Abstract
Scorpion venoms efficiently block the normal neurotransmitter signaling pathway by prejudicing the ion channel operating mechanism in the body system. Besides its negative effect, venoms also possess some beneficial qualities for humans. They have also been shown to exhibit anticancer properties in various cancer types. This unique property of the venom as an anticancer agent is mainly a result of its role in initiating apoptosis and inhibiting several signaling cascade mechanisms that promote cancer cell proliferation and growth. In this study, we examine the effect of venom on phenotypic changes as well as changes at the molecular levels in colorectal and breast cancer cell lines. A dramatic decrease in cell invasion was observed in both cancer cell lines on venom treatment. Additionally, there was decrease in IL-6, RhoC, Erk1/2, and STAT3 in venom-treated cell lines, providing strong evidence of its anticancer properties. Furthermore, decrease in the expression of antiapoptotic proteins and also upregulation of proapoptotic ones by these lines were observed on venom treatment. Moreover, a vivid picture of DNA damage was also detected on venom treatment. In conclusion, scorpion venom possesses significant potential as an anticancer agent against colorectal and breast cancer cell lines.
Keywords: scorpion venom, P53, BID, Bcl-XL, STAT3, IL-6
Introduction
Scorpion stings are painful and not uncommon all over the world, including in the Kingdom of Saudi Arabia.1 Frequent occurrence has been reported in the northwest region of the kingdom, which is in close proximity to the border of Jordan.2,3 Scorpions are believed to be one of the oldest species on earth.4 Furthermore, it has also been reported that the approximately 25 species of scorpion found in this country possess highly toxic venoms.5 The hot climate and outdoor activities of the local dwellers of the county expose them to scorpion attacks. Per the local reporting agency, about 15 000 new cases of scorpion stings have been reported annually in this country.6 Interestingly, the most toxic venom of the scorpion species, which belong to the genus Androctomus, is also found in the Kingdom of Saudi Arabia.7
Scorpion venom causes several alterations in normal physiology of the victims, such as fall in blood pressure, tachycardia, and breathing obstructions.8 Additionally, the inhibition of Na+/K+ ATPase pump, resulting in the impairment and total failure of the nervous system, has also been widely reported.9 Furthermore, about 250 K+ channel ligands have been isolated and identified from scorpion venom. A detailed study on the chemistry of scorpion venom was conducted by Kuzmenkov et al,10 providing an extensive amount of information on the role of scorpion venom in the activation and function of different ion channels.10
In general, scorpion venom is considered to be a major health hazard. However, it has great potential if used in the correct way to fight various types of illness and is also being used by drug designing teams to synthesize an appropriate toxin-neutralizing vaccine.11,12 In addition to this, the anticancer potential of scorpion venom has been reported against pancreatic and blood cancers.13,14 Induced apoptosis and decrease in cell progression as well as disruption in normal architecture of the cancer cells are the characteristic features that were observed when these cells were treated with scorpion venom. Furthermore, the selective action of scorpion venom against cancer cells only, sparing normal cells, has also been reported.15 Therefore, these remarkable properties of the venom provide an opportunity to use them widely against cancer.16
As a study model, we chose colorectal cancer (HCT-8) and breast cancer (MDA-MB-231) cell lines. In our previous published work, we have given detailed information about and rationale for selecting these lines as well as the prevalence of these cancers worldwide as well as among the populations of the Kingdom of Saudi Arabia.17
In the current study, we focused on the effect of scorpion venom on an important parameter of cancer cells: cell invasion. This phenomenon was assessed by Matrigel cell invasion chamber assay. Additionally, we explored the possible mechanism involved in programmed cell death (apoptosis) when the cells were challenged with scorpion venom. Furthermore, the expressions of IL-6 as well as the expressions of various signaling proteins involved in cancer metastasis were also examined.
This study is a continuation of our previous work, where we have shown a significant decrease in phenotypic changes that promotes cancer metastasis, such as colony formation and cell motility in colorectal and breast cancer cell lines.17 The current work places much emphasis on the mechanistic approach to the venoms as well as how they work against cancer progression.
In summary, we have demonstrated a decrease in cell invasion through Matrigel assay, which is a hallmark assay in cancer cell biology. Furthermore, we have shown the upregulation of proapoptotic proteins and downregulation of tumor-promoting proteins. In addition to this, a decrease in IL-6 cytokines as well as downregulation of cancer survival proteins such as RhoC, Erk1/2, and STAT3 in colorectal and breast cancer cell lines were observed. Therefore, it will be postulated that venom therapy could be a novel therapeutic approach against aggressive cancers. To the best of the authors’ knowledge and from literature searches, this study is the first of its kind to explore the mechanism of action of scorpion venoms collected in the Kingdom of Saudi Arabia against colorectal and breast cancer cell lines.
Materials and Methods
Cultivation of Scorpions and Venom Collection
For this study, venom from the medically important scorpions was used. With the prior approval of the Research Ethics Committee (REC), which is also responsible for proper animal care monitoring, at Prince Sultan Military Medical City, this study was conducted. Scorpions were captured and venom extracted in our research facility at Prince Sultan Military Medical City research center. Collection of scorpion species and milking of the venoms were performed as described earlier.17 Venoms were reconstituted in Phosphate Buffered Saline (PBS) (10 mg/mL). In this study, we used whole venom obtained from scorpions cultured in our local serpentarium, and we did not observe any difference in the different batches of venoms used in this study. Furthermore, our proteomics division has been extensively working on fractionation, identification of the active fragments (peptides) of the whole venom, and sequencing them. Our future studies will be elaborating on and incorporating these biochemical and biophysical analyses. For this study, we obtained the venoms from the following species of scorpions (abbreviation in parentheses): Androctonus crassicauda (V1), Androctonus bicolor (V2), and Leiurus quinquestriatus (V3). Our preliminary study showed the optimal dosages of venoms to be around 80 µg/mL, where cells appeared morphologically composed and not much cell destruction was observed. Taking into consideration this optimal dose, all experiments in this study were performed at 80 µg/mL final concentration unless otherwise stated.
