Abstract
Studies have shown that vitamin D could have a role in breast cancer survival; however, the evidence of the relationship between patients’ vitamin D levels and their survival has been inconsistent. This meta-analysis explores possible dose-response relationships between vitamin D levels and overall survival by allowing for differences in vitamin D levels among populations of the various studies. Studies relating vitamin D (25-OH-D [25-hydroxyvitamin D]) levels in breast cancer patients with their survival were identified by searching PubMed and Embase. A pooled HR (hazard ratio) comparing the highest with the lowest category of circulating 25-OH-D levels were synthesized using the Mantel-Haenszel method under a fixed-effects model. A two-stage fixed-effects dose-response model including both linear (a log-linear dose-response regression) and nonlinear (a restricted cubic spline regression) models were used to further explore possible dose-response relationships. Six studies with a total number of 5984 patients were identified. A pooled HR comparing the highest with the lowest category of circulating 25-OH-D levels under a fixed-effects model was 0.67 (95% confidence interval = 0.56-0.79, P < .001). Utilizing a dose-response meta-analysis, the pooled HR for overall survival in breast cancer patients was 0.994 (per 1 nmol/L), Pfor linear trend < .001. At or above a 23.3 nmol/L threshold, for a 10 nmol/L, 20 nmol/L, or 25 nmol/L increment in circulating 25-OH-D levels, the risk of breast cancer overall mortality decreased by 6%, 12%, and 14%, respectively. There was no significant nonlinearity in the relationship between overall survival and circulating 25-OH-D levels. Our findings suggest that there is a highly significant linear dose-response relationship between circulating 25-OH-D levels and overall survival in patients with breast cancer. However, better designed prospective cohort studies and clinical trials are needed to further confirm these findings.
Keywords: vitamin D, breast cancer, overall survival, dose-response relationship, meta-analysis, dose-response meta-analysis
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among females worldwide, with an estimated 1.7 million cases and over half a million deaths in 2012.1 There have been a number of epidemiological studies examining the relationship between low levels of vitamin D and increased breast cancer incidence and decreased patient survival. There are several studies2-7 and 2 meta-analyses8,9 suggesting high circulating levels of 25-OH-D (25-hydroxyvitamin D) are associated with better patient survival. However, the results of such studies are inconsistent with some10-13 showing no significant relationship between higher circulating 25-OH-D levels and better patient survival. Vitamin D could potentially provide an avenue to reduce the burden of breast cancer as vitamin D is a common nutrient in our daily life, which can be easily obtained through sunlight exposure and diet. It is the precursor to the potent steroid hormone calcitriol (1,25-hydroxyvitamin D), which has widespread actions throughout the body and regulates numerous cellular pathways that could have roles in breast cancer survival.14
Varying ranges of vitamin D levels have been reported in different populations. For example, in China, the mean 25-OH-D level was reported to be 48.5 ± 16 nmol/L with 55.9% of the population with less than 50 nmol/L15; in Germany, the mean 25-OH-D level was reported to be 45.6 nmol/L with 61.6% with less than 50 nmol/L16; in Canada, the mean level was 70.36 nmol/L with 20.4% with less than 50 nmol/L.17 In Africa and the Middle East, there have been reports of high vitamin D insufficiency (usually defined as 37.5-50 nmol/L) with ranges from 5% to 80%.18 An earlier meta-analysis8 concerning vitamin D and breast cancer survival reported a pooled HR (hazard ratio) of 0.56 for the highest versus the lowest vitamin D categories. However, such statistical approaches do not take account of differences in the average vitamin D levels of the highest versus lowest categories in different studies. These relate both to differences in populations and also in the particular categories used in the original analyses. In this study, we use an innovative dose-response meta-analysis of relevant cohort studies, focusing on circulating 25-OH-D and overall survival in breast cancer patients, to assess the dose-response relationship between circulating vitamin D levels and breast cancer patient survival. Such an analysis will be statistically more appropriate than the classical meta-analyses as all the population data of each study is utilized rather than just the extreme categories.
Materials and Methods
Protocol and Registration
This meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement and was registered at International Prospective Register of Systematic Reviews (PROSPERO): Number CRD42016038837.
Literature Search
We searched the Embase database (from1974 to 2017, January 24) through OVID, and PubMed (on 2017, January 24) for cohort studies assessing the association between circulating 25-OH-D levels and overall survival in patients with breast cancer, without limitation of language or publication period. Search fields included MeSH terms, entry terms, thesaurus terms, title/abstract, using search terms as follows: (breast cancer) and (vitamin D or cholecalciferol or calcitriol or calcifediol) and (prognosis or outcome or survival or mortality).
Inclusion Criteria
The inclusion criteria were cohort studies. The exposure of interest was circulating 25-OH-D levels in patients with breast cancer. The outcome of interest included overall survival with HRs and corresponding 95% confidence intervals (Cis) corresponding to at least 3 categories of patients based on relative circulating 25-OH-D levels.
