Abstract
Background. Conventional anticancer therapies still cause difficulties with selective eradication and accompanying side effects that reduce patients’ quality of life (QOL). Fucoidan is extracted from seaweeds and has already exhibited broad bioactivities, including anticancer and anti-inflammatory properties, in basic studies. It is expected to enhance therapeutic efficacy and minimize side effects in cancer patients; however, despite its potential benefits, there are very few clinical trials using fucoidans. Therefore, we performed an exploratory clinical study for advanced cancer patients to examine the efficacy of fucoidans, especially focusing on inflammation in relation to QOL scores. Methods. We conducted a prospective, open-label clinical study for advanced cancer patients using fucoidans via oral administration; 20 advanced cancer patients with metastases were recruited and were given 400 mL/d fucoidan (10 mg/mL) for at least 4 weeks. Inflammatory biomarkers, including high-sensitivity C-reactive protein and various cytokines, and QOL scores were monitored before treatment, after 2 weeks, and after 4 weeks of fucoidan ingestion. Results. The main proinflammatory cytokines, including interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) were significantly reduced after 2 weeks of fucoidan ingestion. QOL scores, including fatigue, stayed almost stable without significant changes during the study period. The univariate and multivariate analyses revealed that the responsiveness of IL-1β was a significant independent prognostic factor. Conclusion. This is the first study providing evidence of the anti-inflammatory effects of fucoidans for advanced cancer patients. In future studies, larger blinded, controlled trials are required to establish the efficacy of fucoidan as supportive care for cancer patients, especially those undergoing chemotherapy.
Keywords: fucoidan, cytokine, inflammation, cancer, QOL, IL-1β
Introduction
A close association between cancer and inflammatory responses is now well established. The idea was first postulated by R. Virchow in the nineteenth century and has subsequently been corroborated by a growing number of studies.1-5 Approximately 90% to 95% of cancers are caused by lifestyle and environmental factors such as food, smoking, infectious agents, and stress. When these inflammatory stresses persist for a long time, chronic inflammation may appear, giving rise to tumor development and metastasis.2,3 In addition to inflammatory stresses, cancer itself induces inflammatory reactions, which bring about symptoms such as pain, fever, anorexia and weight loss, fatigue, and cancer cachexia in the terminal stages. The inflammatory reactions may also affect therapeutic efficacy.4-6 Moreover, chemotherapy (Cx) for advanced cancer induces inflammatory reactions, which may result in many side effects and also accelerate tumor growth.7 Inflammatory responses are mediated by multiple factors, notably proinflammatory cytokines, including interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α).1-7 These proinflammatory cytokines have been shown to participate in the various stages of cancer. For example, elevated serum concentrations of IL-1β were detected in cancer patients,8-10 and a shorter life span was reported in advanced cancer patients with high inflammatory cytokine levels.11-13
As mentioned above, cancer and inflammatory responses are closely associated, suggesting the possibility of therapeutic interventions with anti-inflammatory agents.6,7,14-16 Steroids are the standard anti-inflammatory therapy for symptoms, including nausea and fatigue, in patients with advanced cancer.17 However, steroidal and nonsteroidal anti-inflammatory drugs have many serious side effects such as immunosuppression, ulceration, and organ dysfunction, especially for advanced cancer patients.18-20 Because there is currently no way to prevent these side effects, there is a strong demand for improved anti-inflammatory agents in clinic, especially for patients with advanced cancer.6
One promising biomaterial, fucoidan, is mainly present in various species of brown marine macroalgae (or seaweeds) such as mozuku, kombu, bladderwrack, and others.21 Fucoidans are sulfated polysaccharides having complicated chemical structures, consisting of various combinations of fucose, uronic acids, galactose, and xylose.21,22 Isolated fucoidans without further treatments may present in various sizes, for example, 5100 kDa23 and 1600 kDa,24 depending on the methods of extraction and seaweeds used. Various molecular weight derivatives, for example, classified as low-molecular-weight fucoidan (up to 40 kDa), intermediate-molecular-weight fucoidan (110-138 kDa), and high-molecular-weight fucoidan (300-330 kDa), have been examined for their health benefits and were reported to exhibit broad biological activities such as anticancer, antioxidant, anticoagulant, anti-inflammatory, and immune-modulatory effects in in vitro and in vivo studies.21,22,25-27 One of our previous basic science studies revealed that fucoidan can enhance the anticancer activity of chemotherapeutic agents (such as cisplatin, tamoxifen, and paclitaxel).28 There are substantial data showing that the low toxicity of fucoidan makes it a suitable supplemental treatment for cancer patients along with conventional therapies.29,30 Despite its potential benefits, there is little clinical data on the effects of fucoidan; the only available study is a recent prospective randomized clinical trial revealing that fucoidan significantly reduces the general fatigue caused by Cx in advanced colorectal cancer patients.31 Therefore, further research is needed to confirm the efficacy of fucoidan in clinical cancer management.
Here, we hypothesized that fucoidan, as a supportive care agent, could alleviate inflammatory conditions and improve the quality of life (QOL) in advanced cancer patients. In the present study, we conducted an exploratory prospective clinical study to monitor various biomarkers, including proinflammatory cytokines, and QOL scores in advanced cancer patients receiving fucoidan.
