Abstract
Background: Sleep disturbance is the second most bothersome symptom in patients with cancer, and it can significantly impair their quality of life. The aim of this study was to investigate the efficacy and safety of the traditional herbal medicine Gamiguibi-tang (GGBT) in patients with cancer-related sleep disturbance. Methods: We conducted a prospective, randomized, wait-list-controlled, open-label pilot clinical trial on cancer-related sleep disturbance. Patients with cancer experiencing poor sleep quality with a Pittsburgh Sleep Quality Index of at least 6 were randomly assigned to the GGBT and wait-list groups to receive GGBT and conventional care, respectively, for 2 weeks. The primary endpoint was the Insomnia Severity Index (ISI) score. Fatigue, depression, and cognitive impairment were assessed as the secondary endpoints by using the Brief Fatigue Inventory (BFI), Beck Depression Inventory (BDI), and Montreal Cognitive Assessment (MoCA). Results: Thirty participants who met the eligibility criteria were enrolled. Sleep disturbance assessed using the ISI improved significantly more in the GGBT group than in the wait-list group (−5.5 ± 4.4 vs 0.1 ± 1.1, P < .001). Fatigue level determined using the BFI also improved significantly more in the GGBT group than in the wait-list group (−0.8 ± 0.8 vs 0.0 ± 0.3, P = .002). The BDI and MoCA scores showed no significant changes. Adverse events were reported in two patients in the GGBT group and consisted of mild dyspepsia and mild edema. Conclusion: GGBT may be a potential treatment option for cancer-related sleep disturbance. Further research is needed to investigate the efficacy and safety of GGBT.
Keywords: cancer-related sleep disturbance, insomnia, Gamiguibi-tang, Kamikihito, traditional medicine, Cancer symptoms
Introduction
Relative to the general population, patients with cancer are disproportionately affected by sleep disturbance. Sleep disturbance is the second most bothersome symptom in these patients, and the incidence rates of insomnia range from 30% to 60%.1,2 These problems can be a consequence of the psychological, behavioral, and physical effects of a cancer diagnosis, as well as cancer treatment including chemotherapy and surgery.3-5 Sleep disturbance is known to cause fatigue, depression, poor healing, failure of cognitive functioning, impaired work productivity, and poor relationships, as well as increased chances of cancer recurrence, safety issues, and medication misuse/abuse, thereby increasing the health care costs.6 Sleep disturbance has also been recognized as a persisting problem that is not always addressed effectively in cancer care.7,8
The standard approach to treating sleep disturbance in conventional medicine includes nonpharmacological and pharmacological treatments.9 Hypnotics are one of the most commonly prescribed medications for patients with cancer, being prescribed for sleep disturbance in up to 44% of patients.10 In general, improvements in various sleep endpoints with pharmacologic therapy have been modest, with the mean differences in sleep latency being about 15 minutes, waking after sleep onset improving by about 26 minutes, and total sleep time improving by about 40 minutes.11,12 Despite these improvements, hypnotic medications are associated with a number of risks, including residual next-day hypersomnia, dizziness, lightheadedness, impaired mental states, and increased risk of falls and hip fractures, especially in elderly patients.13,14 Therefore, better therapeutic options to improve sleep disturbance are required.
Gamiguibi-tang (GGBT) is the most frequently used traditional herbal formula for the treatment of sleep disturbance, and it has been widely used for thousands of years in traditional oriental medicine.15 Recent systematic reviews have reported that traditional herbal medicine, including GGBT, showed a potential benefit for improving sleep disturbance with mild adverse effects; however, definitive conclusions could not be drawn because of the poor methodological quality of these studies.16 In addition, a systematic review and some biochemical studies suggest that GGBT might be effective in improving depression and cognitive impairment.17-19
Given these research results and the research priority of sleep disturbance in cancer, we performed a clinical trial to investigate the efficacy and safety of GGBT in patients with cancer-related sleep disturbance.