Cell Culture
Cancer cell lines—namely, HCT-8 (derived from the ileocecal adenocarcinoma of a 67-year-old man18 and MDA-MB-231 (obtained from breast mammary glands)19—were maintained according to the standard cell culture protocol as described previously by our research group.17,20 Supernatants were monitored fortnightly for any mycoplasma contamination and treated if needed, as described by Brenner et al.21
Cell Invasion Assay
Cell invasion assays were performed as described earlier using a BD BioCoat Matrigel Invasion Chamber, which was obtained from BD Biosciences, Bedford, MA, USA.22 The procedure followed was according to the manufacturer’s instructions. Briefly, about 2.5 × 105 cells in 2 mL of serum-free Dulbecco’s Modified Eagle Medium (DMEM) was added at the top of the insert and 1 mL of medium was added in the bottom wells of each insert. Fetal bovine serum albumin (FBS) was added in the medium of the lower chamber (final concentration of FBS was 10%, v/v), which acts as a chemoattractant. Cells were incubated for 22 hours in a humidified cell culture incubator, at 37°C, 5% CO2 atmosphere. Next, the noninvading cells at the top of the insert were scraped out with the help of a cotton-tipped swab. The invading cells, which were attached to the underside of the membrane, were fixed in 100% methanol and stained with 1% toluidine prepared in 100% methanol. After repeated washing of the membrane with distilled water, stained cells were allowed to air dry at room temperature before they were visualized under a microscope. Matrigel-invaded cells were counted microscopically at 40× magnification.
Enzyme-Linked Immunosorbent Assay (ELISA)
The assay for the expression of IL-6 in scorpion venom–treated cell lines was performed as described by Duffy et al.23 The assay was performed on precoated human IL-6 ELISA plates procured from R&D Systems, Minneapolis, MN. Experiments were performed per the manufacturer’s protocol. Color intensity was read on the Hidex Sense Micro plate Reader (Turku, Finland) at 450 nm. The expression of cytokine was normalized by the number of cells, and the result is depicted in the form of bar graphs. The concentration of cytokine was reported in picogram per milliliter per million cells.
Western Blot Analysis
The experiments were performed on the W3 Western Workflow complete system (Bio Rad, USA). Equal amounts of protein isolated from breast and colorectal cell lines after treatment with scorpion venom (80 µg/mL) were mixed with 4× Laemmli’s buffer.24 The samples were boiled at 97°C for 7 minutes and then separated on 4% to 12% Sodium Dodecyl Sulfate (SDS) gels. Proteins were transferred onto the pretreated Polyvinylidene Fluoride (PVDF) membranes. After blocking the membranes in 3% BSA for an hour, they were probed with 1:1000 diluted rabbit monoclonal p-Erk1/2, p-STAT3, RhoC, and GAPDH (Ab cam, UK) and BCLXL, BID, and p53 (Sigma Chemical Co, USA). After overnight incubation at 4°C, membranes were washed and blotted for 1 hour with horse radish peroxidase–conjugated antirabbit secondary antibodies (1:2500). Next, protein bands were visualized in a Chemi doc imaging system using ECL detection reagents; band density was calculated using image Lab 5.0 (Bio Rad, USA) and normalized against corresponding total proteins or Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The normalized values are depicted in the form of bar graphs.
Determination of DNA Damage (Comet Assay)
DNA damage was quantified using single-cell gel electrophoresis (or) comet assay.25,26 The assays were performed under dim light at 4°C. Cells used for the comet assay were sampled from a monolayer during the growing phase, 24 hours after seeding. Cells were treated with V1 to V3 for 24 and 48 hours. First, 200 µL of 1% regular agarose in PBS at 65°C was poured gently on to a fully frosted micro slide, covered immediately with a cover slip, and placed over a frozen ice pack for about 5 minutes. The coverslip was removed after the gel had set. The cell suspension from one fraction was mixed with 1% low-melting agarose at below 37°C in a 1:3 ratio. Then, 100 µL of this mixture was applied quickly on top of the gel coated over the micro slide and allowed to set as before. A third coating of 100 µL of 1% low-melting agarose was applied on the gel containing the cell suspension and allowed to set. After solidification of the agarose, the coverslips were removed and the slides were immersed in ice-cold lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, NaOH pH 10, 0.1% Triton X-100) and transferred to a refrigerator at 4°C for 4 hours. All the above procedures were performed in ambient light to avoid additional DNA damage. After being removed from the lysis solution, the slides were placed horizontally in an electrophoresis tank. The reservoirs were filled with electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA pH > 13) until the slides were just immersed in it. Next, the slides were allowed to stand in the buffer for about 20 minutes (to allow DNA unwinding), after which electrophoresis was carried out at 0.8 V/cm for 15 minutes. At the completion of electrophoresis, the slides were removed, washed 3 times in neutralizing buffer (0.4 M Tris, pH 7.5) and gently dabbed to dry. Nuclear DNA was stained with 20 µL of ethidium bromide (10 µg/mL), and the images were captured on a Olympus BX53 fluorescent microscope.
Statistical Analysis
Statistical analyses were performed using Student’s t-test. The means are reported with SDs. Differences were considered to be statistically significant when P values were ≤.05.
Results
Cell Invasion Was Dramatically Decreased in Scorpion Venom–Treated Breast and Colorectal Cancer Cell Lines
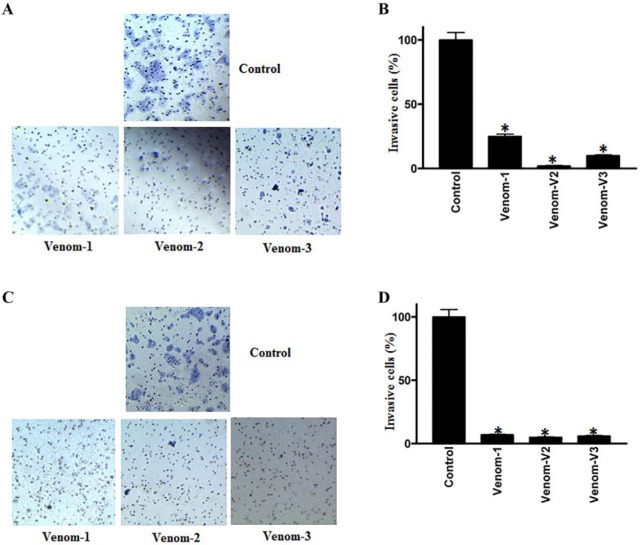
To elucidate the effectiveness of scorpion venom on cell invasion, we performed a Matrigel invasion assay in the absence or presence of scorpion venoms in colorectal (HCT-8) and breast (MDA-MB-231) cancer cell lines. As shown in Figures 1A and 1B, a perceptible decrease in cell invasion was observed on venom treatment. The decrease in invasion was 80% to 90% in venom-treated samples as compared with controls in both breast and colorectal cancer cell lines. It is worth mentioning here that the pores of the membrane also got stained, and they appeared like cancer cells, but in reality, this is not the case.
