Abstract
Objective: To estimate the efficacy of traditional Chinese medicine (Chining decoction, CHIN) for radiation-induced oral mucositis in patients with head and neck cancer. Methods: From May 2014 to December 2015, 70 consecutive patients were randomly assigned to receive CHIN (treatment group) or recombinant human epidermal growth factor (rhEGF) spray (control group) at a 1:1 ratio. CHIN was administered to treatment group from the first day of radiotherapy until the completion of radiotherapy. Simultaneously, the rhEGF spray was administered to control group on the oral mucosa of irradiated area. The clinical benefit was determined by gradation of mucositis (Common Terminology Criteria for Adverse Events v4.0), oral pain, and xerostomia (visual analysis scale) for each week during radiotherapy. Body mass index was evaluated before and after radiotherapy. Results: Patients in the treatment group had prominent remission of oral pain and grade of mucositis on each observing point compared with those in control group (P < .01). Xerostomia was decreased notably in treatment group compared with control group (P < .01). Body mass index in the treatment group exhibited advantage over control group after radiotherapy, but there was no statistical significance (19.8 ± 3.26 vs 18.8 ± 2.5 kg/m2, P = .153, >.05). Conclusions: CHIN presented an obvious advantage in preventing radiation-induced oral mucositis compared with rhEGF spray.
Keywords: traditional Chinese medicine, radiation-induced oral mucositis, mucositis, head and neck cancer, radiotherapy
Introduction
Radiation-induced oral mucositis (OM) is the most notable toxicity for patients receiving head and neck radiotherapy. Severe ulcerative lesions can be extremely painful, leading to decreased intake of food and clinically significant weight loss or interrupted radiation therapy. Approximately 34% of patients who receive conventional radiotherapy (RT) suffer from grade 3 or 4 mucositis, and the incidence rate increases to 56% in patients receiving altered fractionation RT.1 Different therapeutic modalities, such as granulocyte colony-stimulating factor (G-CSF), keratinocyte growth factor (KGF), Mg milk, amphogel, benzydaine, sucralfate, and low-level laser, have been used by researchers.2 Regardless of these proposed treatments, the standard method remains fragmentary. Chining decoction (CHIN), as another oral treatment, may prevent this condition.
CHIN is modified from Liangge San, which is adapted from “Formularies of the Bureau of People’s Welfare Pharmacies,” rated as an epoch-making work in the pharmacopeial history of China. Liangge San, credited with removing heat from the diaphragm, reduces overabundance of pathogenic heat in the upper-energizer and middle-energizer, manifested as constipation and feverish sensation in the chest and epigastrium. The clinical indication for this formula was acute lung injury. Modern pharmacological research shows that it can reduce lung injury, and its mechanism may be to inhibit the release of inflammatory mediators by promoting the secretion of anti-inflammatory mediators, regulating the balance between inflammation and anti-inflammatory response.3 In a recent clinical study, combined Liangge San and Western medicine showed a significant effect on stomatitis patients and reduced the relapse rate.4 We modified the prescription to adapt it for radiation-induced oral mucositis patients.
To explore the potential of CHIN in preventing OM, we designed the present randomized controlled study. We report the efficacy of CHIN in preventing radiation-induced OM in patients with head and neck cancer.
Material and Methods
Study Design
This prospective trial was conducted in Tianjin Medical University Cancer Institute and Hospital (TMUCIH) in China. The ethics committee of TMUCIH approved this study. All patients provided written informed consent. This study was registered at the ClinicalTrials.gov registry (NCT02303197).
Eligibility
Patients were eligible for enrollment if they were 18 to 75 years old, had pathologically confirmed head and neck malignancy, and were prepared for chemoradiotherapy. The key inclusion criteria were estimated survival time of ≥3 months, ECOG (Eastern Cooperative Oncology Group) score of ≤2, no history of antitumor therapies, and no history of oral ulcer and salivary gland diseases. The key exclusion criteria were simultaneous serious complications; submandibular gland pathological changes; taking other drugs for treatment of mucositis; and serious dysfunction of heart, brain, liver, or kidney.
Randomization, Sample Size, Treatment, and Assessment
From May 2014 to December 2015, 70 eligible patients were randomly assigned to receive CHIN (treatment group) or recombinant human epidermal growth factor (rhEGF) spray (control group) at a 1:1 ratio. Computer-assisted randomization was performed. Individuals were assigned to either treatment group or control group by opening an opaque envelope numbered in sequence when informed consent was acquired. An investigator who was not involved with data collection produced the allocation sequence.