Exclusion Criteria
If several publications had overlapping population, only the latest publication was included.
Data Extraction
The following data were extracted from each included study: publication year, first author’s name, country, study design, year of breast cancer diagnosis, timing of blood draw, length of follow-up (years), number of deaths/patients, age at diagnosis, breast cancer stage, average circulating 25-OH-D levels for all participants, range and average circulating 25-OH-D levels in each patient category, number of deaths/patients for each category, HRs and 95% CIs for overall survival for each category, and variables adjusted for in the analysis. The term “category” is used to include different groups divided by both the vitamin D quantiles, or fixed intervals of vitamin D levels, used in the different studies.
Data Estimation
In this report, 25-OH-D concentrations are given in nmol/L. If a study reported concentrations in ng/mL, a conversion factor (1 ng/mL = 2.5 nmol/L) was used. Serum or plasma samples from different studies are referred as “circulating.” To use incidence-rate data to analyze via the Stata data analysis and statistical software possible dose-response relationships, it is necessary to have the following information for each vitamin D concentration category: assigned average 25-OH-D levels, number of deaths, follow-up person-years, adjusted HRs, and 95% CIs. For each category, the means, medians, or midpoints of circulating 25-OH-D levels were assigned to the corresponding HRs. If no means or medians were available and the lowest or highest category was open-ended, the average 25-OH-D levels was assigned at 1.5 times the lower boundary, or the higher boundary divided by 1.5.19 When the number of patient deaths was not directly available from the published data, they were estimated utilizing the total number of deaths, HRs, and the total number of patients in each category using appropriate statistical methods for estimation of missing data.20 For each category of a study, “follow-up person-years” was calculated from the number of patients in each category multiplied by the median follow-up in years. When a study reported HRs and 95% CIs relative to a reference category other than the one with the lowest levels of 25-OH-D, the relevant HRs and 95% CIs were recalculated using the lowest one as reference utilizing a method developed by Hamling et al.21 When necessary, additional information was also obtained by request from the corresponding authors of the original studies.
Assessment of Risk of Bias and Quality
The risk of bias of each included study was assessed by 2 independent investigators and a risk of bias and quality table was generated. Assessment of the quality of the studies was performed by using the Newcastle-Ottawa scale (NOS). A score of 7 or greater was considered high quality. Discrepancies were resolved by discussion until consensus was reached.
Statistical Methods
To compare the highest with the lowest category of circulating 25-OH-D levels, a pooled HR was synthesized with the Mantel-Haenszel method using a fixed-effects model, which considers the heterogeneity both between and within studies. For each study, the HRs comparing the highest with the lowest category were then displayed in a forest plot. A two-stage fixed-effects dose-response model was used to further explore dose-response relationships. HR was analyzed after logarithmic transformation. A log-linear dose-response regression model was used as proposed by Greenland and Longnecker22 and Orsini and colleagues.23 A P value for log-linearity was calculated by testing the null hypothesis that the β vector of regression coefficient is equal to zero. And the potential linear or nonlinear relationship between circulating 25-OH-D and overall survival was assessed using a restricted cubic spline regression model with 4 knots at fixed percentiles (5%, 35%, 65%, and 95%) of the circulating 25-OH-D distribution. A P value for curve linearity or nonlinearity was calculated by testing the null hypothesis that the coefficients of the second and third spline transformations were equal to zero. A 2-sided P < .05 was considered as statistically significant. Curves of linear and nonlinear relationships between overall survival and circulating 25-OH-D levels were generated and only the significant curve is shown. Statistical heterogeneity between studies was evaluated with Cochran’s Q test, with significant heterogeneity defined as P < .10. The heterogeneity between studies was considered to be moderate or high if the I2 statistic24 was greater than 50%. Publication bias was evaluated with the use of the Begg’s test25 and defined significant publication bias as a P < .10. Sensitivity analyses to rule out overrepresentation of results from a single study in the meta-analysis were performed by excluding each study individually from the meta-analysis. All statistical analyses were performed with STATA (version 12.0; StataCorp, College Station, TX).
Results
Study Selection
A total of 210 records were identified through PubMed, and 786 through Embase. A total of 701 citations were screened after removal of duplicates. A total of 603 with irrelevant titles and 73 with irrelevant abstracts were excluded, resulting in 25 potentially relevant studies. Of these, 7 studies13,26-31 were meeting abstracts, 4 studies11,12,32,33 did not present HRs of OS (overall survival), 3 studies34-36 did not give the circulating 25-OH-D levels, 3 studies6,37,38 had less than 3 categories of patient vitamin D levels, 1 study 39 was a case-control study, and 1 study40 included an overlapping population cohort with another study.2 Thus, 6 studies2-5,7,10 fulfilled all the criteria of inclusion. The flowchart of study selection is presented in Figure 1.
Figure 1.