Methods
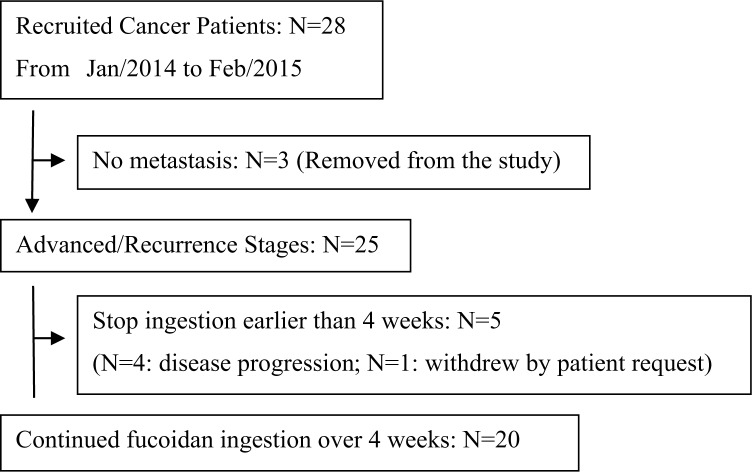
This study was conducted as a prospective, open-label, single-arm clinical study under institutional ethical committee approval. Consecutive cancer patients were recruited from 4 medical clinics in Japan from January 2014 to February 2015. Written informed consent was obtained from all patients who agreed to receive 400 mL/d of fucoidan (10 mg/mL; trade name: Power Fucoidan, Daiichi Sangyo, Co Ltd, Osaka, Japan) for at least 4 weeks. As shown in Figure 1 and Table 1, the total number of 20 patients who met the following inclusion criteria were eligible for this study: (1) clinically diagnosed with inoperable metastatic cancer; (2) age >20 years, (3) ambulatory as an outpatient with normal food intake for at least 4 weeks during the study, and (4) without serious dysfunction of vital organs. Because there was no control group in this exploratory study, we considered setting the baseline for each patient’s status before the administration of fucoidan; then, these data were compared with their status at the second week and fourth week of treatment.
Figure 1.
Flow diagram of patients’ treatment: 20 patients who met all the inclusion criteria were analyzed in this study.
Table 1.
Patient Characteristics.
| Total |
||
|---|---|---|
| N= 20 | N | % |
| Age (mean, range) | 58.9 (18-76) | |
| BMI (mean ± SEM) | 21.0 (±0.9) | |
| Sex | ||
| Male | 12 | 60.0% |
| Female | 8 | 40.0% |
| Primary diagnosis | ||
| Lung | 4 | 20.0% |
| Colon | 4 | 20.0% |
| Liver | 2 | 10.0% |
| Pancreas | 2 | 10.0% |
| Stomach | 2 | 10.0% |
| Sarcoma | 2 | 10.0% |
| Uterus | 1 | 5.0% |
| Breast | 1 | 5.0% |
| Prostate | 1 | 5.0% |
| Head and neck | 1 | 5.0% |
| Histology | ||
| Adenocarcinoma | 13 | 65.0% |
| Squamous cell carcinoma | 3 | 15.0% |
| Others | 4 | 20.0% |
| Anticancer therapy before the trial | ||
| Surgery | 10 | 50.0% |
| Chemotherapy | 18 | 90.0% |
| Radiotherapy | 4 | 20.0% |
| Baseline laboratory data (mean ± SEM) | ||
| WBC (/µL) | 6135 (±787) | |
| Hb (g/dL) | 11.2 (±0.4) | |
| Plt (×104/µL) | 23.1 (±3.0) | |
| Neu (%) | 58.2 (±3.2) | |
| Lym (%) | 29.5 (±3.2) | |
| High-sensitivity CRP (ng/mL) | 20 019 (±7408) | |
Abbreviations: BMI, body mass index; SEM, standard error of the mean; WBC, white blood cells; Hb, hemoglobin; Plt, platelet; Neu, neutrophil; Lym, lymphocyte; CRP, C-reactive protein.
The study was approved by the ethics committee of the Low Molecular Fucoidan Research Association (Reference Number: #20131101-1) and conducted in accordance with the ethical standards as laid down in the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants included in this study.
Materials
The abalone glycosidase-digested fucoidan extract from the brown seaweed Mozuku, Cladosiphon novae-caledoniae Kylin, used in the present study, is commercially available as Power Fucoidan through the Daiichi Sangyo Corporation (Osaka, Japan). The molecular composition of Power Fucoidan is as follows: 72% digested low-molecular-weight fractions with less than 500 Da, and 28% nondigested fractions with peak molecular weight of 800 kDa.28 Therefore, Power Fucoidan consists mostly of a mixture of digested and nondigested fractions of fucoidan and other minor substances.28,32 It is presented as an aqueous solution containing 10 mg/mL of low-molecular-weight fractions as the main constituents.
