Abstract
Hypothesis: Electroacupuncture (EA) has been used as an alternative analgesic therapy for hundreds of years, yet its analgesic potency and therapeutic advantage against bone cancer pain (BCP) in comparison with morphine remains unclear. This study aimed to investigate the effects of EA on mechanical allodynia and cellular immunity of BCP rats, and to further explore the potential mechanism. Methods: The BCP model was established by implanting Walker 256 mammary gland carcinoma cells into the left tibia of adult female Sprague-Dawley rats. EA (dilatational wave, 2/100 Hz, 0.5 mA–1mA–1.5 mA for 10 minutes each intensity) was applied bilaterally to Zusanli (ST 36) and Kunlun (BL 60) for 30 minutes. Both EA stimulation and morphine (10 mg/kg, intraperitoneally) was given once every other day. Naloxone (0.3 mg/kg, intraperitoneally) was injected at 30 minutes prior to EA. Mechanical allodynia were demonstrated by paw withdrawal thresholds (PWTs) which measured by dynamic plantar aesthesiometer. T cell proliferation, percentage of CD3+, CD4+ and CD8+ T lymphocytes in spleen as well as expression of interleukin-2 (IL-2) in plasma were detected by WST-8, flow cytometry, and enzyme-linked immunosorbent assay technique, respectively. Results: An intratibial inoculation of Walker 256 mammary gland carcinoma cells significantly decreased PWTs to mechanical stimuli. EA stimulation alleviated mechanical allodynia in BCP rats, and the analgesic potency of EA was weaker than that of morphine. In contrast to morphine, EA stimulation of BCP rats increased splenic concanavalin A (Con A)-induced T cell proliferation and plasma IL-2 content, as well as increased the percentages of splenic CD3+CD4+ and CD3+CD8+ T cell subsets. Moreover, both the analgesic effect and the partial immunomodulation of EA were suppressed by an intraperitoneal injection of naloxone. Conclusion: EA could significantly alleviate BCP-induced mechanical allodynia. Although the analgesic effect of EA was weaker than that of morphine, EA had an immunomodulation effect on cellular immunity. Both analgesic and immunomodulatory effect of EA might share the same mechanism via the opioid-mediated pathway, which needs further investigation.
Keywords: bone cancer pain, electroacupuncture, morphine, analgesia, mechanical allodynia, cellular immunity, naloxone
Introduction
Bone cancer pain (BCP), which is one of the most common type of cancer pains, always manifests as severe and intractable pain. Pain is the most disruptive cancer-related event to the cancer patient’s quality of life. Opiate drugs, such as morphine, are the gold standard for treating moderate to severe pain or breakthrough pain resulting from cancer, and data of evidence-based medicine have led to opioid therapy being strongly recommended for treating cancer pain.1 Additionally, an increasing number of studies show that opioid-induced immunosuppression is the most significant drug-induced medical problem or side effect of opiate drug administration.2 Morphine, a typical opioid analgesic, has a number of immunosuppressive effects, including inhibiting phagocytosis and neutrophil migration,3 suppressing natural killer cell-mediated cytotoxicity,4,5 decreasing T cell function and reducing the percentages of T lymphocyte subsets in a dose-dependent manner.6,7
Electroacupuncture (EA) has been used as an alternative analgesic therapy for hundreds of years. Numerous studies have shown that EA alleviates multiple painful conditions, such as inflammatory pain8-10 and neuropathic pain.11,12 Moreover, EA has recently been reported to attenuate cancer pain in animal studies.13-16 The results of a systematic meta-analysis suggest that acupuncture is not more effective than drug therapy but that acupuncture plus drug therapy is more effective than drug therapy alone.17 Thus, the exact analgesic potency of EA on cancer pain remains unclear and needs further evaluation.
It is widely known that opioids, such as morphine, suppress the immune system either through a direct pathway by binding to mu-opioid receptors present on immune cells or through an indirect pathway by binding to µ-opioid receptors within the central nervous system.5,18 The finding that EA modulates the release of opioid peptides in the central nervous system is not new. Previous studies reported that the analgesic effect of EA was associated with the release of endogenous opioid peptides, such as β-endorphin, enkephalin and dynorphin.19,20 Moreover, it has been reported that the peripheral opioid system contributes to the analgesic effect of EA.21,22 EA-induced antinociception is blocked by a intraplantar injection of antiopioid peptide antibodies23 or an opioid receptor antagonist,21 and inhibiting the recruitment of opioid-containing macrophages decreases EA-induced antinociception.23 However, the effect of EA on the immune system in a BCP model is unknown, and it is unclear if EA modulates the immune system via the opioid system in a BCP model.
