Abstract
Introduction. Opioid-induced constipation (OIC) is a principal complication secondary to analgesic therapy for cancer pain patients who suffer moderate to severe pain. In this study, we observe the efficacy and safety of transcutaneous acupoint interferential current (IFC) stimulation in those patients with OIC. Methods. A total of 198 patients were randomly allocated to the IFC group and control group in a 1:1 ratio. Finally, 98 patients in the IFC group received 14 sessions administered over 2 weeks, whereas 100 patients in the control group took lactulose orally during the same period. Observation items were documented at management stage and at follow-up stage according to Cleveland Constipation Scales (CCS), pain Numeric Rating Scales (NRS) and Patient Assessment of Constipation Quality of Life (PAC-QoL). Results. The total curative effects of the IFC group and the control group were indistinguishable (76.5% vs 70.0%, P = .299). Regarding CCS and PAC-QoL scores, no significant difference was observed between the 2 groups during the management time and at the follow-up stage of week 3 (P > .05, respectively), but groups were distinguished at the follow-up stage of week 4 (P < .001 and P = .031, respectively). The pain NRS decreased significantly at management stage week 2 and follow-up stage week 3 and week 4 (P = .013, P = .041, P = .011, respectively). Conclusions. Transcutaneous acupoint IFC therapy over acupoints of Tianshu (ST25) and Zhongwan (RN12) may improve constipation and quality of life in cancer patients receiving opiates; further studies are worthwhile.
Keywords: opioid-induced constipation, lactulose, transcutaneous acupoint interferential current therapy, cancer pain, quality of life
Introduction
Pain is a stubborn problem that commonly occurs in midadvanced cancer patients, lengthening hospital stay and increasing the treatment burden.1 Opioid analgesics are the mainstay of cancer pain management for patients who suffer moderate to severe pain.2-4 However, opioid are prescribed conservatively because of their side effects, especially opioid-induced constipation (OIC), the incidence rate of which is between 23% and 85%5,6; worse still, it can persist throughout the course of the prescription. Furthermore, dysphoric and depressive emotions related to OIC may lead to heart and cerebrovascular diseases, and even paralytic intestinal obstruction, critically affecting the patient’s quality of life.4
The mechanism of OIC is still uncertain. The main viewpoints can be summarized as follows: (1) The opioid receptors (predominantly µ, but also κ and δ, which are located in the gut wall of the myenteric plexus and the submucosal plexus) inhibit excitatory and inhibitory neural pathways within the enteric nervous system when activated by endogenous or exogenous opioids. The restraint of excitatory neural pathways depresses peristaltic contractions, whereas the interdiction of inhibitory neural pathways elevates gastrointestinal (GI) muscle activity to nonpropulsive motility patterns7,8; (2) opioids reduce water and electrolyte secretion, increase liquid reabsorption, and also increase sphincter tone, together leading to the dry feces and hard stools, which are difficult to pass through the gut lumen9; and (3) opioids impair the defecation reflex, which reduces sensitivity to distension.6 These mechanisms are generally considered to be responsible for delaying gastric emptying and slowing the intestinal transit.
The oral administration of osmotic agents (usually lactulose) needs to be prescribed in the majority of patients with OIC.3 OIC has an osmotic effect on the colon and stimulates activated neurons in the myenteric and submucosal plexus of the colon, thus reducing the absorption of water and electrolytes by the intraluminal contents.3,10 In recent years, acupuncture and electroacupuncture have been confirmed as effective in the management of GI motility disorders, such as chronic treatment-resistant constipation.11,12 Compared with traditional acupuncture and electrical acupuncture, transcutaneous acupoint interferential current (IFC) therapy has the advantage of being noninvasive as well as amenable to adjustability of stimulation frequency and intensity objectively and quantifiably; therefore, it is increasingly being applied in various GI diseases.13-16 In this study, we assess transcutaneous acupoint IFC stimulation with pad electrodes on the belly (acupoints ST25 and RN12) in cancer pain patients with OIC, compared with oral lactulose administration, to observe its efficacy and safety in this population.