Figure 1.
Cell invasion assay of colorectal and breast cancer cell lines treated with scorpion venoms (A and C); columns (B and D), rates of invasion; bars, 95% CIs, P < .05.
Scorpion Venom Downregulates IL-6 in Breast and Colorectal Cancer Cell Lines
Interleukin-6 (IL-6) is a stress-related cytokine, which is overexpressed in several cancer types, including head and neck squamous cell carcinoma (HNSCC).27 To examine the effect of scorpion venoms on this cytokine, we analyzed the expression of IL-6 by ELISA. As shown in Figures 2A and 2B, a significant decrease in the expression of IL-6 was observed when the lines were treated with V1 and V2. However, the downregulation of IL-6 with V3 treatment did not reach significance, but there was a tendency toward decrease in Il-6 expression.
Figure 2.
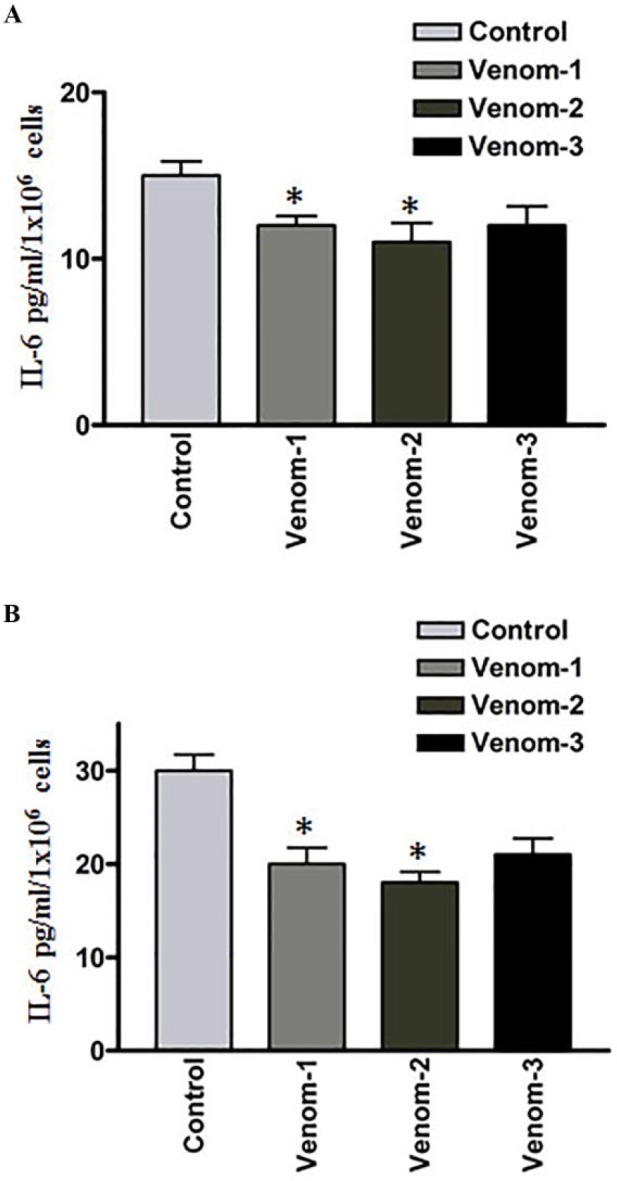
Effect of scorpion venoms on interleukin (IL)-6 expression (A) colorectal and (B) breast cancer cell lines; P < .05.