Endpoints
The primary endpoints of this study were changes in inflammatory biomarkers, including high-sensitivity C-reactive protein (CRP) and proinflammatory cytokines (IL-1β, IL-6, TNF-α). Secondary endpoints were QOL scores using the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30 (EORTC QLQ-C30) and overall survival. These endponts were monitored at outpatient visits using blood sampling and questionnaires before, at the second week, and at the fourth week of fucoidan ingestion.
Blood Collection and Immunological Assessment
Peripheral blood (2 mL) was collected in a tube containing EDTA-2K, and used for a blood count, differential leukocyte counts, detecting high-sensitivity CRP levels, and flow cytometry analysis. Another 8 mL of peripheral blood was collected in a BD Vacutainer CPT Cell Preparation Tube (Becton-Dickinson, CA) for the separation of mononuclear cells to be used for cytokine measurements.
We aimed at identifying T cells (CD3+ cells), B cells (CD20+ cells), natural killer (NK) cells, and NK subsets (CD56+CD16−, CD56+CD16+, CD56−CD16+, CD56+CD16+perforin+granzymeB+,CD56+CD16−perforin+granzymeB+) as well as subpopulations of T cells: CD4+, CD8+, naïve CD4+ (CD4+CD45RA+ cells), memory CD4+ (CD4+CD45RA− cells), and CD8+CD28+ T cells. Therefore, mononuclear cells were stained with a combination of monoclonal antibodies conjugated with chromophores and analyzed using the Navios Flow Cytometer (Beckman Coulter, FL). The following monoclonal antibodies (Beckman Coulter) were used: fluorescein isothiocyanate (FITC)-conjugated anti-CD8, anti-CD20, and anti-CD25; phycoerythrin (RD1)-conjugated anti-CD3 and anti-CD4; phycoerythrin-Texas Red (ECD)-conjugated anti-CD3 and anti-CD45RA; phycoerythrin-cyanin 5.1 (PC5)-conjugated anti-CD28 and CD16; phycoerythrin-cyanin 7 (PC7)-conjugated anti-CD45; and allophycocyanin (APC)-conjugated anti-CD4 and anti-CD56. In addition, the following combinations with monoclonal antibodies were used: (1) CD3-RD1/CD20-FITC/CD16-PC5/CD45-PC7/CD56-APC, (2) CD4-APC/CD8-FITC/CD45RA-ECD/CD28-PC5/CD45-PC7, and (3) CD3-ECD/CD4-PE/CD25-FITC.
The isolated mononuclear cells (1 × 106/well) were cultured for 48 hours in a 24-well culture plate coated with an anti-CD3 monoclonal antibody. The collected culture supernatant was stored at −80°C until cytokine measurement. Cytokines (IL-1β, IL-2, IL-6, IL-12, IL-17, IL-23, interferon-γ, and TNF-α) were measured using a flow cytometer (Navios: Beckman Coulter, Miami, FL) with a BD Cytometric Bead Array (BD CBA: Becton Dickinson). Immunological analysis of peripheral blood was carried out in the Institute for Health & Life Sciences (HLS, Tokyo, Japan) using the analytical technologies patented by Tokyo Medical and Dental University (Patent No.: JP4608704B233; Patent No.: JP5030109B234; Patent No.: US 8,815,524B235).
Statistical Analyses
Data are presented as means ± standard error of the mean (SEM). The significance of differences between time points was determined using the Wilcoxon signed-rank test. The differences between the 2 groups of categorical data were analyzed using the Mann-Whitney U test or Pearson’s χ2 test. Survival curves were plotted using the Kaplan-Meier method, and survival curve comparisons were conducted using the log-rank test and multivariate analysis (Cox’s proportional hazards regression model). A value of P < .05 was considered significant. Analyses were conducted using JMP version 11.0 (SAS Institute Japan, Tokyo, Japan).
Results
Patient Characteristics
In this study, 28 cancer patients were initially recruited in collaboration with 4 clinics in Japan. Finally, 20 patients who met all the inclusion criteria were analyzed. The flow diagram of patient selection for the analyses is shown in Figure 1. The clinical characteristics of all 20 patients are summarized in Table 1. Although the patients’ characteristics were highly variable in their primary origins of cancer, they all had distant metastases, and 90% of the patients had already had standard Cx for advanced stage cancer before the administration of fucoidan.
Changes in Inflammatory Biomarkers
Blood cell counts, including white blood cells, and high-sensitivity CRP were found to stay stable during the study (Table 2). However, the 3 main proinflammatory cytokines, IL-1β, IL-6, and TNF-α, were significantly reduced after 2 weeks of fucoidan administration (Table 2). Other cytokines and all the subsets of lymphocytes except for CD8+CD28+ T cells were stable without significant change during the study period (Table 2).
Table 2.