Patients and Methods
Patients
This trial aimed to assess the efficacy and safety of GGBT in improving sleep in patients with cancer as well as its effect on fatigue, depression, and cognitive function.
Patients with histologically confirmed cancer who were older than 18 years and had sleep disturbance were eligible for enrollment. Sleep disturbance in this trial was defined as Pittsburgh Sleep Quality Index (PSQI) Korean version score of at least 6. Additional enrollment criteria were as follows: Eastern Cooperative Oncology Group (ECOG) performance status of less than 2, survival expectancy of 6 months or longer, and acceptable hematologic, hepatic, and renal function.
Concurrent use of hypnotics was allowed only when the participants maintained the same type and dosage of hypnotics more than 2 weeks before the study initiation. If the participant was prescribed an adjusted dosage or a change in the type of hypnotics during the study period, the participant was withdrawn from the study.
Patients receiving other dietary supplements or herbal medication for sleep disturbance, those with sleep apnea or neuropsychiatric disorder, and pregnant patients were excluded. Patients with known sleep disturbance etiologies such as nighttime hot flushes, uncontrolled pain, or diarrhea were also excluded. All patients provided written informed consent before participating in the study.
Study Design and Treatment
This study was a prospective, randomized, and wait-list-controlled pilot trial. Participants who met the eligibility criteria and voluntarily signed the informed consent were randomly assigned at a 1:1 ratio into the GGBT and wait-list groups. A computer-generated randomization schedule based on a table of random digits was used for group assignment. The random list was concealed in an opaque envelope prior to the first visit of the subject. This trial was a pilot study and we could not find adequate references regarding the sample size; hence, a sample size of 30 patients was determined to be adequate considering a 15% dropout rate.
The GGBT group was administered GGBT for 2 weeks and received sleep hygiene education. The wait-list group received sleep hygiene education alone and was instructed to maintain conventional care for 2 weeks. After the wait-list period, the patients in the wait-list group started receiving GGBT but were not included in the analysis.
GGBT (Gamiguibi-tang in Korean, or Kamikihito in Japanese; Kracie Co, Tokyo, Japan) was a yellow-brown mixture of spray-dried hot water extracts of 14 medicinal plants, including Astragalus root (6.6%), Bupleurum root (9.8%), Ziziphus jujuba (9.8%), Atractylodes lancea rhizome (9.8%), Panax ginseng radix (9.8%), Poria sclerotium (9.8%), Longan aril (9.8%), Polygala root (4.9%), Gardenia fruit (6.6%), Jujube (4.9%), Japanese Angelica sinensis root (6.6%), Glycyrrhiza (3.3%), Zingiber officinale (4.9%), and Saussurea lappa (3.3%). Each herb in GGBT was quality controlled from the places of origin to the final product by the manufacturing company. GGBT (3.75 g) was administered orally with hot water three times a day for 2 weeks. Patients in both groups were educated and instructed to maintain sleep hygiene on the first day of the trial.
This study was reviewed and approved by the institutional review board of Kyung Hee University Hospital at Gangdong (KHNMC-OH-IRB 2016-01-010). The protocol was registered in the Clinical Research Information Service (KCT0001952). All research adhered to the tenets of the Declaration of Helsinki, and the study was performed according to Good Clinical Practice.
Endpoints and Study Assessments
The primary endpoint was the Insomnia Severity Index (ISI) score. The secondary endpoints included the Brief Fatigue Inventory (BFI), Beck Depression Inventory (BDI), and Montreal Cognitive Assessment (MoCA) scores. The ISI comprised 7 questions to assess the severity of sleep disorder and consequent daily life disturbance, and was validated for insomnia in cancer patients with 2 weeks of recall period.20 As the treatment period in this trial was short, with only 2 weeks, ISI was chosen to assess efficacy of GGBT on sleep disturbance instead of PSQI. The BFI measured the severity of fatigue and the influence of fatigue on emotion and physical activities, such as performing daily living activities, walking, and social communication.21 The BDI and MoCA were used to assess the severity of depression and cognitive impairment, respectively. The BDI is a patient-reported questionnaire used to assess depression severity by measuring the cognitive, behavioral, affective, and somatic dimensions of depression.22 The MoCA is a global cognitive assessment used to verify mild cognitive impairment. It has nine domains used to assess mild cognitive impairment and is especially sensitive among all educated individuals.23 All assessments were performed at baseline and after 2 weeks by using validated Korean versions of the questionnaires. The investigator who conducted the allocation and the investigator who conducted the assessment were independent of each other.