We used PASS13.0 to calculate the sample size of our study. Lost to follow-up rate controlled less than 10%. The overall sample size required was 70 participants (2-tailed α = 0.05, 1 − β = 0.84, P1 = 0.9, P2 = 0.6), 35 in the treatment group and 35 in the control group.
All patients received external beam RT treatments to the head and neck using single daily treatment regimens for 5 days a week to total planned cumulative RT doses of at least 5000 cGy. After reviewing the target residual lesions after RT, we increased the radiation dose if required. CHIN is composed of Rhubarb (3 g), Licorice (6 g), Mint (6 g), Scutellaria (10 g), Radix Liriopes (20 g), Red peony root (10 g), Lumbricus (10 g), Radix Scrophulariae (10 g), and Forsythia (10 g). Each component of CHIN was processed into granules packaged in a small tin foil bag from Jiangyin Tianjiang Pharmaceutical Co Ltd (China). The quantitative properties, qualitative properties, and preparation of the Chinese herbs granules are shown in the supplementary material (Supplementary Table 1). The CHIN decoction allocated to the treatment group was repacked as a plastic bag containing granules constituting a single daily dose. The plastic bags were dispensed once a week. It was administered to treatment group from the first day of RT until the completion of RT (intake: mixed with hot water, 200 mL daily, taken morning and evening, 100 mL each time). For the control group, the rhEGF spray was used to the oral mucosa of irradiated area thrice daily.
The oral mucosa evaluations were performed on all patients at the beginning of treatment and continued weekly up to the end of the RT for 7 weeks. The National Cancer Institute Common Toxicity Criteria OM Scale was used for grading mucositis. Oral pain and xerostomia were assessed by the patients with the visual analysis scale (VAS) for 7 weeks. Body mass index (BMI) was used to assess the nutritional status of patients before and after treatment.
Degree of OM was graded according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0). An experienced radiation oncologist evaluated all the patients. The assessor was educated in evaluating the degree of OM. The CTCAE v4.0 grades for OM are defined as follows: grade 0, no mucositis; grade 1, asymptomatic or mild symptoms and intervention not indicated; grade 2, moderate pain not interfering with oral intake and modified diet indicated; grade 3, severe pain interfering with oral intake; grade 4, life-threatening consequences and urgent intervention indicated; and grade 5, death.5
The VAS was used to measure the subjective sensation of oral pain and xerostomia. A 100-mm visual analogue scale was given to patients to keep a record of subjective sensation of oral pain and xerostomia at home. Negative scores for symptoms were on the left (marked as 0), such as not painful (dry). Positive scores for symptoms were on the right (marked as 10), such as very painful (dry), very uncomfortable, or difficult to gauge.6-8
Statistical Analysis
Continuous variables are presented as the mean ± SD and compared using unpaired Student’s t tests. Chi-square tests were used to compare the categorical data between groups. All statistical analyses were performed using SPSS software (Version 13.0, SPSS Inc, Chicago, IL, USA). A 2-sided P < .05 was considered significant.
Results
Patients
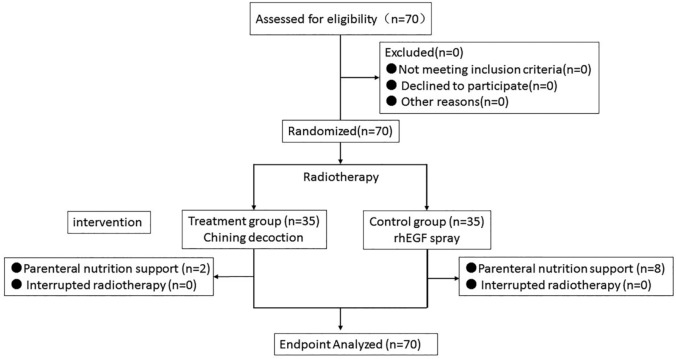
Seventy patients of TMUCIH enrolled in this study from May 2014 to December 2015. All 70 patients completed the study protocol (Figure 1). As shown in Table 1, no statistical difference was detected in mean age, stage, primary site, ECOG, smoking, RT dose, and treatment methods between the 2 groups.
Figure 1.
Flowchart of study selection.
Table 1.