Changes in Inflammatory Biomarkers and QOL Scores.a
| N= 20 | Day 0 | 2 Weeks | 4 Weeks | P Value (Day 0 to 2 Weeks) | P Value (Day 0 to 4 Weeks) | |
|---|---|---|---|---|---|---|
| Baseline laboratory data (mean ± SEM) | WBC (/µL) | 6135 (±787) | — | 6195 (±704) | — | 1.00 |
| Hb (g/dL) | 11.2 (±0.4) | — | 11.4 (±0.4) | — | .98 | |
| Plt (×104/µL) | 23.1 (±3.0) | — | 24.9 (±3.9) | — | .68 | |
| Neu (%) | 58.2 (±3.2) | — | 56.1 (±3.2) | — | .79 | |
| Lym (%) | 29.5 (±3.2) | — | 31.0 (±2.4) | — | .80 | |
| N/L | 2.7 (±0.4) | — | 2.3 (±0.4) | — | .67 | |
| High-sensitivity CRP (ng/mL) | 20 019 (±7408) | 21 494 (±8626) | 17 738 (±8337) | .81 | .90 | |
| Subsets of lymphocytes (%) (mean ± SEM) | T cell (CD3+) | 68.8 (±2.9) | — | 64.7 (±3.2) | — | .08 |
| CD4+ T | 41.4 (±2.4) | — | 37.6 (±2.4) | — | .10 | |
| CD8+ T | 23.2 (±1.0) | — | 24.5 (±1.7) | — | .25 | |
| CD4 naïve T | 32.5 (±3.0) | — | 31.5 (±3.2) | — | .37 | |
| CD4 memory T | 67.5 (±3.0) | — | 68.5 (±3.2) | — | .37 | |
| Treg (CD4+CD25+) | 5.6 (±0.7) | — | 7.1 (±1.0) | — | .24 | |
| CD8+CD28+ T | 58.5 (±4.4) | — | 51.5 (±3.8) | — | .01b | |
| B cell (CD20+) | 11.3 (±2.2) | — | 10.9 (±2.6) | — | .47 | |
| NK cell | 21.2 (±3.0) | — | 25.1 (±2.9) | — | .11 | |
| NK subset, CD56+CD16- | 3.7 (±0.6) | — | 4.2 (±0.8) | — | .31 | |
| NK subset, CD56+CD16+ | 15.9 (±2.5) | — | 19.3 (±2.7) | — | .10 | |
| NK subset, CD56-CD16+ | 1.5 (±0.5) | — | 1.5 (±0.3) | — | .92 | |
| NK (CD56+CD16+) Perforin+Granzyme B+ | 89.8 (±3.4) | — | 89.8 (±2.6) | — | .62 | |
| NK (CD56+CD16-) Perforin+Granzyme B+ | 63.0 (±5.4) | — | 59.0 (±5.1) | — | .28 | |
| Cytokines (pg/mL), (mean ± SEM) | IL-1β | 358.2 (±62.7) | 189.9 (±32.0) | 273.4 (±75.2) | .01b | .16 |
| IL-6 | 2198.6 (±564.3) | 1522.8 (±367.0) | 1624.1 (±301.3) | .02b | .23 | |
| TNF-α | 4819.4 (±772.0) | 3257.2 (±648.6) | 3985.1 (±548.6) | .03b | .08 | |
| IFN-γ | 2060.4 (±285.0) | 1762.8 (±265.3) | 2048.3 (±271.2) | .19 | .65 | |
| IL-2 | 396.5 (±123.8) | 292.3 (±91.2) | 421.4 (±136.4) | .05 | .65 | |
| IL-17 | 81.0 (±16.7) | 92.7 (±21.3) | 101 (±20.0) | .51 | .64 | |
| IL-23 | 138.4 (±19.5) | 97.8 (±15.7) | 100.9 (±18.0) | .06 | .24 | |
| QOL score (mean ± SEM) | ||||||
| Global health status/QoL | 58.3 (±5.3) | 53.5 (±6.8) | 58.3 (±4.8) | .25 | .92 | |
| Functional scales (higher is better) | Physical functioning | 79.7 (±4.5) | 76.8 (±5.4) | 77.7 (±5.0) | .44 | .40 |
| Role functioning | 76.7 (±6.3) | 76.5 (±6.4) | 72.5 (±6.5) | 1.00 | .39 | |
| Emotional functioning | 82.9 (±3.1) | 78.5 (±4.5) | 80.8 (±5.0) | .63 | .91 | |
| Cognitive functioning | 83.3 (±4.5) | 75.4 (±5.9) | 80.0 (±5.2) | .29 | .62 | |
| Social functioning | 86.7 (±4.3) | 76.3 (±6.9) | 81.7 (±5.5) | .19 | .27 | |
| Symptom scales (higher is worse) | Fatigue | 35.0 (±4.7) | 38.6 (±6.3) | 38.6 (±5.5) | .93 | .40 |
| Nausea and vomiting | 6.7 (±2.5) | 4.4 (±2.8) | 8.3 (±5.3) | .50 | 1.00 | |
| Pain | 24.2 (±6.1) | 20.4 (±6.1) | 21.7 (±6.2) | .33 | 1.00 | |
| Dyspnea | 20.0 (±6.1) | 19.3 (±6.4) | 18.3 (±6.2) | 1.00 | 1.00 | |
| Insomnia | 22.8 (±7.7) | 19.3 (±5.9) | 21.7 (±6.5) | 1.00 | .78 | |
| Appetite loss | 25.0 (±6.3) | 29.8 (±6.7) | 23.3 (±6.0) | .53 | .77 | |
| Constipation | 13.3 (±5.6) | 12.3 (±5.8) | 10.0 (±5.5) | 1.00 | .63 | |
| Diarrhea | 23.3 (±7.3) | 26.3 (±7.5) | 21.7 (±5.0) | .80 | .81 | |
| Financial difficulties | 35.0 (±7.0) | 31.6 (±8.2) | 20.0 (±5.6) | .36 | <.01b | |
Abbreviations: QOL, quality of life; SEM, standard error of the mean; WBC, white blood cells; Hb, hemoglobin; Plt, platelet; Neu: neutrophil; Lym, lymphocyte; CRP, C-reactive protein; CD, cluster of differentiation; NK, natural killer; IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ.
