Abstract
Gastric cancer (GC) is a malignancy with few effective treatment options after metastasis occurs. Quercetin (Qu) intake has been associated with reduced incidence and slow development of GC, probably due to its anti-proliferative and apoptotic effects, but it is unclear whether Qu can inhibit the metastatic activity. The urokinase plasminogen activator (uPA)/uPA receptor (uPAR) system plays an important role in cancer metastasis. In this study, we measured both uPA activity and uPAR expression in GC and pericarcinous tissues, and we investigated the correlation between uPAR expression and the migratory and invasive activities of various GC cell lines. GC BGC823 and AGS cells were subjected to treatment with 10 μM Qu for 72 hours and uPAR knockdown, alone or in combination, before evaluating cell metastasis. The results showed that uPA activity and uPAR expression were higher in GC tissues than in pericarcinous tissues. Migratory and invasive activities of GC cell lines positively correlated with uPAR expression. Qu treatment decreased BGC823 and AGS cell migration and invasion, accompanied by reduced uPA and uPAR protein expression. Both Qu treatment and uPAR knockdown decreased matrix metalloproteinase-2 and -9 activity and blocked Pak1-Limk1-cofilin signaling. Qu treatment was associated with inhibition of NF-κb, PKC-δ, and ERK1/2, and with AMPKα activation. Specific inhibitors of NF-κb, PKC, and ERK1/2, and an AMPKα activator suppressed uPA and uPAR expression in GC cells. Collectively, Qu showed an antimetastatic effect on GC cells via the interruption of uPA/uPAR function and modulation of NF-κb, PKC-δ, ERK1/2, and AMPKα. This suggests that Qu is a promising agent against GC metastasis.
Keywords: quercetin, gastric cancer, metastasis, urokinase plasminogen activator, uPA receptor
Introduction
Gastric cancer (GC) is a common malignancy of the digestive tract, with the second highest cancer mortality globally.1,2 Often, GC at earlier stages is asymptomatic, except for anorexia occurring in some of the cases, and therefore, by the time of diagnosis, it has generally developed to advanced stages. At this stage, cancer metastasis is the most characteristic phenomenon of GC.2 This is one of the major factors contributing to mortality, as neither surgical tumor resection nor chemotherapy or other treatment modalities are effective against GC metastasis, and a high proportion of the patients finally succumb due to complications caused by cancer metastasis.2 Thus, cancer metastasis remains a major obstacle in GC management, and the discovery of agents or methods that can effectively inhibit GC metastasis has a high priority.
The urokinase plasminogen activator (uPA)/uPA receptor (uPAR) system plays an important role in metastasis of many types of cancers. uPA is a serine proteinase that is released from cells as an inactive zymogen.3 Its binding to uPAR on the cell surface is considered to be essential for its activation. Activated uPA catalyzes the conversion of the proenzyme plasminogen to the active protease plasmin.3 Plasmin is capable of degrading most extracellular matrix (ECM) components directly or indirectly via the activation of matrix metalloproteinases (MMPs).4 Degradation of ECM is a critical step in cancer metastasis, as it breaks the tether with the primary tumor environment and allows cancer cells to spread to other parts of the body. In addition, a large body of evidence indicates that uPA is beneficial for the function and/or expression of focal adhesion kinase (FAK), αvβ6 integrin, vascular endothelial growth factor (VEGF), and transforming growth factor (TGF)-β,5-8 which are critical factors for cancer metastasis. A recent study implicates the uPA/uPAR system also in the stimulation of p21-activated kinases-1 (Pak1).9 Pak1 is associated with the regulation of the Limk1/cofilin pathway, which is responsible for governing cell motility and morphologic changes, thereby facilitating cancer metastasis.10,11
Quercetin (Qu), a natural constituent abundantly present in vegetables, fruits, tea, and herbs, is known to have potent anti-proliferative and pro-apoptotic effects against diverse GC cells via various mechanisms. A significant negative correlation between Qu and the risk of noncardiac gastric adenocarcinoma was found in a large Swedish population-based case-control study.12 Qu inhibits mitogen-activated protein kinases, including p38, ERK, and JNK, as well as transient receptor potential melastatin channels, which renders it lethal for GC AGS cells.13,14 Moreover, treatment of GC BGC823 cells with Qu decreases the Bcl-2/Bax ratio and increases caspase-3 expression, inducing mitochondrial pathway-related apoptosis.15 A study conducted by Lee et al16 showed that Qu induces apoptosis in GC SNU719 cells, a cell type associated with Epstein-Barr virus infection, thus hindering cell cycle progression and interrupting Epstein-Barr virus infection. There is also evidence indicating that Qu effectively downregulates p-Stat3 and survivin, significantly reducing GC cell viability.17 In addition to its own cytotoxic effects for GC cells, Qu shows synergistic effects with cytostatic drugs (eg, daunorubicin); thus, it is considered as a prospective chemosensitizer to overcome classical resistance in GC cells.18 However, published reports do not support the hypothesis that Qu is a likely inhibitor of GC metastasis. Therefore, the primary objective of this study was to investigate the effect of Qu on GC metastasis and determine whether the uPA/uPAR system is involved in this function of Qu.
Materials and Methods
Tissue Samples and Ethics Statement
GC and pericarcinous tissue samples were collected from 35 patients with GC undergoing surgical resection in the Third Xiangya Hospital of Central South University. All patients provided written informed consent in compliance with the code of ethics of the World Medical Association (Declaration of Helsinki; Ferney-Voltaire, France). This study was approved by the Ethics Committee of Xiangya School of Medicine (Changsha, People’s Republic of China).
Reagents
Qu was purchased from Sigma-Aldrich (No. PHR1488; St Louis, MO). JSH-23, Go6983, and SCH772984 (specific inhibitors of NF-κb, PKC, and ERK1/2 respectively) and A-769662 (specific activator of AMPK) were purchased from Selleck (Houston, TX). Antibodies against uPA (ab131433), uPAR (ab82220), phosphor(p)-Pak1(ab75599), p-Limk1(ab38508), p-cofilin (ab12866), p-NF-κb (p65, S276) (ab106129), p-PKC-α (ab76016), p-PKC-β (ab75657), p-PKC-δ (ab133456), p-ERK1/2 (ab200807), and GAPDH (ab9485) that were used for western blotting were purchased by Abcam (Cambridge, England).
uPA Activity Assay
uPA activity was measured using an uPA Activity Assay Kit (Chemicon, Tokyo, Japan) according to the manufacturer’s instructions. Briefly, samples from GC and pericarcinous tissues were mixed with the assay buffer provided in the assay kit in 96-well plates and incubated with the chromogenic substrate at 37°C for 2 to 24 hours. Subsequently, the optical density at 450 nm was determined using an ELISA plate reader (Model 550; Bio-Rad).
Western Blotting
Western blotting was performed to determine expression levels of total and phosphorylated proteins in GC and pericarcinous tissues, as well as in cells isolated from GC and normal gastric mucosa. Protein extracts were prepared using the RIPA lysis buffer (Beyotime, Shanghai, China). Equal amounts of protein (20 µg) were separated by SDS-polyacrylamide gel electrophoresis on 10% or 12% polyacrylamide Tris-glycine gels, and then electrotransferred onto a nitrocellulose membrane. The membranes were blocked with 5% skim milk for 1 hour at room temperature, followed by incubation with the primary antibodies at 4°C overnight. After incubation in horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature, the immunoreactive bands were visualized using the ECL Western Blotting Substrate (Piece, Carlsbad, CA). Relative protein levels were normalized to that of GAPDH.