Patients’ Characteristics.
| Characteristic | No. of Patients (n = 70) | Treatment Group (n = 35), n (%) | Control Group (n = 35), n (%) | P |
|---|---|---|---|---|
| Age (years) | .276 | |||
| Range | 17-76 | 18-76 | 17-75 | |
| Mean | 52.44 | 52.26 | 52.63 | |
| Gender | .051 | |||
| Male | 53 | 30 (85.7) | 23 (65.7) | |
| Female | 17 | 5 (14.3) | 12 (34.3) | |
| TNM stage | .522 | |||
| II | 13 | 6 (17.1) | 9 (25.7) | |
| III | 24 | 14 (40) | 10 (28.6) | |
| Iva | 31 | 15 (42.9) | 16 (45.7) | |
| Primary site | .952 | |||
| Nasopharyngeal | 43 | 20 (57.1) | 23 (65.7) | |
| Oropharynx | 4 | 3 (8.6) | 1 (2.9) | |
| Salivary glands | 9 | 6 (17.1) | 3 (8.6) | |
| Tonsilla | 2 | 1 (2.9) | 1 (2.9) | |
| Larynx | 5 | 2 (5.7) | 3 (8.6) | |
| Hypopharynx | 7 | 3 (8.6) | 4 (11.4) | |
| ECOG score | .445 | |||
| 0 | 2 | 1 (2.9) | 1 (2.9) | |
| 1 | 56 | 26 (74.3) | 30 (85.7) | |
| 2 | 12 | 8 (22.8) | 4 (11.4) | |
| Smoking | .219 | |||
| >20 pack/year | 25 | 10 (28.6) | 15 (42.8) | |
| <20 pack/year | 18 | 8 (22.9) | 10 (28.6) | |
| Current | 27 | 17 (48.5) | 10 (28.6) | |
| Radiotherapy dose (Gy) | .614 | |||
| 50-60 | 11 | 7 (20) | 4 (11.4) | |
| 60-70 | 57 | 27 (77) | 30 (85.7) | |
| >70 | 2 | 1 (2.9) | 1 (2.9) | |
| Chemotherapy | .766 | |||
| CC | 56 | 29 (82.9) | 27 (77.1) | |
| NC | 14 | 6 (17.1) | (22.9) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; CC, concurrent chemotherapy; NC, no chemotherapy.
Primary Outcome
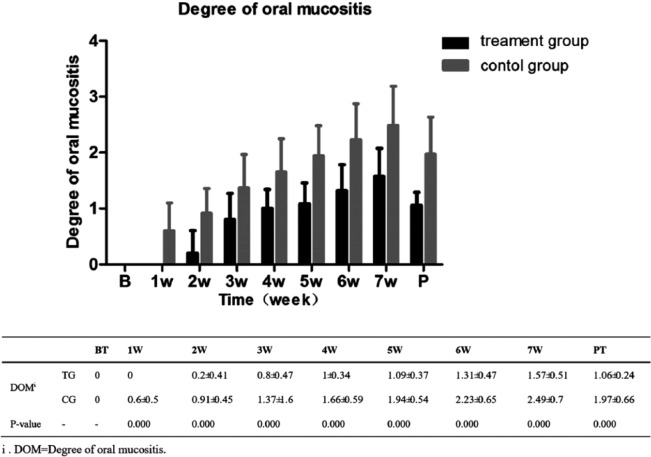
The degrees of OM were 0 before the RT in both groups (Figure 2). Nevertheless, the difference had appeared from the first week after RT and extended gradually, consistent with the increase of radiation dose. At the seventh week of RT, the degrees of OM in the treatment group were 1.57 ± 0.51 versus 2.49 ± 0.7 in the control group (P < .01).
Figure 2.
Degree of oral mucositis at different treatment times.
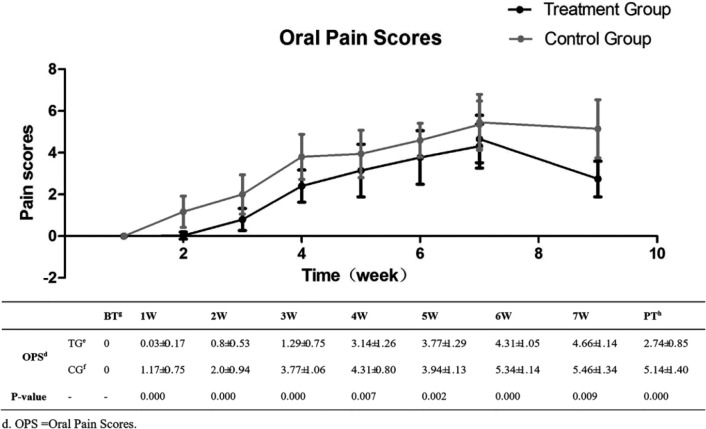
During the entire RT period, the treatment group showed significant effectiveness in remission of oral pain (P < .01). The most serious oral pain appeared at the seventh week; the oral pain score of the treatment group was 4.66 ± 1.14, which was lower than 5.46 ± 1.34 in the control group (P < .01) (Figure 3).
