Abstract
Introduction. Radiation therapy using ionizing radiation is widely used for the treatment of prostate cancer. The intrinsic radiation sensitivity of cancer cells could be enhanced by modulating multiple factors including the capacity to repair DNA damage, especially double-strand breaks (DSBs). We aimed to examine the effect of zerumbone on radiation sensitivity and its protective effects against ionizing radiation–induced DSB in human prostate cancer cells. Materials and Methods. The human prostate cancer PC3 and DU145 cell lines were used. A colony formation assay was performed to analyze the radiation survival of cells. DNA histogram and generation of reactive oxygen species (ROS) were examined using flow cytometry. Western blotting was used to examine the expression of regulatory molecules related to DNA damage repair. Results. Pretreatment with zerumbone enhanced the radiation effect on prostate cancer cells. Zerumbone delayed the abrogation of radiation-induced expression of γ-H2AX, an indicator of DNA DSB. Zerumbone pretreatment markedly reduced ionizing radiation–induced upregulated expression of phosphorylated ATM (ataxia telangiectasia-mutated), which was partially reversed by the ATM agonist methyl methanesulfonate. Ionizing radiation augmented and zerumbone pretreatment reduced the expression of Jak2 and Stat3, which are involved in DNA damage repair signaling. No significant effect on the generation of ROS and expression of ATR was noted after zerumbone treatment. Conclusion: Zerumbone sensitized DU145 and PC3 prostatic cancer cells to ionizing radiation by modulating radiation-induced ATM activation during repair of DNA DSBs.
Keywords: prostate cancer, radiosensitivity, zerumbone, ATM, DNA repair
Introduction
Radiation therapy using ionizing radiation (IR) is a widely accepted treatment for prostate cancer. It is indicated for patients with early-stage prostate cancer with similar prognosis to radical prostatectomy.1 For locally advanced disease and bone metastasis, the role of radiation therapy in local control and palliation has been validated.2 However, the side effects of pelvic radiation therapy in prostate cancer, such as injury to the rectum and urinary bladder, are common.3 The direct contiguity of the rectum, urinary bladder, and prostate inevitably exposes the rectum and urinary bladder to radiation during the delivery of radiation therapy to the prostate. Although advances in radiation therapy techniques have reduced toxicity by decreasing the radiation dose reaching the normal tissues, the radiation dose distributed to the rectum and urinary bladder remains high,4 which induces a considerably high rate of proctitis, cystitis, and fistula. One important strategy to overcome this clinical drawback is to enhance the radiosensitivity of prostate cancer cells, which lowers the needed radiation dose and thereby, reduces the toxicity to surrounding normal tissues.
Multiple factors are involved in the intrinsic radiation sensitivity of cancer cells.5 The capacity to repair DNA damages in response to IR is one of the most important determinants of radiosensitivity.6 Among IR-induced DNA damages, double-strand breaks (DSBs) are regarded as lethal and a critical cause of radiation-induced cell death.7 DSBs of DNA can be repaired by homologous recombination repair and nonhomologous end joining (NHEJ).8 Phosphorylation of H2A histone family, member X (H2AX), a substrate of ATM, to γ-H2AX has been used as an indicator of DNA DSBs, a marker for estimating DNA repair, and a possible target of radiosensitization.9,10
Zerumbone (2,6,10-cy-cloundecatrien-1-one, 2,6,9,9-tetramethyl-,[E,E,E]-), a monocyclic sesquiterpene compound, is the predominant bioactive compound in the rhizomes of Zingiber zerumbet.11-13 The biological properties of zerumbone include anti-inflammatory,14 antitumor,15-17 antiproliferative18 and antiplatelet aggregation.18,19 Zerumbone has a specific pharmacological role as an antagonist of GLI family zinc finger 1 (Gli1) activation in the sonic hedgehog signaling pathway.20 It has been reported to act as a radiosensitizer by promoting reactive oxygen species (ROS)-mediated DNA damage.16,21 In the present study, the detailed mechanism underlying zerumbone-induced decrease in the expression of phosphorylated ataxia telangiectasia-mutated (ATM) kinase in human prostate cancer cells was examined.
