Abstract
Background: Xerostomia (dry mouth) causes many clinical problems, including oral infections, speech difficulties, and impaired chewing and swallowing of food. Many cancer patients have complained of xerostomia induced by cancer therapy. Objective: The aim of this systematic review is to assess the efficacy of herbal medicine for the treatment of xerostomia in cancer patients. Materials and Methods: Randomized controlled trials investigating the use of herbal medicines to treat xerostomia in cancer patients were included. We searched the following 12 databases without restrictions on time or language. The risk of bias was assessed using the Cochrane Risk of Bias Tool. Results: Twenty-five randomized controlled trials involving 1586 patients met the inclusion criteria. A total of 24 formulas were examined in the included trials. Most of the included trials were insufficiently reported in the methodology section. Five formulas were shown to significantly improve the salivary flow rate compared to comparators. Regarding the grade of xerostomia, all formulas with the exception of a Dark Plum gargle solution with normal saline were significantly effective in reducing the severity of dry mouth. Adverse events were reported in 4 trials, and adverse effects of herbal medicine were reported in 3 trials. Conclusions: We found herbal medicines had potential benefits for improving salivary function and reducing the severity of dry mouth in cancer patients. However, methodological limitations and a relatively small sample size reduced the strength of the evidence. More high-quality trials reporting sufficient methodological data are warranted to enforce the strength of evidence regarding the effectiveness of herbal medicines.
Keywords: xerostomia, herbal medicine, traditional East Asian medicine, randomized controlled trials, review
Introduction
Saliva, which consists of water (99%) and many electrolytes, immunoglobulins, proteins, enzymes, mucins, and nitrogenized products,1 serves important functions in maintaining the health of the oral cavity, speech, ingestion, and swallowing.2,3 Xerostomia is the subjective feeling of oral dryness, which is usually associated with insufficient saliva secretion.1,2,4 Although there are differences among individuals, normal unstimulated salivary flow rate (USF) is 0.3 to 0.4 mL/min and stimulated salivary flow rate (SSF) is 1.5 to 2.0 mL/min.5,6 If USF is less than 0.1 mL/min and/or SSF is less than 0.5 to 0.7 mL/min, salivary hypofunction is considered.5,7 Common causes of xerostomia are medications, radiation therapy, systemic diseases, and pathological changes of the salivary glands.2,4,8 Severe xerostomia increases the potential for oral infections and affects the quality of life.9
Because cancer therapies including radiotherapy can induce salivary hypofunction, xerostomia is one of the most common complaints in cancer patients. Radiation therapy in the head and neck region has damaged salivary glands, resulting in altering the volume, consistency, and pH of saliva.3 According to a recent systematic review, about 93% of head and neck cancer patients suffered dry mouth during radiation therapy, and 73.6% to 85.3% complained of xerostomia after radiotherapy ended.10 Also, chemotherapy can induce xerostomia due to its propensity to damage salivary glands.10
The present treatment of xerostomia can be divided into general supportive measures, salivary substitutes, salivary stimulants such as pilocarpine and cevimeline, salivary gland protectors such as amifostine, hyperbaric oxygen therapy, and acupuncture.1,2,4,11 These treatments have been shown to be effective for treating xerostomia, especially cholinergic agonists, which are known to be more effective than hyperbaric oxygen therapy, acupuncture, and salivary substitutes.4,11,12 However, pilocarpine and amifostine also have a potential risk of undesirable adverse effects such as sweating, nausea, diarrhea, hypotension, and dizziness.12,13
In traditional East Asian medicine (TEAM), herbal medicine has been developed based on the theories of Yin and Yang, the 5 elements and visceral manifestation theory. Herbal medicine is prescribed from pattern identification (or syndrome differentiation, pattern classification) based on the aforementioned theories and patients’ conditions.14,15 According to TEAM, xerostomia is considered Yin and thin-fluid deficiency, Yin deficiency and dry heat, Qi deficiency, internal blockage of blood stasis, an accumulation of damp heat, and phlegm accumulation with blood stasis.9,16 Based on these TEAM theories and pattern identification, herbal medicine and acupuncture therapy have been used to treat xerostomia. To our knowledge, some systematic reviews have assessed the efficacy of acupuncture for xerostomia,17-19 while a systematic review assessing herbal medicine for xerostomia has not yet been conducted. Therefore, we aimed to assess the efficacy of herbal medicine for xerostomia in cancer patients.
Methods
Search Strategy
The protocol of this systematic review has been registered in PROSPERO (CRD 42016046420; available from https://www.crd.york.ac.uk/PROSPERO/printPDF.php?RecordID=46420&UserID=23230). A systematic literature search was performed in the following electronic databases regardless of publication date or language: MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, Allied and Complementary Medicine database (AMED), the China National Knowledge Infrastructure (CNKI), the Wanfang database, and 6 Korean medical databases (Korean Studies Information, DBPIA, Korea Institute of Science Technology Information, Research Information Center for Health Database, Korean Medline, and National Assembly Library). In addition, we searched Google Scholar (http://scholar.google.co.kr/).
To identify studies, search strategies were developed and modified for each database based on the search term for MEDLINE (see Appendix A) and CNKI (see Appendix B). Studies were searched until September 12, 2016.
Eligibility Criteria for This Review
All randomized controlled trials (RCTs) testing herbal medicine for xerostomia in cancer patients were included in this review without restrictions on time or language. All adult cancer patients who were diagnosed by histological or clinical evaluation were eligible. Xerostomia was diagnosed by clinical assessment or additional investigation such as scintigraphy, USF, SSF, and sialography. All forms of herbal medicine were included. There were no limitations on the number, administration methods, dosage, or duration of treatment. The comparators were conventional therapeutic agents, placebo, or no treatment. We excluded trials using other types of herbal medicines as a comparison or those that did not use the same baseline therapy.
Types of Outcome Measures
The primary outcome measures were the objective measurement of salivary flow rates, such as USF, SSF, or other standards, and salivary gland scintigraphy. Subjective measurements with observer-based grading or patient self-reported scoring were also included.
Secondary outcome measures were the patients’ quality of life assessed by objective instruments or adverse events.
Data Collection and Assessment of Risk of Bias
Two authors (BP and HN) independently assessed the titles and abstracts of researches retrieved from electronic databases and determined their eligibility for inclusion. Hard copies of the relevant researches were retrieved, and then 2 authors (BP and HN) independently extracted the data using a standard data extraction form. The form included methodology, participants, intervention, duration of treatment, outcomes, and conclusions. The 2 authors (BP and HN) assessed the risk of bias using the Cochrane Risk of Bias Tool.20 The following items were assessed: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other bias. The evaluated domains were assessed as “Yes,” “No,” or “Unclear” according to the criteria. A consensus was reached through discussion in the event of a discrepancy.
Data Synthesis
Data analysis was performed by using Review Manager (RevMan) 5.3 software. If there was sufficient data to pool, a meta-analysis was performed using a random effects model. We used the mean difference (MD) with 95% confidence intervals (CIs) for continuous outcomes. In the case of binary outcomes, a risk ratio (RR) with 95% CI was used. In the case of publication bias and sensitivity analysis, data analysis was not performed since the studies did not meet the qualifications.