Safety Evaluation
Any adverse events were assessed using the Common Terminology Criteria for Adverse Events (CTCAE, version 4.1). Laboratory tests, including those for hemoglobin, white blood cell count, platelet count, aspartate aminotransferase, alanine aminotransferase, total bilirubin, blood urea nitrogen, and serum creatinine, were performed at baseline and at the end of the study. We also followed the WHO-UMC (World Health Organization–Uppsala Monitoring Center) causality assessment system.
Statistical Analysis
The intention-to-treat analysis was used with the last-observation-carried-forward (LOCF) method. All values were presented as mean and standard deviation or n (%) unless stated otherwise. An independent t test was used to compare the changes in the scores after performing the Shapiro-Wilk test to confirm the normality of the distribution. All statistical analyses were 2 sided, and statistical significance was set at .05. The resultant data were analyzed using the corresponding 1-way analysis of covariance, considering the baseline scores as covariates. Baseline variables were compared between the groups by using the independent t test, chi-square test, or Fisher’s exact test. All statistical analyses were performed using PASW Statistics for Windows, Version 18.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism 5 (GraphPad Software Inc, San Diego, CA, USA).
Results
Patient Characteristics
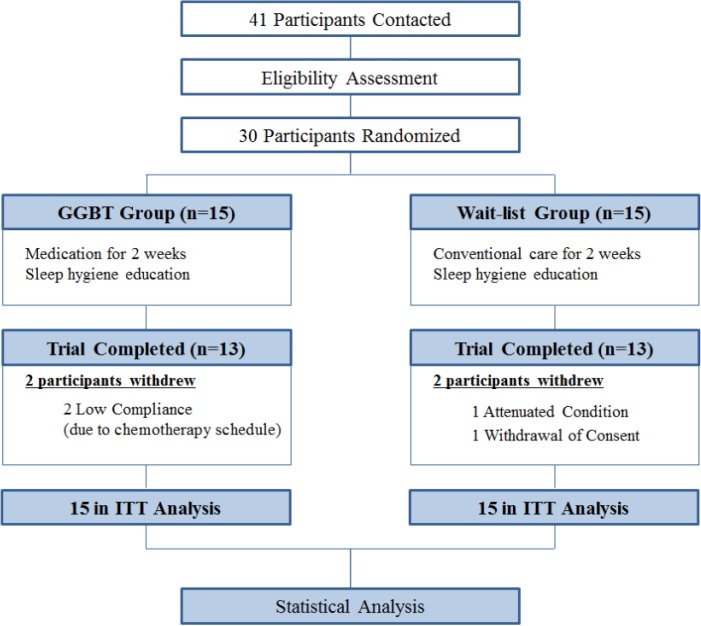
Forty-one patients were contacted, and 30 were enrolled and randomly assigned to the GGBT and wait-list groups between January 2015 and August 2015. Two patients in the GGBT group withdrew from the trial because of low compliance due to chemotherapy, and two patients in the wait-list group withdrew because of attenuated general condition and withdrawal of consent (Figure 1).
Figure 1.
Flow diagram. GGBT, Gamiguibi-tang; ITT, intention-to-treat.