Blood samples from 20 patients were collected at day 0, indicating right before fucoidan ingestion, at 2 weeks, indicating ingestion of fucoidan for 2 weeks, and at 4 weeks, indicating ingestion of fucoidan for 4 weeks, and used for biochemical analyses. QOL scores were calculated using the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30 (EORTC QLQ-C30) submitted by the participants.
P < .05.
QOL Scores
QOL scores using EORTC QLQ-C30 were all stable except for an improvement in the financial difficulty score (Table 3). In contrast to a previous randomized clinical trial, which showed that fucoidan significantly reduces fatigue caused by Cx in advanced colorectal cancer patients,29 the average fatigue score in this study did not improve during the 4-week period.
Table 3.
Univariate and Multivariate Analyses Between the Inflammatory Responsiveness and Overall Survival From the Beginning of Fucoidan Ingestion.a
| Inflammatory Responsiveness | N = 20 |
MST | Log-Rank Test |
Cox’s Hazard Regression |
||
|---|---|---|---|---|---|---|
| Cases | P Value | Hazard Ratio | 95% CI | P Value | ||
| High-sensitivity CRP | ||||||
| Responder (day 0 > 2w) | 10 | 8 | .54 | 1.22 | 0.216-7.078 | .82 |
| Nonresponder (day 0 ≤ 2w) | 10 | 11 | 0.82 | |||
| IL-1β | ||||||
| Responder (day 0 > 2w) | 15 | 13 | .02b | 0.08 | 0.007-0.588 | .01b |
| Nonresponder (day 0 ≤ 2w) | 5 | 5 | 12.87 | |||
| IL-6 | ||||||
| Responder (day 0 > 2w) | 13 | 13 | .28 | 0.55 | 0.108-2.452 | .43 |
| Nonresponder (day 0 ≤ 2w) | 7 | 8 | 1.83 | |||
| TNF-α | ||||||
| Responder (day 0 > 2w) | 14 | 8 | .13 | 5.87 | 0.970-59.162 | .05 |
| Nonresponder (day 0 ≤ 2w) | 6 | 13 | 0.17 | |||
Abbreviations: MST, median survival time; 2w, 2 weeks; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α.
Patients (n = 20) were grouped according to the responsiveness to each inflammatory factor (far left column) after comparing day 0 with 2w, and survival curve comparisons of each factor were conducted with the log-rank test. Multivariate analysis using a Cox proportional hazards regression model was performed to confirm the role of these inflammatory factors related to the overall survival from the beginning of fucoidan ingestion. The univariate and multivariate analyses revealed that the responsiveness of IL-1β was a significant independent prognostic factor (P < .05).
P < .05.
Prognostic Implication of IL-1β Responsiveness in Advanced Cancer Patients
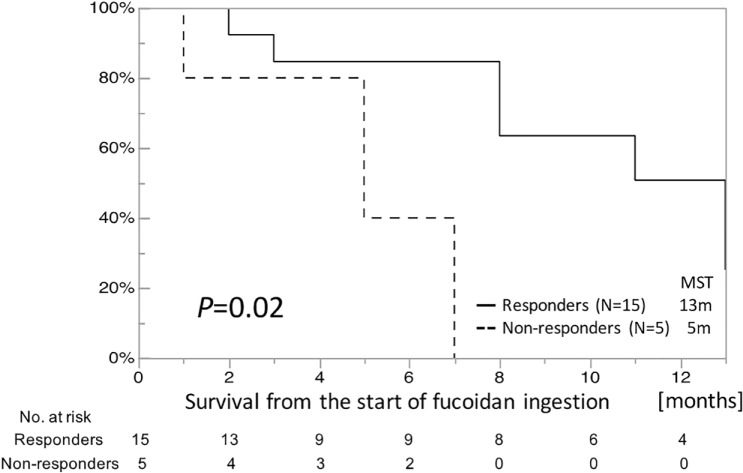
To explore the predictive biomarkers in relation to the clinical responsiveness to fucoidan, a subgroup analysis was conducted between the overall survival (from the start of fucoidan administration) and the response of inflammatory biomarkers. Remarkably, univariate analyses with log-rank tests demonstrated that the patients whose IL-1β level decreased during the first 2 weeks (IL-1β responders) had a significantly longer survival rate compared with the prognoses for IL-1β nonresponders (median survival time = 13.0 vs 5.0 months, respectively; P = .02, Figure 2). Compared with IL-1β, the responsiveness of the other inflammatory biomarkers, including high-sensitivity CRP, IL-6, and TNF-α, were not correlated with survival. Multivariate analysis using a Cox proportional hazards regression model also revealed that the responsiveness of IL-1β was the only significant independent prognostic factor in all the 20 patients (hazard ratio = 0.08, 95% CI = 0.007-0.588, P = .01; Table 3, Figure 2).