Cell Culture
GC cell lines, including MGC803, GC7901, BGC823, AGS, and N87, as well as a gastric mucosa cell line, namely GES-1, were purchased from the American Type Culture Collection (ATCC; Manassas, VA). The cells were cultured in DMEM or RPMI 1640 medium (Sigma, St Louis, MO) at 37°C under a humidified atmosphere of 5% CO2. Both culture media were supplemented with 10% fetal bovine serum and 10% penicillin/streptomycin.
Cell Migration
Cell migration was evaluated by the wound healing assay. Equal numbers of GC and gastric mucosa cells were seeded in 6-well tissue culture plates coated with fibronectin. After a confluent cell monolayer formed, a straight line was scratched on the cell monolayer using a 100-μL pipette tip (wound infliction). Subsequently, the cells were washed and cultured in serum-free medium. Microscopic images of the same area were taken immediately after wound infliction and after 24 hours. Migration rates were calculated using the following equation: (initial distance − final distance/initial distance) × 100.
Cell Invasion
Cell invasion was evaluated in transwell chambers, with upper and lower culture compartments separated by polycarbonate membranes of 8-μm pore size (Millicell Insert; Millipore, Billerica, MA). GC and gastric mucosa cells were placed in the upper chamber, and following a 12-hour incubation, the cells on the upper surface of the membrane were scraped, and the cells that invaded to the bottom surface were fixed with 95% ethanol, stained with 0.1% crystal violet, and counted under a light microscope (200×; Nikon, Tokyo, Japan) at 5 randomly chosen areas.
Short Hairpin RNA (shRNA) uPAR Knockdown
BGC823 and AGS cells were cultured to 60% to 70% confluence and were transfected with an shRNA targeting uPA (shRNA-uPA; GenePharma Co, Ltd, Shanghai, China) using Lipofectamine 2000 (Life Technologies Inc, Gaithersburg, MD) according to the manufacturer’s instructions. Stable transfectant clones with a low expression of the target protein were identified by Western blotting.
Cell Viability Assay
Cell viability was evaluated by the methyl thiazolyl tetrazolium (MTT) assay. BGC823 and AGS cells were seeded in a 96-well plate. After any relevant treatment, 10 μL MTT was added to each well and the cells were cultured for 4 hours. Subsequently the medium was removed, 150 μL dimethylsulfoxide was added per well, and the plate was shaken for 10 minutes to terminate the reaction. Afterwards, the optical density at 570 nm was measured.
Gelatin Zymography Assay
Gelatin-coated polyacrylamide gel electrophoresis for MMP-2 and MMP-9 activity assay was performed. After any relevant treatment, the cell medium was centrifuged and the supernatants were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on 10% gels with 1 mg/mL gelatin (Sigma-Aldrich) at 100 V for 2 hours at 4°C. After electrophoresis, the gels were washed twice in 2.5% Triton X-100 (Sigma-Aldrich) for 30 minutes at room temperature and then incubated for 16 hours in zymography developing buffer containing 50 mM Tris-HCl and 10 mM CaCl2 (pH 7.5; Sigma-Aldrich). The gels were subsequently stained with Commassie Brilliant Blue R-250 and band densities were determined by the Quantity One 4.6.3 software (Bio-Rad Laboratories, Inc, Berkeley, CA).
Statistical Analysis
The experimental data were analyzed by one-way analysis of variance (ANOVA) with post hoc testing, using the version 12.0 of the SPSS software (IBM, Armonk, NY). The results are presented as mean ± SD. Differences were considered significant when P < .05.
Results
uPA Activity, uPAR Expression, and Pak1 Phosphorylation in GC and Pericarcinous Tissues
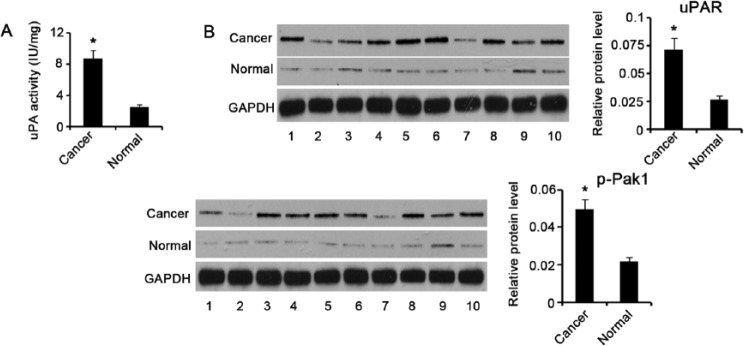
We initially examined uPA activity in GC and pericarcinous tissues using a commercial detection kit, and we found that uPA activity was remarkably elevated in GC tissues compared with pericarcinous tissues (P < .05; Figure 1A). uPA binding to its receptor, uPAR, on the cell surface is essential for its catalytic activity. Thus, knowledge of uPAR expression in tissues contributes to an understanding of uPA activation. Western blotting showed that uPAR expression was higher in GC tissues than in pericarcinous tissues (P < .05; Figure 1B). Pak1 is one of the key downstream targets of the uPA/uPAR system, which controls signals involved in cell movement and invasion. Similar to uPAR upregulation, Pak1 phosphorylation was dramatically increased in GC tissues compared to pericarcinous tissues (P < .05).
Figure 1.