Baseline characteristics were well balanced, and no statistical differences were present between the 2 groups (Table 1). Among the patients, 14 were men (46.7%) and the mean age was 54.2 years (range, 23.0-73.0 years). The primary cancer was gastrointestinal in 13 (43.3%) patients, breast in 8 (26.7%), lung in 3 (10%), and other cancer in 6 (20%). The TNM stage was IV in 14 (46.7%) and III in 8 (26.7%) patients. Chemotherapy, radiotherapy, and hormonal therapy were concurrently given to 8 (26.7%), 3 (10.0%), and 3 (10.0%) patients, respectively. Mean duration after anticancer therapies, including chemotherapy, radiotherapy, and hormonal therapy was 1.4 ± 3.7 years in GGBT group and 2.2 ± 3.2 years in the wait-list group (P = .559). The baseline PSQI sore was 14.3 (2.7). Hypnotics were administered to 6 (20.0%) patients. The ECOG performance status was 1 in 21 (70.0%) and 0 in 7 (23.3%) patients.
Table 1.
Patient Characteristics at Baseline.
| GGBT Group | Wait-List Group | P a | |
|---|---|---|---|
| Age, median (range) | 55.7 (23.0-70.0) | 52.6 (38.0-73.0) | .407 |
| Men, n (%) | 8 (53.5) | 6 (40.0) | .481 |
| BMI, kg/m2, mean (SD) | 23.1 (3.2) | 23.3 (3.7) | .855 |
| Primary Tumor, n (%) | .751 | ||
| Breast | 5 (33.3) | 3 (20.0) | |
| Lung | 2 (13.3) | 1 (6.7) | |
| Gastrointestinal | 6 (40.0) | 7 (46.7) | |
| Other | 2 (13.3) | 4 (26.7) | |
| TNM Stage, n (%) | .218 | ||
| I | 4 (26.7) | 2 (13.3) | |
| II | 2 (13.3) | 0 (0.0) | |
| III | 2 (13.3) | 6 (40.0) | |
| IV | 7 (46.7) | 7 (46.7) | |
| Concurrent treatments, n (%) | |||
| Chemotherapy | 5 (33.3) | 3 (20.0) | .426 |
| Radiotherapy | 2 (13.3) | 1 (6.7) | .559 |
| Hormonal therapy | 3 (20.0) | 3 (20.0) | .999 |
| Hypnotics | 2 (13.3) | 4 (26.7) | .379 |
| PSQI Score, mean (SD) | 14.1 (2.8) | 14.5 (2.6) | .640 |
| ECOG PS, n (%) | .359 | ||
| 0 | 3 (20.0) | 4 (26.7) | |
| 1 | 10 (66.7) | 11 (73.3) | |
| ≥2 | 2 (13.3) | 0 (0.0) |
Abbreviations: GGBT, Gamiguibi-tang; BMI, body mass index; PSQI, Pittsburgh Sleep Quality Index; ECOG PS, Eastern Cooperative Oncology Group performance status.
P value was calculated using the independent t test, chi-square test, or Fisher’s exact test.
Efficacy
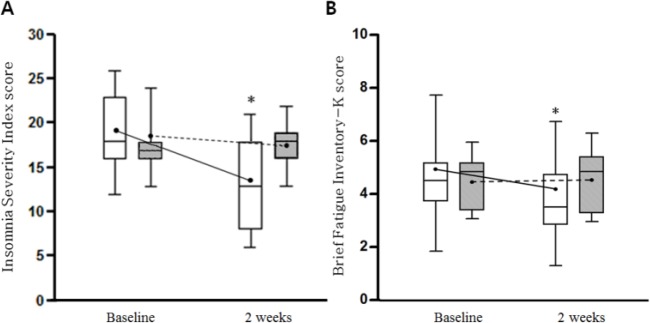
After 2 weeks of treatment, the ISI and BFI scores decreased significantly in the GGBT group, but not in the wait-list group (P < .001), which indicated that GGBT administration improved the sleep disturbance and fatigue in these patients (Table 2, Figure 2). The BDI and MoCA scores did not change significantly in either group.
Table 2.