In this article, the BCP model was established by an intra-tibial injection of Walker 256 mammary gland carcinoma cells. We simultaneously evaluated the effects of EA on mechanical allodynia and T cell-mediated immunity in a rat model of BCP and compared the effects of EA and morphine to further clarify the potential advantages of using EA as a complementary and alternative therapy in the treatment of cancer pain.
Materials and Methods
Experimental Animals
Adult female Sprague-Dawley rats weighing 150 to 180 g were used as the BCP model. All rats were housed in temperature- and light-controlled rooms (24°C ± 2°C, 12-/12-hour light/dark cycle) with access to food and water ad libitum. All experimental procedures were approved by the Animal Ethics Committee of the Zhejiang Chinese Medical University and were in accordance with the guidelines of the Care and Use of Laboratory Animals of the National Institutes of Health.
Surgery
The BCP model was established by implanting Walker 256 mammary gland carcinoma cells into the left tibia using a procedure similar to that described by Wang et al.24 Briefly, suspensions of carcinoma cells were collected from the ascitic fluid of the peritoneal cavity, and the percentage of cellular activity (more than 95%) was calculated using a TC10 Automated Cell Counter (Bio-Rad Laboratories, Inc, Irvine, CA, USA). Injecting the carcinoma cells (3 × 105 cells in 5 µL) into the medullary cavity of the left tibia under anesthesia with 10% chloral hydrate (0.35 mL/100 g) induced bone cancer in the rats. To prevent leakage of the tumor cells, the syringe (10 µL, Hamilton Co, Bonaduz, Switzerland) was kept in position for 2 minutes, and the injection hole was quickly sealed with bone wax. Penicillin (20 000 units/rat, intramuscularly) was given once per day for 5 days to avoid infection. In the sham BCP group, the same volume of boiled carcinoma cells was injected using a similar procedure, and the rats in the Control group did not receive any intervention.
Experimental Groups and Design
Part 1
The rats were divided randomly into 5 groups as follows: (1) a Control group without any intervention; (2) a Sham BCP group, which received an intratibial inoculation of 5 µL boiled carcinoma cells suspension; (3) a BCP group, which received an intratibial inoculation of Walker 256 mammary gland carcinoma cells (3 × 105 cells in 5 µL); (4) a BCP + EA group, which received an intratibial inoculation of carcinoma cells (same as the BCP group) and EA treatment (2/100 Hz, once every other day, 8 times in total); and (5) a BCP + Mor group, which received an intratibial inoculation of carcinoma cells (same as the BCP group) and morphine treatment (10 mg/kg, intraperitoneally, once every other day, 8 times in total).
Part 2
The BCP rats were divided randomly into 2 groups as follows: (1) the EA + NS group and (2) the EA + NAL group, which received an intraperitoneal injection of sterile normal saline (200 µL) or naloxone (0.3 mg/kg) prior to EA treatment, respectively. Both drug and EA treatment were given once every other day, 8 times in total.
EA Treatment
For EA, the rats were gently immobilized using a special cotton retainer designed by our laboratory (Patent No. ZL 2014 2 0473579.9, State Intellectual Property Office of the People’s Republic of China). Stainless steel acupuncture needles (0.25 mm diameter,13 mm length, Suzhou Medical Appliance Factory, Suzhou, China) were inserted to a depth of 5 mm at ST36 (Zusanli) bilaterally (5 mm lateral to and below the anterior tubercule of the tibia) and BL60 (Kunlun) bilaterally (at the level of the ankle joint, between the tip of the lateral malleolus and the Achilles tendon). The 2 homolateral needles were connected to the output terminals of a HANS Acupuncture Point Nerve Stimulator (Hans-100, Huawei Co, Ltd, Beijing, China). The EA parameters were set as follows: constant square wave current output (pulse width: 0.6 ms at 2 Hz, 0.2 ms at 100 Hz); intensities of 0.5, 1 and 1.5 mA (10 minutes each, total 30 minutes); and alternating frequencies of 2 Hz and 100 Hz (automatically shifting between 2 Hz and 100 Hz stimulation every 3 seconds). The EA stimulation was given once every other day for 30 min and was started after the completion of the behavioral test from day 6 to day 20 postinoculation, 8 times in total.