uPA activity, uPAR expression, and Pak1 phosphorylation in GC and pericarcinous tissues. (A) uPA activity in gastric cancer (GC) and pericarcinous tissues (n = 35) was examined using a commercial detection kit. uPA activity was remarkably elevated in GC tissues compared to pericarcinous tissues. (B) Representative Western blot images show the relative protein levels of uPAR and p-Pak1 in GC and pericarcinous tissues (n = 35). uPAR and p-Pak1 had higher expression in GC tissues than in pericarcinous tissues. *P < .05 versus control group. Cancer, GC tissues; Normal, pericarcinous tissues of GC; uPA, urokinase plasminogen activator; uPAR, uPA receptor; Pak1, p21-activated kinases-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Correlation Between uPAR and p-Pak1 Protein Levels and Migration and Invasion of GC Cells
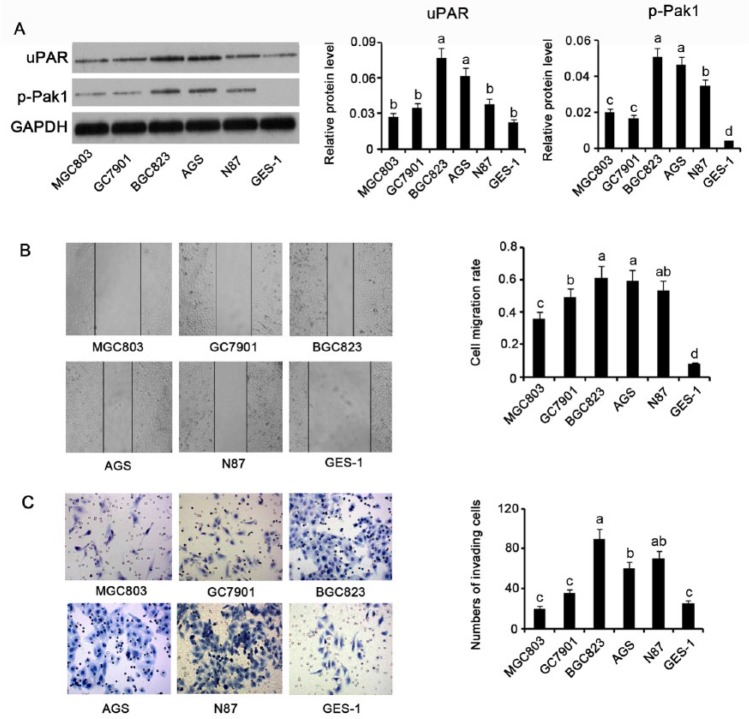
To understand the correlation between uPAR and Pak1 and GC migration and invasion, we measured uPAR expression and Pak1 phosphorylation levels in various GC cells by Western blotting. uPAR expression was higher in GC cell lines compared to the gastric mucosa cell line GES-1, with different cell lines showing different degrees of uPAR expression increase; the highest levels were observed in BGC823 and AGS cells, which exhibited a 2.2- and 1.5-fold increase, respectively (both P < .05; Figure 2A). Pak1 phosphorylation showed a nearly 9- and 8-fold increase in BGC823 and AGS cells, respectively, compared to GES-1 cells (P < .01). N87, MGC803, and GC7901 GC cells displayed approximately 6- (P < .01), 3- (P < .05), and 2.6-fold (P < .01) increase in Pak1 phosphorylation, respectively, compared to GES-1 cells. Cell migration rate as determined by a wound healing assay was used as a measure of the migratory ability of GC and gastric mucosa cells. Of all tested cells, BGC823 and AGS cells showed the highest and second highest migration rates, respectively, followed by N87, GC7901, MGC803, and GES-1 cells, in this order (Figure 2B). In the cell invasion assay, a higher number of cells passing through the transwell membrane generally means a stronger cell invasion activity. We observed that the MGC803 cells processed the strongest invasion activity among all the cells tested (Figure 2C). The invasion activity of N87 and AGS cells was slightly weaker than that of the MGC803 cells, but much stronger than that of the GC7901, GES-1, and MGC803 cells (P < .05). Therefore, BGC823 and AGS cells, considered the ideal cell lines for our study, were used for further experimentation.
Figure 2.
Correlation between uPAR and p-Pak1 protein levels and in GC cell migration and invasion. (A) uPAR and p-Pak1 protein levels in GC cell lines, including MGC803, GC7901, BGC823, AGS, and N87, as well as gastric mucosa cell line, GES-1. (B) Wound healing assay showing migration of GC and gastric mucosa cells. (C) Transwell chamber assay showing invasion of GC and gastric mucosa cells. Migration and invasion of GC cell lines positively correlated with uPAR and p-Pak1 expression. Bars representing the average of data from 3 independent tests. Bars not sharing a common letter differ (P < .05). uPAR, uPA receptor; Pak1, p21-activated kinases-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Qu Treatment Inhibits GC Cell Viability Probably Independent of uPAR
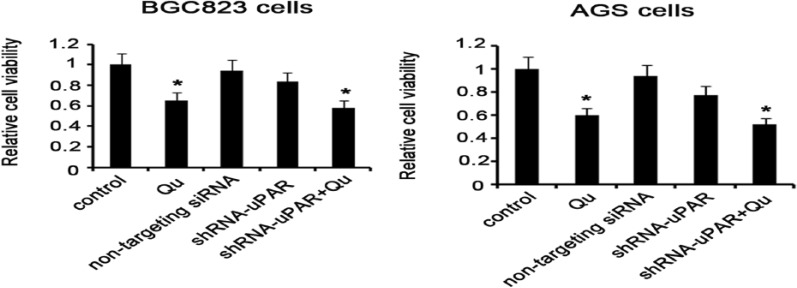
Qu is a plant-derived polyphenol, exhibiting inhibitory effects on proliferation and development of diverse cancers. In this study, GC BGC823 and AGS cells were treated with 10 μM Qu for 72 hours (the dose and time were based on a pilot experiment) to determine the impact of Qu on cell viability. The MTT assay showed that the viability of both BGC823 and AGS cells underwent dramatic decrease following exposure to Qu (P < .05; Figure 3). To determine whether the inhibitory effect of Qu on cell viability could be related to uPA/uPAR function, shRNA-mediated uPAR knockdown was performed before Qu treatment. BGC823 and AGS cell viability was not impaired by uPAR knockdown, but decreased by uPAR knockdown in combination of the Qu treatment (P < 0.05 vs control). It is possible that the inhibitory effect of Qu on cell viability is independent of the uPA/uPAR system.
Figure 3.
Treatment with Qu inhibits GC cell viability probably independent of uPAR. GC BGC823 and AGS cells were treated with 10 μM Qu for 72 hours to determine the impact of Qu on cell viability. shRNA-mediated uPAR knockdown in the presence or absence of the Qu was performed to determine whether the inhibitory effect of Qu on cell viability is related to uPA/uPAR function. MTT assay was performed to evaluate cell viability. GC BGC823 and AGS cell viability was significantly decreased after exposure to 10 μM Qu for 72 hours. uPAR knockdown failed to impair cell viability. Bars representing the average of data from 5 independent tests. *P < .05 versus control group. Qu, quercetin; non-targeting siRNA, the cells were transfected with non-targeting small iRNA; ShRNA-uPAR, uPAR was knocked down via ShRNA-uPAR transfection; ShRNA-uPAR + Qu, uPAR was knocked down via ShRNA-uPAR transfection before the Qu treatment.