Outcome Measurements for the GGBT and Wait-List Groups.
| GGBT Group | Wait-List Group | P | ||
|---|---|---|---|---|
| ISI | Baseline | 18.7 ± 4.3 | 17.5 ± 2.6 | <.001a |
| 2 weeks | 13.1 ± 4.8 | 17.7 ± 2.3 | ||
| Difference | −5.5 ± 4.4 | 0.1 ± 1.1 | <.001b | |
| BFI | Baseline | 4.5 ± 1.4 | 4.6 ± 1.0 | .001a |
| 2 weeks | 3.8 ± 1.7 | 4.6 ± 1.1 | ||
| Difference | −0.8 ± 0.8 | 0.0 ± 0.3 | .002b | |
| BDI | Baseline | 8.6 ± 5.8 | 7.2 ± 4.3 | .855a |
| 2 weeks | 7.3 ± 6.2 | 6.8 ± 4.1 | ||
| Difference | −1.3 ± 7.0 | -0.4 ± 0.7 | .617b | |
| MoCA | Baseline | 25.9 ± 2.4 | 26.8 ± 2.7 | .251a |
| 2 weeks | 25.8 ± 2.9 | 27.1 ± 1.9 | ||
| Difference | −0.1 ± 1.6 | 0.3 ± 1.3 | .454b |
Abbreviations: GGBT, Gamiguibi-tang; ISI, Insomnia Severity Index; BFI, Brief Fatigue Inventory; BDI, Beck Depression Inventory; MoCA, Montreal Cognitive Assessment.
P values are based on the analysis of covariance, with the baseline level as a covariate.
P values are based on the independent t test.
Figure 2.
Changes in the ISI (A) and BFI (B) scores in the GGBT and wait-list groups. The ends of whiskers represent the upper and lower limit of the values and columns represent 25th and 75th percentile with the median value. The connecting lines represent changes of mean values. *Represents change that was statistically significant (P < .05). White columns represent GGBT group, whereas gray columns represent wait-list group. ISI, Insomnia Severity Index; BFI, Brief Fatigue Inventory; GGBT, Gamiguibi-tang.
The changes in the ISI and BFI scores between baseline and 2 weeks were −5.5±4.4 versus 0.1 ± 1.1 (P < .001) and −0.8 ± 0.8 versus 0.0 ± 0.3 (P = .002), respectively, which showed a significant intergroup difference. The changes in the BDI and MoCA scores were −1.3 ±7.0 versus −0.4 ± 0.7 and −0.1 ± 1.6 vs 0.3 ± 1.3, respectively, which showed no significant intergroup differences.
Safety
No serious adverse events related to GGBT administration occurred during the study period. Laboratory results, including liver and renal function, were not significantly different after GGBT administration. However, one participant in the GGBT group who received gynecological intervention the day before trial enrollment experienced grade 1 lower leg edema, which spontaneously subsided within 3 days. Another participant in the GGBT group complained of mild dyspepsia, assessed as grade 1 on the CTCAE scale. The causality according to the WHO-UMC causality assessment system was possible.
Discussion
This study showed that GGBT significantly improved sleep quality and fatigue level but not depression and cognitive impairment in patients with cancer after 2 weeks of intervention without serious adverse events.
Patients with cancer have difficulty maintaining good sleep quality because of their psychosocial and physical stresses. However, standard nonpharmacological and pharmacological approaches to alleviate sleep disturbance in patients with cancer are still lacking. The duration and depth of sleep in these patients is affected by physical problems such as respiratory failure or pain, stressful decisions about cancer therapies and therapy-induced adverse events, as well as circadian disturbance caused by the cancer itself.7,24 In addition, sleep insufficiency arouses immunosuppression and increases the incidence of cardiac, metabolic, and inflammatory diseases.25,26 However, cognitive behavior therapy—one of the nonpharmacological approaches and the current standard recommendation—requires sufficient amount of time and workforce to administer. The possibilities of impaired liver or renal function due to chemotherapies or cancer-related issues call for safer pharmacological approaches for patients with cancer and survivors of cancer.