Materials and Methods
Cell Culture
The human prostate cancer PC3 and DU145 cell lines were purchased from the American Type Culture Collection (ATCC, CRL-1435 and HTB-81). The PC3 cells were maintained in F-12K medium supplemented with 1% (v/v) penicillin and streptomycin (all Gibco) and 10% (v/v) fetal bovine serum (Biological Industries) at 37°C in 5% CO2. For DU145 cells, F-12K was replaced with minimum essential medium (MEM, Gibco).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) Assay
Cell viability was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. The cells were seeded at a density of 104 cells/well in 96-well plates. After 16 hours, cells were exposed to zerumbone (Sigma-Aldrich) for the indicated times. MTT solution was added to the well at 37°C for 2 hours. After removing the medium, dimethyl sulfoxide was used to end the reaction, and the formazan product was quantified by measuring the absorbance of the resultant solution at 570 nm using a spectrophotometer.
Colony Formation Assay
The cells were plated in 6-well plates, cultured overnight, zerumbone was added to the culture medium for 24 hours, and then washed out with phosphate-buffered saline (PBS). After adding fresh medium, the cells were irradiated with different doses of radiation (0-6 Gy), cultured for 14 days and then the colonies were stained with crystal violet and counted.
Cell Cycle Analysis by Flow Cytometry
Cells were treated with zerumbone for 24, 48, and 72 hours, and then they were harvested and fixed at 4°C for 1 hour with 70% ethanol. The cells were stained with propidium iodide (PI) solution (PI, 0.5 mg/mL and RNAse, 0.1 mg/mL) in a CycleTEST plus DNA reagent kit (Becton Dickinson, Lincoln Park, NJ, USA). Analysis of the DNA content was performed using a fluorescence-activated cell sorting (FACS) caliber flow cytometer (Becton Dickinson, Lincoln Park, NJ, USA). The data of the cell analysis were collected and analyzed using the ModFit software (Becton Dickinson, Lincoln Park, NJ, USA).
Western Blot Analysis
Cells were treated as in the colony formation assay, their lysates were subsequently collected using a cell lysis buffer (Cell Signaling Technology) supplemented with a protease and phosphatase inhibitor cocktail (Thermo) 1 hour after irradiation, and then quantified using the Pierce bicinchoninic acid (BCA) protein assay kit (Thermo). The total proteins were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with 5% skim milk, the proteins on the membrane were probed using the following primary antibodies, anti-phospho-ATM (Ser1981), anti-ATM (both Cell Signaling Technology), anti-actin, and anti-tubulin (both Millipore), followed by incubation with peroxidase-conjugated secondary antibody (Jackson Immuno Research). The bound antibodies were visualized using chemiluminescence (Opti-ECL, Bioman).
Results
Effect of Zerumbone Treatment on the Viability of Prostate Cancer Cells
To evaluate the radiosensitizing activity of zerumbone in prostate cancer cells, nontoxic doses (with cell viability >90%) were estimated in vitro before the radiation experiments were performed. After a 24-hour exposure, the highest nontoxic dose of zerumbone in DU145 and PC3 cells was determined to be 10 µM (Figure 1).
Figure 1.