Qu Treatment and uPAR Knockdown Inhibit the Metastatic Activity of GC Cells
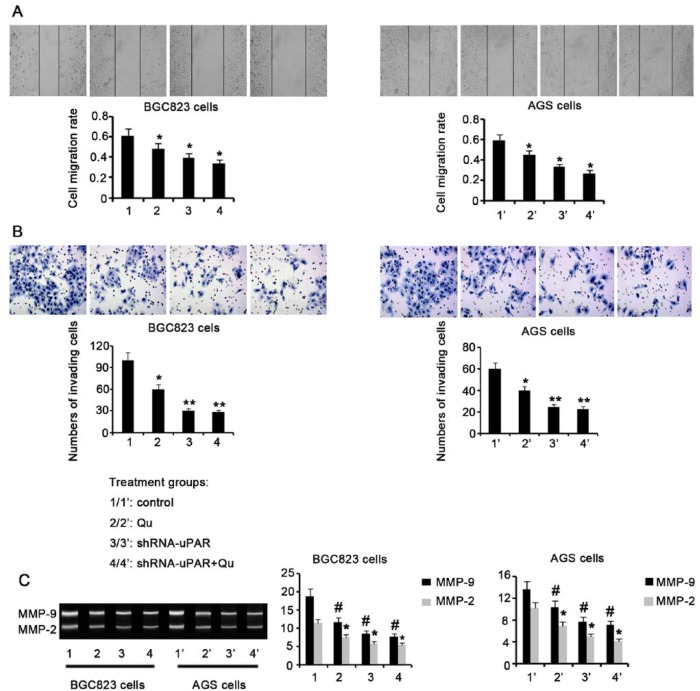
GC cell migration and invasion are usually used to assess metastatic potential. Here we evaluated these activities after various cell treatments. Exposure to 10 μM Qu for 72 hours resulted in a remarkable decrease in migration for both BGC823 and AGS cells, compared to untreated control cells (P < .05; Figure 4A). uPAR knockdown in BGC823 and AGS cells also attenuated cell migration (P < .05). Combination of uPAR knockdown with Qu exposure resulted in a further attenuation of BGC823 and AGS cell migration (P < .05 vs control). Incubation of BGC823 and AGS cells with 10 μM Qu for 72 hours significantly inhibited cell invasion (P < .05; Figure 4B). In addition, on uPAR knockdown, BGC823 and AGS cell invasion was severely impaired, compared to that of control cells (P < .01). uPAR knockdown before Qu treatment caused very poor BGC823 and AGS cell invasion (P < .01 vs control). ECM degradation induced by MMP-2 and MMP-9 strongly contributes to cancer metastasis; thus, MMP-2 and MMP-9 are important targets in cancer management. We found that both MMP-2 and MMP-9 activities were significantly attenuated in BGC823 and AGS cells after Qu treatment (P < .05, Figure 4C). uPAR knockdown also reduced MMP-2 and MMP-9 activity in BGC823 and AGS cells (P < .05 vs control). Combination of uPAR knockdown with Qu treatment resulted in remarkably attenuated MMP-2 and MMP-9 activity in BGC823 and AGS cells (P < .05 vs control).
Figure 4.
Treatment with Qu and uPAR knockdown inhibit the metastatic activity of GC cells. GC BGC823 and AGS cells were treated with 10 μM Qu for 72 hours. shRNA-mediated uPAR knockdown was performed in the presence or absence of Qu treatment. (A) Wound healing assay showing BGC823 and AGS cell migration following various treatments. (B) Transwell chamber assay showing BGC823 and AGS cell invasion following various treatments. (C) Gelatin zymography assay showing MMP-2 and MMP-9 activities in BGC823 and AGS cells following various treatments. BGC823 and AGS cell migration and invasion were decreased by Qu treatment and uPAR knockdown, alone or in combination. Bars representing the average of data from 3 independent tests. *P < .05, **P < .01 versus control group. Qu, quercetin; ShRNA-uPAR, uPAR was knocked down via ShRNA-uPAR transfection; ShRNA-uPAR + Qu, uPAR was knocked down via ShRNA-uPAR transfection before the Qu treatment.
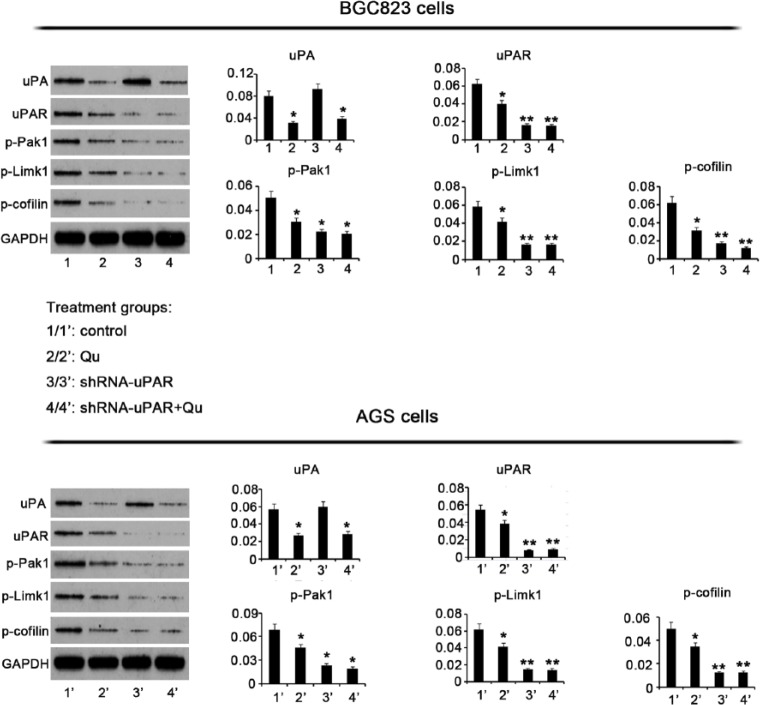
Qu Inhibits Expression of uPA, uPAR, and Their Downstream Targets
To gain insights into the regulatory effects of Qu on uPA, uPAR, and their downstream targets, we evaluated their expression by Western blotting. Incubation of BGC823 cells with 10 μM Qu for 72 hours resulted in decreased uPA expression, compared to control cells (P < 0.05, Figure 5). uPA protein expression was not affected by uPAR knockdown, but decreased significantly by the combination of uPAR knockdown and Qu treatment (P < .05). Exposure of BGC823 cells to Qu lead to decreased uPAR expression as well. A predominant decrease in uPAR expression was observed with uPAR knockdown alone or in combination with subsequent Qu treatment (P < .01). PAK1 phosphorylation also decreased after Qu treatment (P < .05). Moreover, PAK1 phosphorylation decreased on shRNA-mediated uPAR knockdown (P < .01). uPAR knockdown followed by Qu treatment resulted in a significant inhibition of PAK1 phosphorylation (P < .01). Limk1 is downstream to PAK1. Administration of Qu inhibited Limk1 phosphorylation. uPAR knockdown also led to the reduction in the levels of Limk1 phosphorylation (P < .05), regardless of subsequent treatment with the Qu. Cofilin phosphorylation is under the regulation of Limk1. Exposure of BGC823 cells to Qu inhibited cofilin phosphorylation relative to control (P < .05). Cofilin phosphorylation was dramatically reduced after uPAR knockdown (P < .01). The combination of uPAR silencing with Qu treatment resulted in a significant inhibition of cofilin phosphorylation (P < .01).
Figure 5.
Qu has inhibitory effects on the expression of uPA, uPAR, and their downstream targets. GC BGC823 and AGS cells were treated with 10 μM Qu for 72 hours. shRNA-mediated uPAR knockdown was performed in the presence or absence of Qu treatment. Western blotting was performed to determine the expression levels of the indicated proteins after various treatments. Both Qu treatment and uPAR knockdown decreased MMP-2 and -9 activities and blocked Pak1-Limk1-cofilin signaling. Bars representing the average of data from 3 independent tests. *P < .05, **P < .01 versus control group. Qu, quercetin; ShRNA-uPAR, uPAR was knocked down via ShRNA-uPAR transfection; ShRNA-uPAR + Qu, uPAR was knocked down via ShRNA-uPAR transfection before the Qu treatment; uPA, urokinase plasminogen activator; uPAR, uPA receptor; Pak1, p21-activated kinases-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Similar to BGC823 cells, AGS cells showed markedly reduced uPA protein expression following exposure to 10 μM Qu for 72 hours. Silencing uPAR had no effect on uPA protein expression, whereas the combination of uPAR knockdown with Qu treatment caused a significant reduction in uPA protein expression. uPAR expression was also reduced in AGS cells after Qu treatment (P < .05). uPAR silencing led to a dramatic reduction in uPAR expression, regardless of subsequent Qu treatment. PAK1, Limk1, and cofilin phosphorylation was significantly lower in AGS cells treated with Qu than in the nontreated cells (P < .05). Furthermore, there was a strong inhibition of PAK1 (P < .05), Limk1 (P < .01), and cofilin (P < .01) phosphorylation on uPAR knockdown. The combination of Qu treatment with uPAR silencing resulted in a remarkable inhibition of PAK1 (P < .05), Limk1 (P < .01), and cofilin (P < .01) phosphorylation.