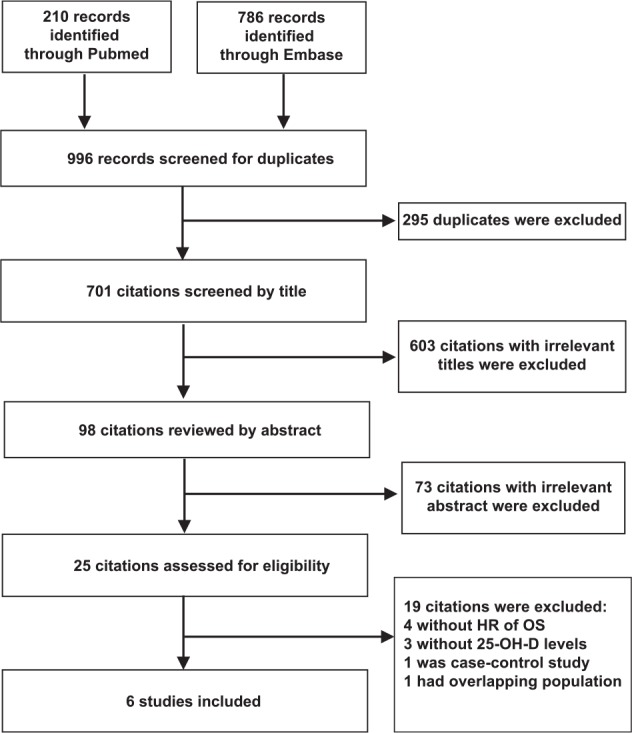
Flowchart of study selection for inclusion in meta-analysis.
Characteristics of the Studies
The 6 studies2-5,7,10 fully meeting the inclusion criteria were included in this dose-response meta-analysis, with a total number of 5984 breast cancer patients and 1024 deaths (Figure 1). Five were prospective cohort designs and one was retrospective cohort study, and patients were diagnosed from years 1984 to 2013. The time between breast cancer diagnosis and blood draw ranges from a median of 33 days to a mean of 30 months. Patients’ 25-OH-D average levels range from 44.9 to 69.7 nmol/L. Patients were divided into 3, 4, or 5 vitamin D categories in each study, and each category has different range and average of 25-OH-D level, corresponding HR of OS and 95% CIs after multivariate adjustment. Details of the characteristics of these studies are provided in Table 1. All studies are considered as of very high quality with a NOS score of 8, except the study of Tretli et al,4 which got a NOS score of 7 due to its unclear risk of selection bias (Table 2).
Table 1.
Characteristics of Studies Included in the Meta-Analysis.
| Year | Author | Country | Study Type | Year of Diagnosis | Timing of Blood Draw | Follow-up Years | No. of Deaths/Patients | Age at Diagnosis | Stage | 25-OH-D Average (nmol/L) | Category (nmol/L) |
HR (95%CI) for Overall Survival | Adjustment in Multivariate Model | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Average | |||||||||||||
| 2009 | Goodwin et al5 | Canada | PC | 1989-1996 | Mean 58.1 days postdiagnosis; postoperative; pre-chemotherapy | 11.6 | 106/512 | 50.4 ± 9.7 | T1-3, N0-1, M0 | 58.1 ± 23.4 | 8-38 | 30.5 | 1 | NA |
| 39-50 | 44 | 0.85 (0.53, 1.36) | ||||||||||||
| 51-61 | 56 | 0.75 (0.50, 1.13) | ||||||||||||
| 61-76 | 68 | 0.68 (0.47, 0.97) | ||||||||||||
| 77-177 | 87 | 0.58 (0.36, 0.92) | ||||||||||||
| 2012 | Tretli et al4 | Norway | RC | 1984-2004 | Median 33 days postdiagnosis | 4-24 | 98/251 | 53.6 (36-75) | Local, RM, DM | NA | <46 | 30.7 | 1 | Sex, AAD, and season |
| 46-61 | 53.5 | 0.55 (0.32, 0.95) | ||||||||||||
| 61-81 | 71 | 0.41 (0.23, 0.74) | ||||||||||||
| >81 | 121.5 | 0.37 (0.21, 0.67) | ||||||||||||
| 2013 | Villasenor et al3 | USA | PC | 1996-1999 | Mean 30 months postdiagnosis | 9.2 | 110/585 | 55.8 ± 10.8 | In situ or stage I-IIIa | 62 ± 25.8 | <50 | 34.75 | 1 | AAD, stage, BMI, race, tamoxifen use, season, treatment, physical activity, smoking status |
| 50-75 | 63.75 | 1.07 (0.66, 1.75) | ||||||||||||
| >75 | 91.5 | 0.90 (0.50, 1.61) | ||||||||||||
| 2014 | Vrieling et al2 | Germany | PC | 2001-2005 | Median 116 days postdiagnosis | 5.3 | 274/2136 | 62.8 ± 5.5 | In situ or stage I-IV | 44.9 | <35 | 23.3 | 1 | AAD, study center, season, tumor size, nodal status, metastases, grade, ER/PR status, diabetes, CD, smoking, HRT, detection mode |
| 35-55 | 44.9 | 0.58 (0.41, 0.81) | ||||||||||||
| >55 | 82.5 | 0.72 (0.53, 1.00) | ||||||||||||
| 2015 | Lohmann et al10 | Canada | PC | 2000-2005 | Pre-chemotherapy | 9.2 | 186/934 | NA | T1-4, N0-2,M0 | 69.7 | <40 | 26.7 | 1 | Treatment and factors with significant imbalances |
| 40-49 | 44.5 | 1.44 (0.73, 2.83) | ||||||||||||
| 50-124 | 87 | 1.03 (0.58, 1.85) | ||||||||||||