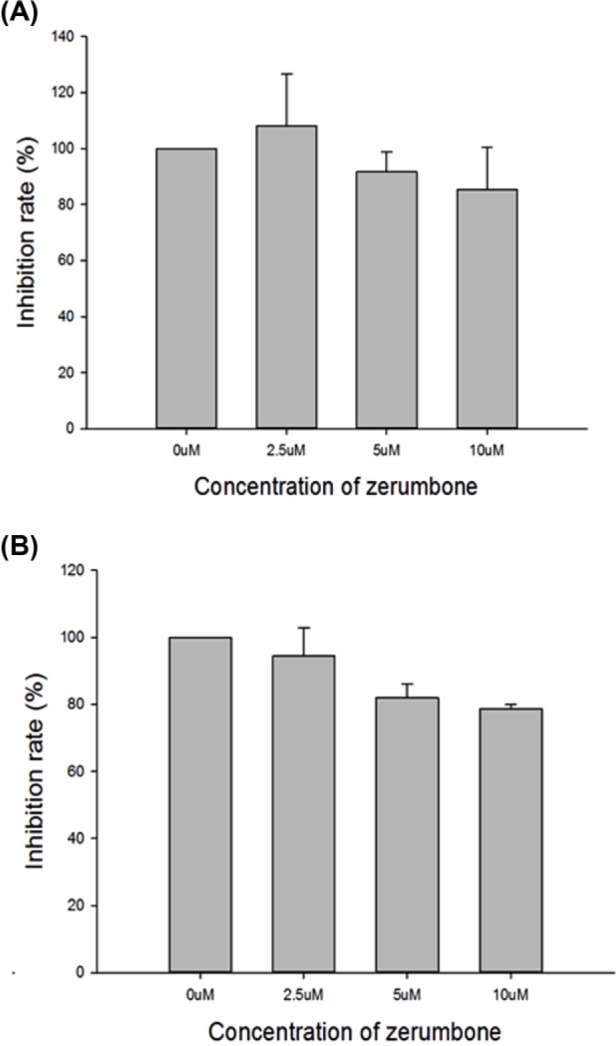
Effect of zerumbone on viability of human prostate cancer cells. Cell viability was evaluated using an MTT assay to examine the dose-dependent effects of zerumbone. After 24-hour exposure, the highest nontoxic dose of zerumbone in DU145 and PC3 cells was estimated as 10 μM. (A) DU145 and (B) PC3 cells.
Effect of Zerumbone Pretreatment on Radiosensitivity of Prostate Cancer Cells
Irradiation of untreated DU145 cells at a dose of 0 to 6 Gy reduced the survival rate to 19% while pretreatment with zerumbone 10 µM markedly decreased the survival of irradiated tumor cells (Figure 2). SERs were 1.4 and 2.1 for DU145 and PC3 cells, respectively.
Figure 2.
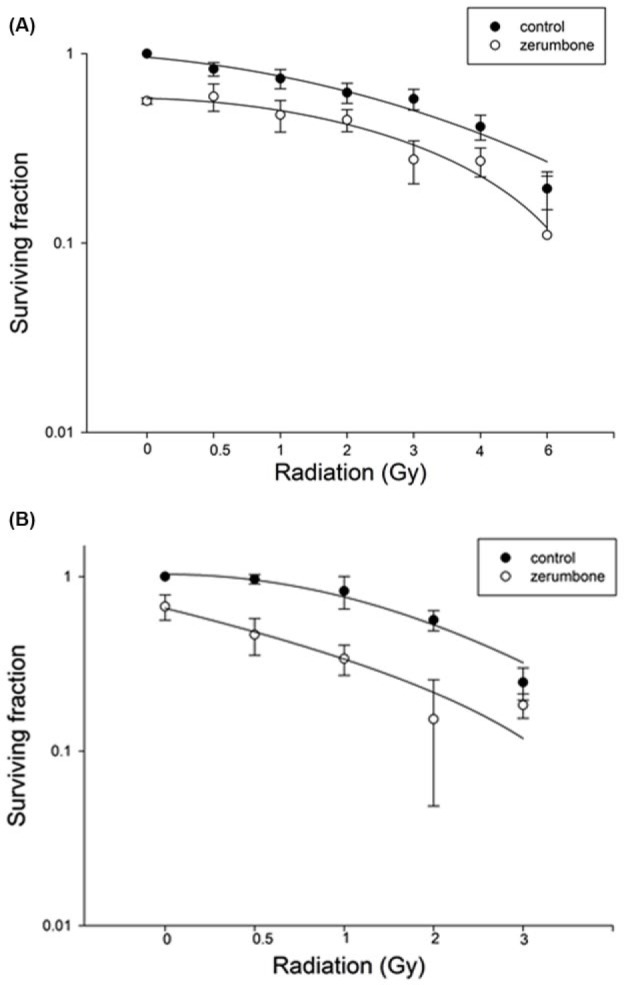
Effect of zerumbone on surviving irradiated prostate cancer cells. Effect of zerumbone pretreatment on the radiosensitivity of prostate cancer cells was evaluated using a colony formation assay. Pretreatment with zerumbone 10 µM significantly decreased the survival of irradiated tumor cells. (A) DU145 and (B) PC3 cells.