Methods
Patient Collection
A hospital-based prospective study was performed that consisted of consecutive cancer pain patients who were admitted to the Oncology Department, Pain Department, and Gastrointestinal Department of Tongde Hospital in Zhejiang province (China) from July 2013 to June 2016. Patients were included in this study if they (1) were diagnosed with cancer and given analgesic therapy, with daily use of oxycodone hydrochloride delayed-release tablets ≥80 mg; (2) met the Rome III diagnostic criteria for constipation17; (3) were aged between 18 and 80 years old; (4) received no other management for constipation for 2 weeks before enrollment, except rescue symptomatic treatment for acute intestinal obstruction or gas; and (5) had no language barrier and signed the informed consent. The exclusion criteria were as follows: (1) secondary constipation caused by metabolic, endocrine, nervous, or postoperative diseases or intestinal organic diseases (such as Crohn’s disease, intestinal tuberculosis, etc); (2) serious cardiovascular, hepatic, renal, or psychiatric diseases, influencing the cooperation, examination, or follow-up process of IFC; and (3) pregnancy or lactation. Patients were withdrawn if they (1) had severe adverse reactions or complications that meant they could not tolerate the trial; (2) withdrew the informed consent at any time; and (3) were out of touch or died within the follow-up stage. The research protocol was approved by Clinical Trial Ethics Committee of Tongde Hospital of Zhejiang Province (Protocol No. [2013] 071-008) and registered in Chinese Clinical Trial Registry (ChiCTR, registration No. ChiCTR-IPR-15007105).
Design and Protocols
Based on our preliminary work, the efficacy of transcutaneous acupoint IFC in treating constipation was about 80%, and according to the literature, the efficacy of lactulose oral solution was about 75%. To detect a significant difference (P = .05) between the groups with 90% power, and assuming a drop-out rate of 15%, at least 100 patients were required for each group. Patients were randomly assigned to the IFC group and control group in a ratio of 1:1 according to a basic random sampling method using SPSS 20.0 software (SPSS Inc, Chicago, IL), and clinical research coordinators assigned them through the random numbers to receive treatment accordingly. Patients in the IFC group received 14 sessions of IFC therapy administered over 2 weeks. Interferential stimulators (TT-900-6A, Guangzhou, China) delivered IFC using electrodes (4 cm × 4 cm, CM4040, Hangzhou, China) with a carrier frequency of 4 kHz, an adjustable intensity of 25 to 30 mA, and a beat frequency of 80 to 120 Hz. The intensity of the stimulator was increased gradually until patients felt that it was “strong but comfortable.” In each session, 30 minutes of IFC per day was applied to the patients at the acupoints of Tianshu (ST25, 2 cm to the side of the belly button) and Zhongwan (RN12, 4 cm above the belly button). In Figure 1, the electrode on the top left is connected to the electrode on the lower right, and the electrode on the top right is connected to the electrode on the lower left. Patients in the control group were treated with lactulose oral solution, 10 mL 3 times per day for 2 weeks.
Figure 1.
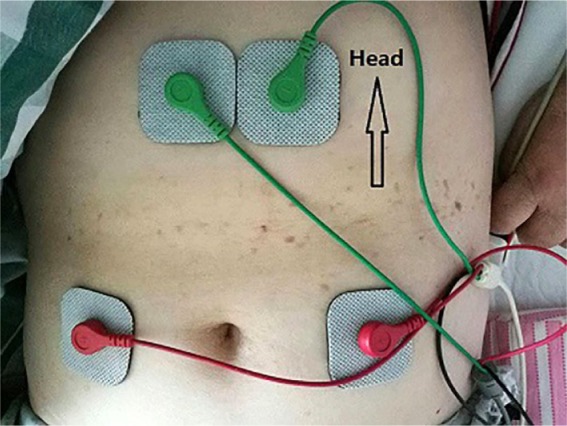
Positions of transcutaneous acupoint interferential current stimulation.