GGBT was originally prescribed for insomnia with heart and spleen deficiency type, and then generally prescribed for all types of chronic insomnia. Currently, GGBT is the most common herbal formula prescribed for sleep disturbance in East Asia.15 One of active components in GGBT—spinosin from Ziziphus jujube—has a sedative-hypnotic effect, especially on REM sleep via the serotonergic 5-HT receptor.27,28 A meta-analysis by Tong et al16 showed that GGBT administration for 2 to 4 weeks showed better efficacy than conventional therapy in improving insomnia. The findings of these previous studies are consistent with the results of the current trial showing the efficacy of GGBT against cancer-related sleep disturbance.
Patients with cancer usually experience a cluster of symptoms, including sleep disturbance, fatigue, depression, and anxiety.2 Sleep disturbance is closely correlated with cancer-related fatigue,29 but pharmacological intervention in conventional medicine has clinical limitations in improving fatigue in patients with cancer or survivors of cancer with insomnia.30 In our study, fatigue was alleviated in addition to sleep improvement. This result suggests that GGBT may be beneficial in improving the symptom cluster and quality of life in patients with cancer.
A meta-analysis by Tian et al19 showed that GGBT administration for 4-8 weeks improved depression effectively and safely. In a preclinical study,17 GGBT enhanced the memory function in a rat model. However, in the current trial, GGBT administration for 2 weeks did not improve depression and cognitive impairment in patients with cancer. Further long-term studies should investigate the efficacy of GGBT, focusing on depression and cognitive impairment in patients with cancer.
Pharmacological interventions against insomnia in conventional medicine are associated with a number of risks, including residual next-day hypersomnia, dizziness, lightheadedness, impaired mental states, and increased risk of falls and hip fractures.9-14 Tian et al19 with a total of 9 randomized controlled trials involving 893 cases reported that the incidence of adverse reactions to GGBT with or without antidepressants was significantly lower than that of antidepressants alone. In this study, only 2 cases of mild adverse events with National Cancer Institute–Common Toxicity Criteria grade 1 occurred in the GGBT group. This suggests that GGBT may be a safe therapeutic option for vulnerable patients with cancer-related sleep disturbance.
This study has several limitations. First, the intervention period was only 2 weeks without follow-up, and this short intervention period could have biased the results regarding the efficacy and safety of GGBT against cancer-related symptoms. Second, a wait-list control was used instead of a placebo control in this trial. This was because creating a placebo in the manufacturing company was not feasible, and a placebo trial could have reduced the compliance of the patients with cancer. Third, pattern identification of GGBT according to traditional Korean medicine was not applied as an inclusion criterion in this trial. This was because although GGBT was originally prescribed to patients with insomnia diagnosed with heart and spleen deficiency, GGBT is the most commonly used formula for insomnia regardless of pattern diagnosis.15 Finally, concurrent use of anticancer therapies, including chemotherapy, radiotherapy, and hormonal therapy in some patients in this pilot study may act as confounders. Future studies should minimize these confounders to clarify the effects of GGBT.
Despite these limitations, the current study highlights the potential benefits of GGBT for patients with cancer-related sleep disturbance. More methodologically rigorous studies with larger sample sizes, longer term treatment, and placebo control should be performed in the future to confirm the current findings.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant of Comprehensive and Integrative Medicine R&D project through Comprehensive and Integrative Medicine Institute (CIMI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: 090-091-3000-3038-301-320-01), and Traditional Korean Medicine R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (HB16C0067).