| >125 | 187.5 | 0.50 (0.14, 1.77) | ||||||||||||
| 2016 | Yao et al7 | USA | PC | 2006-2013 | Median 69 days postdiagnosis | 7 | 250/1566 | 58.7 ± 12.4 | Stage I-IV | 53.8 | <42 | 27.92 | 1 | AAD, race, BMI, season, tumor stage, grade, subtype |
| 42-63 | 52.31 | 0.78 (0.59, 1.04) | ||||||||||||
| >63 | 94.1 | 0.72 (0.54, 0.98) | ||||||||||||
Abbreviations: PC, prospective cohort; RC, retrospective cohort; RM, regional metastasis; DM, distant metastasis; BMI, body mass index; AAD, age at diagnosis; ER, estrogen receptor; PR, progesterone receptor; HRT, hormone replacement therapy; CD, cardiovascular disease; NA, not available.
Table 2.
Assessment of Risk of Bias and Quality for Included Studies.
| Bias | Goodwin et al (2009)5 | Tretli et al (2012)4 | Villasenor et al (2013)3 | Vrieling et al (2014)2 | Lohmann et al (2015)10 | Yao et al (2016)7 |
|---|---|---|---|---|---|---|
| Selection bias | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk |
| Lost to follow-up bias | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Information bias | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Confounding bias | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| NOS score | 8 | 7 | 8 | 8 | 8 | 8 |
Abbreviations: NOS, Newcastle-Ottawa scale.
Pooled HR of Breast Cancer Overall Survival by the Highest Versus the Lowest Category of Vitamin D Levels
There was no significant heterogeneity (I2 = 16.5%, P = .31) between the studies. Utilizing a fixed-effects model with the Mantel-Haenszel method to compare the highest with the lowest category in vitamin D levels in each study, the pooled HR of breast cancer overall survival was 0.67 (95% CI = 0.56-0.79, P < .001; see Figure 2).
Figure 2.
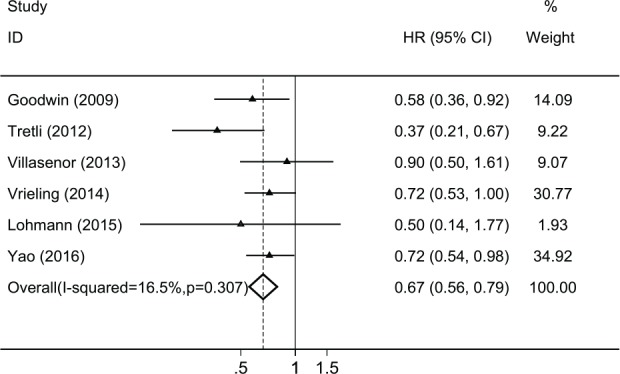
Forest plot of pooled HR of overall survival by the highest versus the lowest category of vitamin D levels in each study.
Dose-Response Meta-Analysis
There was no significant heterogeneity between the studies (χ2 = 4.62, degrees of freedom [df] = 5, P = .46); hence, a fixed-effects model was used. The two-stage fixed-effects dose-response model showed that the pooled HR for overall survival in breast cancer patients was 0.994 per 1 nmol/L (Z = −4.95, Pfor linear trend < .001). At or above a 23.3nmol/L threshold, the pooled HR for overall survival in breast cancer patients was 0.94 (95% CI = 0.92-0.96, P < .001) per 10 nmol/L increment in circulating 25-OH-D levels, 0.88 (95% CI = 0.84-0.93, P < .001) per 20 nmol/L and 0.86 (95% CI = 0.81-0.91, P < .001) per 25 nmol/L increment in circulating 25-OH-D levels. There is no significant nonlinearity in the relationship between overall survival and circulating 25-OH-D levels (χ2 = 4.07, Pfor nonlinearity = 0.13). Estimates of the linear trend in this dose-response meta-analysis are displayed in Table 3, and the curve of linear relationship between overall survival and circulating 25-OH-D levels is plotted in Figure 3. There was no indication of publication bias in the literature on circulating 25-OH-D levels and overall survival in patients with breast cancer (Z = 0.38, Begg’s P = .71). A sensitivity analysis to examine the impact of outliers was performed by excluding each study individually and recalculating the pooled HR. Similar results were obtained in each analysis (data not shown).