Figure 2.
Comparison of the overall survival from the beginning of fucoidan administration, between IL-1β responders and nonresponders. Overall survival from the beginning of fucoidan administration was monitored. Responders were patients whose IL-1β level decreased during the first 2 weeks. Nonresponders were patients whose IL-1β level did not decrease during the first 2 weeks.
Abbreviation: IL, interleukin; MST, median survival time.
Discussion
The present exploratory clinical study revealed that the levels of the major proinflammatory cytokines, including IL-1β, significantly decreased during the first 2 weeks after the administration of fucoidan in the advanced cancer patients. Although we have to be cautious about overestimating these results because of the lack of a control group and a small number of patients, this exploratory clinical study revealed that there are some responders who can benefit from using fucoidan by achieving an anti-inflammatory effect in a short time.
Targeting inflammation in cancer patients is a promising approach not only for alleviating cancer-related symptoms as supportive care but also for enhancing clinical efficacy.2-7,11 Inflammatory responses are mediated by multiple factors, such as proinflammatory cytokines IL-1β, IL-6, and TNF-α.1-7 These cytokines play a pivotal role in establishing a suitable microenvironment for tumor development and metastasis4,5 and also in diminishing therapeutic efficacy.7 Among proinflammatory cytokines, IL-1β is thought to be a key factor in the initiation of inflammatory cascades.36,37 It activates multiple intracellular pathways to initiate the production of factors such as IL-6, IL-8, matrix metalloproteinases, and vascular endothelial growth factor, which promote tumor growth and metastasis.11,38-40 Clinically, it has been shown that elevated serum concentrations of IL-1β were detected in patients with infectious or inflammatory conditions, including cancer.8-10 In particular, the survival time was reported to be shorter in advanced cancer patients with high proinflammatory cytokine levels.11-13 Furthermore, IL-1β was more closely associated with symptoms of cancer cachexia such as loss of appetite, weight loss, and sarcopenia when compared with IL-6 or TNF-α.12 Therefore, the therapeutic approach of IL-1β blockade in cancer patients is expected to be an effective intervention in various cancer progression stages.11,37 Moreover, fucoidan inhibits the viability and invasiveness, and induces apoptosis in IL-1β–treated human rheumatoid arthritis fibroblast synoviocytes.41 Combining these studies with our present results, fucoidan could well be one of the therapeutic options targeting inflammation in advanced cancer patients. Recently, the effect of a combined use of low-molecular-weight fucoidan fractions (mainly 760 Da) with gemcitabine and cisplatin, 2 substances that are standard Cx agents for advanced bladder cancer, was studied, and it was observed that fucoidan could ameliorate cachexia-associated muscle atrophy in bladder cancer–bearing mice. Additionally, these researchers found that inflammatory cytokines such as TNF-α, IL-6, and IL-1β were suppressed by fucoidan, which is in line with our results.42
Fatigue in advanced cancer patients is associated with proinflammatory cytokines such as IL-1β, IL-6, and TNF-α.43 In contrast to our expectation and although a previous randomized clinical trial showed that fucoidan alleviated Cx-induced fatigue,31 in the present study, QOL scores, including fatigue, were almost stable and did not show an important improvement in spite of the significant cytokine reduction. Such discrepant results are likely a result of the individual patient status in fatigue development and the type of questionnaire used. In this exploratory study, the EORTC QLQ-C30 questionnaire was chosen to evaluate the general and comprehensive grades of various aspects of patients’ QOL because the recruited patients represented the diverse symptomatic conditions associated with advanced cancer progression stages. On the other hand, in a previous trial,31 the National Cancer Institute Common Toxicity Criteria assessed the grades of the specific toxicity caused by Cx for advanced colorectal cancer patients. Additionally, Cx is known to induce inflammatory reactions via proinflammatory cytokines, which are associated with Cx-related side effects, including fatigue.43-46 In future studies, it may be needed to focus specifically on Cx-related fatigue and also choose an appropriate questionnaire to detect the change in Cx-related side effects.
It is noteworthy that univariate and multivariate analyses of the data obtained in this study showed a correlation between prognosis and IL-1β responsiveness. However, because of the design of this study, which included a heterogeneous population of patients without a control group, it is difficult to draw any definitive conclusions because various biases cannot be excluded. Nevertheless, there was no significant difference in the characteristic baseline variables of the patients between the group of IL-1β responders and those of nonresponders, except for sex distribution (Online Supplement 1). Interestingly, the levels of the other major proinflammatory cytokines, TNF-α and IL-6, also significantly decreased in the group of IL-1β responders (P < .05) but not in the group of IL-1β nonresponders (Online Supplement 2). High serum concentrations of these proinflammatory cytokines are usually associated with a reduced survival rate.11,13 In addition, cancer patients often exhibit higher IL-1β plasma levels, suggesting a systemic role of this factor.39 It has also been shown that tumor cells secrete IL-1β, triggering an indirect upregulation of IL-6 and other multiple factors, thereby promoting tumor growth and metastasis.4,39 Taken together with these facts, the results obtained in this study seem feasible and may lead us to believe that the responsiveness of IL-1β in the first 2 weeks might predict a prolonged survival as a result of fucoidan administration. It is also interesting that the QOL score relating to the financial difficulty was significantly improved only in the group of IL-1β responders (Online Supplement 2). Accordingly, the previous randomized clinical trial already reported a similar favorable prognosis in the fucoidan group with Cx.31 Because inflammatory reactions induced by Cx usually result in many side effects and also affect therapeutic efficacy,7 fucoidan could be used to target proinflammatory cytokine effects as supportive care for cancer patients especially undergoing Cx.11,22,47 However, further prospective, larger randomized clinical studies will be needed to clarify whether the responsiveness of IL-1β is a reliable predictive biomarker for patients with advanced cancer receiving fucoidan.