Assessments and Follow-up
A series of face-to-face questionnaires was documented at treatment stages week 1 and week 2 and follow-up stages week 3 and week 4. The Cleveland Constipation Scale18 (CCS) was used to assess constipation, which includes 8 main evaluation items consisting of defecation frequency, defecation straining, complete emptying, abdominal pain, defecation time, defecation help, failed defecation frequency per 24 hours, and constipation course. The Patient Assessment of Constipation Quality of Life19 (PAC-QoL) questionnaire was adopted to evaluate patients’ quality of life. It involves 28 items in 4 subscales: physical discomfort, psychosocial discomfort, worries and concerns, and satisfaction. A Numeric Rating Scale20 (NRS) for pain (0 = no pain and 10 = worst possible pain) was applied to evaluate the patient’s pain intensity. Overall prognosis of constipation was assessed according to the Ministry of Health of Peoples Republic of China Guidelines for clinical study of new Chinese medicines21 as follows: (1) obvious efficacy: constipation symptoms are improved totally or significantly, defecation interval and stool quality are normal or close to normal (the stool is slightly dry and defecation interval is within 72 hours), and all or most other symptoms disappear; (2) effective: defecation interval is shortened by 1 day, the symptom of dry feces is improved, and other symptoms are ameliorated; and (3) invalid: no improvement in constipation and other symptoms.
Statistical Analysis
The continuous variables for data were expressed as mean ± SD, and the categorical variables were expressed as a percentage or frequency. The independent-sample Student’s t-test was used for analysis of continuous data; if the P value of the homogeneity test of variance was less than .05, the Wilcoxon signed-rank test was applied. The χ2 test was used to identify categorical data. All statistical analyses were performed using SSPS version 20.0 software (SPSS, Inc, Chicago, IL), and data were considered statistically significant when P ≤.05.
Results
Among the patients enrolled in this study, 6 dropped out at the management stage because of adverse effects, and 3 individuals withdrew; 2 were withdrawn from the trial as lost to follow-up, and 1 patient died at the follow-up stage owing to the advanced cancer. Finally, 98 patients completed the ICF therapy, whereas 100 patients finished the lactulose oral solution protocol. There were no statistically significant differences in the baseline characteristics between the 2 groups (Table 1).
Table 1.
The Baseline Clinical Characteristics of the 2 Groups (Mean ± SD).
| IFC Group (n = 98) | Control Group (n = 100) | P Value | |
|---|---|---|---|
| Age (year) | 57.12 ± 9.93 | 59.54 ± 10.77 | .087 |
| Sex (M/F) | 52/46 | 45/55 | .257 |
| Physical discomfort | 6.05 ± 0.98 | 5.89 ± 0.86 | .221 |
| Psychosocial discomfort | 9.97 ± 1.15 | 10.22 ± 1.45 | .180 |
| Worries and concerns | 11.69 ± 1.47 | 12.02 ± 1.55 | .130 |
| Satisfaction | 10.46 ± 1.30 | 10.79 ± 1.55 | .106 |
| CCS | 14.16 ± 2.03 | 13.93 ± 1.83 | .397 |
| NRS | 3.29 ± 0.66 | 3.40 ± 0.65 | .221 |
| Daily dosage of oxycodone hydrochloride | 96.02 ± 16.97 | 94.00 ± 13.78 | .359 |
Abbreviations: IFC, interferential current; M, male; F, female; CCS, Cleveland Constipation Scales; NRS, pain Numeric Rating Scales.
The total curative effects of the 2 therapeutic methods are analogous (IFC group 76.5% vs control group 70.0%, P = .299), which is displayed in detail in Table 2. There is no significant difference in comparison with obvious remission, effective remission, and limited remission between the 2 groups (P > .05, respectively).
Table 2.
The Comparison of Treatment Efficacy for OIC Between the 2 Groups.
| IFC Group (n = 58) | Control Group (n = 60) | P Value | |
|---|---|---|---|
| Obvious remission | 30 (30.6%) | 21 (21.0%) | .144 |
| Effective remission | 45 (45.9%) | 49 (49.0%) | .664 |
| Limited remission | 23 (23.5%) | 30 (30.0%) | .229 |
| Total remissiona | 75 (76.5%) | 70 (70.0%) | .299 |
Abbreviations: OIC, opioid-induced constipation; IFC, interferential current.
Total remission = Obvious remission + Effective remission.