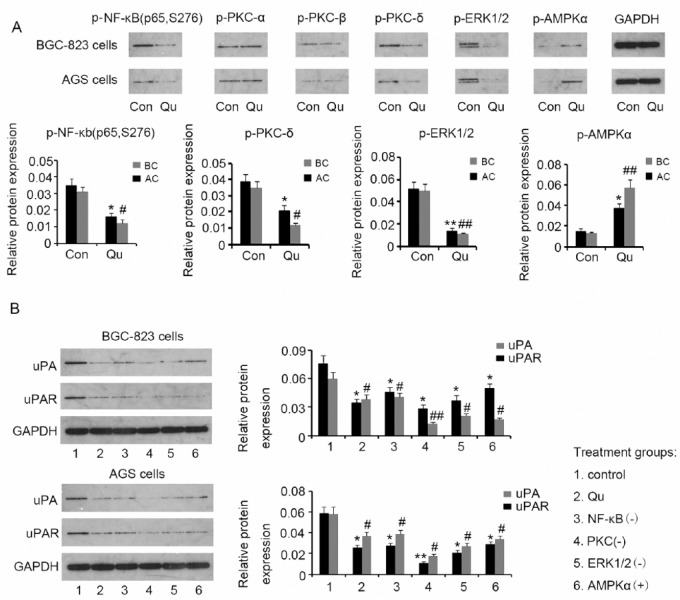
Suppression of the uPA/uPAR System by Qu Could Be Mediated by NF-κb, PKC-δ, ERK1/2, and AMPKα
The ability of Qu to modulate multiple signaling pathways is probably involved in the suppression of the uPA/uPAR system. Based on previous studies, we hypothesized that NF-κb, PKC, ERK1/2, and AMPKα are potential candidates that could mediate the suppression of the uPA/uPAR system by Qu. We evaluated NF-κb (p65), PKC-α, PKC-β, PKC-δ, ERK1/2, and AMPKα phosphorylation in BGC823 and AGS cells by Western blotting, after treatment with 10 μM Qu for 72 hours. We observed that Qu decreased NF-κb (p65) (P < .05), PKC-δ (P < .05), and ERK1/2 (P < .01) phosphorylation in both BGC823 and AGS cells, but not PKC-α and PKC-β phosphorylation (Figure 6A). Conversely, AMPKα phosphorylation was increased by Qu (P < .05 in BGC823 cells and P < .01 in AGS cells). Furthermore, we used specific inhibitors of NF-κb (p65), PKC, and ERK1/2, as well as activators of AMPKα to assess the role of these molecules in regulating uPA and uPAR. BGC823 and AGS cells were incubated with 9 μM JSH-23, 10 nM Go6983, 12 μM SCH772984, or 4.5 μM A-769662 for 72 hours. Similar to Qu, inhibitors of NF-κb (p65), PKC, and ERK1/2, and the AMPKα activator decreased uPA expression (all P < .05) in BGC823 cells. uPAR expression was also dramatically reduced after treatment with Qu (P < .05), JSH-23 (P < .05), Go6983 (P < .01), SCH772984 (P < .05), and A-769662 (P < .05). Similarly, AGS cells showed decreased uPA expression following exposure to Qu (P < .05), JSH-23 (P < .05), Go6983 (P < .01), SCH772984 (P < .05), and A-769662 (P < .05). These substances also lowered uPAR expression in AGS cells (all P < .05). These data indicate that suppression of the uPA/uPAR system by Qu could proceed through NF-κb, PKC-δ, ERK1/2, and AMPKα regulation.
Figure 6.
Suppression of the uPA/uPAR system by Qu may be mediated by NF-κb, PKC-δ, ERK1/2, and AMPKα. (A) Phosphorylation levels of NF-κb (p65), PKC-α, PKC-β, PKC-δ, ERK1/2, and AMPKα were evaluated in BGC823 and AGS cells by Western blotting, after treatment with 10 μM Qu for 72 hours. Qu treatment was associated with inhibition of NF-κb, PKC-δ, and ERK1/2 activities and with AMPKα activation. (B) Specific inhibitors of NF-κb (p65), PKC, and ERK1/2, as well as activators of AMPKα were used to assess their roles in uPA and uPAR regulation. BGC823 and AGS cells were incubated with 9 μM JSH-23, 10 nM Go6983, 12 μM SCH772984, or 4.5 μM A-769662 for 72 hours. Similar to Qu, specific inhibitors of NF-κb, PKC, and ERK1/2, and an AMPKα activator suppressed uPA and uPAR expression in GC cells. Bars representing the average of data from 3 independent tests. *P < .05, **P < .01, #P < .05, ##P < .01 versus control group. Qu, quercetin; uPA, urokinase plasminogen activator; uPAR, uPA receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NF-κb, nuclear factor-κb; PKC, protein kinase C; ERK1/2, extracellular signal-regulated kinase 1/2; AMPKα, adenosine monophosphate activated protein kinase α; NF-κb(−), NF-κb inhibition with the inhibitor; PKC(−), PKC inhibition with the inhibitor; ERK1/2(−), ERK1/2 inhibition with the inhibitor; AMPKα(+), AMPKα activation with the activator.
Discussion
The critical importance of the uPA/uPAR system in GC metastasis has been revealed in previous studies. An immunohistochemical study using cancer tissues from 101 GC patients showed a positive correlation between uPA and uPAR expression and various clinicopathological factors, including tumor size, differentiation, depth of tumor invasion, as well as lymphatic and vascular invasion.19 Based on the analysis to 105 GC specimens, Zhang et al20 found that uPA and uPAR mRNA expression rates in infiltrating-type cases, stage III-IV, vessel invasion, lymphatic metastasis, and distant metastasis were significantly higher than those in expanding-type cases, stage I-II, non–vessel invasion, non–lymphatic metastasis, and non–distant metastasis, respectively, thereby suggesting that uPA and uPAR expressions can serve as prognostic markers of GC. Semiquantitative RT-PCR and ELISA measurements conducted by Ding et al21 showed higher expressions of uPA and uPAR in peritoneal metastatic lesions than in normal peritoneal tissues. High levels of uPA and uPAR may predict an adverse outcome in patients with GC. Consistent with previous data, the present study displayed enhanced uPA activity and upregulated uPAR protein level in GC tissues. Furthermore, we revealed a strong correlation between uPAR expression and GC metastatic potency, by evaluating uPAR expression, migration, and invasion in various GC cell lines. Our data provide additional evidence that the uPA/uPAR system is critical in promoting GC metastasis.