Drug Administration
Rats in the BCP + Mor group received an intraperitoneal injection of 10 mg/kg morphine hydrochloride (C81004-2, Northeast Pharmaceutical Group Shenyang No.1 Pharmaceutical Co, Ltd, China) once every other day starting after the completion of the behavioral test from day 6 to day 20 postinoculation. Naloxone hydrochloride dihydrate (0.3 mg/kg, Sigma-Aldrich, St Louis, MO, USA) was injected intraperitoneally into the rats in the EA + NAL group 30 minutes prior to the EA stimulation once every other day. The rats in the EA + NS group were injected intraperitoneally with the same volume of sterile normal saline.
Paw Withdrawal Threshold Measurements
All procedures were performed in quiet conditions by the same experimenter in a blind manner. The rats were allowed to acclimate to the test room and the experimental cages for 30 minutes per day for 3 days prior to the experiments. Mechanical allodynia was evaluated in the rats on the day before the intra-tibial inoculation with the Walker 256 cancer cells (Base), on postinoculation day 6 (D6, Before EA), 30 minutes after the completion of the EA treatment on D6 (After EA) and on days 10, 16, and 20 postinoculation (D10, D16, D20). The pain thresholds from D6 (After EA) to D20 were measured within a half an hour after the completion of the EA stimulation. The mechanical stimulus (force 0 to 50 g, slope 20 seconds) was delivered to the central plantar surface of the left hind paw using a dynamic plantar aesthesiometer (37450, UGO Basile, Italy). When the animal withdrew its hind paw, the mechanical stimulus automatically stopped, and the force at which the animal withdrew its paw was recorded as the paw withdrawal threshold PWT. The withdrawal responses were taken from the average of 4 consecutive trials, with at least 3 minutes between the trials. The rats in which the PWT did not decrease on post-inoculation day 6 (Before EA) were eliminated.
Preparation of Splenic Monocytes
On day 20 postinoculation, the rats from the different groups were sacrificed by cervical dislocation with chloral hydrate anesthesia (350 mg/kg, intraperitoneally). All spleens were aseptically removed from the rats and teased onto a 200-mesh stainless steel screen immersed in chilled sterile phosphate-buffered saline. The cell suspensions were collected, and then 3 to 5 volumes of red blood cell lysis buffer were added (Beyotime Biotechnology, Shanghai, China) to lyse the erythrocytes. After centrifuging at 1000 × g for 10 minutes, the remaining cells were suspended in RPMI 1640 (Gibco, Gaithersburg, MD, USA) containing 10% fetal bovine serum, 100 U/mL penicillin G, and 100 μg/mL streptomycin, and the number of cells was adjusted based on the experimental design.
Splenic T Lymphocyte Proliferation Assays
Splenic T lymphocyte proliferation was assessed using a WST-8 assay and a Cell Counting Kit-8 (Beyotime Biotechnology). Briefly, splenic monocytes were seeded into 96-well plates at 2 × 105 cells/well (in triplicate) in 200 μL of culture medium with or without 10 μg/mL Con A (Sigma-Aldrich) and cultured at 37°C for 72 hours. Then, 20 μL of the WST-8 mixture was added to each well, and the whole plate was incubated in a CO2 incubator at 37°C for 4 hours. The optical density (OD) was determined at 450 nm with the reference wavelength set at 650 nm using a microplate reader (Spectra Max M4, Molecular Devices, Sunnyvale, CA, USA). The values for splenic T lymphocyte proliferation were calculated by (mean OD of Con A–stimulated cells/mean OD of nonstimulated cells) × 100%.
Flow Cytometry
Flow cytometry analysis was performed using a BD FACSCalibur flow cytometer (BD Biosciences, Franklin lakes, NJ, USA) to determine the proportion of different splenic T cell phenotypes (CD3+, CD3+CD4+, CD3+CD8+). Briefly, 1 to 5 × 105 cells were incubated at 4°C for 30 minutes with a FITC-conjugated anti-rat CD3 monoclonal antibody, an APC-conjugated anti-rat CD4 monoclonal antibody, and a PE-conjugated anti-rat CD8a monoclonal antibody. Then, the cells were fixed with 1% paraformaldehyde, washed, and measured by flow cytometry. FITC anti-mouse IgG3, APC anti-mouse IgG2a K, and PE anti-mouse IgG1 K isotypes were used as the controls. All antibodies mentioned in this experiment were purchased from eBioscience company (eBioscience, Inc, San Diego, CA, USA). The data were analyzed for 5000 events with BD FACSDiva software version 6.1.3 (BD Biosciences, Franklin Lakes, NJ, USA).