Results
Description of Studies
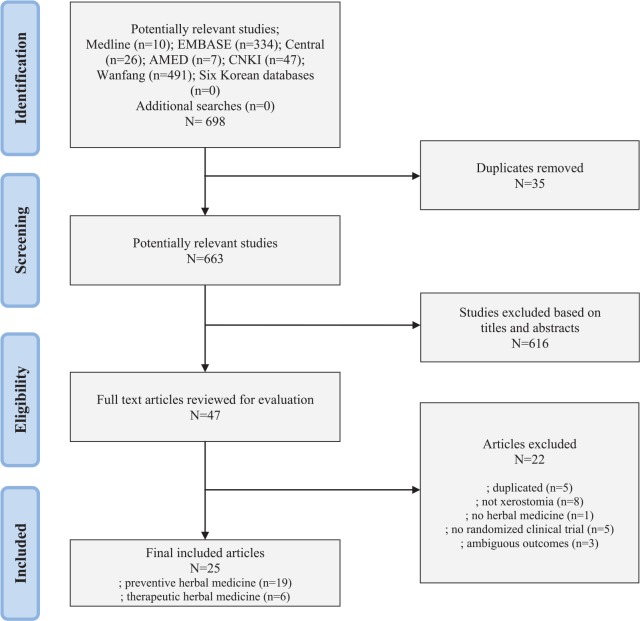
From the 11 electronic databases, 698 articles were identified (Figure 1). After excluding duplicate studies and articles that did not meet the inclusion criteria on the basis of reading the title and abstract, full texts of 47 articles were retrieved and evaluated. By browsing full text articles, 22 articles were excluded; 5 were duplicates, 8 did not concern xerostomia, 1 was not herbal medicine, 5 were not randomized, and 3 had ambiguous outcomes. As a result, a total of 25 trials were included for this review process and data analysis (see Tables 1 and 2).21-45
Figure 1.
Flow diagram of study selection.
Table 1.
Characteristics of the Included Randomized Controlled Trials of Herbal Medicines (Preventative Effect) for Xerostomia in Cancer Patients.
| Author (Year, Reference) | Sample Size (dropouts); Type of Cancer | Treatment Method |
Main Outcomes | Results | Effect Estimate (MD, 95% CI) | |
|---|---|---|---|---|---|---|
| Intervention Group (Number) | Control Group (Number) | |||||
| Herbal medicine versus symptomatic therapy | ||||||
| Zhou Y (2015a, 22) | 64 (n.r.); Head and neck cancer | (A) R-Tx. plus Yangyinqingre decoction during R-Tx. (n = 32) | (B) R-Tx. plus vitamin B12 infusion (0.5 mg/day) during R-Tx. (n = 32) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 1. Grade 0/1/2/3 | ||||||
| 0/19/11/2 vs 0/8/17/7 (P < .05) | ||||||
| He Y (2016, 23) | 70 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. and C-Tx. plus gargle solution of herbal decoction combined with plastering acupoint (n = 35) | (B) R-Tx. and C-Tx. with Dobell’s solution (n = 35) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 1. Grade 0/1/2/3/4 | ||||||
| 0/6/9/20/0 vs 0/1/2/30/2 (P < .05) | ||||||
| Herbal medicine plus symptomatic therapy versus symptomatic therapy | ||||||
| Wang Q (1998, 24) | 50 (n.r.); Head and neck cancer | (A) R-Tx. with Dobell’s gargle solution and nebulization for 6-7 weeks plus Houxueshengjin decoction bid during R-Tx. (n = 24) | (B) R-Tx. with Dobell’s gargle solution and nebulization for 6-7 weeks (n = 26) | 1. Grade of dry mouth (VAS) | (A) versus (B) | |
| 2. USF (mL/min) | 1. 1.56 vs 2.67 (P < .05) | |||||
| 3. Secretion of salivary amylase (µ ×103/min) | 2. 0.20 ± 0.10 vs 0.11 ± 0.10 (P < .01) | 2. 0.09 [0.03, 0.15] | ||||
| 3. 112.0 ± 60.9 vs 61.9 ± 49.4 (P < .01) | 3. 50.1 [19.21, 80.99] | |||||
| Lai Z (2005, 25) | 62 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. with artificial salvia plus Xuanchaishaodihuang decoction, qd or qod (n = 32) | (B) R-Tx. with artificial salvia (n = 30) | 1. Grade of dry mouth (own standard) | (A) versus (B) | |
| 2. UR of 99mTcO4 scintigraphy | 1. Incidence rate of moderate/severe | |||||
| 3. ER of 99mTcO4 scintigraphy | 1.1. End of R-Tx.: 15/32 vs 22/30 (P < .05) | |||||
| 4. Adverse effects | 1.2. 1-year after R-Tx.: 12/32 vs 20/30 (P < .05) | |||||
| 2.1. End of R-Tx.: 1.27 ± 0.41 vs 1.02 ± 0.35 (P > .05) | 2.1. 0.25 [0.06, 0.44] | |||||
| 2.2. 1-year after R-Tx.: 1.85 ± 0.50 vs 1.22 ± 0.44 (P < 0.05) | 2.2. 0.63 [0.40, 0.86] | |||||
| 3.1. End of R-Tx.: 1.88 ± 0.53 vs 1.43 ± 0.45 (P > .05) | 3.1. 0.45 [0.21, 0.69] | |||||
| 3.2. 1-year after R-Tx.: 2.95 ± 0.61 vs 1.62 ± 0.47 (P < .05) | 3.2. 1.33 [1.06, 1.60] | |||||
| 4. No adverse effects | ||||||
| Zeng M (2014, 26) | 80 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. and C-Tx. (DDP, docetaxel) with gargle solution (boric acid, lidocaine, dexamethasone, chymotrypsin) plus Shennongbaijie decoction from beginning of R-Tx. to 4 weeks after R-Tx. (n = 40) | (B) R-Tx. and C-Tx. (DDP, docetaxel) with gargle solution (boric acid, lidocaine, dexamethasone, chymotrypsin) (n = 40) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 2. USF (mL/min) | 1. Grade 1/2/3/4 | |||||
| 7/19/10/4 vs 0/11/21/8 (P < .01) | ||||||
| 2. 0.25 ± 0.13 vs 0.11 ± 0.12 (P < .01) | 2. 0.14 [0.09, 0.19] | |||||
| Herbal medicine versus no treatment (or water) | ||||||
| Hu Y (2005, 27) | 140 (n.r.); Head and neck cancer | (A) R-Tx. plus Shenqifanghou decoction bid during R-Tx. (n = 70) | (B) R-Tx. (n = 70) | 1. Grade of dry mouth (own standard) | (A) versus (B) | |
| 2. Adverse effects | 1. Slight/moderate/severe: 46/16/8 vs 9/40/21 (P < .01) | |||||
| 2. No adverse effects | ||||||
| Li H (2009, 28) | 40 (n.r.); Head and neck cancer | (A) R-Tx plus Xuanmaizengyehuadu decoction tid during R-Tx (n = 20) | (B) R-Tx. plus mineral water (n = 20) | 1. Grade of dry mouth (VAS) | (A) versus (B) | |
| 2. Salivary flow time (determined by the time of the filter paper wetted) | 1. 6.52 vs 9.95 (P < .01) | |||||
| 2. 128 ± 29 vs 221 ± 39 (P < .01) | 2. −93.00 [−114.30, −71.70] | |||||
| Wei B (2009, 29) | 46 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx and C-Tx. (DDP, 5-FU, total 1 or 2 cycles) plus Sarcandra Glabra decoction qd during R-Tx (n = 21) | (B) R-Tx. and C-Tx. (DDP, 5-FU, total 1 or 2 cycles) (n = 25) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 1. Grade 1/2/3/4 | ||||||
| 5/12/3/1 vs 0/6/15/4 (P < .01) | ||||||