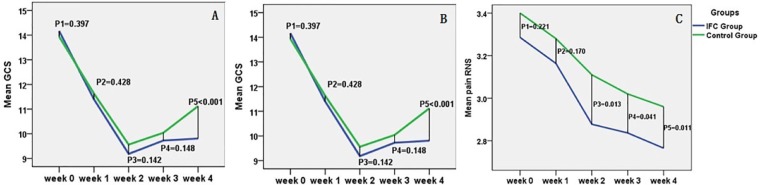
Differences in CCS, PAC-QoL, and NRS scores between the 2 groups at the management and follow-up stages are illustrated in Table 3. Regarding CCS scores, there was no significant difference in the scores between the 2 groups, either during the management time or at the follow-up stage of week 3 (P = .397, P = .428, P = .142, and P = .148, respectively). However, we found significantly lower CCS statistics in the IFC group than the control group at the follow-up stage of week 4 (P = .000). Interestingly, we found comparable outcomes for the PAC-QoL (P = .688, P = .130, P = .119, P = .195, and P = .031, respectively) questionnaire, suggesting that IFC therapy may have sustained effects and lead to a better quality of life for longer than lactulose treatment. In the analysis of pain sensation, NRS statistics showed that patients in the IFC group achieved significant pain remission at the management stage of week 2 and the follow-up stage of week 3 and week 4 (P = .013, P = .0413, and P = .011, respectively), but there was no significant difference at the management stage of week 1 (P = .221), suggesting that IFC might alleviate patients’ pain (Figure 2). In within-group comparisons, the results revealed that both CCS and QoL changed significantly from week 1, whereas pain NRS showed significant change from week 2 (respectively, P < .01).
Table 3.
Comparison of CCS, PAC-QoL, and Pain NRS Between the 2 Groups (Mean ± SD).
| Week 0a | Week 1b | Week 2b | Week 3c | Week 4c | ||
|---|---|---|---|---|---|---|
| CCS | IFC group (n = 58) | 14.16 ± 2.03 | 11.40 ± 2.13 | 9.18 ± 1.81 | 9.72 ± 1.59 | 9.81 ± 1.67 |
| Control group (n = 60) | 13.93 ± 1.83 | 11.63 ± 1.98 | 9.56 ± 1.79 | 10.04 ± 1.47 | 11.12 ± 1.73 | |
| P1 value | .397 | .428 | .142 | .148 | .000 | |
| PAC-QoL | IFC group (n = 58) | 39.15 ± 4.83 | 32.47 ± 4.84 | 27.78 ± 5.89 | 29.15 ± 6.16 | 29.83 ± 6.64 |
| Control group (n = 60) | 38.89 ± 4.37 | 33.53 ± 4.97 | 29.10 ± 6.02 | 30.36 ± 6.88 | 31.87 ± 6.58 | |
| P2 value | .688 | .130 | .119 | .195 | .031 | |
| NRS | IFC group (n = 58) | 3.29 ± 0.66 | 3.16 ± 0.60 | 2.88 ± 0.63 | 2.84 ± 0.62 | 2.77 ± 0.49 |
| Control group (n = 60) | 3.40 ± 0.65 | 3.28 ± 0.59 | 3.11 ± 0.68 | 3.02 ± 0.64 | 2.96 ± 0.57 | |
| P3 value | .221 | .170 | .013 | .041 | .011 |
Abbreviations: CCS, Cleveland Constipation Scales; PAC-QoL, Patient Assessment of Constipation Quality of Life; NRS, pain Numeric Rating Scales; IFC, interferential current.
Outcomes at baseline.
Outcomes of treatment on.
Outcomes of treatment off.
Figure 2.
A. The outcomes of CCS at different stages for the 2 groups. B. The outcomes of PAC-QoL at different stages for the 2 groups. C. The outcomes of NRS at different stages for the 2 groups.a
Abbreviations: CCS, Cleveland Constipation Scales; PAC-QoL, Patient Assessment of Constipation Quality of Life; NRS, pain Numeric Rating Scales; IFC, interferential current.
a P1, P value at baseline; P2, P3, P values of treatment on; P4, P5, P values of treatment off.
