroke. Exp Neurol. 2017;290:63–73. [DOI] [PubMed] [Google Scholar]
- 38. Schmidt A, Diederich K, Strecker JK, Geng B, Hoppen M, Duning T, Schäbitz WR, Minnerup J. Progressive cognitive deficits in a mouse model of recurrent photothrombotic stroke. Stroke. 2015;46(4):1127–1131. [DOI] [PubMed] [Google Scholar]
- 39. Silva B, Sousa L, Miranda A, Vasconcelos A, Reis H, Barcelos L, Arantes R, Teixeira A, Rachid MA. Memory deficit associated with increased brain proinflammatory cytokine levels and neurodegeneration in acute ischemic stroke. Arq Neuropsiquiatr. 2015;73(8):655–659. [DOI] [PubMed] [Google Scholar]
- 40. Song J, Christian KM, Ming GL, Song H. Modification of hippocampal circuitry by adult neurogenesis. Dev Neurobiol. 2012;72(7):1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sah N, Peterson BD, Lubejko ST, Vivar C, van Praag H. Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci Rep. 2017;7(1):10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annu Rev Psychol. 2015;66:53–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329(3):409–420. [DOI] [PubMed] [Google Scholar]
- 44. Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25(44):10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quadrato G, Di Giovanni S. Gatekeeper between quiescence and differentiation: p53 in axonal outgrowth and neurogenesis. Int Rev Neurobiol. 2012;105:71–89. [DOI] [PubMed] [Google Scholar]
- 46. Armesilla-Diaz A, Bragado P, Del Valle I, Cuevas E, Lazaro I, Martin C, Cigudosa JC, Silva A. p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience, 2009;158(4):1378–1389. [DOI] [PubMed] [Google Scholar]
- 47. Bond AM, Ming GL, Song H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. 2015;17(4):385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–417. [DOI] [PubMed] [Google Scholar]
- 49. Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H. Running-induced systemic cathepsin b secretion is associated with memory function. Cell Metab. 2016;24(2):332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shioda N, Han F, Fukunaga K. Fukunaga, Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int Rev Neurobiol. 2009;85:375–387. [DOI] [PubMed] [Google Scholar]
- 52. Abe K, Tanzi RE, Kogure K. Selective induction of Kunitz-type protease inhibitor domain-containing amyloid precursor protein mRNA after persistent focal ischemia in rat cerebral cortex. Neurosci Lett. 1991;125(2):172–174. [DOI] [PubMed] [Google Scholar]
- 53. Webster NJ, Green KN, Peers C, Vaughan PF. Altered processing of amyloid precursor protein in the human neuroblastoma SH-SY5Y by chronic hypoxia. J Neurochem. 2002;83(6):1262–1271. [DOI] [PubMed] [Google Scholar]
- 54. Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2002;1(1):1–31. [DOI] [PubMed] [Google Scholar]
- 55. Aydin D, Weyer SW, Müller UC. Functions of the APP gene family in the nervous system: insights from mouse models. Exp Brain Res. 2012;217(3-4):423–434. [DOI] [PubMed] [Google Scholar]
- 56. Kögel D, Deller T, Behl C. Roles of amyloid precursor protein family members in neuroprotection, stress signaling and aging. Exp Brain Res. 2012;217(3-4):471–479. [DOI] [PubMed] [Google Scholar]
- 57. Rogers JT, Lahiri DK. Metal and inflammatory targets for Alzheimer’s disease. Curr Drug Targets. 2004;5(6):535–551. [DOI] [PubMed] [Google Scholar]
- 58. Zhang F, Eckman C, Younkin S, Hsiao KK, Iadecola C. Increased susceptibility to ischemic brain damage in transgenic mice overexpressing the amyloid precursor protein. J Neurosci. 1997;17(20):7655–7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu F, Previti ML, Van Nostrand WE. Van Nostrand, Increased severity of hemorrhage in transgenic mice expressing cerebral protease nexin-2/amyloid beta-protein precursor. Stroke. 2007;38(9):2598–2601. [DOI] [PubMed] [Google Scholar]
- 60. Acosta SA, Tajiri N, Sanberg PR, Kaneko Y, Borlongan CV. increased amyloid precursor protein and tau expression manifests as key secondary cell death in chronic traumatic brain injury. J Cell Physiol. 2017;232(3):665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yokota M, Saido TC, Tani E, Yamaura I, Minami N. Cytotoxic fragment of amyloid precursor protein accumulates in hippocampus after global forebrain ischemia. J Cereb Blood Flow Metab. 1996;16(6):1219–1223. [DOI] [PubMed] [Google Scholar]
- 62. Hachinski V. Stroke and Alzheimer disease: fellow travelers or partners in crime? Arch Neurol. 2011;68(6):797–798. [DOI] [PubMed] [Google Scholar]
- 63. Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. Int J Stroke. 2012;7(1):61–73. [DOI] [PubMed] [Google Scholar]
- 64. Yang LY, Chu YH, Tweedie D, Yu QS, Pick CG, Hoffer BJ, Greig NH, Wang JY. Post-trauma administration of the pifithrin-alpha oxygen analog improves histological and functional outcomes after experimental traumatic brain injury. Exp Neurol. 2015;269:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang LY, Greig NH, Huang YN, Hsieh TH, Tweedie D, Yu QS, Hoffer BJ, Luo Y, Kao YC, Wang JY. Post-traumatic administration of the p53 inactivator pifithrin-alpha oxygen analogue reduces hippocampal neuronal loss and improves cognitive deficits after experimental traumatic brain injury. Neurobiol Dis. 2016;96:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hoffer BJ, Pick CG, Hoffer ME, Becker RE, Chiang YH, Greig NH. Repositioning drugs for traumatic brain injury - N-acetyl cysteine and Phenserine. J Biomed Sci. 2017;24(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH. A new treatment strategy for parkinson’s disease through the gut-brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplant. 2017;26(9):1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166(5):1586–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]