Figure 3.
Degree of oral mucositis: oral pain scores at different treatment times.
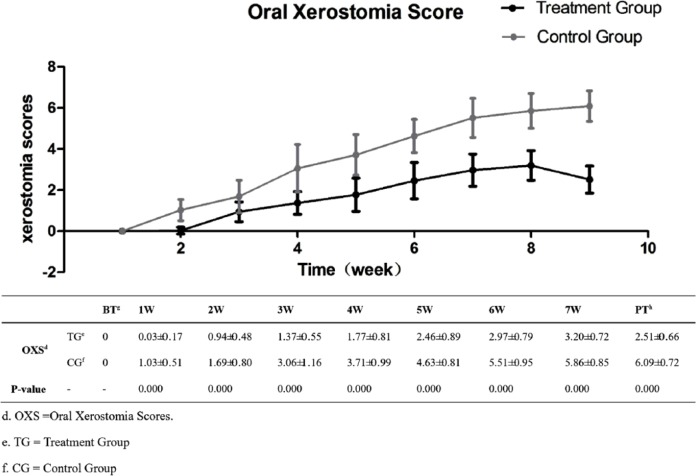
Before the treatment, no difference was noted in the degree of oral xerostomia between the 2 groups (Figure 4). However, during the entire treatment, the treatment group displayed superiority in preventing oral xerostomia (P < .01). In addition, the difference continued to extend until the week after the RT, 2.51 ± 0.66 in the treatment group in contrast with 6.09 ± 0.72 in the control group (P < .01).
Figure 4.
Xerostomia scores at different treatment times.
No statistical difference was observed between the 2 groups in terms of the BMI before treatment (P = .412, >.05). The BMI was 20.8 ± 2.7 kg/m2 in the treatment group and 21.1 ± 2.6 kg/m2 in the control group. The difference between the 2 groups became obvious after treatment (P = .153, >.05). The BMI was 19.8 ± 3.26 kg/m2 in the treatment group and 18.8 ± 2.5 kg/m2 in the control group (Figure 5).
Figure 5.
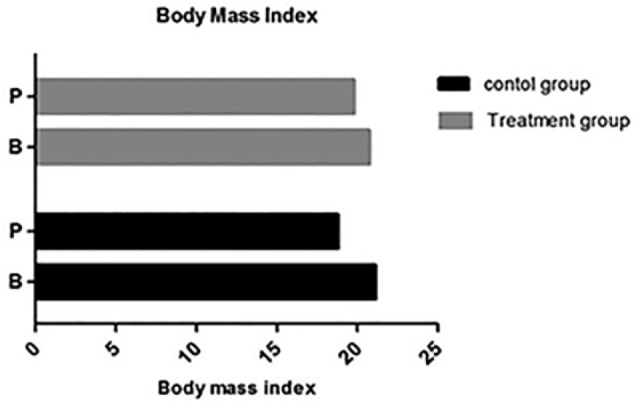
Body mass index (BMI) in the different groups.