No severe related adverse events, such as intestinal bleeding, acute abdominal pain, coma, frequent nausea or vomiting, local amyasthenia, or infection, were observed in the IFC group patients during treatment and follow-up visits. One patient died from a deteriorating condition associated with cancer that was foreign to the transcutaneous acupoint IFC therapy in this trial.
Discussion
Up to 80% of patients with cancer suffer moderate to severe pain, and most of these patients take strong opioids for pain remission.3 Because of metatrophia, reduced activity, oncotherapy, and the fear associated with cancer, these patients suffer not only intractable constipation, but also psychosocial distress, including global distress and specific anticipatory anxiety, which may increase over time and could be a prominent barrier to effective constipation management.1
Laxatives, such as lactulose, are frequently used to treat chronic constipation and are recommended in the oncological clinical practice guidelines by the National Comprehensive Cancer Network (NCCN),22 which even proposes it as a prophylactic strategy for OIC.23,24 Opioid receptor antagonists such as naltrexone and naloxone have been used to treat OIC recently, but the side effects of the analgesia limit their efficiency, and the degree to which the outcome is acceptable is difficult to determine.25 Cancer accompanied with constipation is complicated, and it is unlikely that viewing it as a single entity in cancer will advance our understanding of its pathophysiology. It may be that opioids have only a modest adverse influence on the severity of constipation in cancer patients but are not the main culprit.26 So far, OIC in cancer pain patients is still poorly managed.3,27 Thus, it seems that some other effective therapeutic approaches are worth exploring.
According to traditional Chinese medicine (TCM), the human body is run by the circulation of meridians via equilibrating the elements of qi, Blood, yin, and yang. Opioids are warm-scorching drugs that can deplete yin and body fluids and lead to heat accumulation, resulting in constipation.28 Acupoints are the special epidermatous sites that accumulate affluent blood capillaries and teleneurons and are considered the hinges of the meridians. They are polymorphous and diverse in spatial structures, which are of uncertain size, possibly depending on structure domains, location, function, dominating visceral organs, and so on. Unlike electric acupuncture, IFC stimulation acts on a larger region of body skin because of its higher frequency and higher current. However, regardless of whether it is electric acupuncture or IFC, the point of action they perform is the whole acupoint structure, not only some certain point or area on the body skin. Acupuncture has been used to treat constipation for thousands of years in China and is also recommended by the World Health Organization for chronic constipation.29 In the past decade, transcutaneous IFC has been increasingly applied in several disorders, including muscle strengthening, soft-tissue mobilizing, detrusor instability, and slow-transit constipation.13-16,30,31 IFC is a noninvasive transcutaneous electrical type of stimulation, which produces 2 sinusoidal currents that cross within the body.30 In this study, we adopted the Tianshu (ST25) and Zhongwan (RN12) points, which are the specific acupoints prescribed for GI dysfunction diseases, compared with oral lactulose administration, to confirm the efficacy and safety of transcutaneous acupoint IFC therapy for OIC innovatively. We reviewed multiple literature sources and TCM to determine the electrode positions at the design stage of our trial and came to the conclusion that the positions adopted in our study are definitely effective. Hence, we concentrated on Tianshu and Zhongwan acupoints in our study temporarily; other positions might be considered in our next step. Similar to other literature,12 the IFC frequency in our study was also set to 4 kHz, with an adjustable intensity and a beat frequency sweep covering 80 to 120 Hz. The intensity of the stimulator was increased gradually until the patient reported that a further rise would cause discomfort.
In the present clinical trial, we showed preliminarily that the curative effects of transcutaneous acupoint IFC are similar to that of lactulose oral solution in cancer pain patients with OIC, and its effects may even be longer lasting. Patients in the IFC group were given a treatment cycle of 14 sessions over 2 weeks, which is similar to a previous study.12 Judging by the preliminary results, after undergoing this treatment cycle, our research testified that this therapy duration yielded a satisfactory outcome. Whether a longer treatment cycle would achieve more efficacy needs to be clarified in the future.