Finally, we must also address the fact that IL-1β, IL-6, and TNF-α levels decreased after 2 weeks of fucoidan administration but returned to pretreatment levels after 4 weeks. Perhaps the fucoidan dose was not enough, or the period of treatment was too short, or the monitoring time point at 4 weeks may just be a transition phase from cytokine markers to other markers (eg, lymphocyte subsets; Table 2). Still another likely alternative to explain this cytokine fluctuation is the speculation that fucoidan’s effect on some cell surface receptors is weakened at around 4 weeks post–fucoidan ingestion because fucoidan has been postulated to downregulate some transcription factors involved in survival, proliferation, apoptosis, invasion, metastasis, and angiogenesis via receptors (eg, EGF receptor and receptor tyrosine kinase).21 However, the reasons for this instability remain to be solved.
Conclusions
This exploratory prospective clinical study for advanced cancer patients revealed that levels of important proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, were significantly reduced after a short period of fucoidan administration. Interestingly, a subgroup analysis showed that the responsiveness of IL-1β was significantly correlated with the overall survival and suggested that this responsiveness might be a useful prognostic biomarker for advanced cancer patients receiving fucoidan. To the best of our knowledge, this is the first study to provide evidence of the anti-inflammatory effects of fucoidan for advanced cancer patients. In the future, larger controlled trials are required to establish the efficacy of fucoidan, especially for advanced cancer patients undergoing Cx as supportive care.
Acknowledgments
The authors express appreciation to the participants, investigators, and all other individuals involved in the present study.
Footnotes
Authors’ Note: Trial Registration Number: UMIN000024066; retrospectively registered on September 15, 2016 (UMIN-CTR: http://www.umin.ac.jp/ctr/index.htm). The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Daiichi Sangyo Co, Ltd, Osaka, Japan. The funder provided support in the form of research materials but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Supplemental Material: The Online Supplementary files are available at http://journals.sagepub.com/doi/suppl/10.1177/1534735417692097
References
- 1. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [DOI] [PubMed] [Google Scholar]
- 2. Mantovani A, Allavena1 P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [DOI] [PubMed] [Google Scholar]
- 3. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831-3852. [DOI] [PubMed] [Google Scholar]
- 5. Fernandes JV, Cobucci RN, Jatobá CA, Fernandes TA, de Azevedo JW, de Araújo JM. The role of the mediators of inflammation in cancer development. Pathol Oncol Res. 2015;21:527-534. [DOI] [PubMed] [Google Scholar]
- 6. Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruchard M, Mignot G, Derangère V, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57-64. [DOI] [PubMed] [Google Scholar]
- 8. Gasiorowska A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Małecka-Panas E. Subclinical inflammation and endothelial dysfunction in patients with chronic pancreatitis and newly diagnosed pancreatic cancer. Dig Dis Sci. 2016;61:1121-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellebæk MB, Baatrup G, Gjedsted J, Fristrup C, Qvist N. Cytokine response in peripheral blood indicates different pathophysiological mechanisms behind anastomotic leakage after low anterior resection: a pilot study. Tech Coloproctol. 2014;18:1067-1074. [DOI] [PubMed] [Google Scholar]
- 10. Qian N, Chen X, Han S, et al. Circulating IL-1beta levels, polymorphisms of IL-1β, and risk of cervical cancer in Chinese women. J Cancer Res Clin Oncol. 2010;136:709-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheede-Bergdahl C, Watt HL, Trutschnigg B, et al. Is IL-6 the best pro-inflammatory biomarker of clinical outcomes of cancer cachexia? Clin Nutr. 2012;31:85-88. [DOI] [PubMed] [Google Scholar]
- 13. Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33-40. [DOI] [PubMed] [Google Scholar]
- 14. Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomized and observational studies. Lancet. 2007;369:1603-1613. [DOI] [PubMed] [Google Scholar]
- 15. Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131-2142. [DOI] [PubMed] [Google Scholar]
- 16. Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12-21. [DOI] [PubMed] [Google Scholar]
- 17. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31:3076-3082. [DOI] [PubMed] [Google Scholar]
- 18. Lesovaya E, Yemelyanov A, Swart AC, Swart P, Haegeman G, Budunova I. Discovery of compound A: a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity. Oncotarget. 2015;6:30730-30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lau PM, Stewart K, Dooley M. The ten most common adverse drug reactions (ADRs) in oncology patients: do they matter to you? Support Care Cancer. 2004;12:626-633. [DOI] [PubMed] [Google Scholar]