Qu, a plant-derived polyphenol, has been shown to have anti-proliferative and apoptotic effects on GC cells. This property of Qu was also validated in our cell experiments, where GC BGC823 and AGS cell viability was significantly decreased after exposure to 10 μM Qu for 72 hours. However, less information is currently available as to whether Qu is able to inhibit metastasis. Since GC has often developed to an advanced stage when it is diagnosed, cancer metastasis is a common event in GC patients.2 Patients presented with metastatic GC suffer from limited treatment options and rapid disease progression; thus, identification of new substances and methods that can retard GC metastatic progression is urgent. In this study, we found that GC BGC823 and AGS cells have potent migratory and invasive abilities, which, however, were severely impaired after treatment with Qu, suggesting an inhibitory effect of Qu on GC metastasis. Melanoma is another malignant tumor with a high propensity for metastasis. Studies showed that Qu inhibits melanoma cell migration and invasion through the inhibition of hepatocyte growth factor/c-Met and STAT3 signaling.22 In pancreatic cancer, treatment with Qu suppresses the migratory activity induced by transforming growth factor-β, basic fibroblast growth factor, and vascular endothelial growth factor-A.23 Moreover, numerous researches have revealed a positive correlation between Qu treatment and invasion inhibition in prostate cancer. The mechanisms by which Qu antagonizes prostate cancer invasiveness involve suppression of transcriptional repressors (eg, Snail, Slug, and Twist), inhibition of epidermal growth factor receptor/PI3K/Akt/ERK1/2 pathway, and the vascular and endothelial growth factor-R2/Akt/mTOR/P70S6K signaling pathway.24,25
The present work revealed that uPA and uPAR protein levels in GC cells were downregulated by Qu. uPAR knockdown in GC cells resulted in decreased migration and invasion, while uPAR knockdown in combination with Qu treatment had an additive effect. These results indicate that uPA and uPAR downregulation is an important mechanism by which Qu can inhibit GC migration and invasion. uPA and uPAR downregulation are also observed upon Qu treatment of highly invasive prostate cancer.26 Although cancer metastasis involves a series of complex processes, degradation and destruction of ECM and basement membrane is an essential step, which requires the participation of several proteolytic enzyme systems, such as the uPA/uPAR system and MMPs. After binding to uPAR, uPA is activated and is capable of converting plasminogen into plasmin, which then can degrade several ECM components.
MMP-2 and MMP-9 are 2 major MMPs responsible for the degradation of ECM and basement membrane. High levels of MMP-2 and MMP-9 have been associated with the malignant behavior of GC and acted as predictors of adverse outcomes.27 In the present study, gelatin zymography showed that MMP-2 and MMP-9 activities were significantly reduced in GC BGC823 and AGS cells after Qu treatment, which suggests that Qu can inhibit MMP-2 and MMP-9 activities. A reduction in MMP-2 and MMP-9 activities on Qu treatment has also been observed in human glioma, fibrosarcoma, and epidermal cancer.28-30 A previous study showed that Qu decreased the expression of MMP-2 and MMP-9 in a dose-dependent manner in prostate cancer.31 Since MMP-2 and MMP-9 activity is modulated by the uPA/uPAR system, as demonstrated by the present and previous studies,4 it is highly possible that inactivation of MMP-2 and MMP-9 is due to the Qu-induced downregulation of uPA and uPAR. Moreover, Lee et al28 have shown that Qu inhibited the formation of reactive oxygen species (ROS) induced by phenazine methosulfate (PMS) in human fibrosarcoma cells, resulting in the interruption of PMS-induced stimulation of MMP-2 and MMP-9. In the absence of PMS, Qu also decreased intracellular ROS and MMP-2 and MMP-9 activities. Hence, they suggested that the Qu-induced inhibition of MMP-2 and MMP-9 is likely the result of suppression of intracellular ROS formation. There are also studies that attribute the Qu-mediated MMP-2 and MMP-9 inactivation to PI3K/Akt signaling inhibition.32,33 These studies reveal that Qu can directly bind to PI3K and then lower Akt phosphorylation, leading to reduced MMP-2 and MMP-9 activities. Thus, Qu-mediated inactivation of MMP-2 and MMP-9 in GC cells may be dependent on or independent of the uPA/uPAR system.
In addition to ECM degradation, cytomorphologic changes and motility are required for cancer cell penetration into neighboring tissues and metastasis to distant organs. Cofilin is an important actin-modulating protein that controls cytoskeleton remodeling and cell lamellipodia formation, and thus it is related to cytomorphologic changes and cell movement.34,35 Inactivation of cofilin by phosphorylation results in the polymerization of actin filaments, which leads to increased migration and invasion. Cofilin is phosphorylated mainly by Limk1.34,35 Limk1 activity relies on Pak1-induced phosphorylation. A recent study has established that the uPA/uPAR system has an impact on the phosphorylation and activation of Pak1,9 which suggests another important mechanism by which the uPA/uPAR system regulates cancer migration and invasion. Our histological research showed that increased uPA activity and uPAR upregulation in GC was accompanied by elevated p-Pak1 protein levels. In experiments with various GC cell lines, p-Pak1 showed a similar expression profile to that of uPAR. Selectively targeting uPAR by shRNA interference attenuated phosphorylation of Pak1 and its downstream effectors, Limk1 and cofilin-1. These results suggest that the uPA/uPAR system serves as an important activator of Pak1/Limk1/cofilin signaling. This study showed that GC cell lines with higher p-Pak1 levels had stronger migratory and invasive capabilities, consistent to previous findings that show correlation between high expression and phosphorylation of Pak1 and increased cancer metastasis.36 Furthermore, we found that phosphorylation of Pak1, Limk1, and cofilin in GC BGC823 and AGS cells was significantly decreased following treatment with Qu. Based on these data, we suggest that Qu-induced uPA and uPAR downregulation inhibits the Pak1/Limk1/cofilin signaling pathway, leading to cell migration and invasion inhibition.
Qu has been shown to regulate multiple signaling molecules acting upstream of uPA and uPAR, which may be an important mechanism underlying the suppression of uPA and uPAR by Qu. The p65 subunit of NF-κB binds the promoter of the uPA gene, facilitating the activation of uPA transcriptional.37 A substantial body of evidence suggests that most polyphenols are inhibitors of NF-κb, thereby processing anti-inflammatory properties.38 Our study showed that p65 phosphorylation in GC cells was effectively reduced by Qu. Suppression of NF-κB via a specific inhibitor had similar inhibitory effects on uPA and uPAR expression as Qu treatment did. This suggests that NF-κB is involved in the regulation of uPA and uPAR by Qu. Signaling pathways involving protein kinase C (PKC) are also influenced by Qu and various other natural polyphenols as well.39 One of the mechanisms by which polyphenols exert antioxidant properties is the regulation of antioxidant enzyme gene transcription via PKC signaling.39 The regulation of PKC signaling involved complicated mechanisms. Which isoform of PKC is regulated and whether PKC is activated or inhibited depend not only on the polyphenol form but also on cell and tissue types and local environments.39 Qu has been shown to dramatically decrease PKC-δ phosphorylation, whereas it has minor effects on PKC-α and PKC-β phosphorylation. An in vivo study found that inhibition of PKC-δ largely blocks sepsis-induced expression of uPAR.40 In the present study, uPA and uPAR expression in GC cells was decreased following treatment with a PKC inhibitor. These results indicate that PKC-δ may participate in the mechanisms underlying the regulation of uPA and uPAR by Qu. In addition, we suggest that ERK1/2 is also an important regulator, asERK1/2 inhibition by a specific inhibitor or Qu was associated with decreased expression of uPA and uPAR. A report documents that p-ERK is substantially decreased by siRNA interference of uPAR in pancreatic adenocarcinoma cells, and ERK1/2 inactivation suppresses uPAR protein expression, suggesting the presence of a positive feedback loop between uPAR and ERK1/2.41 In previous studies, Qu has been shown to have both inhibitory and promoting effects on ERK1/2, possibly depending on cell and tissue type.42-44 The effect of Qu on AMPK signaling is positive in various cells. Qu augments AMPK signaling, blocking the AKT/mTOR cascades and reducing breast cancer growth and metastasis in nude mice.45 In this study, Qu and an AMPK specific activator showed a similar result with respect to AMPK activation, which was concomitant with lower uPA and uPAR expression. AMPK thus may be a potential signaling molecule that mediates uPA and uPAR regulation by Qu.