Discussion
Radiation and chemotherapy as mainstay regimens play an important role in treating patients with head and neck cancer. Nevertheless, chemoradiation-related complications induce serious effects on quality of life and may interfere with cancer therapy. Chemoradiation-related OM has been raised as a concern by oncologists because of its high incidence and excruciating oral pain. Clinically, OM is characterized by mucosal breakdown resulting in extensive, deep ulcerations, severe function-altering pain, increased risk of secondary infection, bacteremia and sepsis, and expanded need for gastrostomy feedings, and ambulatory and in-patient supportive care.9 Despite this concern, no consensus has been reached with regard to protocol for preventing and treating OM, although various treatments have been reported.10
CHIN mainly comprises Radix Ophiopogonis and Scutellaria baicalensis in the ratio of 2:1. Rhubarb, Licorice, Mint, Red Peony root, Lumbricus, Radix Scrophulariae, and Forsythia are secondary materials. CHIN is modified from Liangge san, which was reported as inhibiting the expression of inflammatory factors by inhibiting expression of lipopolysaccharide (LPS)-induced p65 and promoting detoxification of the organism.11-13 In modifying the Liangge San decoction, we reserved the active components of heat-clearing and detoxifying, but omitted the mirabilite and Gardenia jasminoides Ellis for severe diarrhea. Licorice and Red Peony root were supplements to the method of treating yin deficiency by reinforcing body fluid and nourishing the blood. We abandoned benzydamine mouthwashes for the control group because of the difficulty of obtaining the product in our district. Given the efficiency of rhEGF spray, we chose this method in treating the patients in control group.14-18
Our clinical results revealed that CHIN had a prominent clinical benefit on preventing OM and reducing oral pain. The results also demonstrated that CHIN could relieve oral xerostomia and improve physical condition. Nevertheless, the mechanism of CHIN on remission OM has yet to be completely defined. Radix Liriopes, one of the dominant drugs, was ascertained to have antioxidative and anti-inflammatory activities.19,20 Multiple in vitro studies demonstrate that ruscogenin, the major constituent of Radix Liriopes, had a significant anti-inflammatory effect by inhibiting proinflammatory cytokine (eg, tumor necrosis factor–α [TNF-α], interleukin-6 [IL-6], and IL-1) overexpression and suppressed nuclear factor–κB (NF-κB) activation.21 A plethora of evidence also shows that Scutellaria baicalensis inhibits the production of inflammatory cytokines (eg, TNF-α, IL-6) by negatively regulating the NF-κB signaling pathway under LPS simulation.22 In addition, baicalin has an especially favorable inhibition of bacterial growth of fungi and bacteria.23 These research results reveal the possible mechanisms of CHIN on prevention of OM. We deduce that the active ingredient of CHIN protects against OM by regulating the NF-κB signaling pathway and oral flora regulation. However, compelling evidence in this regard is currently lacking.
Our study has certain limitations. First, the study was performed at a single center, and the sample size was small. Second, the study was not double-blind trial. Third, only short-term observation was performed, and thus the long-term effects were not noted in this study. Nevertheless, our findings provide preliminary support for the possibility that CHIN is efficacious for the management of mucositis in patients receiving head and neck RT. We will focus on buccal mucosal cells and levels of salivary inflammatory factors to explore the mechanism at the molecular level and extend other clinical symptom control, such as xerostomia, in our future studies. In conclusion, to our knowledge, CHIN exhibited an obvious advantage in prophylaxis of radiation-induced OM compared with rhEGF spray.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Medical Seed Foundation for Cancer Transformation of Tianjin Medical University Cancer Institute and Hospital and National Key Clinical Specialist Construction Programs of China (No. 2013-544).
References
- 1. Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253-262. [DOI] [PubMed] [Google Scholar]
- 2. Mallick S, Benson R, Rath GK. Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol. 2016;273:2285-2293. doi: 10.1007/s00405-015-3694-6. [DOI] [PubMed] [Google Scholar]
- 3. Yu LZ, Hu KY. Effect of Liangge san on inflammation in acute lung injury rat induced by endotoxin. Chin J Tradit Chin Med Pharm. 2009;11:1433-1436. [Google Scholar]
- 4. Yang YH, Wang RF, Chen XY. Efficacy and recurrence western medicine of combined with Liangg san fiery flaming type stomatitis. Chin Arch Tradit Chin Med. 2015;33:1470-1472. [Google Scholar]
- 5. Common Terminology Criteria for Adverse Events [EB/OL]. CTCAE Version 4.0. http://www.calgb.org/Public/meetings/presentations/2009/summer_group/cra_cont_ed/06a_CTCAE-Setser_062009.pdf. Accessed July 26, 2017.