References
- 1. Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group symptom outcomes and practice patterns study. Cancer. 2013;119:4333-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jim HS, Evans B, Jeong JM, et al. Sleep disruption in hematopoietic cell transplantation recipients: prevalence, severity and clinical management. Biol Blood Marrow Transplant. 2014;20:1465-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester cancer center-community clinical oncology program. J Clin Oncol. 2010;28:292-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vale TC, Fernandes do Prado LB, do Prado GF, Povoas Barsottini OG, Pedroso JL. Rapid eye movement sleep behavior disorder in paraneoplastic cerebellar degeneration: improvement with immunotherapy. Sleep. 2015;29:117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Otte JL, Carpenter JS, Manchanda S, et al. Systematic review of sleep disorders in cancer patients: can the prevalence of sleep disorders be ascertained? Cancer Med. 2015;4:183-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895-908. [DOI] [PubMed] [Google Scholar]
- 8. Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864-5866. [DOI] [PubMed] [Google Scholar]
- 9. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504. [PMC free article] [PubMed] [Google Scholar]
- 10. Stiefel FC, Kornblith AB, Holland JC. Changes in the prescription patterns of psychotropic drugs for cancer patients during a 10-year period. Cancer. 1990;65:1048-1053. [DOI] [PubMed] [Google Scholar]
- 11. Barbera J, Shapiro C. Benefit-risk assessment of zaleplon in the treatment of insomnia. Drug Saf. 2005;28:301-318. [DOI] [PubMed] [Google Scholar]
- 12. Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233. [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson LC, Chernik DA. Sedative-hypnotics and human performance. Psychopharmacology. 1982;76:101-113. [DOI] [PubMed] [Google Scholar]
- 14. Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303-3307. [PubMed] [Google Scholar]
- 15. Yeung WF, Chung KF, Poon MM, et al. Chinese herbal medicine for insomnia: a systematic review of randomized controlled trials. Sleep Med Rev. 2012;16:497-507. [DOI] [PubMed] [Google Scholar]
- 16. Tong D, Yang W, Li J, et al. A systematic review for efficacy of Jiajian Guipi decoction for insomnia. Guangming J Chin Med. 2013;28:2233-2236. [Google Scholar]
- 17. Oh MS, Huh Y, Bae H, Ahn DK, Park SK. The multi-herbal formula Guibi-tang enhances memory and increases cell proliferation in the rat hippocampus. Neurosci Lett. 2005;379:205-208. [DOI] [PubMed] [Google Scholar]
- 18. Park H, Hwang YH, Yang HJ, Kim HK, Song KS, Ma JY. Acute toxicity and genotoxicity study of fermented traditional herb formula Guibi-tang. J Ethnopharmacol, 2014;156:182-189. [DOI] [PubMed] [Google Scholar]
- 19. Tian J, Wen Z, Guo X, Li Y, Lv Z. Systematic review of efficacy and safety of the treatment of Guipi decoction for depression. Chin J Inform TCM. 2016:23:36-40. [Google Scholar]
- 20. Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14:429-441. [DOI] [PubMed] [Google Scholar]
- 21. Yun YH, Wang XS, Lee JS, et al. Validation study of the Korean version of the Brief Fatigue Inventory. J Pain Symp Manage. 2005;29:165-172. [DOI] [PubMed] [Google Scholar]
- 22. Song YM, Lee HK, Kim JW, Lee K. Reliability and validity of the Korean version of Beck Depression Inventory II via the internet: results from a university student sample. J Korean Neuropsychiatr Assoc. 2012;51:402-408. [Google Scholar]
- 23. Lee JY, Lee DW, Cho SJ, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008;21:104-110. [DOI] [PubMed] [Google Scholar]
- 24. Pires GN, Bezerra AG, Tufik S, Andersen ML. Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep Med. 2016;24:109-118. [DOI] [PubMed] [Google Scholar]
- 25. Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao JX, Zhang QY, Cui SY, et al. Hypnotic effect of jujubosides from Semen Ziziphi Spinosae. J Ethnopharmacol. 2010;130:163-166. [DOI] [PubMed] [Google Scholar]
- 28. Wang LE, Cui XY, Cui SY, et al. , Potentiating effect of spinosin, a C-glycoside flavonoid of Semen Ziziphi spinosae, on pentobarbital-induced sleep may be related to postsynaptic 5-HT(1A) receptors. Phytomedicine. 2010;17:404-409. [DOI] [PubMed] [Google Scholar]
- 29. Peoples AR, Roscoe JA, Block RC, et al. Nausea and disturbed sleep as predictors of cancer-related fatigue in breast cancer patients: a multicenter NCORP study. Support Care Cancer. 2017;25:1271-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer. 2016;24:2059-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]