Expression of Cancer Survival Proteins Is Downregulated in Venom-Treated Breast and Colorectal Cancer Cell Lines
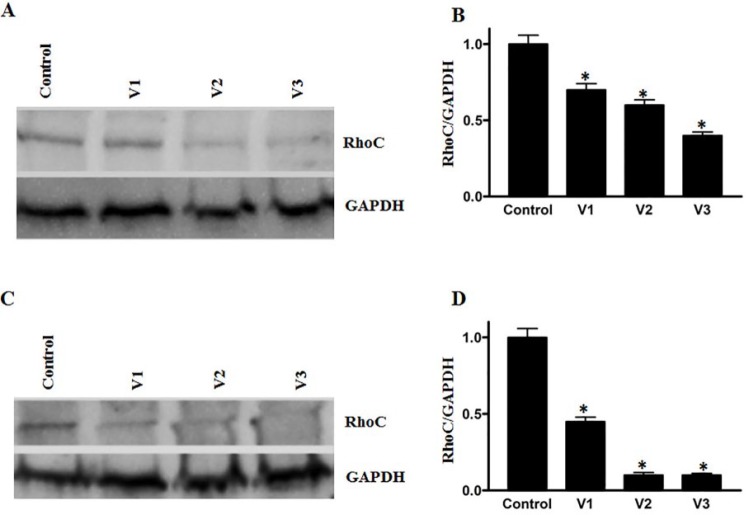
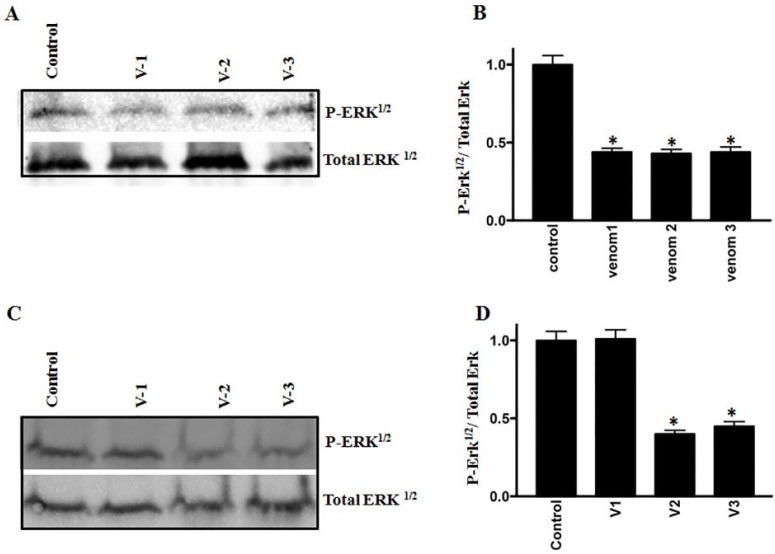
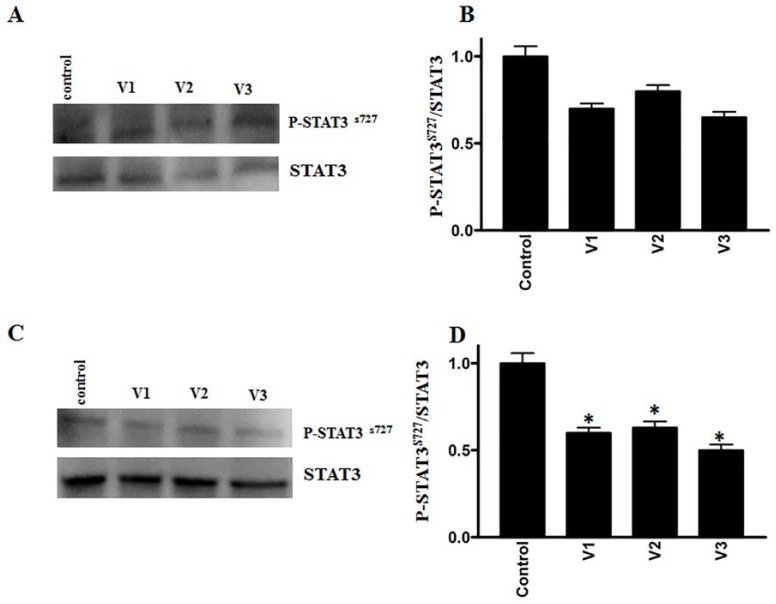
RhoC, a prometastatic oncogene, is constitutively active in several cancers, including breast and HNSCCs.28-30 We evaluated the expression of RhoC after challenging the breast and colorectal cancer cell lines with scorpion venoms. As shown in Figures 3A to 3D, a significant downregulation of RhoC was detected after venom treatment. Additionally, the phosphorylation and, hence, the activation of cancer promoting signaling protein Erk½ showed significant downregulation after venom treatment (Figures 4A-4D). Furthermore, we also analyzed the activation pattern of another signaling protein—STAT3. The role of STAT3 activation in cancer stem cell progression is well established and leads to cancer metastasis.31 As shown in Figures 5A to 5D a downregulation in the activation of STAT3 was observed after scorpion venom treatments.
Figure 3.
Effect of scorpion venoms on RhoC expression in (A and C) colorectal and breast cancer cell lines: a significant decrease was observed in RhoC expression after venom treatment. Columns (B and D) show the densitometric analysis of the RhoC band with corresponding GAPDH (P < .05).
Figure 4.
Western blot analyses show the phosphorylation of Erk1/2 on colorectal (A) and breast (C) cancer cell lines. Columns (B and D) show the densitometric analysis of the phosphorylated Erk with corresponding total Erk (P < .05). Extent of inhibition was more pronounced in the colorectal cancer cell line as compared with breast cancer cell line.
Figure 5.
Western blot analyses show the phosphorylation of STAT3 on colorectal (A) and breast (C) cancer cell lines after scorpion venom treatment. Columns (B and D) show the densitometric analysis of the phosphorylated STAT3 with corresponding total STAT3 (P < .05). Extent of inhibition was more pronounced in colorectal cancer as compared with the breast cancer cell line.
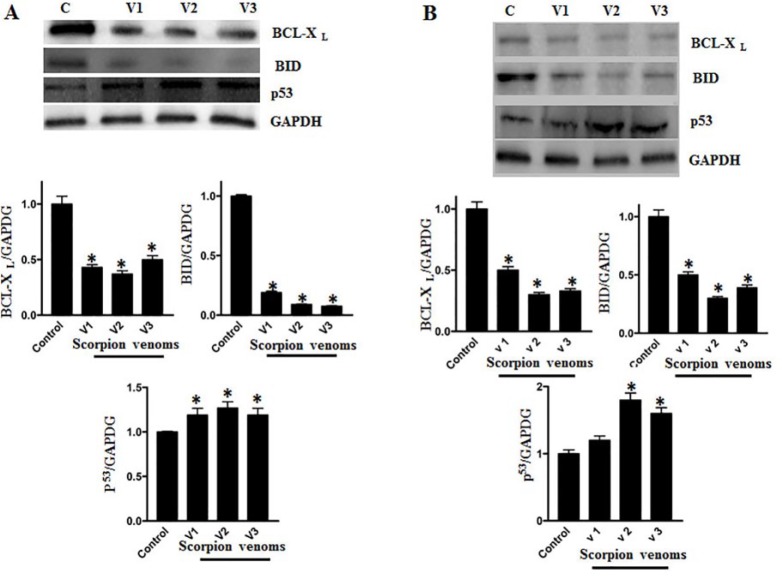
Scorpion Venom Modulates the Expression Pattern of Apoptotic Markers in Breast and Colorectal Cancer Cell Lines
As shown in Figures 6A and 6B, a perceptible change in the expression of apoptotic marker proteins was observed when they were analyzed by Western blot analyses after venom treatments to breast and colorectal cancer cell lines. Downregulation of antiapoptotic proteins Bcl-XL and BID and upregulation of proapoptotic protein p53 was observed after scorpion venom treatment in breast and colorectal cancer cell lines.