| Yin L (2009, 30) | 60 (n.r.); Head and neck cancer | (A) R-Tx. for 7 weeks plus Baiying decoction tid from the beginning of R-Tx. to 4 weeks after R-Tx. (n = 30) | (B) R-Tx. for 7 weeks plus mineral water (n = 30) | 1. Grade of dry mouth (NRS) | (A) versus (B) | |
| 1. 5.8 ± 1.33 vs 6.9 ± 1.85 (P < .05) | 1. −1.10 [−1.92, −0.28] | |||||
| Zhuang M (2010, 31) | 60 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. plus nebulization of Houxueshengjin decoction qd or bid and rh-EGF qd or tid (n = 30) | (B) R-Tx. plus normal saline gargle solution (n = 30) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 1. Grade 0/1/2 | ||||||
| 17/11/1 vs 5/23/2 | ||||||
| Huang H (2011, 32) | 42 (n.r.); Lung and esophageal cancer | (A) R-Tx. plus Fuzhengjiandu granule tid during R-Tx. (n = 40) | (B) R-Tx. (n =40) | 1. Grade of dry mouth (own standard) | (A) versus (B) | |
| 2. QoL (KPS) | 1. 0.90 vs 1.93 (P < .01) | |||||
| 2. Increased more than 10/unchanged/decreased more than 10; 12/25/3 vs 8/25/7 | ||||||
| Wang L (2011, 33) | 65 (5); Head and neck cancer | (A) R-Tx. plus Zengye decoction tid for 7 weeks (n = 33) | (B) R-Tx. plus normal saline gargle solution tid for 7 weeks (n = 27) | 1. Grade of dry mouth (VAS) | (A) versus (B) | |
| 2. UR of 99mTcO4 scintigraphy | 1. Under 2.5/2.5 to 5/5 to 7.5/over 7.5 | |||||
| 3. ER of 99mTcO4 scintigraphy | 4/17/9/3 vs 2/5/12/7 (P < .01) | |||||
| 4. Adverse effects | 2. 4.07 ± 2.19 vs 3.68 ± 1.43 (P < .01) | 2. 0.39 [−.0.53, 1.31] | ||||
| 3. 0.21 ± 0.29 vs 0.11 ± 0.08 (P < .01) | 3. 0.10 [0.00, 0.20] | |||||
| 4. Leukopenia; 5 vs 7 | ||||||
| Guo Y (2012, 34) | 64 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. plus Niancianchuanqipipa gel 10 mL and Yunnanbaiyao capsule 0.5 g daily from beginning of R-Tx. to 4 weeks after R-Tx. (n = 34) | (B) R-Tx. (n = 30) | 1. Grade of dry mouth (RTOG and Nishioka T) | (A) versus (B) | |
| 2. SSF (mL/min) | 1. Grade 0/1/2/3 | |||||
| 3. USF (mL/min) | 7/17/7/3 vs 0/9/14/7 (P < .01) | |||||
| 4. UR of 99mTcO4 scintigraphy | 2. 0.92 ± 0.24 vs 0.36 ± 0.22 (P < .05) | 2. 0.56 [0.45, 0.67] | ||||
| 5. ER of 99mTcO4 scintigraphy | 3. 0.07 ± 0.05 vs 0.09 ± 0.06 (P > .05) | 3. −0.02 [−0.05, 0.12] | ||||
| 6. Adverse effects | 4. 4.00 ± 0.12 vs 3.99 ± 0.97 (P > .05) | 4. 0.04 [0.00, 0.08] | ||||
| 5. 0.15 ± 0.66 vs 0.12 ± 0.05 (P < .05) | 5. −0.86 [−0.88, −0.84] | |||||
| 6. No adverse effects | ||||||
| Huang D (2013, 35) | 100 (2); Nasopharyngeal carcinoma | (A) R-Tx. and C-Tx. (DDP, 5-FU) plus Sarcandra Glabra decoction qd during R-Tx. (n = 50) | (B) R-Tx. and C-Tx. (DDP, 5-FU) (n = 48) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 1. Grade 1/2/3 | ||||||
| 29/16/5 vs 18/19/11 (P < .01) | ||||||
| Wu X (2014, 36) | 64 (n.r.); Head and neck cancer | (A) R-Tx. plus nebulization of Kangfuxin liquid 10 g tid and vitamin C 1 g tid from beginning of R-Tx. to 30 days after R-Tx. (n = 32) | (B) R-Tx. (n = 32) with nebulization | 1. Grade of dry mouth (LENT SOMA) | (A) versus (B) | |
| 1. Grade 1/2/3/4 | ||||||
| 11/16/5/0 vs 3/11/14/4 (P < .01) | ||||||
| Yang X (2014, 37) | 60 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. plus Zengye decoction tid for 7 weeks (n = 33) | (B) R-Tx. plus normal saline gargle solution tid for 7 weeks (n = 27) | 1. Clinical effectiveness (own standard) | (A) versus (B) | |
| 1. CR/PR/NC | ||||||
| 12/15/3 vs 0/7/23 (P < .01) | ||||||
| Wang J (2015a, 38) | 60 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. plus Jiaweizengye decoction bid during R-Tx. (n = 30) | (B) R-Tx. (n = 30) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 2. SSF (mL/min) | 1. Grade 0/1/2/3/4 | |||||
| 13/10/6/1/0 vs 0/0/7/12/11 (P < .05) | ||||||
| 2. 0.54 ± 0.13 vs 0.24 ± 0.10 (P < .05) | 2. 0.30 [0.24, 0.36] | |||||
| Yu F (2015, 39) | 60 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. plus nebulization of Houxueliyan decoction qd or bid and rh-EGF qd or tid | (B) R-Tx. plus gargle solution of normal saline | 1. Grade of dry mouth (own standard) | (A) versus (B) | |
| 1. Grade 1/2/3/4 | ||||||
| 11/1/0/0 vs 13/10/2/0 (P < .05) | ||||||
| Zhang W (2015, 40) | 62 (n.r.); Nasopharyngeal carcinoma | (A) R-Tx. plus Jiaweizengye decoction from beginning of R-Tx. to 4 weeks after R-Tx. (n = 32) | (B) R-Tx. (n = 30) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 2. SSF (mL/min) | 1. Grade 0/1/2/3/4 | |||||
| 4/12/10/5/1 vs 0/0/8/10/12 (P < .05) | ||||||
| 2. 0.53 ± 0.25 vs 0.25 ± 0.18 (P < .05) | 2. 0.28 [0.24, 0.35] | |||||
Abbreviations: MD, mean difference; CI, confidence interval; n.r., not reported; R-Tx., radiation therapy; RTOG, Radiation Therapy Oncology Group; C-Tx., chemotherapy; bid, twice a day; VAS, Visual Analogue Scale; USF, unstimulated salivary flow rate; SSF, stimulated salivary flow rate; qd, once a day; qod, every other day; UR, uptake rate; ER, excretion rate; DDP, cisplatin; tid, 3 times a day; 5-FU, 5-fluorouracil; NRS, Numerical Rating Scale; Rh-EGF, recombinant human epidermal growth factor; KPS, Karnofsky Performance Scale; LENT, late effects of normal tissue; SOMA, subjective, objective, management criteria with analytic laboratory and imaging procedure.
Table 2.
Characteristics of the Included Randomized Controlled Trials of Herbal Medicines (Therapeutic Effect) for Xerostomia in Cancer Patients.