Effect of Zerumbone on Cell Cycle Distribution of Prostate Cancer Cells
The effect of zerumbone on the distribution of cells in the cell cycle under the same conditions that affected radiosensitivity was estimated using DNA histograms. After a 2-hour treatment with zerumbone 10 µM, there was no obvious difference in the distribution of cells in the cell cycle (Table 1).
Table 1.
Two-Hour Treatment With Zerumbone 10 μM Showed No Obvious Difference in Distribution of Cells in Cell Cycle for DU145 and PC3 cells. Zerumbone Had No Effect on Distribution of Cells in Cell Cycle.
| Cell Cycle Phase |
|||
|---|---|---|---|
| G0/G1 | G2/M | S | |
| DU145 cells | |||
| Control | 53.21 ± 6.21 | 18.65 ± 1.72 | 28.15 ± 4.84 |
| 10 μM zerumbone | 55.10 ± 11.03 | 24.49 ± 7.28 | 20.41 ± 7.62 |
| PC3 cells | |||
| Control | 50.41 ± 2.01 | 24.12 ± 0.62 | 25.46 ± 1.64 |
| 10 μM zerumbone | 43.99 ± 4.38 | 31.43 ± 5.35 | 24.57 ± 2.65 |
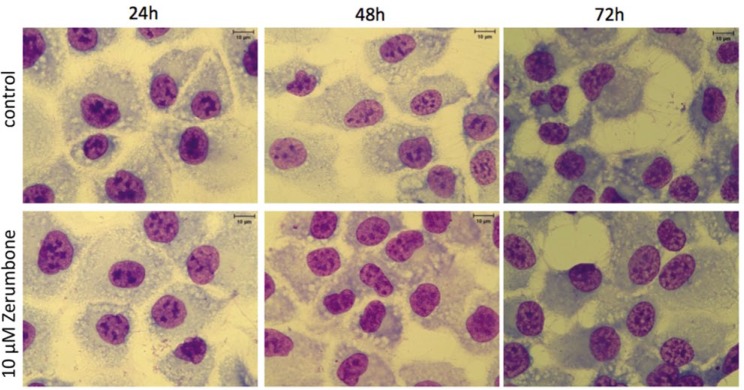
Effect of Zerumbone on Morphology of Prostate Cancer Cells
After 24-, 48-, and 72-hour pretreatments of prostate cancer cells with zerumbone 10 µM, no obvious difference was observed in cell morphology (Figure 3).
Figure 3.
Effect of zerumbone on cell morphology. Cells were collected after 24-, 48-, and 72-hour treatment with zerumbone 10 µM for Liu’s staining. Magnification, 400×.
Effect of Zerumbone on ROS Generation of Prostate Cancer Cells
Pretreatment with zerumbone under the same conditions used for the radiosensitization experiment had no effect on ROS generation in prostate cancer cells (Table 2).
Table 2.