Figure 6.
Western blot shows the downregulation of the antiapoptotic proteins (Bcl2)/Bcl-XL and BID, whereas upregulation of proapoptotic protein p53 was observed both in colorectal (A) and (B) breast cancer cell lines. Protein bands were normalized using corresponding GAPDH Schema showingband density (P < .05).
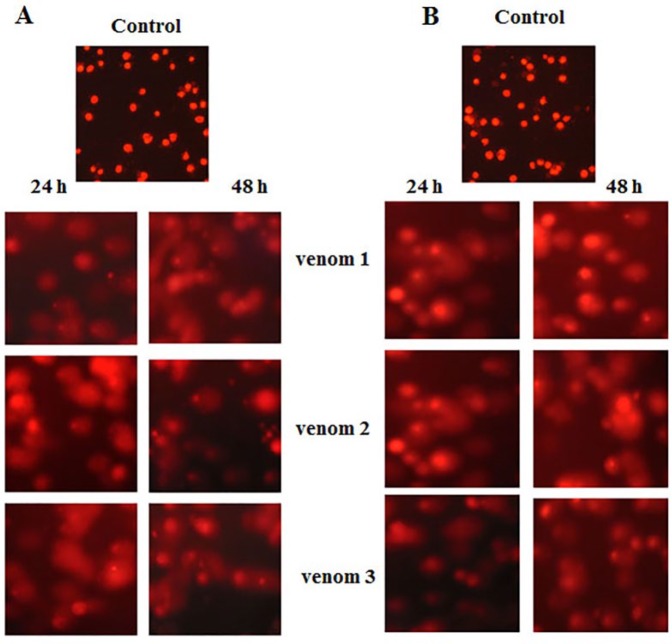
Scorpion Venom Causes DNA Damage and Membrane Blubbing in Breast and Colorectal Cancer Cell Lines
DNA damage and apoptosis go hand in hand. To assess the extent of DNA damage as a consequence of venom treatments, we performed the comet assay. As shown in Figures 7A and 7B, considerable DNA damage was clearly seen in venom-treated cell lines.
Figure 7.
Comet assay showing the extent of DNA damage after scorpion venom treatment. Note a membrane disruption and DNA fragmentation in treated cell lines both in colorectal (A) and (B) breast cancer cell lines.
Discussion
The anticancer properties of scorpion venom can be attributed to its chemical composition, comprising various type of proteins and small peptides. In this study, we have shown that the venom demonstrated anticancer properties by decreasing cell invasion, increasing the expression of proapoptotic protein, and downregulating the cancer survival proteins that are involved in the signaling mechanism that leads to cell growth and metastasis.
Although venoms contain various harmful chemicals, the beneficial aspects of the venoms cannot be ruled out, especially in the treatment of chronic diseases.32,33 As reported, scorpion venom has been shown to be effective in reducing cancer progression efficiently.34
Given its positive role in inhibiting cancer cell growth, we have investigated the effect of scorpion venom on 2 different cancer cell lines—namely, HCT-8 (colorectal) and MDA-MB-231 (breast) cancer cell lines—as described earlier.17 In the same vein, we have investigated the effect of these venoms (described in the Materials and Methods section) on Matrigel-based cancer cell invasion property. Because Matrigel provides 3-dimensional matrixes similar to human tissue, this approach to cell invasion analysis is considered to be more natural and mimics human cells. As shown in Figures 1A to 1D, a significant decrease in cell invasion, which varies from 70% to 90% in colorectal and breast cancer cell lines, was observed. Our results are in good agreement with our previously published work (Islam et al22), where we have shown that knocking down of RhoC, a prometastatic oncogene causes a decrease in cell invasion in HNSCC cell lines. This implies that reduction in cell invasion is a manifestation of the impairment of metastatic protein(s).
Furthermore, to investigate the cause of decrease in cell invasion after being treated with scorpion venom, we looked at the expression levels of several cytokines that are known to play a significant role in cancer progression. Among these is IL-6, which we earlier reported to be a prognostic marker of HNSCC (Figures 2A-2B).23,35 Decrease in IL-6 expression in venom-treated cell lines is an indication that scorpion venom can also act as an anti–IL-6 expressing molecule and, consequently, can be used as an effective tool in cancer therapy. Furthermore, previously, we have reported the role of RhoC in downregulating the expression of IL-6 and also how IL-6 is modulating STAT3 dimerization and phosphorylation in HNSCC.31 Taking these aspects into consideration, we examined the extent of inhibition of RhoC in colorectal and breast cancer cell lines. As shown in Figures 3A to 3D, a significant decrease was observed in RhoC expression after scorpion venom treatment. This implies an inhibitory role of these venoms against RhoC, resulting in the downregulation of IL-6 expression.
To elucidate the molecular mechanism of the signaling cascade in scorpion venom–treated cells, we examined the activation of an important signaling molecule, Erk1/2. The cancer proliferative role of MAP kinase (Erk1/2) is a well-established phenomenon.31 Additionally, Erk1/2 controls cell survival and also promotes apoptosis by regulating the antiapoptotic and proapoptotic proteins.36 We have shown a significant downregulation in p-Erk½ in the colorectal cancer cell line. However, in breast cancer, V1 was not effective, but we observed a significant downregulation in V2 and V3 treatments. The failure of V1 to downregulate this protein could be attributed to the differential uptake of the venom in this particular experimental setup (Figures 4A-4D). Our results are in good agreement with the previously published work of one of the coauthors of this article, where the role of MAP kinase in HNSCC metastasis was demonstrated.22 Interestingly, the exact molecular mechanism of scorpion venom in the signaling cascade has not been well demonstrated. However, we can hypothesize an intermediate role of RhoC in downregulating the p-Erk1/2 activation after scorpion venom treatment because Erk is downstream of RhoC. It is important to note that the band appearance of Erk in this study is in a singlet form rather than doublets. In our recent study, we have already explained this anomaly.37
One of the coauthors of this article has earlier shown the role of atorvastatin (a HMG Co-A- reductase inhibitor) in RhoC activation. The authors reported the inactivation of RhoC in the presence of atorvastatin as a result of the lack of posttranslational modification (gerynyl-geranylation of RhoC).38 This is in line with the previous results of Chai et al,39 who isolated Bumarsin, a scorpion venom protein that acts as the inhibitor of HMG Co-A- reductase activity. Thus, we can correlate these 2 previous findings with those of our current study and state that the active components of scorpion venom are the causative agents for the downregulation of total RhoC protein.