Enzyme-Linked Immunosorbent Assay for Plasma IL-2
Plasma for the analysis of interleukin-2 (IL-2) was collected on day 20 postinoculation. An enzyme-linked immunosorbent assay (ELISA) was performed using a Quantikine Rat IL-2 ELISA kit (R&D Systems Europe, Ltd, Abingdon, UK) and following the instruction manual. Each sample was measured in triplicate, and the OD values were measured at 450 nm using a Spectra Max M4 microplate reader (Molecular Devices).
Statistical Analysis
All data are expressed as the means ± standard error of the mean (SEM). The PWTs were analyzed using a 2-way analysis of covariance with repeated measures. To analyze the other data, the differences between the groups were compared with a 1-way analysis of variance followed by an least significant difference post hoc analysis. P < .05 was considered statistically significant.
Results
EA Stimulation Alleviated Mechanical Allodynia in BCP Rats and Its Analgesic Potency Was Less Than That of Morphine
PWTs to mechanical stimuli were significantly decreased by an intratibial inoculation of Walker 256 mammary gland carcinoma cells, as shown in Figure 1. Among 5 groups, the basal PWTs to the mechanical stimuli were not different (P > .05). However, the PWTs in the rats with implanted cancer cells were clearly lower than those of the Control group and the Sham BCP group (P < .01); the PWTs in the BCP group began to decrease at day 6 and remained low through at least day 20 postinoculation (the endpoint of this study). No significant changes were observed between the ipsilateral PWTs of the Control group and the Sham BCP group at any of the observed time points (P > .05). At D6 (Before EA), the ipsilateral PWTs of the BCP group, the BCP + EA group and the BCP + Mor group were significantly lower than those of the Control and Sham BCP groups (P < .05 or P < .01). After the treatments, the ipsilateral PWTs of the BCP + EA group were significantly higher than those of the BCP group (P < .01), and there was no difference between the BCP + EA group and the Sham BCP group or the Control group (P > .05). However, the ipsilateral PWTs of the BCP + Mor group increased dramatically after the morphine injection and were much higher than those of the other 4 groups at all subsequent time points (P < .01).
Figure 1.
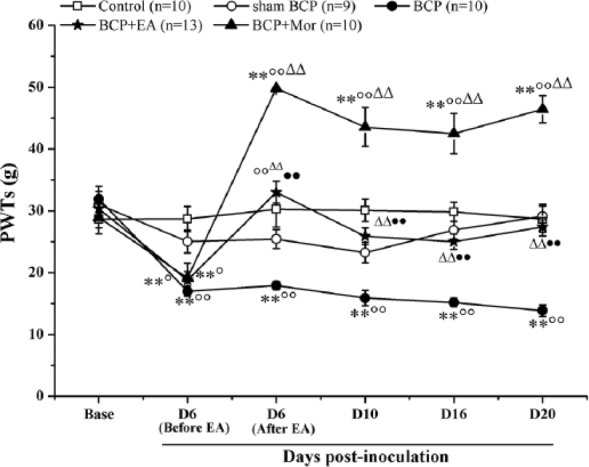
Electroacupuncture (EA) stimulation increased the mechanical pain threshold of rats with bone cancer pain (BCP). The effect of EA or morphine treatment on the ipsilateral paw withdrawal threshold (PWT), which represents the mechanical pain threshold, was measured preinoculation (Base) and on days 6 (Before EA and After EA), 10, 16, and 20 postinoculation. At D6, EA stimulation or a morphine injection were given immediately after completing the behavioral measurements. The ipsilateral PWTs at D6 (After EA) were measured 30 minutes after EA stimulation. The value at each point represents the mean ± SEM. *P < .05, **P < .01 versus Control; ○P < .05, ○○P < .01 versus Sham BCP; ∆∆P < .01 versus BCP; ●●P < .01 versus BCP + Mor.