| Author (Year, Reference) | Sample Size (Dropouts); Type of Cancer | Treatment Method |
Main Outcomes | Results | Effect Estimate (MD, 95% CI) | |
|---|---|---|---|---|---|---|
| Intervention Group (Number) | Control Group (Number) | |||||
| Herbal medicine versus symptomatic therapy | ||||||
| Cao Y (2009, 41) | 41 (n.r.); Nasopharyngeal carcinoma with xerostomia | (A) Sanganhuayin decoction 6 to 10 times daily for 4 weeks (n = 23) | (B) Vitamin C 0.2 g tid for 4 weeks (n = 18) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 2. SSF (mL/min) | 1. 2.27 ± 0.67 vs 3.67 ± 0.49 (P < .01) | 1. −1.40 [−1.76, −1.04] | ||||
| 2. 0.57 ± 0.18 vs 0.23 ± 0.09 (P < .01) | 2. 0.34 [0.26, 0.42] | |||||
| Zhou Y (2015b, 42) | 28 (n.r.); Nasopharyngeal carcinoma with xerostomia | (A) R-Tx. plus Jiaweishengmai decoction during R-Tx. for 30 days (n = 14) | (B) R-Tx. Plus vitamin C 0.2 g tid for 30 days (n = 14) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 2. SSF (mL/min) | 1. 2.27 ± 0.56 vs 3.12 ± 0.56 (P < .05) | 1. −0.85 [−1.26, −0.44] | ||||
| 2. 0.52 ± 0.11 vs 0.30 ± 0.09 (P < .05) | 2. 0.22 [0.15, 0.29] | |||||
| Ameri A (2016, 43) | 75 (13); Nasopharyngeal carcinoma with xerostomia | (A) Malva sylvestris L and Alcea digitata powder for 4 weeks (n = 32) | (B) Artificial saliva for 4 weeks (n = 30) | 1. Grade of dry mouth (VAS) | (A) versus (B) | |
| 2. Grade of dry mouth (own standard) | 1. 4.24 vs 3.43 (P < .05) | |||||
| 2. No significant difference between groups | ||||||
| Herbal medicine versus no treatment (or water) | ||||||
| Zhang C (2011, 44) | 65 (n.r.); Nasopharyngeal carcinoma with xerostomia | (A) Herbal decoction based on pattern identification more than 6 months (n = 33) | (B) no treatment (n = 32) | 1. Grade of dry mouth (VAS) | (A) versus (B) | |
| 2. Grade of dry mouth (RTOG) | 1. No data | |||||
| 2. Grade 1/2/3/4 | ||||||
| 0/15/16/2 vs 0/8/19/5 (P < .05) | ||||||
| Wang W (2013, 45) | 68 (n.r.); Head and neck cancer with xerostomia | (A) Liriopes Radix tea with 3 L water daily for 2 weeks (n = 36) | (B) 3 L water daily (n = 32) | 1. Grade of dry mouth (VAS) | (A) versus (B) | |
| 1. 6.857 ± 1.418 vs 3.333 ± 1.362 (P < .01) | 1. 3.25 [2.59, 3.92] | |||||
| Wang J (2015b, 46) | 60 (7); Head and neck cancer with xerostomia | (A) Dark Plum and normal saline gargle solution tid for 7 weeks (n = 27) | (A) Normal saline gargle solution tid for 7 weeks (n = 26) | 1. Grade of dry mouth (RTOG) | (A) versus (B) | |
| 2. USF (mL/min) | 1. Grade 1/2/3/4 | |||||
| 3. SSF (mL/min) | 1/4/13/9 vs 1/4/9/12 (P > .05) | |||||
| 2. 0.12 ± 0.054 vs 0.10 ± 0.0832 (P > .05) | 2. 0.02 [−0.02, 0.06] | |||||
| 3. 0.19 ± 0.084 vs 0.15 ± 0.089 (P > .05) | 3. 0.04 [−0.01, 0.09] | |||||
Abbreviations: MD, mean difference; CI, confidence interval; n.r., not reported; RTOG, Radiation Therapy Oncology Group; tid, 3 times a day; SSF, stimulated salivary flow rate; R-Tx., radiation therapy; VAS, Visual Analogue Scale; USF, unstimulated salivary flow rate.
Characteristics of Included Studies
A total of 1586 cancer patients were included, and 22 patients dropped out. Nine trials tested patients with head and neck cancer, 15 trials studied nasopharyngeal cancer, and 1 trial investigated lung and esophageal cancer. Two trials did not report that baseline characteristics were comparable between the intervention group and the control group.36,44 The mean sample size was 63.44 patients (range = 28-140 patients), but no trial reported the calculation sequence of the sample size. The duration of treatment ranged from 2 weeks to 6 months.
Twenty-four formulations in 25 trials were investigated. Some form of decoction was used in 22 trials (Table 3). Of the 22 trials, 17 trials applied decoction orally, 2 trials applied herbal medicine through gargling, and 3 trials used nebulization. Each granule, powder, liquid, capsule, and gel was tested in 3 trials. Standardized formulations were tested in 23 trials, one trial chose 1 of 3 herbal formulas based on the individuals’ pattern identification,43 and the other trial used a standardized formula with herbs added according to the individuals’ pattern identification.36 Of the 24 formulations, Jiaweizengye decoction37,39 and Sarcandra glabra decoction28,35 were tested in 2 trials each.
Table 3.
Preparation of Herbal Formulas for Xerostomia in This Review.
| First Author (Year) | Name of Herbal Medicines | Compositions (g) |
|---|---|---|
| Wang Q (1998) | Houxue shengjin decoction | Polygonati Odorati Rhizoma (30), Liriopes Radix (20), Persicae Semen (24), Dendrobii Herba (30), Lycii Fructus (30), Rehmanniae Radix (40), Rehmanniae Radix Preparat (40), Panaciis Quinquefolii Radix (30), Carthami Flos (20), Ligustici Rhizoma (20) |
| Hu Y (2005) | Shenqifanghou decoction | Codonopsis Pilosulae Radix (30), Astragali Radix (30), Poria (30) Dioscoreae Rhizoma (30), Oldenlandiae Diffusae Herba (30), Scutellaria Herba (30), Puerariae Radix (30), Polygonati Odorati Rhizoma (10), Ligustri Lucidi Fructus (10), Bombycis Corpus cum Batryticatus (10), Tribuli Fructus (10), Acori Graminei Rhizoma (10), Atractylodis Rhizoma Alba (10), Coicis Semen (50), Citri Pericarpium (6), 6g, Paridis Rhizoma (20), Scrophulariae Radix (15), Anemarrhenae Rhizoma (15), Uncariae Ramulus cum Uncis (15), Scorpio (5), Notoginseng Radix (5), Glycyrrhizae Radix (5) |
| Lai Z (2005) | Chaishaodihuang decoction | Rehmanniae Radix (24), Corni Fructus (12), Dioscoreae Tuber (12), Moutan Cortex (12), Alismatis Rhizoma (9), Poria (9), Bupleuri Radix (9), Schizandrae Fructus (6), Paeoniae Radix Alba (9), Cinnamomi Cortex Spissus (6) |
| Cao Y (2009) | Sanganhuayin decoction | Mume Fructus (10), Schizandrae Fructus (6), Paeoniae Radix Alba (15), Glycyrrhizae Radix (10), Rehmanniae Radix (15), Liriopes Radix (15), Dendrobii Herba (15), Pseudostellariae Radix (15), Lycii Fructus (15), Ligustri Lucidi Fructus (15), Puerariae Radix (15), Ligustici Rhizoma (6), Moutan Cortex (10), Bombycis Corpus cum Batryticatus (10) Pheretimae Corpus (15) |
| Li H (2009) | Xuanmaizengyehuadu decoction | Astragali Radix (30), Panaciis Quinquefolii Radix (5), Scrophulariae Radix (15), Liriopes Radix (15), Lycii Fructus (15), Polygonati Odorati Rhizoma (10), Corni Fructus (10), Dendrobii Herba (15), Houttuyniae Herba (30), Oldenlandiae Diffusae Herba (20), Scorpio (5), Notoginseng Radix (5), Glycyrrhizae Radix (10) |
| Wei B (2009) | Sarcandra Glabra decoction | Sarcandra Glabra (20) |
| Yin L (2009) | Baiying decoction | Solani Herba, Lilii Bulbus, Cordyceptis Vermis, Asparagi Radix, Houttuyniae Herba (dosage not available) |
| Zhuang M (2010) | Houxueshengjin decoction | Platycodi Radix (10), Arctii Fructus (10), Paeoniae Radix Rubra (15), Sophorae Tonkinensis Radix (15), Paridis Rhizoma (15), Glycyrrhizae Radix (3) |