Table 3.
Estimates of Linear Trend Between 25-OH-D Levels and Breast Cancer Overall Survival From Two-Stage Fixed-Effects Dose-Response Meta-Analysis.
| HR | 95% CI | Linear Trend | Nonlinearity | Heterogeneity | Publication Bias |
|---|---|---|---|---|---|
| 0.994 | 0.991-0.996 | Z = −4.95, P < .001 | χ2 = 4.07, P = .13 | χ2 = 4.62, df = 5, P = .46 | Z = 0.38, P = .71 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Figure 3.
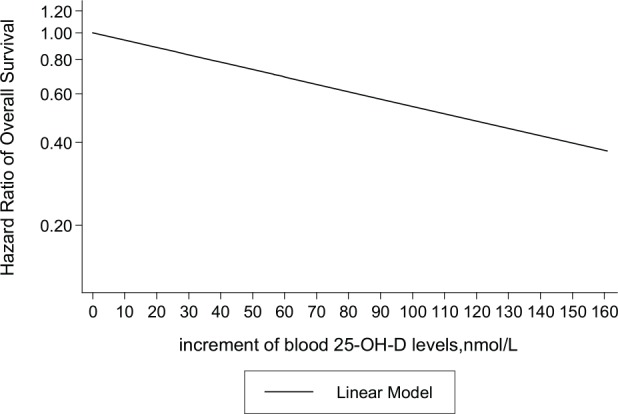
The linear trend between circulating 25-OH-D levels and overall survival in patients with breast cancer.
Plotted on the x-axis are increments of circulating 25-OH-D levels from 23.3 nmol/L, the lowest average level of 25-OH-D from all of the included study categories. Plotted on the y-axis are hazard ratios of overall survival on a log scale. The solid line represents the linear relationship.
Discussion
The evidence of a relationship between the vitamin D levels of breast cancer patients and their subsequent survival has been inconsistent.2-6,10,11,27,34-37,39,40 Our meta-analysis identified 5984 breast cancer patients from 5 prospective studies and 1 retrospective study, where patient overall survival has been related to different categories of vitamin D levels. We have utilized a two-stage, fixed-effects model to explore a dose-response relationship between vitamin D levels and breast cancer survival. The average vitamin D levels of the categories in the 6 studies ranged from 23.3 nmol/L to 187.5 nmol/L of vitamin D (Table 1). Overall, there was a highly significant linear relationship between circulating 25-OH-D levels and overall survival. We conclude that at, or above, a 23.3 nmol/L threshold, for a 10 nmol/L increment in circulating 25-OH-D levels, the rate of breast cancer overall mortality decreased by 6%, and for a 25 nmol/L increment in circulating 25-OH-D levels, the rate of breast cancer overall mortality decreased by 14%.
There are a number of possible limitations of this meta-analysis. First, the included studies only used a single measurement of circulating 25-OH-D levels; however, the circulating levels of 25-OH-D levels have been reported to remain relatively stable over time,41 so this may not cause significant bias. The season of blood sampling can be significantly associated with serum 25-OH-D3 concentration.42 In the 6 studies the season of the blood sampling varied; however, 4 of the 6 studies have adjusted for this factor while one study found little evidence that vitamin D varied during the calendar year and the other one did not report season. All the studies measured circulating 25-OH-D levels at varying times postdiagnosis of breast cancer, and it is possible that patients may initiate the use of vitamin D supplements following such a diagnosis. Assuming that vitamin D levels postdiagnosis can have an impact on survival, this factor may result in reducing the magnitude of the effect of vitamin D on patient survival.
Since patient serum vitamin D levels were measured at various times postdiagnosis of breast cancer, a further potential bias is the finding that serum vitamin D levels may decrease during neoadjuvant chemotherapy.43 In one study vitamin D levels were measured before commencement of chemotherapy, while 3 of the 6 studies adjusted for treatment of breast cancer. Such an adjustment could not be undertaken on the entire cohort due to limitations of the data presented in the published studies.
The various molecular subtypes of breast cancer have different rates of mortality,44 and according to published findings,45-47 breast cancer patients with a more aggressive molecular subtype tend to have lower 25-OH-D levels. In the 6 studies, the different molecular subtypes are not taken into consideration and may vary in frequency among different studies. Due to the limited information presented in the studies, it was not possible to perform a subgroup analysis according to breast cancer tumor stage, treatment pattern, molecular subtype, or menopausal status.
TNM staging can also provide prognostic information. Of the 6 studies, 3 adjusted for TNM staging. As the TNM staging of all the studies were not available, it was not possible to adjust for any differential prognostic effect of TNM staging within the different vitamin D intervals of the studies. It should also be noted that due to limitations of the data presented in the 6 studies, not all the relevant data for our analyses could be directly obtained and in some cases estimates were required.