Furthermore, Islam et al31 have reported the downregulation of STAT3 in RhoC knockdown HNSCC cell lines. In this study, we have also shown the downregulation of STAT3, which could be a manifestation of RhoC inactivation as a result of lack of geranylation in the presence of scorpion venom. Our results are in agreement with those of previous published work.31
Additionally, Siddiquee et al40 reported that targeting STAT3 leads to an increase in apoptosis in solid and hematological tumors. In this study, we have also shown the downregulation of antiapoptotic and upregulation of proapoptotic marker proteins in STAT3 downregulated cell lines when treated with scorpion venom. Furthermore, this could be a manifestation of the downregulation of STAT3 in breast and colorectal cancer cell lines.
The JAk-STAT signaling pathway is involved in many cellular responses as well as in the activation of both the cancer-related signaling cascade and stem cell transcription factor activation, nanog. One of the coauthors of this article as well as others have also demonstrated the role of IL-6 in STAT3 modulation and, consequently, decrease in cancer-related cellular processes.31,41,42 In this study, we have shown a downtrend in the activation of STAT3ser727 in both cell lines used in this study (Figures 5A-5D). However, in our experimental setup, the effect of venoms are more pronounced in the breast cancer cell line as compared with the colorectal cancer cell line. One possible reason for the differential action of the venom between colorectal and breast cancer cell lines could be the receptor-mediated uptake potential of the venom. Our results are also in accordance with the published work, where STAT3 showed a proliferative property in thyroid and breast cancers.43,44
Next, an important biological question arises at this point as to how these signaling proteins downregulate the cell invasion and cellular progression? There could be 3 possible reasons: (a) reduction in cell growth; (b) inhibition of cell differentiation; and (c) cell death or apoptosis. To investigate this important question, in this study, we focused on programmed cell death (apoptosis) in venom-treated cell lines. Previously, it has been shown that scorpion venom has a role in causing apoptosis in brain cancer.45 Our extensive studies on venom-treated cell lines (breast and colorectal) showed the downregulation of antiapoptotic proteins (Bcl-2/Bcl-XL and BID) and a significant upregulation in proapoptotic/tumor suppressor marker protein, p53 (Figures 6A and 6B).
These findings suggest a proactive role of scorpion venom in initiating apoptosis, using the sequestration in colorectal and breast cancer cell lines to proliferate and invade. To further elaborate our findings on apoptosis, we investigated the extent of DNA damage after venom treatment using the comet assay. As shown in Figures 7A and 7B, perceptible DNA damage and membrane blubbing was observed in the venom-treated cell lines when compared with their respective controls. Our study also supports the fact that several different scorpion venoms are known to contain substances that act as DNA-damaging molecules in breast and colorectal cancers.14,46 Furthermore, because of the consistent stress of venom on the cells, cells accumulate excessive reactive oxygen species (ROS), which is one of the causative factors for DNA damage. Therefore, the DNA damage in this study under the influence of scorpion venom could be the manifestation of the formation of ROS, leading to DNA damage.47 Our preliminary study shows the overproduction of ROS in scorpion venom–treated cell lines (data not shown). Therefore, we can conclude that generation of ROS in scorpion venom–treated cell lines is what causes DNA damage, leading to upregulation of proapoptotic genes and downregulation of antiapoptotic genes, initiating apoptosis in breast and colorectal cancer cell lines.
A schema showing the 2 possible arms of cellular apoptosis while treating with scorpion venoms, either through STAT3 or ERK pathway or simultaneously promoting apoptosis via NFKB pathways, is given in Figure 8.
Figure 8.
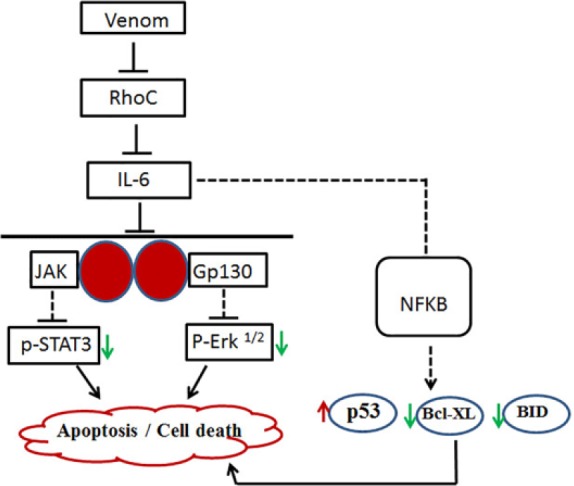
Schema showing the probable pathways through which scorpion venom acts as an apoptotic agent in colorectal and breast cancer cell lines: the solid line indicates our findings, and the dotted line suggests the probable steps involved in this cascade.
The aim of this study was to reduce the cost of traditional cancer treatment by replacing with venom therapy. In sum, the venom therapy may open new vistas not only for cancer therapy, but also for other chronic diseases.48-50 In addition to this, the new techniques of liposomal delivery to target-specific cells will be an additional approach for venom therapy.
In conclusion, the findings presented in this study demonstrate that reduced cell invasion, IL-6 expression, and signaling proteins, coupled with the proper modulation of apoptotic markers and DNA damage, correlates positively with the inhibitory role of scorpion venom against colorectal and breast cancer cell lines. Furthermore, these finding suggest that venom therapy will be an important step toward a more specific treatment for aggressive forms of cancer. Additionally, to the best of the author’s knowledge, this study is the first of its kind to demonstrate some of the detailed phenotypic and molecular aspects exhibited by the cancer cells on exposure to scorpion venom.