| Huang H (2011) | Fuzhengjiandu granule | Astragali Radix, Rehmanniae Radix, Sanguisorbae Radix, Scutellariae Radix, Trichosanthis Radix |
| Wang L (2011) | Zengye decoction | Scrophulariae Radix (20), Liriopes Radix (20), Rehmanniae Radix (20), Lonicerae Flos (15), Oldenlandiae Diffusae Herba (15), Platycodi Radix (15), Belamcandae Rhizoma (10), Trichosanthis Radix (15), Atractylodis Rhizoma Alba (15), Glycyrrhizae Radix (5) |
| Zhang C (2011) | Herbal decoction based on pattern identification | (1) Clear heat nourish yin method: Rehmanniae Radix, Scrophulariae Radix, Liriopes Radix, Terminaliae Fructus, Cicadae Periostracum, Sterculiae Semen, Adenophorae Radix, Isatidis Radix, Taraxci Herba (dosage not available) |
| (2) Fortify the spleen nourish yin method: Codonopsis Pilosulae, Radix Atractylodis, Rhizoma Alba, Poria, Aucklandiae Radix, Amomi Fuctus, Polygonati Rhizoma, Isatidis Radix, Glycyrrhizae, Radix Praeparata (dosage not available) | ||
| (3) Nourish the kidney nourish yin method: Rehmanniae Radix, Dioscoreae Rhizoma, Corni Fructus, Scrophulariae Radix, Glehniae Radix, Liriopes Radix, Schizandrae Fructus (dosage not available) | ||
| Guo Y (2012) | Yunnanbaiyao capsule | Ajuga Forrestii Diels 85 mg, Dioscoreae Parviflora Ting 30 mg, Herba Geranli & Herba Erodii 36 mg, Herba Inulae Cappae 25 mg, Rhizoma Dioscoreae Nipponicae 57.5 mg, Rhizoma Dioscoreae 66.5 mg, Radix Notoginseng 200 mg, Proprietary Blend 500 mg |
| Ninjiompeipakoa | Fritillariae Cirrhosae Bulbus, Eriobotryae Folium, Adenophorae Radix, Poria, Citri Rubrum Exocarpium, Platycodi Radix, Pinelliae Rhizoma, Schizandrae Fructus, Trichosanthis Semen, Farfarae Flos, Polygalae Radix, Armeniacae Semen, Zingiberis Rhizoma Recens, Glycyrrhizae Radix (dosage not available) | |
| Huang D (2013) | Sarcandra Glabra decoction | Sarcandra Glabra (20) |
| Wang W (2013) | Liriopes Radix tea | Liriopes Radix (20) |
| Wu X (2014) | Kangfuxin liquid | An ethanolic extract of Periplanetae (dosage not available) |
| Yang X (2014) | Zengye decoction | Scrophulariae Radix (30), Liriopes Radix (24), Rehmanniae Radix (24) |
| (1) Addition based on pattern identification: Polygonati Odorati Rhizoma, Puerariae Radix, Trichosanthis Radix, Dendrobii Herba, Phragmitis Rhizoma, Lonicerae Flos, Scutellariae Radix, Forsythiae Fructus | ||
| Zeng M (2014) | Shennongbaijie decoction | Baijie (10), Gynostemmae Herba (10), Paederiae Herba 10 g, Lonicerae Flos (10), Isatidis Radix (10), Chrysanthemi lndici Flos (10), Lophatheri Herba (10), Ginseng lead (6), Scoparia dulcis L (6), Cordyceptis Vermis (10), Glycyrrhizae Radix (6) |
| Wang J (2015a) | Jiaweizengye decoction | Glycyrrhizae Radix (15), Trichosanthis Radix (15), Scrophulariae Radix (15), Liriopes Radix (15), Adenophorae Radix (15), Dendrobii Herba (20), Mume Fructus (20), Puerariae Radix (30), Rehmanniae Radix (30) |
| Wang J (2015b) | Dark Plum gargle | Mume Fructus (30), Glycyrrhizae Radix (6) |
| Yu F (2015) | Houxueliyan decoction | Sophorae Tonkinensis Radix (15), Paeoniae Radix Rubra (15), Paridis Rhizoma (15), Arctii Fructus (10), Platycodi Radix (10), Glycyrrhizae Radix (3) |
| Zhang W (2015) | Jiaweizengye decoction | Scrophulariae Radix (15), Rehmanniae Radix (30), Liriopes Radix (15), Dendrobii Herba (20), Adenophorae Radix (15), Trichosanthis Radix (15), Puerariae Radix (30), Glycyrrhizae Radix (15), Mume Fructus (20) |
| Zhou Y (2015a) | Yangyinqingre mixture | Adenophorae Radix (15), Glehniae Radix (15), Astragali Radix (30), Trichosanthis Radix (20), Rehmanniae Radix (15), Prunellae Spica (10), Scrophulariae Radix (15), Lilii Bulbus (10), Lonicerae Flos (20), Liriopes Radix (15), Asparagi Radix (10) |
| Zhou Y (2015b) | Jiaweishengmai decoction | Ginseng Radix (10), Liriopes Radix (15), Schizandrae Fructus (10), Adenophorae Radix (30), Belamcandae Rhizoma (10), Phyllostachydis Folium (10), Rehmanniae Radix (30), Dendrobii Herba (20), Trichosanthis Radix (15), Lonicerae Flos (20), Glycyrrhizae Radix Praeparata (6) |
| Ameri A (2016) | Malva sylvestris L and Alcea digitata powder | Malva sylvestris L (2), Alcea digitata (2) |
| He Y (2016) | Herbal gargle | Lonicerae Flos (15), Forsythiae Fructus (15), Scutellariae Radix (10), Bupleuri Radix (10), Scrophulariae Radix (10), Gynostemmae Herba (15), Ilicis Radix (20) |
Symptomatic treatments, which were artificial saliva,24,42 Dobell’s solution,22,23 vitamin B12,21 vitamin C,40,41 and gargling with boric acid, lidocaine, dexamethasone, and chymotrypsin25 were used as comparators. Nineteen trials investigated preventive effects for xerostomia during radiotherapy with or without chemotherapy.21-39 Of the 19 trials, 2 trials compared herbal medicine with symptomatic treatments,21,22 3 trials compared herbal medicine plus symptomatic treatments with symptomatic treatments,23-25 and 14 trials compared herbal medicine with water supplementation or no treatment.26-39 Six trials studied the therapeutic effects of herbal medicine in cancer patients with xerostomia.40-45 Of them, 3 trials compared herbal medicine with symptomatic treatments,40-42 and 3 trials compared herbal medicine with water supplementation or no treatment.43-45
Regarding outcome measurements, 9 trials reported salivary flow rate,23,25,27,33,37,39-41,45 3 trials reported the uptake rate (UR) and excretion rate (ER) of scintigraphy,24,32,33 all trials reported the grade of xerostomia or clinical improvement, and 1 trial reported the patients’ quality of life. Only 4 trials reported adverse events.24,26,32,33
Risk of Bias in Included Studies
Four trials mentioned the appropriate generation of a random sequence.22,32,37,45 Two trials reported inadequate randomization methods.21,25 Six trials reported concealment using envelopes; however, only one of these mentioned using a sealed, opaque envelope.45 All studies had a high risk of bias in the blinding of participants and personnel, because no details were provided about a double-blind test. Regarding incomplete outcome data, 4 trials reported dropouts.32,34,42,45 Because dropouts were not clearly reported, we assessed 21 trials as “unclear risk of bias” in spite of the inclusion of outcome data. Additionally, one trial conducted “as-treated analysis,” despite substantial dropouts in the experimental group.42 Since none of the trials were found to have preregistered protocols in the electronic databases, we basically assessed “selective reporting” as “unclear risk of bias.” By checking the methods and results sections from each trial, we assessed that 2 trials reported inappropriate outcomes that were not prespecified in the methods,26,34 and 2 trials did not report the results that were mentioned in the methods or reported inaccurate results.30,45 In addition, 2 trials did not report the participants’ characteristics, which were sex, age, and type of cancer36,44 (Figure 2).
Figure 2.