EA Stimulation Increased Splenic Concanavalin A (Con A)–Induced T Cell Proliferation in BCP Rats
Splenic T cell proliferation was analyzed using a WST-8 assay. In the BCP model, there was a decline in splenic T cell proliferation, but there were no obvious differences between the BCP group and the Control group or the Sham BCP group (P > .05 each). Compared with the Sham BCP group, intraperitoneal morphine significantly inhibited Con A–induced T cell proliferation (158.01 ± 10.23 vs 115.73 ± 10.44, P < .05). EA stimulation enhanced the proliferation of splenic T lymphocytes by 33.1% compared with the BCP + Mor group (154.05 ± 11.34 vs 115.73 ± 10.44, P < .05) (Figure 2), which was similar to the Control and Sham BCP groups.
Figure 2.
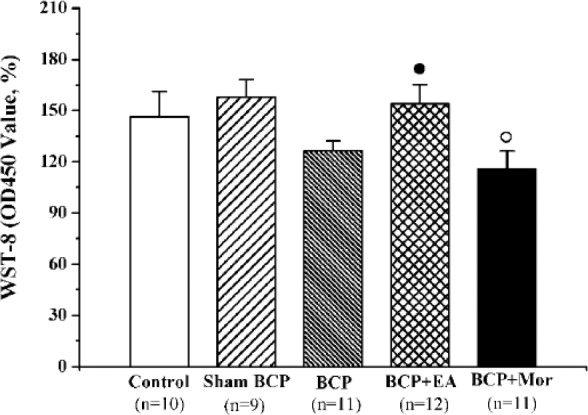
Electroacupuncture (EA) stimulation enhanced splenic concanavalin A (Con A)–induced T cell proliferation in rats with bone cancer pain (BCP). Splenic monocytes from each of the 5 different groups were cultivated on day 20 postinoculation, stimulated with 10 μg/mL Con A and compared with nonstimulated cells. The values are shown as the mean ± SEM of triplicate wells. ○P < .05 versus Sham BCP; ●P < .05 versus BCP + Mor.
EA Stimulation Increased the Plasma IL-2 Content of BCP Rats
IL-2, which is a T cell growth factor, is secreted by T lymphocytes and participates in the initiation and maintenance of T cell activation. Compared with the Control group, the plasma IL-2 concentrations of the BCP and BCP + Mor groups were significantly lower (452.50 ± 32.97 vs 368.64 ± 13.24, P < .01 and 380.09 ± 5.80, P < .05, respectively). Moreover, there were no significant differences between the plasma IL-2 content of the BCP + EA group and the Control and Sham BCP groups (P > .05). However, the plasma IL-2 content of the BCP + EA group was higher than those of the BCP and BCP + Mor groups (468.31 ± 17.50 vs 368.64 ± 13.24 and 380.09 ± 5.80, respectively, P < .01) (Figure 3).
Figure 3.
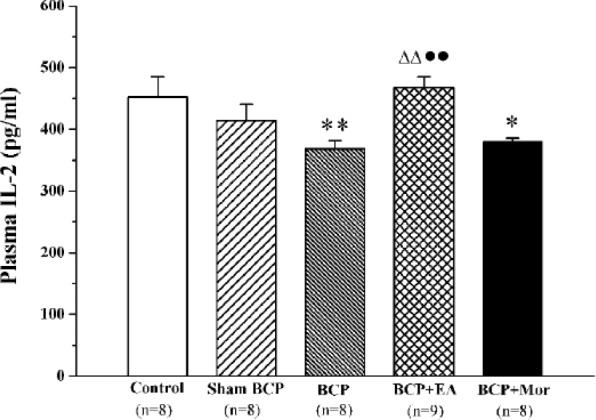
Electroacupuncture (EA) stimulation increased the plasma interleukin-2 (IL-2) content of rats with bone cancer pain (BCP). Plasma from each of the 5 different groups was collected on day 20 postinoculation, and the plasma IL-2 content was measured using ELISA. The values are shown as the mean ± SEM of duplicate wells. *P < .05, **P < .01 versus Control; ∆∆P < .01 versus BCP; ●●P < .01 versus BCP + Mor.