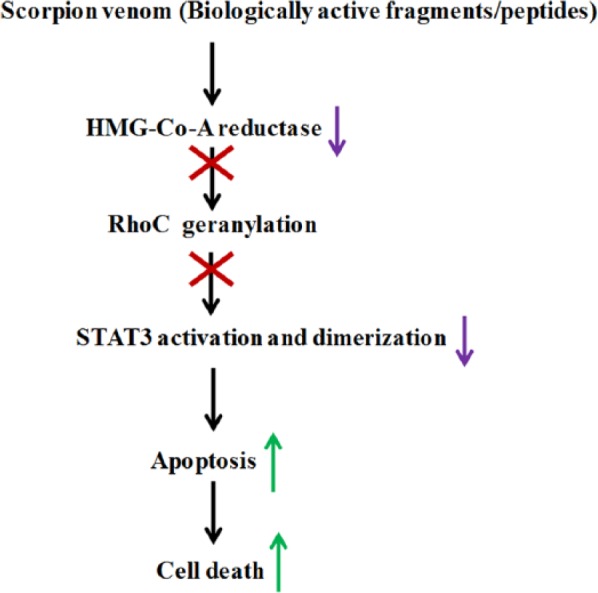
A schematic figure showing a summary of the probable mechanism of action of scorpion venom against breast and colorectal cancer cell lines.
Acknowledgments
The authors would like to thank the Research Center of Prince Sultan Military Medical City Hospital and King Abdulaziz City for Science and Technology (KACST) for providing the necessary facilities and financial support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors would like to thank the Research Center of Prince Sultan Military Medical City Hospital and King Abdulaziz City for Science and Technology (KACST) for providing the necessary facilities and financial support.
References
- 1. Al-Asmari AK, Al-Saif AA. Scorpion sting syndrome in a general hospital in Saudi Arabia. Saudi Med J. 2004;25:64-70. [PubMed] [Google Scholar]
- 2. Mahaba HM, El Sayed SA. Scorpion sting: is it a health problem in Saudi Arabia? Evaluation of management of 820 cases. Saudi Med J. 1996;17:15-21. [Google Scholar]
- 3. Mahaba HM. Scorpion sting syndrome: epidemiology, clinical presentations and management of 2240 cases. East Med Health J. 1997;3:82-89. [Google Scholar]
- 4. Simard MJ, Watt DD. Venom and Toxin. Palo Alto, CA: Stanford University Press; 1990. [Google Scholar]
- 5. Al Asmari AK, Khan HA, Manthiri RA, Al Yahya KM, Al Otaibi KE. Effects of Echis pyramidum snake venom on hepatic and renal antioxidant enzymes and lipid peroxidation in rats. J Biochem Mol Toxicol. 2014;28:407-412. [DOI] [PubMed] [Google Scholar]
- 6. Al Asmari AK, Al Zahrani AG, Al Jowhary S, Arshaduddin M. Clinical aspects and frequency of scorpion stings in the Riyadh region of Saudi Arabia. Saudi Med J. 2012;33:852-858. [PubMed] [Google Scholar]
- 7. Ozkan O, Filazi A. The determination of acute lethal dose-50 (LD50) levels of venom in mice, obtained by different methods from scorpions, Androctonus crassicauda (Oliver 1807). Acta Parasitol Turc. 2004;28:50-53. [Google Scholar]
- 8. Bawaskar HS, Bawaskar PH. Scorpion sting: update. J Assoc Physicians India. 2012;60:46-55. [PubMed] [Google Scholar]
- 9. Petricevich VL. Effect of Tityus serrulatus venom on cytokine production and the activity of murine macrophages. Mediators Inflamm. 2002;11:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuzmenkov A, Grishin E, Vassilevski A. Diversity of potassium channel ligands: focus on scorpion toxins. Biochemistry (Mosc). 2015;80:1764-1799. [DOI] [PubMed] [Google Scholar]
- 11. Andreotti N, Jouirou B, Mouth L, Sabatier JM. Comprehensive Natural Products II. Oxford, UK: Elsevier; 2010. [Google Scholar]
- 12. Ahn MY, Ryu KS, Lee YW, Kim YS. Cytotoxicity and l-amino acid oxidase activity of crude insect drugs. Arch Pharm Res. 2000;23:477-481. [DOI] [PubMed] [Google Scholar]
- 13. El-Ghlban S, Kasai T, Shigehiro T, et al. Chlorotoxin-Fc fusion inhibits release of MMP-2 from pancreatic cancer cells. Biomed Res Int. 2014;2014:152659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta SD, Gomes A, Debnath A, Saha A, Gomes A. Apoptosis induction in human leukemic cells by a novel protein Bengalin, isolated from Indian black scorpion venom: through mitochondrial pathway and inhibition of heat shock proteins. Chem Biol Interact. 2010;183:293-303. [DOI] [PubMed] [Google Scholar]
- 15. Díaz-García A, Morier-Díaz L, Frión-Herrera Y, et al. In vitro anticancer effect of venom from Cuban scorpion Rhopalurus junceus against a panel of human cancer cell lines. J Venom Res. 2013;4:5. [PMC free article] [PubMed] [Google Scholar]
- 16. Ding J, Chua P-J, Bay B-H, Gopalakrishnakone P. Scorpion venoms as a potential source of novel cancer therapeutic compounds. Exp Biol Med. 2014;239:387-393. [DOI] [PubMed] [Google Scholar]
- 17. Al-Asmari AK, Islam M, Al-Zahrani AM. Analysis of the anticancer properties of scorpion venom in colorectal and breast cancer cell lines. Oncol Lett. 2016;11:1256-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vermeulen SJ, Chen TR, Speleman F, Nollet F, Van Roy FM, Mareel MM. Did the four human cancer cell lines DLD-1, HCT-15, HCT-8, and HRT-18 originate from one and the same patient? Cancer Genet Cytogenet. 1998;107:76-79. [DOI] [PubMed] [Google Scholar]
- 19. Cailleau R, Young R, Olive M, Reeves WJ., Jr. Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974;53:661-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Asmari AK, Albalawi SM, Athar MT, Khan AQ, Al-Shahrani H, Islam M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS One. 2015;10:e0135814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brenner JC, Graham MP, Kumar B, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Islam M, Lin G, Brenner JC, et al. RhoC expression and head and neck cancer metastasis. Mol Cancer Res. 2009;7:1771-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duffy SA, Taylor JM, Terrell JE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750-757. [DOI] [PubMed] [Google Scholar]
- 24. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [DOI] [PubMed] [Google Scholar]