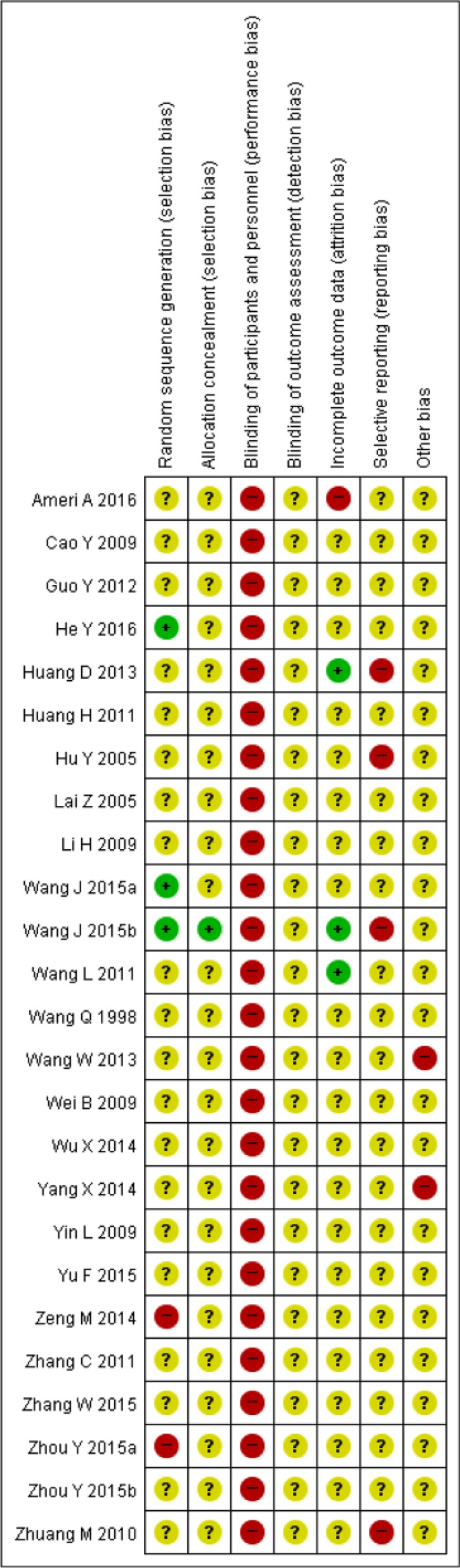
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. “+”: low risk of bias; “?”: unclear risk of bias; “-”: high risk of bias.
Effects of Interventions
The analyzed data from the 25 included trials were divided into 2 categories:
Preventative category: Herbal medicine was investigated to prevent xerostomia in cancer patients receiving radiation therapy with or without chemotherapy (19 RCTs; Table 1).
Therapeutic category: Herbal medicine was investigated as a therapeutic agent in cancer patients diagnosed with xerostomia (6 RCTs; Table 2).
The 19 trials that investigated herbal medicine as preventive agents against xerostomia were analyzed as 3 subgroups: herbal medicine versus symptomatic therapy; herbal medicine plus symptomatic therapy versus symptomatic therapy; and herbal medicine versus no treatment or water supplementation. In addition, the 6 trials testing herbal medicines as therapeutic agents were analyzed as 2 subgroups: herbal medicine versus symptomatic therapy and herbal medicine versus no treatment or water supplementation. We performed meta-analysis for the trials investigating Jiaweizengye decoction,37,39 while the other 23 trials were evaluated descriptively since different herbal formulas or different outcome measures were used.
Preventive Effects: Herbal Medicine Versus Symptomatic Therapy
Two trials investigated herbal medicine in comparison with symptomatic therapy during radiation therapy with or without chemotherapy.21,22 Two trials reported the grade of dry mouth according to Radiation Therapy Oncology Group (RTOG) criteria.21,22 Yangyinqingre decoction and gargling with an herbal decoction were shown to have a significant effect on improving the severity of xerostomia, compared to vitamin B12 and Dobell’s solution, respectively. The salivary flow rate, scintigraphy, and the quality of life were not reported.
Preventive Effects: Herbal Medicine Plus Symptomatic Therapy Versus Symptomatic Therapy
Three trials were conducted to evaluate the preventive effects of herbal medicine plus symptomatic therapy in comparison with symptomatic therapy while receiving radiotherapy with or without chemotherapy.23-25 Two trials reported on salivary flow rate.23,25 Houxueshengjin decoction (mL/min, MD 0.09; 95% CI 0.03 to 0.15) and Shennongbaijie decoction (mL/min, MD 0.14; 95% CI 0.09 to 0.19) were found to have a significant effect on improving USF when compared with the control group. In addition, Houxueshengjin decoction was found to improve the secretion of salivary amylase (µ ×103/min, MD 50.1; 95% CI 19.21 to 80.99). One trial reported the UR and ER of 99mTcO4 scintigraphy.24 The results showed that the UR and ER were significantly higher in the Xuanchaishaodihuang decoction group than in the control group 1 year after radiation therapy (UR: MD 0.63, 95% CI 0.40 to 0.86; ER: MD 1.33, 95% CI 1.06 to 1.60). Three trials reported the grade of dry mouth using different standards.23-25 Houxueshengjin decoction, Xuanchaishaodihuang decoction, and Shennongbaijie decoction were found to have a significant effect on lowering the severity of xerostomia in the intervention group compared with the control group. The quality of life was not reported.
Preventive Effects: Herbal Medicine Versus No Treatment or Water Supplementation
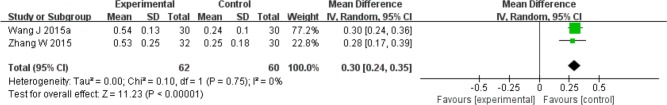
Fourteen trials tested the preventive effects of herbal medicine compared to no treatment or water supplementation during radiation therapy with or without chemotherapy.26-39 Administered herbal formulas were Shenqifanghou decoction, Xuanmaizengyehuadu decoction, Sarcandra glabra decoction, nebulization of Houxueshengjin decoction, Fuzhengjiandu granule, Zengye decoction, Yunnanbaiyao capsule combined with Niancianchuanqipipa gel, nebulization of Kangfuxin liquid, and Jiaweizengye decoction. Four trials reported on the salivary flow rate.27,33,37,39 The meta-analysis of 2 trials comparing Jiaweizengye decoction with no treatment showed a significant effect in improving SSF (mL/min, MD 0.30; 95% CI 0.24 to 0.35; Figure 3).37,39 Yunnanbaiyao capsule combined with Niancianchuanqipipa gel was found to significantly improve SSF (mL/min, MD 0.56; 95% CI 0.45 to 0.67), while no significant difference was found in the USF compared to no treatment. In addition, Xuanmaizengyehuadu decoction significantly decreased salivary flow time compared to mineral water (seconds, MD −93.00; 95% CI −114.30 to −71.70). Two trials presented the UR and ER of 99mTcO4 scintigraphy.32,33 One trial testing Zengye decoction32 reported that the UR and ER were significantly higher in the intervention group compared to no treatment (UR: MD 0.39, 95% CI −0.53 to 1.31; ER: MD 0.10, 95% CI 0.00 to 0.20). The other trial investigating Yunnanbaiyao capsule combined with Niancianchuanqipipa gel did not show a significant difference in the UR and ER when compared to no treatment. Fourteen trials reported the grade of dry mouth using RTOG criteria; the Late Effects of Normal Tissue (LENT)–subjective, objective, management criteria with analytic laboratory and imaging procedure (SOMA) criteria; the Visual Analogue Scale (VAS); the Numerical Rating Scale (NRS); and the trials’ own standards.26-39 All trials reported a significant effect of lowering the severity of xerostomia in the intervention groups compared to the control groups. Regarding the quality of life, no significant difference was found between the 2 groups on the Karnofsky performance scale for Fuzhengjiandu granule.31
Figure 3.
Forest plot for stimulated salivary flow rate: Jianweizengye decoction versus no treatment.
Therapeutic Effects: Herbal Medicine Versus Symptomatic Effects
Three trials investigated the therapeutic effects of herbal medicine compared to symptomatic therapy in cancer patients with xerostomia.40-42 Regarding the salivary flow rate, Jiaweishengmai decoction was tested in comparison with vitamin C, and a significant improvement of SSF was found (mL/min, MD 0.22; 95% CI 0.15 to 0.29).41 Three trials reported on the grade of dry mouth using RTOG or VAS, with the severity of dry mouth significantly reduced.40-42 Scintigraphy and the quality of life were not reported.
Therapeutic Effects: Herbal Medicine Versus No Treatment (or Water Supplementation)
Three trials investigated the therapeutic effects of herbal medicine compared to no treatment or water supplementation in cancer patients with xerostomia.43-45 One trial reported on the salivary flow rate.45 A gargling solution of Dark Plum with normal saline was found to make no significant difference in the USF and SSF compared to gargling with normal saline. Three trials reported on the grade of dry mouth using RTOG or VAS.43-45 Herbal formulas based on pattern identification and Liriopes Radix tea were shown to have a significant effect on reducing the severity of dry mouth, while a gargling solution of Dark Plum with normal saline did not. Scintigraphy and the quality of life were not reported.