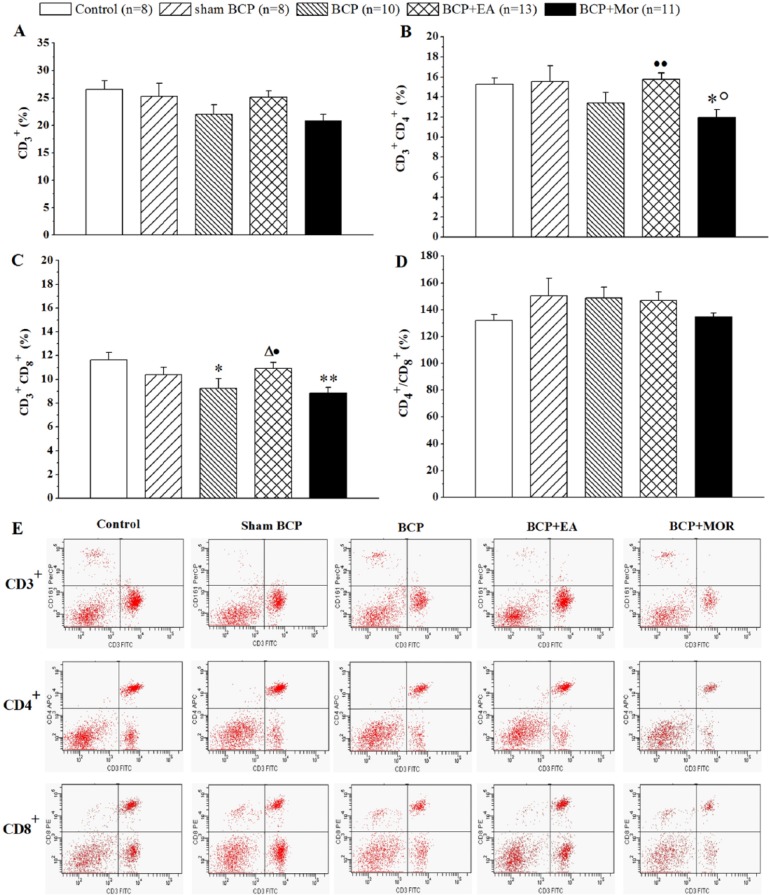
EA Stimulation Increased the Percentages of Splenic T Cell Subsets in BCP Rats, but Morphine Decreased It
To verify the effect of EA stimulation on peripheral T lymphocytes, the percentages of CD3+, CD3+CD4+ and CD3+CD8+ T cells in the spleen mononuclear cells were analyzed using flow cytometry. As shown in Figure 4, no significant difference in the percentage of splenic lymphocytes and their subsets was found between the Control and Sham BCP groups (P > .05). The percentage of splenic CD3+CD8+ T cell subsets was clearly lower in the BCP group compared with the Control groups (9.24 ± 0.81 vs 11.65 ± 0.61, P < .05). Intraperitoneal morphine significantly reduced the percentage of the CD3+CD4+ T cell subset compared with the Control and Sham BCP groups (11.96 ± 0.77 vs 15.29 ± 0.62 and 15.56 ± 1.54, respectively, both P < .05). Moreover, the percentage of the splenic CD3+CD8+ T cell subset in the BCP + Mor group was lower than that of the Control group (8.83 ± 0.49 vs 11.65 ± 0.61, P < .01). After EA stimulation, the percentage of the splenic CD3+CD8+ T cell subset was greater than those of both the BCP group and the BCP + Mor group (10.91 ± 0.50 vs 9.24 ± 0.81 and 8.83 ± 0.49, respectively, P < .05 each). EA stimulation also increased the percentage of the CD3+CD4+ T cell subset compared with that of the BCP + Mor group (15.75 ± 0.66 vs 11.96 ± 0.77, P < .01). Although the same tendency in the percentage of the CD3+ T cell subset was observed among the groups, neither the CD3+ T cell percentages nor the CD4+/CD8+ ratio showed a significant difference (P > .05).
Figure 4.
Electroacupuncture (EA) stimulation increased the percentages of the splenic CD4+ and CD8+ T cell subsets. On day 20 postinoculation, the spleens from the rats in each of the 5 groups were collected, and the spleen mononuclear cells were prepared. (A-D) The histograms show the differences in the percentages of the CD3+ (A), CD3+CD4+ (B), CD3+CD8+ T cells (C), and CD4+/CD8+ cells (D) in the splenic monocytes in the 5 groups. The values are shown as the mean ± SEM of duplicate wells. *P < .05, **P < .01 versus Control; ○P < .05 versus Sham BCP; ∆P < .05 versus BCP; ●P < .05, ●●P < .01 versus BCP + Mor. (E) Representative quadrant dot-plots of the splenic T cell subsets are shown.