Pretreatment With Zerumbone Under Same Condition for Radiosensitization Had No Effect on Reactive Oxygen Species (ROS) Generation in Prostate Cancer Cells.
| 0 min | 10 min | 20 min | 30 min | 40 min | 50 min | 60 min | |
|---|---|---|---|---|---|---|---|
| DU145 | |||||||
| Control | 101.98 ± 8.15 | 152.69 ± 25.65 | 173.07 ± 22.36 | 199.83 ± 34.14 | 212.17 ± 46.74 | 221.33 ± 38.08 | 246.83 ± 39.09 |
| 10 μM zerumbone | 101.98 ± 8.15 | 135.04 ± 19.07 | 174.03 ± 16.01 | 201.36 ± 37.35 | 229.83 ± 31.45 | 235.88 ± 39.44 | 261.66 ± 45.05 |
| PC3 | |||||||
| Control | 103.35 ± 0.65 | 118.22 ± 11.80 | 137.12 ± 12.49 | 148.98 ± 14.29 | 164.96 ± 7.81 | 183.48 ± 4.91 | 187.08 ± 3.87 |
| 10 μM zerumbone | 103.35 ± 0.65 | 114.82 ± 15.81 | 126.19 ± 21.06 | 139.16 ± 19.73 | 154.22 ± 12.10 | 162.42 ± 8.24 | 176.56 ± 2.50 |
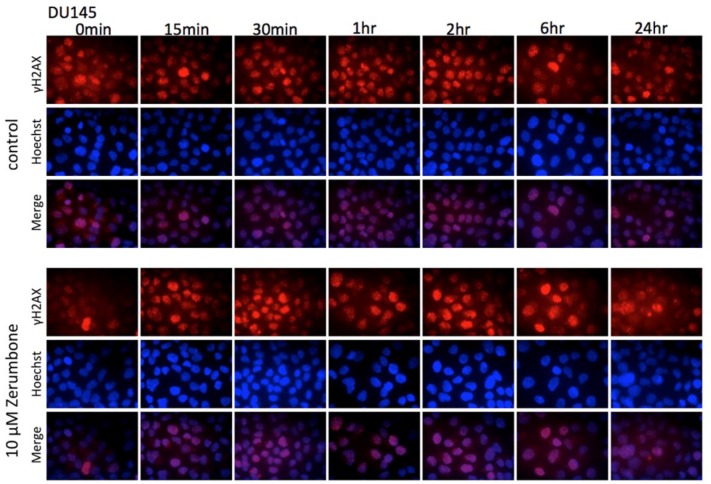
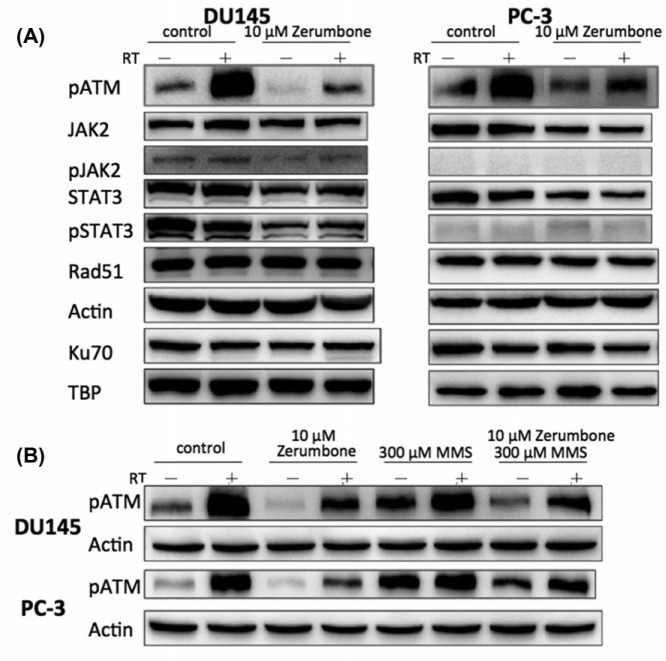
Effect of Zerumbone on Regulation of DNA Damage Repair Machinery
Immunofluorescence showed that pretreatment with zerumbone delayed the abrogation of radiation-induced expression of γ-H2AX, an indicator of DNA DSBs. This observation suggests that the action mechanism of zerumbone may involve radiosensitization (Figure 4). Western blotting showed that IR induced marked expression of phosphorylated ATM kinase in both DU145 and PC3 cells. Pretreatment with zerumbone 10 µM for 24 hours markedly reduced the upregulated expression of phosphorylated ATM (Figure 5A). The inhibition of ATM phosphorylation by zerumbone was partially reversed by the ATM agonist, methyl methanesulfonate (MMS, Figure 5B). IR augmented and zerumbone pretreatment reduced the expression of Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3), which are also DNA damage-repair molecules (Figure 5A). Zerumbone had no effect on the expression of Rad51 or Ku70 in irradiated cells.