- 20. Lanas A, Hunt R. Prevention of anti-inflammatory drug-induced gastrointestinal damage: benefits and risks of therapeutic strategies. Ann Med. 2006;38:415-428. [DOI] [PubMed] [Google Scholar]
- 21. Senthilkumar K, Manivasagan P, Venkatesan J, Kim S-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int J Biol Macromol. 2013;60:366-374. [DOI] [PubMed] [Google Scholar]
- 22. de Jesus Raposo MF, de Morais AM, de Morais RM. Marine polysaccharides from algae with potential biomedical applications. Mar Drugs. 2015;13:2967-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang C, Chung D, Shin IS, et al. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int J Biol Macromol. 2008;43:433-437. [DOI] [PubMed] [Google Scholar]
- 24. Ruperez P, Ahrazem O, Leal JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem. 2002;50:840-845. [DOI] [PubMed] [Google Scholar]
- 25. Park HY, Kim GY, Moon SK, Kim WJ, Yoo YH, Choi YH. Fucoidan inhibits the proliferation of human urinary bladder cancer T24 cells by blocking cell cycle progression and inducing apoptosis. Molecules. 2014;19:5981-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang TH, Chiu YH, Chan YL, et al. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar Drugs. 2015;13:1882-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Azuma K, Ishihara T, Nakamoto H, et al. Effects of oral administration of fucoidan extracted from Cladosiphon okamuranus on tumor growth and survival time in a tumor-bearing mouse model. Mar Drugs. 2012;10:2337-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Z, Teruya K, Yoshida T, Eto H, Shirahata S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar Drugs. 2013;11:81-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwak JY. Fucoidan as a marine anticancer agent in preclinical development. Mar Drugs. 2014;12:851-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fitton JH. Therapies from fucoidan: multifunctional marine polymers. Mar Drugs. 2011;9:1731-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ikeguchi M, Yamamoto M, Arai Y, et al. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol Lett. 2011;2:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye J, Li Y, Teruya K. Enzyme-digested fucoidan extracts derived from seaweed Mozuku of Cladosiphon novae-caledoniae kylin inhibit invasion and angiogenesis of tumor cells. Cytotechnology. 2005;47:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirokawa K, Utsuyama M, Kitagawa M. inventors; National University Corporation, Tokyo Medical and Dental University, assignee. Immunity evaluation method, immunity evaluation apparatus, The immunity evaluation program. Japanese patent JP4608704B2. Issed 12 January 2011. https://www.google.com/patents/US8815524. Accessed January 26, 2017.
- 34. Hirokawa K, Utsuyama M, Kitagawa M. inventors; National University Corporation, Tokyo Medical and Dental University, assignee. Immunity evaluation method, apparatus, and program. Japanese patent JP5030109B2. Issued 9 September 2012. [Google Scholar]
- 35. Hirokawa K, Utsuyama M, Kitagawa M, inventors; National University Corporation, Tokyo Medical and Dental University, assignee. Immunity evaluation method, immunity evaluation apparatus, immunity evaluation program and data recording medium having the immunity evaluation program stored therein. US patent US 8815524B2. Issued 26 August 2014. https://www.google.com/patents/US8815524. Accessed January 26, 2017.
- 36. Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 2012;13:343-351. [DOI] [PubMed] [Google Scholar]
- 38. Saijo Y, Tanaka M, Miki M, et al. Proinflammatory cytokine IL-1β promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469-475. [DOI] [PubMed] [Google Scholar]
- 39. Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1β mediated up-regulation of HIF-1α via an NFkB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115-2117. [DOI] [PubMed] [Google Scholar]
- 41. Shu Z, Shi X, Nie D, Guan B. Low-molecular-weight fucoidan inhibits the viability and invasiveness and triggers apoptosis in IL-1β-treated human rheumatoid arthritis fibroblast synoviocytes. Inflammation. 2015;38:1777-1786. [DOI] [PubMed] [Google Scholar]
- 42. Chen MC, Hsu WL, Hwang PA, Chen YL, Chou TC. Combined administration of fucoidan ameliorates tumor and chemotherapy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget. 2016;7:51608-51618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain Behav Immun. 2001;15:371-387. [DOI] [PubMed] [Google Scholar]
- 44. Kelley KW, Bluth R-M, Dantzer R. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(suppl 1):112-118. [DOI] [PubMed] [Google Scholar]
- 45. Mills PJ, Parker B, Dimsdalea JE, Sadler GR, Ancoli-Israel S. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol. 2005;69:85-96. [DOI] [PubMed] [Google Scholar]
- 46. Tsuji E, Hiki N, Nomura S, et al. Simultaneous onset of acute inflammatory response, sepsis-like symptoms and intestinal mucosal injury after cancer chemotherapy. Int J Cancer. 2003;107:303-308. [DOI] [PubMed] [Google Scholar]
- 47. Zhang W, Oda T, Yu Q, Jin JO. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar Drugs. 2015;13:1084-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]