- 25. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184-191. [DOI] [PubMed] [Google Scholar]
- 26. Alapetite C, Wachter T, Sage E, Moustacchi E. Use of the alkaline comet assay to detect DNA repair deficiencies in human fibroblasts exposed to UVC, UVB, UVA and gamma-rays. Int J Radiat Biol. 1996;69:359-369. [DOI] [PubMed] [Google Scholar]
- 27. Duffy SA, Taylor JM, Terrell JE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750-757. [DOI] [PubMed] [Google Scholar]
- 28. Islam M, Lin G, Brenner JC, et al. RhoC expression and head and neck cancer metastasis. Mol Cancer Res. 2009;7:1771-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleer CG, Teknos TN, Islam M, et al. RhoC GTPase expression as a potential marker of lymph node metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2006;12:4485-4490. [DOI] [PubMed] [Google Scholar]
- 30. Van Golen KL, Wu Z-F, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832-5838. [PubMed] [Google Scholar]
- 31. Islam M, Sharma S, Teknos TN. RhoC regulates cancer stem cells in head and neck squamous cell carcinoma by overexpressing IL-6 and phosphorylation of STAT3. PLoS One. 2014;9:e88527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomes A, Bhattacharjee P, Mishra R, et al. Anticancer potential of animal venoms and toxins. Indian J Exp Biol. 2010;48:93-103. [PubMed] [Google Scholar]
- 33. Lewis RJ, Garcia ML. Therapeutic potential of venom peptides. Nat Rev Drug Discov. 2003;2:790-802. [DOI] [PubMed] [Google Scholar]
- 34. D’Suze G, Rosales A, Salazar V, Sevcik C. Apoptogenic peptides from Tityus discrepans scorpion venom acting against the SKBR3 breast cancer cell line. Toxicon. 2010;56:1497-1505. [DOI] [PubMed] [Google Scholar]
- 35. Allen C, Duffy S, Teknos T, et al. Nuclear factor-κB–related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13:3182-3190. [DOI] [PubMed] [Google Scholar]
- 36. Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and-independent mechanisms. Science. 1999;286:1358-1362. [DOI] [PubMed] [Google Scholar]
- 37. Al-Asmari AK, Riyasdeen A, Al-Shahrani MH, Islam M. Snake venom causes apoptosis by increasing the reactive oxygen species in colorectal and breast cancer cell lines. Onco Targets Ther. 2016;9:6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Islam M, Sharma S, Kumar B, Teknos TN. Atorvastatin inhibits RhoC function and limits head and neck cancer metastasis. Oral Oncol. 2013;49:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chai S, Armugam A, Strong P, Jeyaseelan K. Characterization of bumarsin, a 3-hydroxy-3-methylglutaryl-coenzyme reductase inhibitor from Mesobuthus martensii Karsch venom. Toxicon. 2012;60:272-279. [DOI] [PubMed] [Google Scholar]
- 40. Siddiquee KAZ, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chung Y-J, Park B-B, Kang Y-J, Kim T-M, Eaves CJ, Oh I-H. Unique effects of Stat3 on the early phase of hematopoietic stem cell regeneration. Blood. 2006;108:1208-1215. [DOI] [PubMed] [Google Scholar]
- 42. Suzuki M, Yamamoto M, Sugimoto A, Nakamura S, Motoda R, Orita K. Delta-4 expression on a stromal cell line is augmented by interleukin-6 via STAT3 activation. Exp Hematol. 2006;34:1142-1149. [DOI] [PubMed] [Google Scholar]
- 43. Plaza-Menacho I, van der Sluis T, Hollema H, et al. Ras/ERK1/2-mediated STAT3 Ser727 phosphorylation by familial medullary thyroid carcinoma-associated RET mutants induces full activation of STAT3 and is required for c-fos promoter activation, cell mitogenicity, and transformation. J Biol Chem. 2007;282:6415-6424. [DOI] [PubMed] [Google Scholar]
- 44. Yeh YT, Ou-Yang F, Chen IF, et al. STAT3 ser727 phosphorylation and its association with negative estrogen receptor status in breast infiltrating ductal carcinoma. Int J Cancer. 2006;118:2943-2947. [DOI] [PubMed] [Google Scholar]
- 45. Wang W-X, Ji Y-H. Scorpion venom induces glioma cell apoptosis in vivo and inhibits glioma tumor growth in vitro. J Neurooncol. 2005;73:1-7. [DOI] [PubMed] [Google Scholar]
- 46. Zargan J, Umar S, Sajad M, Naime M, Ali S, Khan HA. Scorpion venom (Odontobuthus doriae) induces apoptosis by depolarization of mitochondria and reduces S-phase population in human breast cancer cells (MCF-7). Toxicol In Vitro. 2011;25:1748-1756. [DOI] [PubMed] [Google Scholar]
- 47. Aitken JB, Naumovski N, Curry B, Grupen CG, Gibb Z, Aitken RJ. Characterization of an l-amino acid oxidase in equine spermatozoa. Biol Reprod. 2015;92:125. [DOI] [PubMed] [Google Scholar]
- 48. Park MH, Jo M, Won D, et al. Snake venom toxin from Vipera lebetina turanica induces apoptosis of colon cancer cells via upregulation of ROS-and JNK-mediated death receptor expression. BMC Cancer. 2012;12:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jo M, Park MH, Kollipara PS, et al. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258:72-81. [DOI] [PubMed] [Google Scholar]
- 50. Park MH, Choi MS, Kwak DH, et al. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-κB. Prostate. 2011;71:801-812. [DOI] [PubMed] [Google Scholar]