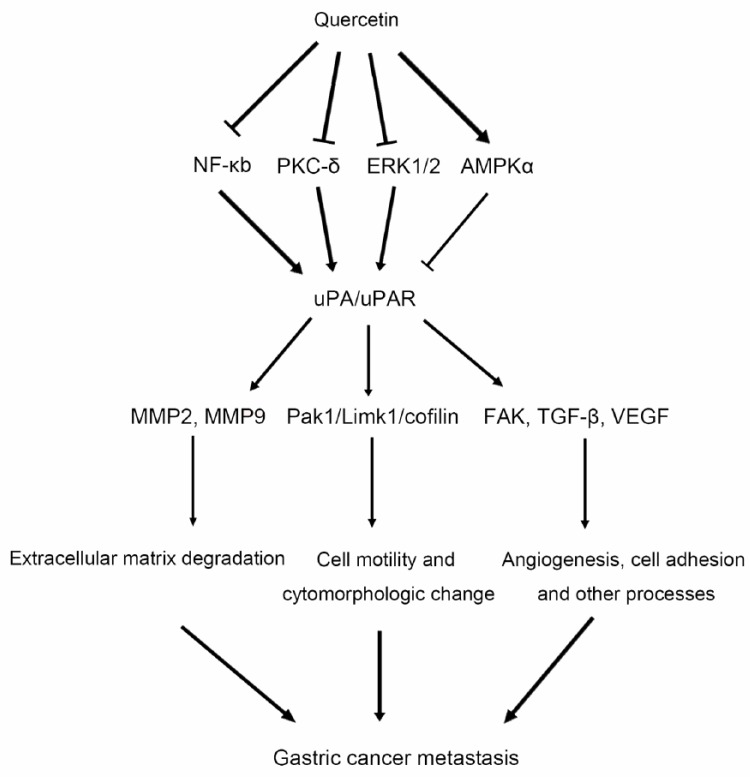
In summary, this study revealed that Qu has antimetastatic effects on GC via the interruption of uPA/uPAR-dependent pathways. The uPA/uPAR system is involved in multiple processes that are essential for GC metastasis, including ECM degradation, cell motility, cytomorphologic changes, and angiogenesis, by regulating MMPs, the Pak1/Limk1/cofilin signaling pathway, and FAK, TGF-β, and VEGF (Figure 7). The suppression of uPA/uPAR by Qu may be related to NF-κb, PKC-δ, and ERK1/2 inhibition, as well as AMPKα activation. The use of Qu in adjunctive therapy in patients with metastatic GC might be considered, although further studies of Qu in animal experiments and clinical trials are needed.
Figure 7.
Possible mechanisms by which Qu inhibits GC metastasis. Qu suppresses uPA/uPAR function probably via inhibition of NF-κb, PKC-δ, and ERK1/2, as well as AMPKα activation. The uPA/uPAR system is involved in multiple processes essential for GC metastasis, including ECM degradation cell motility, cytomorphologic changes, and angiogenesis, by regulating MMPs, the Pak1/Limk1/cofilin signaling pathway, and FAK, TGF-β, and VEGF. Thus, Qu suppresses uPA/uPAR function, resulting in GC metastasis inhibition. Qu, quercetin; uPA, urokinase plasminogen activator; uPAR, uPA receptor; MMP, matrix metalloproteinase; Pak1, p21-activated kinases-1; FAK, focal adhesion kinase; VEGF, vascular endothelial growth factor; TGF-β, transforming growth factor-β; NF-κb, nuclear factor-κb; PKC, protein kinase C; ERK1/2, extracellular signal-regulated kinase 1/2; AMPKα, adenosine monophosphate activated protein kinase α.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lee KH, Kim SW, Kim JR. Reactive oxygen species regulate urokinase plasminogen activator expression and cell invasion via mitogen-activated protein kinase pathways after treatment with hepatocyte growth factor in stomach cancer cells. J Exp Clin Cancer Res. 2009;28:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Zeng J, Pan J, et al. MiR-320a inhibits gastric carcinoma by targeting activity in the FoxM1-P27KIP1 axis. Oncotarget. 2016;7:29275-29286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding Y, Zhang H, Zhong M, et al. Clinical significance of the uPA system in gastric cancer with peritoneal metastasis. Eur J Med Res. 2013;18:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noh H, Hong S, Huang S. Role of urokinase receptor in tumor progression and development. Theranostics. 2013;3:487-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briassouli P, Rifkin D, Clancy RM, Buyon JP. Binding of anti-SSA antibodies to apoptotic fetal cardiocytes stimulates urokinase plasminogen activator (uPA)/uPA receptor-dependent activation of TGF-β and potentiates fibrosis. J Immunol. 2011;187:5392-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uhrin P, Breuss JM. uPAR: a modulator of VEGF-induced angiogenesis. Cell Adh Migr. 2013;7:23-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahn SB, Mohamedali A, Anand S, et al. Characterization of the interaction between heterodimeric αvβ6 integrin and urokinase plasminogen activator receptor (uPAR) using functional proteomics. J Proteome Res. 2014;13:5956-5964. [DOI] [PubMed] [Google Scholar]
- 8. Lino N, Fiore L, Rapacioli M, et al. uPA-uPAR molecular complex is involved in cell signaling during neuronal migration and neuritogenesis. Dev Dyn. 2014;243:676-689. [DOI] [PubMed] [Google Scholar]
- 9. Prager GW, Mihaly J, Brunner PM, Koshelnick Y, Hoyer-Hansen G, Binder BR. Urokinase mediates endothelial cell survival via induction of the X-linked inhibitor of apoptosis protein. Blood. 2009;113:1383-1390. [DOI] [PubMed] [Google Scholar]
- 10. Mashiach-Farkash E, Rak R, Elad-Sfadia G, et al. Computer-based identification of a novel LIMK1/2 inhibitor that synergizes with salirasib to destabilize the actin cytoskeleton. Oncotarget. 2012;3:629-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jain P, Baranwal S, Dong S, Struckhoff AP, Worthylake RA, Alahari SK. Integrin-binding protein nischarin interacts with tumor suppressor liver kinase B1 (LKB1) to regulate cell migration of breast epithelial cells. J Biol Chem. 2013;288:15495-15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekström AM, Serafini M, Nyrén O, Wolk A, Bosetti C, Bellocco R. Dietary quercetin intake and risk of gastric cancer: results from a population-based study in Sweden. Ann Oncol. 2011;22:438-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang K, Liu R, Li J, et al. Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy. 2011;7:966-978. [DOI] [PubMed] [Google Scholar]
- 14. Kim MC, Lee HJ, Lim B, et al. Quercetin induces apoptosis by inhibiting MAPKs and TRPM7 channels in AGS cells. Int J Mol Med. 2014;33:1657-1663. [DOI] [PubMed] [Google Scholar]
- 15. Wang P, Zhang K, Zhang Q, et al. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol In Vitro. 2012;26:221-282. [DOI] [PubMed] [Google Scholar]
- 16. Lee M, Son M, Ryu E, et al. Quercetin-induced apoptosis prevents EBV infection. Oncotarget. 2015;6:12603-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pandey A, Vishnoi K, Mahata S, et al. Berberine and curcumin target survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutr Cancer. 2015;67:1293-1304. [DOI] [PubMed] [Google Scholar]
- 18. Borska S, Chmielewska M, Wysocka T, Drag-Zalesinska M, Zabel M, Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: targeting cancer cells death and MDR. Food Chem Toxicol. 2012;50:3375-3383. [DOI] [PubMed] [Google Scholar]