The mechanism of action of IFC on the motor control of the GI tract is still quite unclear; it might be synthesized via the functional activities of muscle cells, pacemaker cells (Cajal, etc), nerves (somatic and visceral), and hormones (gastrin, etc). Improvements have been confirmed at different frequencies on different positions on the body’s surface.12 According to TCM, the acupoints responsible for the GI system primarily contain He-Sea acupoints, Front-Mu acupoints, and Back-Shu acupoints. At present, no definite conclusion has been arrived at yet on which acupoints are optimal stimulation positions or the specific location of neuroregulation (IFC or electroacupuncture). In the previous IFC studies,13,31 the electrodes were unambiguously positioned on the belly or belly and back; unlike these studies, the positions in our study were determined definitely according to acupoint theory. However, different acupoints were also used in some other research for patients with constipation11,32,33; for example, the He-Sea points (Zusanli, ST36; Shangjuxu, ST37, etc). Wu et al32 argued that He-Sea acupoint prescription is the best choice for the treatment of functional constipation in comparison with other acupoints. Nevertheless, Peng et al33 demonstrated that the individual deep puncture at Tianshu (ST 25) is also effective in the treatment of slow transit constipation, and besides, it can achieve satisfactory long-term efficacy. Later, Sun et al34 testified that an incremental expression of colonic interstitial cells of Cajal on Tianshu (ST 25) may be responsible for its efficacy in improving slow transit constipation in animal experiments. Therefore, it could be inferred that the effective positions on the body surface for stimulation are multiple point, and the different effects could be a result of the stimulation of different types of nerve fibers.13,30,31 Stimulation by IFC may dominate the parasympathetic and sympathetic efferent fibers directly or indirectly by stimulation of the afferent fibers. However, sympathetic innervation inhibits bowel motility, whereas parasympathetic innervation activates the bowel.35
In previous studies, connections between the intestinal nerve cell network and the central nervous system via the splanchnic, spinal, and pelvic nerves have been established.36,37 Based on our study, we hypothesize the following: (1) IFC stimulation on the belly might target afferent somatic and visceral fibers, affecting somatovisceral reflexes or regulation of intestinal mucosa; this conjecture was tested by Vitton et al38 in animal experimentation, in which they stimulated sacral roots (S1, S2, S3) and somatic (radial and sciatic) nerves on the colon and internal anal sphincter to analyze the electromyographic activity in cats and concluded that current stimulation on sacral spinal roots may improve the defecation reflex by inhibiting colonic activity and enhancing internal anal sphincter activity via a somatosympathetic reflex; (2) the somatic nerves innervate the anal sphincter muscle and sensory fibers to the central nervous system, whereas the spinal nerves target parasympathetic outflow to the rectum and internal anal sphincter; this could explain the mechanism of action of sacral neuromodulation on both anal incontinence and constipation; and (3) the acupoint sites that we selected in this study hinted at a better therapeutic effect compared with former research,31 which might be explained by more efficacious neuromuscular regulation and vasoganglion within these positions.
Besides, we observed that transcutaneous acupoint IFC may relieve cancer patients with constipation of pain. This is similar to the findings by Coban et al,14 who conducted a clinical trial using vacuum IFC to treat patients with irritable bowel syndrome (IBS) and concluded that IFC therapy can significantly improve symptoms and quality of life in these patients, such as abdominal distension and pain.
However, there are several limitations to this study: (1) research participants were not blinded in this trial; the patients knew which treatment prescription they would receive and that could have led to selection bias; (2) a relatively short treatment period, suboptimal acupoint selection, deficiency of related biomarkers, and patients’ subjective higher treatment expectations might also have contributed to the absence of significant beneficial effects in outcome assessments.
Conclusion
This preliminary study demonstrated that the efficacy of transcutaneous acupoint IFC stimulation with pad electrodes is equivalent to that of lactulose therapy. Furthermore, it seems to be free of adverse effects and to assist with pain relief, and may be more beneficial to quality of daily life in the long term. Optimal utility of this procedure would require identifying the most suitable electrode positions, the most reasonable current settings, and the treatment frequency that is most likely to be beneficial. Future research should be aimed at conducting a larger clinical trial with longer visit duration to further investigate these concerns.