Figure 4.
Effect of zerumbone on repair of radiation-induced DNA double-strand break (DSB) in prostate cancer DU145 cells. Immunofluorescence of γ-H2AX was used to detect DNA DSB. Red and blue fluorescence, γ-H2AX and Hoechst 33342, respectively.
Figure 5.
Effect of zerumbone on expression of proteins involved in DNA damage repair. Proteins extracted from cells exposed to various treatment were subjected to western blotting. (A) Pretreatment with zerumbone 10 μM for 24 hours markedly reduced upregulated expression of phosphorylated ATM. (B) Inhibition of ATM phosphorylation by zerumbone was partially reversed by ATM agonist, methyl methanesulfonate (MMS) 300 µM.
Discussion
The biological activities of zerumbone include anti-inflammation,14 antitumor,15-17 antiproliferation,18 and antiplatelet aggregation.18,19 In a previous study, zerumbone was also found to enhance the radiosensitivity of WOX1-overexpressed U373MG and U87MG cells22 by downregulating the Gli-1/B-cell lymphoma 2 (Bcl-2) pathway. In addition, it has been reported to act as a radiosensitizer by promoting ROS-mediated DNA damage.16,21 Our results demonstrate that zerumbone sensitized prostatic cancer cells to IR, which involved inhibition of radiation-induced activation of ATM and modulation of repair of radiation-induced DNA damage.
The activation of ATM in response to DNA damage is known to induce coordinated cellular responses to initiate DNA repair.23-25 Several investigations have shown that modulation of DNA damage repair enhances the response of cancer cells to radiation. Small interfering RNA (siRNA)-silenced ATM has been reported to sensitize SiHa cervical cancer cells to radiation.26 Caffeine has been reported to radiosensitize RKO cells by suppressing the radiation-induced activation of ATM kinase, activation of Chk2 kinase and accumulation of human colorectal cancer RKO cells in the G2 phase.27
In our study, an obvious increase in ATM phosphorylation was noted in irradiated prostate cancer cells. Zerumbone inhibited ATM phosphorylation, and this effect was attenuated by the ATM activator MMS (Figure 5B). While zerumbone inhibited ATM activation, no significant changes were observed on ATR expression, indicating there was no cross-linkage to the ATR axis of the repair machinery of DNA DSBs. This suggests that zerumbone may potentially act as a novel inhibitor of ATM activation. The regulatory pathways downstream of ATM include homologous recombination and NHEJ for DNA damage repair, especially for the DSB. In the present study, we demonstrated there were no significant alterations in the expression of Rad51 and Ku70, which are regarded as key molecules in homologous recombination and NHEJ signaling, respectively. However, the expression of other proteins involved in these pathways remains to be evaluated. In further studies, more detailed in vitro investigations of genes related to ATM downstream signaling along with validating in vivo experiments will be performed.