- 6. Breivik EK, Skoglund LA. Comparison of present pain intensity assessments on horizontally and vertically oriented visual analogue scales. Methods Find Exp Clin Pharmacol. 1998;20:719-724. [DOI] [PubMed] [Google Scholar]
- 7. Reed MD, Van Nostran W. Assessing pain intensity with the visual analog scale: a plea for uniformity. Clin Pharmacol. 2014;54:241-244. [DOI] [PubMed] [Google Scholar]
- 8. Pai S, Ghezzi EM, Ship JA. Development of a visual analogue scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:311-316. [DOI] [PubMed] [Google Scholar]
- 9. Chen P, Mancini M, Sonis ST, et al. A novel peptide for simultaneously enhanced treatment of head and neck cancer and mitigation of oral mucositis. PLoS One. 2016;11:e0152995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moslemi D, Nokhandani AM, Otaghsaraei MT, Moghadamnia Y, Kazemi S, Moghadamnia AA. Management of chemo/radiation-induced oral mucositis in patients with head and neck cancer: a review of the current literature. Radiother Oncol. 2016;120:13-20. [DOI] [PubMed] [Google Scholar]
- 11. Jiang AD, Yu LZ, Gong XW, Deng P, Ma XD. Effect of Liangge san on lipopolysaccharide-induced nuclear factor kappa B activation in cultured mouse macrophages [in Chinese]. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:619-622. [PubMed] [Google Scholar]
- 12. Wang B, Cao SH, Wang YQ. Effects of modified Liangge powder contained serum on LPS stimulated TLR4 expression and release of cytokines in mouse platelets [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:681-684. [PubMed] [Google Scholar]
- 13. Yu LZ, Jiang AD, Cheng YY, Lin H, Qin QH, Ma XD. Liangge san effects the expression of CD14 and scaverger receptor in the Kupffer cells of liver of endotoxemia mice [in Chinese]. Zhongguo Zhong Yao Za Zhi. 2006;31:220-223. [PubMed] [Google Scholar]
- 14. Kim KI, Kim JW, Lee HJ, et al. Recombinant human epidermal growth factor on oral mucositis induced by intensive chemotherapy with stem cell transplantation. Am J Hematol. 2013;88:107-112. [DOI] [PubMed] [Google Scholar]
- 15. Hong JP, Lee SW, Song SY, et al. Recombinant human epidermal growth factor treatment of radiation-induced severe oral mucositis in patients with head and neck malignancies. Eur J Cancer Care (Engl). 2009;18:636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu HG, Song SY, Kim YS, et al. Therapeutic effect of recombinant human epidermal growth factor (RhEGF) on mucositis in patients undergoing radiotherapy, with or without chemotherapy, for head and neck cancer: a double-blind placebo-controlled prospective phase 2 multi-institutional clinical trial. Cancer. 2009;115:3699-3708. [DOI] [PubMed] [Google Scholar]
- 17. Ryu SH, Kang KM, Moon SY, et al. Therapeutic effects of recombinant human epidermal growth factor (rhEGF) in a murine model of concurrent chemo- and radiotherapy-induced oral mucositis. J Radiat Res. 2010;51:595-601. [DOI] [PubMed] [Google Scholar]
- 18. Yahya S, Benghiat H, Nightingale P, Tiffany M, Sanghera P, Hartley A. WITHDRAWN: does dose to an oral mucosa organ at risk predict the duration of grade 3 mucositis after intensity-modulated radiotherapy for oropharyngeal cancer [published online April 5, 2016]. Clin Oncol (R Coll Radiol). doi: 10.1016/j.clon.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 19. Sun W, Hu W, Meng K, et al. Activation of macrophages by the ophiopogon polysaccharide liposome from the root tuber of Ophiopogon japonicus. Int J Biol Macromol. 2016;91:918-925. [DOI] [PubMed] [Google Scholar]
- 20. Fan Y, Ma X, Ma L, Zhang J, Zhang W, Song X. Antioxidative and immunological activities of ophiopogon polysaccharide liposome from the root of Ophiopogon japonicus. Carbohydr Polym. 2016;135:110-120. [DOI] [PubMed] [Google Scholar]
- 21. Huang YL, Kou JP, Ma L, Song JX, Yu BY. Possible mechanism of the anti-inflammatory activity of ruscogenin: role of intercellular adhesion molecule-1 and nuclear factor-kappaB. J Pharmacol Sci. 2008;108:198-205. [DOI] [PubMed] [Google Scholar]
- 22. Wang J, Luo H, Yang L, Li Y. Baicalein induces apoptosis and reduces inflammation in LPS-stimulated keratinocytes by blocking the activation of NF-κB: implications for alleviating oral lichen planus. Cell Mol Biol (Noisy-le-grand). 2016;62:55-60. [PubMed] [Google Scholar]
- 23. Yang D, Hu H, Huang S, Chaumont JP, Millet J. Study on the inhibitory activity, in vitro, of baicalein and baicalin against skin fungi and bacteria. Zhong Yao Cai. 2000;23:272-274. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.