Adverse Events
Four trials reported adverse events.24,26,32,33 One trial reported 5 cases of leukopenia in the intervention group compared to 7 in the control group,32 while 3 trials reported no adverse events. The remaining 21 trials did not provide information about adverse effects.
Discussion
We identified 25 trials that included 1586 cancer patients on the preventive or therapeutic effectiveness of herbal medicines against xerostomia, used either individually or combined with symptomatic therapies. Four main outcomes were analyzed to assess the effectiveness of herbal medicines compared to different comparators. From this review, 5 formulas, namely, Shennongbaijie decoction, Xuanmaizengyehuadu decoction, Yunnanbaiyao capsule with Niancianchuanqipipa gel, Jiaweizengye decoction, and Sanganhuayin decoction, were shown to significantly improve the salivary flow rate compared to comparators. In addition, Xuanchaishaodihuang decoction, Zengye decoction,32 and Yunnanbaiyao capsule with Niancianchuanqipipa gel were found to significantly affect, either partially or entirely, the UR and ER of scintigraphy. Regarding the grade of xerostomia, all formulas, with the exception of a gargling solution of Dark Plum with normal saline, showed significant effectiveness for reducing the severity of dry mouth. Adverse events were reported in 4 trials, with one reporting adverse effects from herbal medicine. The adverse events were relatively rare; however, we cannot conclude that the use of herbal medicines for xerostomia is safe since 21 of the 25 included trials did not report on adverse events. The documentation of adverse effects should be fully compliant to draw conclusions on safety.
Symptomatic therapies used for comparison in this review were artificial saliva, Dobell’s solution, vitamin B12, vitamin C, and gargling with boric acid, lidocaine, dexamethasone, and chymotrypsin. As already known, conventional therapies for xerostomia consist of salivary substitutes, salivary stimulants such as pilocarpine and cevimeline, and salivary gland protectors such as amifostine. To assess more accurately the effects of herbal medicine, it is necessary to use other conventional medicines that are known to be more effective than the symptomatic therapies used in this review.
For the number of trials included in this review, relatively many formulas were investigated. In fact, individualized herbal medicine is a representative feature of TEAM. Based on pattern identification, diverse herbal medicines are prescribed for the same disease. Furthermore, modifying the herbs in a particular formula is very common. These features, which are diverse formulas, forms, and medication methods, made it difficult to conduct meta-analyses. Thus, we assessed the evidence in accordance with each formula individually, instead of conducting an integrative analysis of all the herbal medicines.
From this review, we can assume that herbal medicines used individually or in combination with other therapies potentially have preventive or therapeutic effects for xerostomia in cancer patients. However, some limitations make it difficult to draw clear conclusions. First, most of the included trials had methodological limitations. The biggest problem was that most trials provided insufficient data to assess methodological quality. Information on randomization sequencing, allocation concealment, blinding, and dropouts was deficient. Consequently, we thought that potential biases might have occurred in selection, performance, attrition, and reporting. Second, due to clinical heterogeneity induced by the different herbal formulas tested in each trial, it was not possible to draw conclusions for global herbal medicine. Furthermore, the sample size was too small to draw conclusions about each herbal formula for clinical practitioners.
Conclusions
Herbal medicines were found to potentially improve salivary function and to reduce the severity of dry mouth in cancer patients, and they were shown to be relatively safe. However, methodological limitations and a relatively small sample size reduced the strength of the evidence. In the future, more high-quality trials reporting sufficient methodological data, more clinically homogeneous trials, and further evidence of safety are warranted to draw definitive conclusions concerning the effectiveness of herbal medicines.
Appendix A
MEDLINE (via Ovid) Search Strategy
#1. exp Neoplasms/
#2. (cancer or oncolog* or neoplasm* or malignan* or tumor or tumour or carcinoma* or adenocarcinoma* or osteosarcoma* or sarcoma* or leukemi* or lymphoma* or teratoma* or metastat* or (head and neck neoplasms) or radiation or irradiation or radiotherapy or chemotherapy or (antineoplastic agents)).mp.
#3. #1 or #2
#4. Exp Xerostomia/
#5. ((Dry mouth) or (oral dryness) or xerostomia or hyposalivation or (salivary gland dysfunction)).mp.
#6. #4 or #5
#7. exp Drugs, Chinese herbal/
#8. exp Herbal Medicine/
#9. exp Plants, Medicinal/
#10. exp Medicine, Chinese Traditional/
#11. (traditional Korean medicine or traditional Chinese medicine or Traditional oriental medicine or Kampo medicine or alternative medicine or complementary medicine or herb* or herbal* or decoction* or botanic*).mp.
#12. or/#7 - #11
#13. exp Randomized Controlled Trials as Topic/
#14. exp controlled clinical trials as topic/
#15. (randomized controlled trial* or controlled clinical trial$ or randomized* or randomly* or placebo or clinical trial* or controlled trial*).mp.
#16. or/#13 - #15
#17. #3 and #6 and #12 and #16
Appendix B
CNKI Search Strategy
(癌 or 恶性 or 肿瘤 or 肉瘤 or 白血病 or 淋巴瘤 or 放疗 or 化疗) and (口腔干燥 or 口干症 or 口干燥症 or 口內干燥) and (中医药 or 中医 or 中西医结合 or 汉方 or 东洋医学 or 中药 or 中草药 or 汤 or 丸 or 散 or 注射液 or 口服液 or 中成药 or 饮) and (随机 or 对照)
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Farre M. Salivary secretory disorders, inducing drugs, and clinical management. Int J Med Sci. 2015;12:811-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Napenas JJ, Brennan MT, Fox PC. Diagnosis and treatment of xerostomia (dry mouth). Odontology. 2009;97:76-83. [DOI] [PubMed] [Google Scholar]
- 3. Bhide SA, Miah AB, Harrington KJ, Newbold KL, Nutting CM. Radiation-induced xerostomia: pathophysiology, prevention and treatment. Clin Oncol (R Coll Radiol). 2009;21:737-744. [DOI] [PubMed] [Google Scholar]
- 4. Visvanathan V, Nix P. Managing the patient presenting with xerostomia: a review. Int J Clin Pract. 2010;64:404-407. [DOI] [PubMed] [Google Scholar]
- 5. Villa A, Wolff A, Aframian D, et al. World Workshop on Oral Medicine VI: a systematic review of medication-induced salivary gland dysfunction: prevalence, diagnosis, and treatment. Clin Oral Investig. 2015;19:1563-1580. [DOI] [PubMed] [Google Scholar]
- 6. Greenfield S, Aronow HU, Elashoff RM, Watanabe D. Flaws in mortality data. The hazards of ignoring comorbid disease. JAMA. 1988;260:2253-2255. [PubMed] [Google Scholar]
- 7. Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50:140-161. [DOI] [PubMed] [Google Scholar]
- 8. Tanasiewicz M, Hildebrandt T, Obersztyn I. Xerostomia of various etiologies: a review of the literature. Adv Clin Exp Med. 2016;25:199-206. [DOI] [PubMed] [Google Scholar]
- 9. Murakami M, Wei MX, Ding W, Zhang QD. Effects of Chinese herbs on salivary fluid secretion by isolated and perfused rat submandibular glands. World J Gastroenterol. 2009;15:3908-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen SB, Pedersen AM, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18:1039-1060. [DOI] [PubMed] [Google Scholar]