Footnotes
Authors’ Note: Jun Kong and Cong-bin Peng are co-corresponding authors; they contributed equally to the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a program funded by the Traditional Chinese Medicine Bureau of Zhejiang province (No. 2014ZA014).
References
- 1. Dhingra L, Shuk E, Grossman B, et al. A qualitative study to explore psychological distress and illness burden associated with opioid-induced constipation in cancer patients with advanced disease. Palliat Med. 2013;27:447-456. [DOI] [PubMed] [Google Scholar]
- 2. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433. [DOI] [PubMed] [Google Scholar]
- 3. Droney J, Ross J, Gretton S, Welsh K, Sato H, Riley J. Constipation in cancer patients on morphine. Support Care Cancer. 2008;16:453-459. [DOI] [PubMed] [Google Scholar]
- 4. Chen CM, Lin LZ, Zhang EX. Standardized treatment of Chinese medicine decoction for cancer pain patients with opioid-induced constipation: a multi-center prospective randomized controlled study. Chin J Integr Med. 2014;20:496-502. [DOI] [PubMed] [Google Scholar]
- 5. Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain. 2001;93:247-257. [DOI] [PubMed] [Google Scholar]
- 6. Abramowitz L, Béziaud N, Labreze L, et al. Prevalence and impact of constipation and bowel dysfunction induced by strong opioids: a cross-sectional survey of 520 patients with cancer pain: DYONISOS study. J Med Econ. 2013;16:1423-1433. [DOI] [PubMed] [Google Scholar]
- 7. Davis MP. The opioid bowel syndrome: a review of pathophysiology and treatment. J Opioid Manag. 2005;1:153-161. [DOI] [PubMed] [Google Scholar]
- 8. Reimer K, Hopp M, Zenz M, et al. Meeting the challenges of opioid-induced constipation in chronic pain management—a novel approach. Pharmacology. 2009;83:10-17. [DOI] [PubMed] [Google Scholar]
- 9. Panchal SJ, Müller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61:1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leppert W. The impact of opioid analgesics on the gastrointestinal tract function and the current management possibilities. Contemp Oncol (Pozn). 2012;16:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong F, Wang Y, Li SQ, Tian M, Zheng CH, Huang GY. Clinical study of electro-acupuncture treatment with different intensities for functional constipation patients. J Huazhong Univ Sci Technolog Med Sci. 2014;34:775-781. [DOI] [PubMed] [Google Scholar]
- 12. Southwell BR. Medical devices to deliver transcutaneous electrical stimulation using interferential current to treat constipation. Expert Rev Med Devices. 2013;10:701-704. [DOI] [PubMed] [Google Scholar]
- 13. Queralto M, Vitton V, Bouie M, Abysique A, Portier G. Interferential therapy: a new treatment for slow transit constipation: a pilot study in adults. Colorectal Dis. 2013;15:e35-e39. [DOI] [PubMed] [Google Scholar]
- 14. Coban Ş, Akbal E, Köklü S, et al. Clinical trial: transcutaneous interferential electrical stimulation in individuals with irritable bowel syndrome—a prospective double-blind randomized study. Digestion. 2012;86:86-93. [DOI] [PubMed] [Google Scholar]
- 15. Vitton V, Benezech A, Honoré S, et al. CON-COUR study: interferential therapy in the treatment of chronic constipation in adults: study protocol for a randomized controlled trial. Trials. 2015;16:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Queralto M, Vitton V, Bouvier M, Abysique A, Portier G. Interferential therapy: a new treatment for slow transit constipation. A pilot study in adults. Colorectal Dis. 2013;15:e35-e39. [DOI] [PubMed] [Google Scholar]
- 17. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [DOI] [PubMed] [Google Scholar]
- 18. American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(suppl 1):S1-S4. [DOI] [PubMed] [Google Scholar]
- 19. Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40:540-551. [DOI] [PubMed] [Google Scholar]