Our study has several strengths. To better reflect the prognosis of breast cancer patients, we used overall survival instead of relapse-free survival (RFS) or disease-free survival (DFS) as the end point of our study because the definition of RFS and DFS are not consistent among the 6 studies. In addition, all the 6 studies are well-designed cohort studies, 5 prospective and 1 retrospective. By using an innovative dose-response meta-analysis of relevant cohort studies, our analysis utilizes all the population data of each study rather than just the extreme categories. This is statistically more appropriate than the classical meta-analyses where the differences in vitamin D levels between populations are not included in the analyses.
We identified a statistically significant dose-response relationship in breast cancer between patient survival and circulating levels of 25-OH-D. A protective effect of higher vitamin D levels have also been shown in skin cancer,48 ovarian cancer,49 prostate cancer,50 and colorectal cancer.51 As shown in Table 1 and Figure 3, the linear relationship encompasses vitamin D levels from 23.3 to 187.5 nmol/L. It should be noted that due to limited data presented in the studies the highest 3 vitamin D levels (187.5, 121.5, and 94.1 nmol/L) were all estimated and therefore the linear relationship at higher levels of vitamin D are likely to be unreliable. Additional well-designed studies that address the limitations of the presently reported studies are required, of particular importance will be to define the level of vitamin D in women with breast cancer that provides them with the maximum protective effect.
Vitamin D converts to its active form calcitriol through endocrine, paracrine or autocrine pathway. Calcitriol plays its anticancer role mediated by the vitamin D receptor, via both genomic and nongenomic actions.52 In the context of cancer, calcitriol induces apoptosis, inhibits cell proliferation and angiogenesis,53 promotes cell differentiation, and acts as anti-inflammatory factor within the tumor microenvironment.54 Besides, calcitriol may have a specific role in breast cancer through estrogen receptor (ER) signaling pathways, like the suppression of aromatase expression in breast adipose tissue55,56 and the suppression of ER expression and estrogen-mediated signaling by calcitriol in breast cancer cells.57 Interestingly, calcitriol can also induce ER expression and restore antiestrogen responsiveness in ER-negative breast cancer cells.58 A preclinical study with murine models shows a tumor autonomous effect of vitamin D signaling to suppress breast cancer metastases via regulation of inhibitor of differentiation 1 (ID1).59 Through its broad anticancer actions and specific signaling pathways in breast cancer, and according to epidemiological evidence, vitamin D may have a role in protecting against breast cancer mortality.
Vitamin D is a nutrient with low cost and easy access, and according to our findings, adding vitamin D supplements to traditional breast cancer therapy would be expected to be an economical and safe way to improve overall survival of breast cancer patients. This will require comprehensive clinical supplementation trials since there are a number of factors to be addressed, for example, can short-term increases in vitamin D levels following breast cancer diagnosis influence patient survival.
Conclusions
In conclusion, our findings suggest that there is a highly significant linear dose-response relationship between circulating 25-OH-D levels and overall survival in patients with breast cancer. At, or above, a 23.3 nmol/L threshold, for a 10 nmol/L, 20 nmol/L, 25 nmol/L increment in circulating 25-OH-D levels, the risk of breast cancer overall mortality decreased by 6%, 12%, and 14%, respectively. However, better designed prospective cohort studies and clinical trials are needed to further confirm these findings.
Acknowledgments
The authors thank Dr Pamela Jean Goodwin (Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, University of Toronto, Canada) for her kind assistance in providing additional data.