- 11. Shiboski CH, Hodgson TA, Ship JA, Schiodt M. Management of salivary hypofunction during and after radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(suppl):S66.e61-19. [DOI] [PubMed] [Google Scholar]
- 12. Lovelace TL, Fox NF, Sood AJ, Nguyen SA, Day TA. Management of radiotherapy-induced salivary hypofunction and consequent xerostomia in patients with oral or head and neck cancer: meta-analysis and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:595-607. [DOI] [PubMed] [Google Scholar]
- 13. Koukourakis MI, Maltezos E. Amifostine administration during radiotherapy for cancer patients with genetic, autoimmune, metabolic and other diseases. Anticancer Drugs. 2006;17:133-138. [DOI] [PubMed] [Google Scholar]
- 14. Lu A, Bensoussan A, Liu J, Bian Z, Cho WC. TCM Zheng classification and clinical trials. Evid Based Complement Alternat Med. 2013;2013:723659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang M, Lu C, Zhang C, et al. Syndrome differentiation in modern research of traditional Chinese medicine. J Ethnopharmacol. 2012;140:634-642. [DOI] [PubMed] [Google Scholar]
- 16. Hsu PY, Yang SH, Tsang NM, et al. Efficacy of traditional Chinese medicine in xerostomia and quality of life during radiotherapy for head and neck cancer: a prospective pilot study. Evid Based Complement Alternat Med. 2016;2016:8359251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jedel E. Acupuncture in xerostomia–a systematic review. J Oral Rehabil. 2005;32:392-396. [DOI] [PubMed] [Google Scholar]
- 18. Zhuang L, Yang Z, Zeng X, et al. The preventive and therapeutic effect of acupuncture for radiation-induced xerostomia in patients with head and neck cancer: a systematic review. Integr Cancer Ther. 2013;12:197-205. [DOI] [PubMed] [Google Scholar]
- 19. O’Sullivan EM, Higginson IJ. Clinical effectiveness and safety of acupuncture in the treatment of irradiation-induced xerostomia in patients with head and neck cancer: a systematic review. Acupunct Med. 2010;28:191-199. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Y, Tang Y. Application research of Yangyinqingre mixture for teatment of xerostomia after radiotherapy of head and neck cancer. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu. 2015;13:52-53. [Google Scholar]
- 22. He Y, Yao J, Liang F. Evaluation of efficacy of an herbal compound on dry mouth in patients with head and neck cancers: a randomized clinical trial. Hushi Jinxiu Zazhi. 2016;31:1348-1350. [DOI] [PubMed] [Google Scholar]
- 23. Wang Q, Liu H, Qiao N. Effects of traditional Chinese medicine on salivary glands in the patients with head and neck cancer during radiotherapy. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;18:662-664. [PubMed] [Google Scholar]
- 24. Lai Z, Cheng Z, Xiao Y, Yu Z. The effect of artificial saliva and Chinese traditional medicine on prevention and treatment of radioactive xerostomia. Zhonghua Zhongliu Zazhi. 2005;35:973-975. [Google Scholar]
- 25. Zeng M, Chen Q, Liu Z, Cheng H. Clinical observation of Shennong baijie decoction in prevention and treatment of radioactive dry mouth disease. Hainan Yixue. 2014;25:2069-2071. [Google Scholar]
- 26. Hu Y, Wu C, Liu Y, et al. Clinical observation on effect of Shenqi Fanghou recipe in preventing and treating radiation injury in patients with head and neck tumor. Zhongguo Zhongxiyi Jiehe Zazhi. 2005;25:623-625. [PubMed] [Google Scholar]
- 27. Li H, Cheng HZ, Hu YW, Tian DL, Yu ZC, Wang HF. Clinical outcome of traditional Chinese medicine for patients with xerostomia during radiotherapy in head and neck cancer. Liaoning Zhongyi Zazhi. 2009;36:1355-1357. [Google Scholar]
- 28. Wei B, Wang R, Qin J, Zhang Y, Teng J. The clinical observation of Sarcandra Glabra extracts against oxidative damage induced by radiation. Guangxi Yike Daxue Xuebao. 2009;26:206-208. [Google Scholar]
- 29. Yin L, Huang X, Zhou R. Clinical study of prevention and treatment on head and neck neoplasms radiotherapy injury with baiyingtang. Shizhen Guoyi Guoyao Zazhi. 2009;20:2827-2828. [Google Scholar]
- 30. Zhuang M, Wang W, Huang Q. Lixuetang and Ginyinati in the treatment of mucositis and dry mouth syndrome. Xiandai Yiyuan. 2010;10:28-30. [Google Scholar]
- 31. Huang H, Guo M, Xu P, Wei P. The effect of Chinese herbal medicine in the treatment of lung and esophagus cancer treated with radiotherapy. Xiandai Zhougliu Yixue. 2011;19:1134-1136. [Google Scholar]
- 32. Wang L. Clinical Study of Effect of Zengye Fluid to Xerostomia in Patients With Nasopharyngeal Carcinoma After Radiation Therapy and Quantitative Evaluation of Salivary Gland Function by SPECT Radioactive Dynamic Imaging [dissertation]. Guangzhou: Guangzhou University of Chinese Medicine; 2011. [Google Scholar]
- 33. Guo Y, Yang Y, Ma C, Qi J, Jiao Z. Clinical observation of Niancianchuanqipipa gel and Yunnanbaiyao capsule in prevention and treatment of radiation-induced salivary gland dysfunction. Yiyao Fangzhi Zhongliu Xueshu Nianhui. 2012:247-253. [Google Scholar]
- 34. Huang D, Huang H, Lu Y. Clinical observation of Sarcandra Glabra combined chemoradiotherapy for treating patients with local advanced nasopharyngeal carcinoma. Zhongguo Zhongxiyi Jiehe Zazhi. 2013;33:456-458. [PubMed] [Google Scholar]
- 35. Wu X, Yang Q, Jiang L, Yang W, Liu X. Vitamin C and Kangfuxin liquid alleviate radiation-induced oral mucosal injury in patients with neck and head cancer under radiotherapy. Shiyong Zhongliu Zazhi. 2014;29:466-469. [Google Scholar]
- 36. Yang X, Li J, Wu L. Clinical observation of therapeutic of Zengye fluid to radiation-induced xerostomia. Shiyong Zhongyiyao Zazhi. 2014;30:398. [Google Scholar]
- 37. Wang J, Zhan J, Li X. Efficacy of Jiaweizengye decoction for the treatment of xerostomia after radiotherapy in patients with nasopharyngeal carcinoma. Zhongguo Weishen Biaozhun Guanli. 2015;6:123-125. [Google Scholar]
- 38. Yu F. Efficacy of Houxueliyan decoction and Ginyinati in the treatment of mucositis and xerostomia. Quanke Kouqiang Yizue Zazhi. 2015;2:117-118. [Google Scholar]
- 39. Zhang W, Zhang M. Observation of Jiaweizengye decoction for treatment of xerostomia after radiation therapy in nasopharyngeal carcinoma patients. Xiandai Zhongxiyi Jiehe Zazhi. 2015;24:308-309. [Google Scholar]
- 40. Cao Y, Cai C, Wei B, Chen L. Effect of ‘sour and sweet flavors transforming into Yin’ therapy on xerostomia in patients with nasopharyngeal carcinoma after radiotherapy. Zhongguo Yiyao Daobao. 2009;6:50-51. [Google Scholar]
- 41. Zhou Y. Observation of Jiaweishengmai decoction for treatment of radiation-therapy induced xerostomia in 28 patients of nasopharyngeal carcinoma. Shiyong Zhongyiyao Zazhi. 2015;31:901-902. [Google Scholar]
- 42. Ameri A, Heydarirad G, Rezaeizadeh H, Choopani R, Ghobadi A, Gachkar L. Evaluation of efficacy of an herbal compound on dry mouth in patients with head and neck cancers: a randomized clinical trial. Evid Based Complement Alternat Med. 2016;21:30-33. [DOI] [PubMed] [Google Scholar]
- 43. Zhang C, Zeng C, Huang Z, Li C, Wu X, Zhang X. Clinical observation treatment of xerostomia after radiotherapy of nasopharyngeal carcinoma with Chinese drugs. Zhongguo Zhongliu Linchuang Yu Kangfu. 2011;18:374-376. [Google Scholar]
- 44. Wang W. Effects of Ophiopogon japonicus tea on radiotherapy induced xerostomia. Tianjin Huli. 2013;21:211. [Google Scholar]
- 45. Wang J. Clinical Study of Gargle of Dark Plum to Postradiation Xerostomia in Patients With Head and Neck Tumors [dissertation]. Guangzhou: Guangzhou University of Chinese Medicine; 2015. [Google Scholar]