Zerumbone-induced radiosensitization was noted in DU145 and PC3 cells, which are both categorized as androgen-independent prostate cancer cells.28,29 DU145, but not PC3 cells harbor wild-type p53.30,31 Therefore, zerumbone could serve as a radiosensitizer of hormone-refractory prostate cancer cells independent of wild-type p53 expression. Zerumbone has been reported to be an antagonist of Gli1 activation in the sonic hedgehog signaling pathway20 and, therefore, we examined its effect on DU145 and PC3 cells. The results showed that zerumbone had no effect on the expression of sonic hedgehog signaling proteins including Patch1, Smo, Shh, and Gli1 (Supplemental Figure 1). Therefore, zerumbone may enhance the radiation effect in these prostate cancer cells independent of the sonic hedgehog signaling pathway.
In conclusion, zerumbone sensitized DU145 and PC3 prostatic cancer cells to IR and this effect may involve the modulation of radiation-induced ATM activation during repair of DNA DSBs. Further animal studies are needed to validate the radiosensitization effect and toxicity of zerumbone in prostatic cancer.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an MOST 103-2314-B-195-013-MY3 grant from the Ministry of Science Technology as well as MMH-E-106-13 and MMH-E-105-13 grants from Mackay Memorial Hospital of Taiwan.
References
- 1. Bellavita R, Scricciolo M, Bini V, et al. Radiotherapy for early-stage prostate cancer in men under 70 years of age. Tumori. 2016;102:209-216. [DOI] [PubMed] [Google Scholar]
- 2. Bortolus R. Radiation therapy in locally advanced and/or relapsed urological tumors. Urologia. 2013;80:212-224. [DOI] [PubMed] [Google Scholar]
- 3. Spratt DE, Pei X, Yamada J, Kollmeier MA, Cox B, Zelefsky MJ. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colaco RJ, Hoppe BS, Flampouri S, et al. Rectal toxicity after proton therapy for prostate cancer: an analysis of outcomes of prospective studies conducted at the University of Florida Proton Therapy Institute. Int J Radiat Oncol Biol Phys. 2015;91:172-181. [DOI] [PubMed] [Google Scholar]
- 5. Chang L, Graham PH, Hao J, et al. Emerging roles of radioresistance in prostate cancer metastasis and radiation therapy. Cancer Metastasis Rev. 2014;33:469-496. [DOI] [PubMed] [Google Scholar]
- 6. Yu S, Wang M, Ding X, et al. Testicular orphan nuclear receptor 4 is associated with the radio-sensitivity of prostate cancer. Prostate. 2015;75:1632-1642. [DOI] [PubMed] [Google Scholar]
- 7. van Oorschot B, Hovingh SE, Moerland PD, et al. Reduced activity of double-strand break repair genes in prostate cancer patients with late normal tissue radiation toxicity. Int J Radiat Oncol Biol Phys. 2014;88:664-670. [DOI] [PubMed] [Google Scholar]
- 8. Ceccaldi R, Rondinelli B, D’Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016;26:52-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene. 2003;22:5792-5812. [DOI] [PubMed] [Google Scholar]
- 10. Hu S, Fu S, Xu X, et al. The mechanism of radiosensitization by YM155, a novel small molecule inhibitor of survivin expression, is associated with DNA damage repair. Cell Physiol Biochem. 2015;37:1219-1230. [DOI] [PubMed] [Google Scholar]
- 11. Chan ML, Liang JW, Hsu LC, Chang WL, Lee SS, Guh JH. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:1223-1236. [DOI] [PubMed] [Google Scholar]
- 12. Sidahmed HM, Hashim NM, Abdulla MA, et al. Antisecretory, gastroprotective, antioxidant and anti-Helicobacter pylori activity of zerumbone from Zingiber zerumbet (L.) Smith. PLoS One. 2015;10:e0121060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Fusi F, Durante M, Sgaragli G, et al. In vitro vasoactivity of zerumbone from Zingiber zerumbet. Planta Med. 2015;81:298-304. [DOI] [PubMed] [Google Scholar]