- 20. Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149-158. [DOI] [PubMed] [Google Scholar]
- 21. Ministry of Health of the People’s Republic of China. Guideline for Clinical Study of New Chinese Medicines. Beijing, China: Ministry of Health of the People’s Republic of China; 1993:131-133. [Google Scholar]
- 22. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100:936-971. [DOI] [PubMed] [Google Scholar]
- 23. Ishihara M, Ikesue H, Matsunaga H, et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain. 2012;28:373-381. [DOI] [PubMed] [Google Scholar]
- 24. Wang Z, Qian JX, Jiao XD, et al. Clinical observation of prophylactic lactulose for prevention of oral morphine-induced constipation [in Chinese]. Zhonghua Yi Xue Za Zhi. 2012;92:2968-2971. [DOI] [PubMed] [Google Scholar]
- 25. Liu M, Wittbrodt E. Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J Pain Symptom Manage. 2002;23:48-53. [DOI] [PubMed] [Google Scholar]
- 26. Davis MP. Cancer constipation: are opioids really the culprit? Support Care Cancer. 2008;16:427-429. [DOI] [PubMed] [Google Scholar]
- 27. Tack J, Mueller-Lissner S, Dubois D, Schenck F. Only 27% of European patients with chronic constipation are satisfied with current treatment options. Gut. 2009;58(II):A1811. [Google Scholar]
- 28. Gu YJ, Chen J, Sun YY, Zhang X. Clinical observation on Jiangni Kuanchang decoction for opioid-related constipation. Liaoning J Tradit Chin Med (Chin). 2009;36:233-234. [Google Scholar]
- 29. Liu ZS, Liu J, Zhao Y, et al. The efficacy and safety study of electro-acupuncture for severe chronic functional constipation: study protocol for a multicenter, randomized, controlled trial. Trials. 2013;14:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chase J, Robertson VJ, Southwell B, Hutson J, Gibb S. Pilot study using transcutaneous electrical stimulation (interferential current) to treat chronic treatment-resistant constipation and soiling in children. J Gastroenterol Hepatol. 2005;20:1054-1061. [DOI] [PubMed] [Google Scholar]
- 31. Köklü S, Köklü G, Ozgüçlü E, Kayani GU, Akbal E, Hasçelik Z. Clinical trial: interferential electric stimulation in functional dyspepsia patients—a prospective randomized study. Aliment Pharmacol Ther. 2010;31:961-968. [DOI] [PubMed] [Google Scholar]
- 32. Wu JN, Zhang BY, Zhu WZ, Du RS, Liu ZS. Comparison of efficacy on functional constipation treated with electroacupuncture of different acupoint prescriptions: a randomized controlled pilot trial [in Chinese]. Zhongguo Zhen Jiu. 2014;34:521-528. [PubMed] [Google Scholar]
- 33. Peng WN, Wang L, Liu ZS, et al. Analysis on follow-up efficacy and safety of slow transit constipation treated with individualized deep puncture at Tianshu (ST 25): a multi-central randomized controlled trial [in Chinese]. Zhongguo Zhen Jiu. 2013;33:865-869. [PubMed] [Google Scholar]
- 34. Sun JH, Guo H, Chen L, et al. Effect of electroacupuncture at “Tianshu” (ST25) on colonic smooth muscle structure and interstitial cells of cajal in slow transit constipation rats. Zhen Ci Yan Jiu. 2011;36:171-175. [PubMed] [Google Scholar]
- 35. MacDonagh RP, Sun WM, Smallwood R, Forster D, Read NW. Control of defecation in patients with spinal injuries by stimulation of sacral anterior nerve roots. BMJ. 1990;300:1494-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanner JA. Reversible blocking of nerve conduction by alternating-current excitation. Nature. 1962;195:712-713. [DOI] [PubMed] [Google Scholar]
- 37. Bowman BR, McNeal DR. Response of single alpha motoneurons to high-frequency pulse trains: firing behavior and conduction block phenomenon. Appl Neurophysiol. 1986;49:121-138. [DOI] [PubMed] [Google Scholar]
- 38. Vitton V, Abysique A, Gaigé S, Leroi AM, Bouvier M. Colonosphincteric electromyographic responses to sacral root stimulation: evidence for a somatosympathetic reflex. Neurogastroenterol Motil. 2008;20:407-416. [DOI] [PubMed] [Google Scholar]