Footnotes
Authors’ Note: All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants of included studies.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [DOI] [PubMed] [Google Scholar]
- 2. Vrieling A, Seibold P, Johnson TS, et al. Circulating 25-hydroxyvitamin D and postmenopausal breast cancer survival: influence of tumor characteristics and lifestyle factors? Int J Cancer. 2014;134:2972-2983. [DOI] [PubMed] [Google Scholar]
- 3. Villasenor A, Ballard-Barbash R, Ambs A, et al. Associations of serum 25-hydroxyvitamin D with overall and breast cancer-specific mortality in a multiethnic cohort of breast cancer survivors. Cancer Causes Control. 2013;24:759-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tretli S, Schwartz GG, Torjesen PA, Robsahm TE. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control. 2012;23:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757-3763. [DOI] [PubMed] [Google Scholar]
- 6. Hatse S, Lambrechts D, Verstuyf A, et al. Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis. 2012;33:1319-1326. [DOI] [PubMed] [Google Scholar]
- 7. Yao S, Kwan ML, Ergas IJ, et al. Association of serum level of vitamin D at diagnosis with breast cancer survival: a case-cohort analysis in the pathways study. JAMA Oncol. 2017;3:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohr SB, Gorham ED, Kim J, Hofflich H, Garland CF. Meta-analysis of vitamin D sufficiency for improving survival of patients with breast cancer. Anticancer Res. 2014;34:1163-1166. [PubMed] [Google Scholar]
- 9. Rose AA, Elser C, Ennis M, Goodwin PJ. Blood levels of vitamin D and early stage breast cancer prognosis: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141:331-339. [DOI] [PubMed] [Google Scholar]
- 10. Lohmann AE, Chapman JA, Burnell MJ, et al. Prognostic associations of 25 hydroxy vitamin D in NCIC CTG MA.21, a phase III adjuvant randomized clinical trial of three chemotherapy regimens in high-risk breast cancer. Breast Cancer Res Treat. 2015;150:605-611. [DOI] [PubMed] [Google Scholar]
- 11. Clark AS, Chen J, Kapoor S, et al. Pretreatment vitamin D level and response to neoadjuvant chemotherapy in women with breast cancer on the I-SPY trial (CALGB 150007/150015/ACRIN6657). Cancer Med. 2014;3:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs ET, Thomson CA, Flatt SW, et al. Vitamin D and breast cancer recurrence in the Women’s Healthy Eating and Living (WHEL) study. Am J Clin Nutr. 2011;93:108-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piura E, Chapman JW, Lipton A, et al. Serum 1-OH vitamin D (D) and prognosis of postmenopausal breast cancer (BC) patients: NCIC-CTG MA14 trial [Abstract]. J Clin Oncol. 2009;27(15 suppl 1):A534. [Google Scholar]
- 14. Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342-357. [DOI] [PubMed] [Google Scholar]
- 15. Yu S, Fang H, Han J, et al. The high prevalence of hypovitaminosis D in China: a multicenter vitamin D status survey. Medicine (Baltimore). 2015;94:e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabenberg M, Scheidt-Nave C, Busch MA, Rieckmann N, Hintzpeter B, Mensink GB. Vitamin D status among adults in Germany: results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health. 2015;15:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greene-Finestone LS, Berger C, de Groh M, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011;22:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green RJ, Samy G, Miqdady MS, et al. Vitamin D deficiency and insufficiency in Africa and the Middle East, despite year-round sunny days. S Afr Med J. 2015;105:603-605. [DOI] [PubMed] [Google Scholar]
- 19. Rong Y, Chen L, Zhu T, et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ. 2013;346:e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu C, Liu TZ, Kuang XY, Zhang YG, Weng H, Zhang C. How to estimate the missing data and transform the effect measure in dose-response meta-analysis. Chin J Evid-Based Med. 2015;15:984-987. [Google Scholar]
- 21. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954-970. [DOI] [PubMed] [Google Scholar]
- 22. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301-1309. [DOI] [PubMed] [Google Scholar]
- 23. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40-57. [Google Scholar]
- 24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [DOI] [PubMed] [Google Scholar]
- 25. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] [Google Scholar]
- 26. Habib JG, Espirito J, Harrell R, et al. Vitamin D levels, triple-negative breast cancer, and geography: a retrospective analysis of a large database of oncology practices in the United States. Cancer Res. 2015;75(9 suppl 1):P6-09-02. [Google Scholar]
- 27. Yao S, Hong CC, Kwan ML, et al. The association of serum 25-hydroxyvitamin D with breast cancer characteristics and prognosis in the Pathways Study. Cancer Res. 2014;74(19 suppl 1):4126-4126. [Google Scholar]
- 28. Habib JG, Espirito JL, Harrell RK, et al. Vitamin D levels, triple-negative histology, and geography in breast cancer: a retrospective analysis of a large database of oncology practices in the United States. J Clin Oncol. 2014;32(26 suppl 1):17. [Google Scholar]
- 29. Coleman RE, Rathbone EJ, Marshall HC, et al. Vitamin D, but not bone turnover markers, predict relapse in women with early breast cancer: an AZURE translational study. Cancer Res. 2012;72(24 suppl. 3). [Google Scholar]
- 30. Cauley J, Wactawski-Wende J, Robbins J, et al. The women’s health initiative (WHI) calcium plus vitamin D supplementation trial: health outcomes 5 years after trial completion. J Bone Miner Res. 2013;22:915-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rickles A, Peppone L, Huston A, Piazza K, Skinner K. Serum 25-hydroxyvitamin D and prognostic tumor characteristics in breast cancer patients. Ann Surg Oncol. 2011;18:S159. [Google Scholar]