- 14. Sulaiman MR, Perimal EK, Akhtar MN, et al. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia. 2010;81:855-858. [DOI] [PubMed] [Google Scholar]
- 15. Tsuboi K, Matsuo Y, Shamoto T, et al. Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol Rep. 2014;31:57-64. [DOI] [PubMed] [Google Scholar]
- 16. Prasannan R, Kalesh KA, Shanmugam MK, et al. Key cell signaling pathways modulated by zerumbone: role in the prevention and treatment of cancer. Biochem Pharmacol. 2012;84:1268-1276. [DOI] [PubMed] [Google Scholar]
- 17. Wahab SIA, Abdul AB, Yeel HC, Alzubairi AS, Elhassan MM, Syam MM. Anti-tumor activities of analogues derived from the bioactive compound of Zingiber zerumbet. Int J Cancer Res. 2008;4:154-159. [Google Scholar]
- 18. Rahman HS, Rasedee A, Yeap SK, et al. Biomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trials. Biomed Res Int. 2014;2014:920742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jantan I, Raweh SM, Sirat HM, et al. Inhibitory effect of compounds from Zingiberaceae species on human platelet aggregation. Phytomedicine. 2008;15:306-309. [DOI] [PubMed] [Google Scholar]
- 20. Sun Y, Sheng Q, Cheng Y, et al. Zerumbone induces apoptosis in human renal cell carcinoma via Gli-1/Bcl-2 pathway. Pharmazie. 2013;68:141-145. [PubMed] [Google Scholar]
- 21. Hu Z, Zeng Q, Zhang B, Liu H, Wang W. Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells. Biochimie. 2014;107(pt B):257-262. [DOI] [PubMed] [Google Scholar]
- 22. Chiang MF, Chen HH, Chi CW, et al. Modulation of Sonic hedgehog signaling and WW domain containing oxidoreductase WOX1 expression enhances radiosensitivity of human glioblastoma cells. Exp Biol Med (Maywood). 2015;240:392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo Y, Feng W, Sy SM, Huen MS. ATM-dependent phosphorylation of the Fanconi anemia protein PALB2 promotes the DNA damage response. J Biol Chem. 2015;290:27545-27556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reddy V, Wu M, Ciavattone N, et al. ATM inhibition potentiates death of androgen receptor-inactivated prostate cancer cells with telomere dysfunction. J Biol Chem. 2015;290:25522-25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhandaru M, Martinka M, McElwee KJ, Rotte A. Prognostic significance of nuclear phospho-ATM expression in melanoma. PLoS One. 2015;10:e0134678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li W, Jian W, Xiaoping X, Yingfeng L, Tao X, Xiaoyan X. Enhanced radiation-mediated cell killing of human cervical cancer cells by small interference RNA silencing of ataxia telangiectasia-mutated protein. Int J Gynecol Cancer. 2006;16:1620-1630. [DOI] [PubMed] [Google Scholar]
- 27. Choi EK, Ji IM, Lee SR, et al. Radiosensitization of tumor cells by modulation of ATM kinase. Int J Radiat Biol. 2006;82:277-283. [DOI] [PubMed] [Google Scholar]
- 28. Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979;17:16-23. [PubMed] [Google Scholar]
- 29. Carrión-Salip D, Panosa C, Menendez JA, et al. Androgen-independent prostate cancer cells circumvent EGFR inhibition by overexpression of alternative HER receptors and ligands. Int J Oncol. 2012;41:1128-1138. [DOI] [PubMed] [Google Scholar]
- 30. Simone CB, 2nd, John-Aryankalayil M, Palayoor ST, et al. mRNA expression profiles for prostate cancer following fractionated irradiation are influenced by p53 status. Transl Oncol. 2013;6:573-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aryankalayil MJ, Makinde AY, Gameiro SR, et al. Defining molecular signature of pro-immunogenic radiotherapy targets in human prostate cancer cells. Radiat Res. 2014;182:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]