- 19. Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci. 2003;94:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L, Zhao ZS, Ru GQ, Ma J. Correlative studies on uPA mRNA and uPAR mRNA expression with vascular endothelial growth factor, microvessel density, progression and survival time of patients with gastric cancer. World J Gastroenterol. 2006;12:3970-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ding Y, Zhang H, Lu A, et al. Effect of urokinase-type plasminogen activator system in gastric cancer with peritoneal metastasis. Oncol Lett. 2016;11:4208-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao HH, Cheng CY, Su T, et al. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer. 2015;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee J, Lee J, Kim SJ, Kim JH. Quercetin-3-O-glucoside suppresses pancreatic cancer cell migration induced by tumor-deteriorated growth factors in vitro. Oncol Rep. 2016;35:2473-2479. [DOI] [PubMed] [Google Scholar]
- 24. Pratheeshkumar P, Budhraja A, Son YO, et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS One. 2012;7:e47516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhat FA, Sharmila G, Balakrishnan S, et al. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25:1132-1139. [DOI] [PubMed] [Google Scholar]
- 26. Senthilkumar K, Arunkumar R, Elumalai P, et al. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem Funct. 2011;29:87-95. [DOI] [PubMed] [Google Scholar]
- 27. Agnantis NJ, Goussia AC, Batistatou A, Stefanou D. Tumor markers in cancer patients. An update of their prognostic significance. Part II. In Vivo. 2004;18:481-488. [PubMed] [Google Scholar]
- 28. Lee DE, Chung MY, Lim TG, Huh WB, Lee HJ, Lee KW. Quercetin suppresses intracellular ROS formation, MMP activation, and cell motility in human fibrosarcoma cells. J Food Sci. 2013;78:H1464-H1469. [DOI] [PubMed] [Google Scholar]
- 29. Song NR, Chung MY, Kang NJ, et al. Quercetin suppresses invasion and migration of H-Ras-transformed MCF10A human epithelial cells by inhibiting phosphatidylinositol 3-kinase. Food Chem. 2014;142:66-71. [DOI] [PubMed] [Google Scholar]
- 30. Pan HC, Jiang Q, Yu Y, Mei JP, Cui YK, Zhao WJ. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem Int. 2015;80:60-71. [DOI] [PubMed] [Google Scholar]
- 31. Vijayababu MR, Arunkumar A, Kanagaraj P, Venkataraman P, Krishnamoorthy G, Arunakaran J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol Cell Biochem. 2006;287:109-116. [DOI] [PubMed] [Google Scholar]
- 32. Hwang MK, Song NR, Kang NJ, Lee KW, Lee HJ. Activation of phosphatidylinositol 3-kinase is required for tumor necrosis factor-alpha-induced upregulation of matrix metalloproteinase-9: its direct inhibition by quercetin. Int J Biochem Cell Biol. 2009;41:1592-1600. [DOI] [PubMed] [Google Scholar]
- 33. Lai WW, Hsu SC, Chueh FS, et al. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013;33:1941-1950. [PubMed] [Google Scholar]
- 34. Zhang HH, Lechuga TJ, Tith T, Wang W, Wing DA, Chen DB. S-nitrosylation of cofilin-1 mediates estradiol-17β-stimulated endothelial cytoskeleton remodeling. Mol Endocrinol. 2015;29:434-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu A, Zhou Z, Dang R, et al. Neuroligin 1 regulates spines and synaptic plasticity via LIMK1/cofilin-mediated actin reorganization. J Cell Biol. 2016;212:449-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hammer A, Diakonova M. Prolactin-induced PAK1 tyrosyl phosphorylation promotes FAK dephosphorylation, breast cancer cell motility, invasion and metastasis. BMC Cell Biol. 2016;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zou Z, Zeng F, Xu W, et al. PKD2 and PKD3 promote prostate cancer cell invasion by modulating NF-κB- and HDAC1-mediated expression and activation of uPA. J Cell Sci. 2012;125(pt 20):4800-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karunaweera N, Raju R, Gyengesi E, Münch G. Plant polyphenols as inhibitors of NF-κB induced cytokine production-a potential anti-inflammatory treatment for Alzheimer’s disease? Front Mol Neurosci. 2015;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Das J, Ramani R, Suraju MO. Polyphenol compounds and PKC signaling. Biochim Biophys Acta. 2016;1860:2107-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang XS, Liu MY, Zhang HM, Xue BZ, Shi H, Liu DX. Protein kinase C-δ mediates sepsis-induced activation of complement 5a and urokinase-type plasminogen activator signaling in macrophages. Inflamm Res. 2014;63:581-589. [DOI] [PubMed] [Google Scholar]
- 41. Xue A, Xue M, Jackson C, Smith RC. Suppression of urokinase plasminogen activator receptor inhibits proliferation and migration of pancreatic adenocarcinoma cells via regulation of ERK/p38 signaling. Int J Biochem Cell Biol. 2009;41:1731-1738. [DOI] [PubMed] [Google Scholar]
- 42. Lee BK, Jung YS. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via pkc-ϵ inactivation/ERK1/2 activation. Oxid Med Cell Longev. 2016;2016:2495624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim W, Yang C, Park S, Bazer FW, Song G. Inhibitory effects of quercetin on progression of human choriocarcinoma cells are mediated through PI3K/AKT and MAPK signal transduction cascades [published online October 7, 2016]. J Cell Physiol. doi: 10.1002/jcp.25637. [DOI] [PubMed] [Google Scholar]
- 44. Si TL, Liu Q, Ren YF, et al. Enhanced anti-inflammatory effects of DHA and quercetin in lipopolysaccharide-induced RAW264.7 macrophages by inhibiting NF-κB and MAPK activation. Mol Med Rep. 2016;14:499-508. [DOI] [PubMed] [Google Scholar]
- 45. Rivera A, Castillo-Pichardo L, Gerena Y, Dharmawardhane S. Anti-breast cancer potential of quercetin via the Akt/AMPK/mammalian target of rapamycin (mTOR) signaling cascade. PLoS One. 2016;11:e0157251. [DOI] [PMC free article] [PubMed] [Google Scholar]