- 32. Santini D, Galluzzo S, Vincenzi B, et al. Longitudinal evaluation of vitamin D plasma levels during anthracycline- and docetaxel-based adjuvant chemotherapy in early-stage breast cancer patients. Ann Oncol. 2010;21:185-186. [DOI] [PubMed] [Google Scholar]
- 33. Neuhouser ML, Sorensen B, Hollis BW, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeichner SB, Koru-Sengul T, Shah N, et al. Improved clinical outcomes associated with vitamin D supplementation during adjuvant chemotherapy in patients with HER2+ nonmetastatic breast cancer. Clin Breast Cancer. 2015;15:e1-e11. [DOI] [PubMed] [Google Scholar]
- 35. Poole EM, Shu X, Caan BJ, et al. Postdiagnosis supplement use and breast cancer prognosis in the after Breast Cancer Pooling Project. Breast Cancer Res Treat. 2013;139:529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saquib J, Rock CL, Natarajan L, et al. Dietary intake, supplement use, and survival among women diagnosed with early-stage breast cancer. Nutr Cancer. 2011;63:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lim ST, Jeon YW, Suh YJ. Association between alterations in the serum 25-hydroxyvitamin D status during follow-up and breast cancer patient prognosis. Asian Pac J Cancer Prev. 2015;16:2507-2513. [DOI] [PubMed] [Google Scholar]
- 38. Nechuta S, Lu W, Chen Z, et al. Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:262-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yao S, Sucheston LE, Millen AE, et al. Pretreatment serum concentrations of 25-hydroxyvitamin D and breast cancer prognostic characteristics: a case-control and a case-series study. PloS One. 2011;6(2):e17251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vrieling A, Hein R, Abbas S, Schneeweiss A, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res. 2011;13:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McKibben RA, Zhao D, Lutsey PL, et al. Factors associated with change in 25-hydroxyvitamin D levels over longitudinal follow-up in the ARIC study. J Clin Endocrinol Metab. 2015;101:33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fohner AE, Wang Z, Yracheta J, et al. Genetics, diet, and season are associated with serum 25-hydroxycholecalciferol concentration in a Yup’ik study population from southwestern Alaska. J Nutr. 2016;146:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Charehbili A, Hamdy NA, Smit VT, et al. Vitamin D (25-0H D3) status and pathological response to neoadjuvant chemotherapy in stage II/III breast cancer: data from the NEOZOTAC trial (BOOG 10-01). Breast. 2016;25:69-74. [DOI] [PubMed] [Google Scholar]
- 44. Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rainville C, Khan Y, Tisman G. Triple negative breast cancer patients presenting with low serum vitamin D levels: a case series. Cases J. 2009;2:8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peppone L, Rickles A, Huston A, et al. The association between prognostic demographic and tumor characteristics of breast carcinomas with serum 25-OH vitamin D levels. Cancer Epidemiol Biomarkers Prev. 2011;20:717. [Google Scholar]
- 47. Peppone LJ, Rickles AS, Janelsins MC, Insalaco MR, Skinner KA. The association between breast cancer prognostic indicators and serum 25-OH vitamin D levels. Ann Surg Oncol. 2012;19:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caini S, Boniol M, Tosti G, et al. Vitamin D and melanoma and non-melanoma skin cancer risk and prognosis: a comprehensive review and meta-analysis. Eur J Cancer. 2014;50:2649-2658. [DOI] [PubMed] [Google Scholar]
- 49. Webb PM, de Fazio A, Protani MM, et al. Circulating 25-hydroxyvitamin D and survival in women with ovarian cancer. Am J Clin Nutr. 2015;102:109-114. [DOI] [PubMed] [Google Scholar]
- 50. Shui IM, Mondul AM, Lindstrom S, et al. Circulating vitamin D, vitamin D-related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer. 2015;121:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morales-Oyarvide V, Meyerhardt JA, Ng K. Vitamin D and physical activity in patients with colorectal cancer: epidemiological evidence and therapeutic implications. Cancer J. 2016;22:223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hii CS, Ferrante A. The non-genomic actions of vitamin D. Nutrients. 2016;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garcia-Quiroz J, Rivas-Suarez M, Garcia-Becerra R, et al. Calcitriol reduces thrombospondin-1 and increases vascular endothelial growth factor in breast cancer cells: implications for tumor angiogenesis. J Steroid Biochem Mol Biol. 2014;144(Pt A):215-222. [DOI] [PubMed] [Google Scholar]
- 54. Diaz L, Diaz-Munoz M, Garcia-Gaytan AC, Mendez I. Mechanistic effects of calcitriol in cancer biology. Nutrients. 2015;7:5020-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D. Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology. 2010;151:32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Swami S, Krishnan AV, Wang JY, et al. Inhibitory effects of calcitriol on the growth of MCF-7 breast cancer xenografts in nude mice: selective modulation of aromatase expression in vivo. Horm Cancer. 2011;2:190-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Swami S, Krishnan AV, Peng L, Lundqvist J, Feldman D. Transrepression of the estrogen receptor promoter by calcitriol in human breast cancer cells via two negative vitamin D response elements. Endocr Relat Cancer. 2013;20:565-577. [DOI] [PubMed] [Google Scholar]
- 58. Santos-Martinez N, Diaz L, Ordaz-Rosado D, et al. Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: a potential new therapeutic approach. BMC Cancer. 2014;14:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Williams JD, Aggarwal A, Swami S, et al. Tumor autonomous effects of vitamin D deficiency promote breast cancer metastasis. Endocrinology. 2016;157:1341-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]