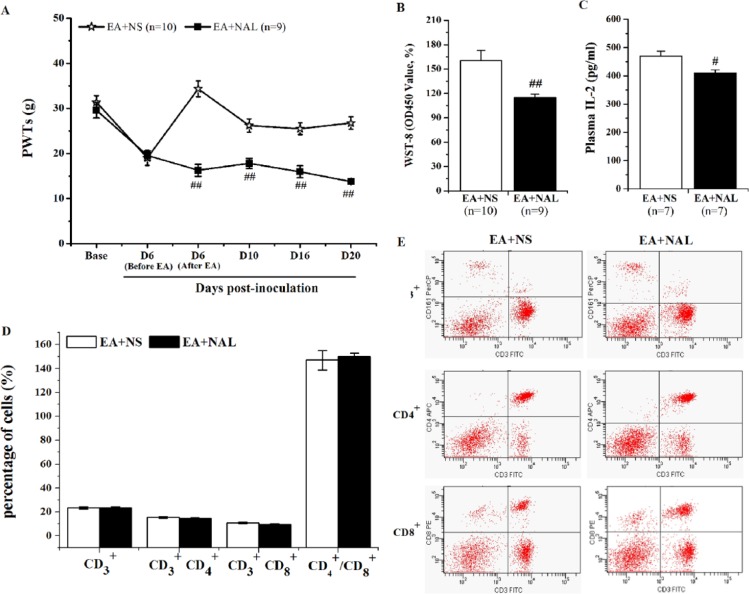
Both the Analgesic Effect and the Partial Immunoenhancement of EA Were Suppressed by an Intraperitoneal Naloxone Injection
Although it is widely known that the opioid receptor is involved in the analgesic effect of EA, it is not known how the receptor contributes to the immune enhancement of EA. To elucidate this, the opioid receptor antagonist naloxone was delivered systematically before each EA treatment, and then the cellular immunity function was measured. Figure 5A illustrates that the PWTs of the EA + NAL group decreased significantly compared with those of the EA + NS group (P < .01). Furthermore, there was a significant decline in splenic Con A–induced T cell proliferation and plasma IL-2 content in the EA + NAL group (160.20 ± 12.45 vs 115.23 ± 4.08, P < .01 and 470.02 ± 16.94 vs 410.33 ±10.86, P < .05, Figure 5B and C). However, the percentage of the splenic T cell subsets and CD4+/CD8+ did not differ between the 2 groups (P > .05, Figure 5D and E).
Figure 5.
Intraperitoneal injection of naloxone reversed the analgesia of electroacupuncture (EA) and partially antagonized its immunoenhancement. Naloxone (NAL, 0.3 mg/kg) or a sterile normal saline (NS) solution was injected intraperitoneally 30 minutes prior to EA stimulation. Both the drug injection and the EA stimulation were given once every other day. (A) The analgesia of EA was reversed by an intraperitoneal injection of NAL. (B, C) An intraperitoneal injection of NAL significantly reduced splenic Con A–induced T lymphocyte proliferation and plasma IL-2 content, which were measured using a WST-8 assay and an ELISA, respectively. (D) The percentages of CD3+, CD3+CD4+, CD3+CD8+ T cell subsets, and CD4+/ CD8+ in the splenocytes were not obviously changed after the naloxone treatment. (E) Representative quadrant dot-plots of splenic T cell subsets in the EA + NS and EA + NAL groups are shown. All data are shown as the mean ± SEM. #P < .05, ##P < .01 versus EA + NS.
Discussion
EA has been used worldwide as a complementary and alternative method for treating chronic pain. In recent years, research has increasingly focused on the analgesic effect of EA on cancer pain. Because of a lack of sufficient clinical evidence, it is still unclear whether acupuncture is effective for treating cancer pain in adults.25 Contrary to the result of clinical study, a few evidences show that EA alleviates hyperalgesia and allodynia in animal models of cancer pain, which was induced by an intratibial injection of Walker 256 mammary gland carcinoma cells26 or AT-3.1 prostate cancer cells15,16 and by an intraplantar injection of B16-BL6 melanoma cells.14 However, the analgesic potency of EA on bone cancer pain has not been exactly evaluated. One of the main aims of this study was to evaluate that of EA on BCP using morphine as a control. In the present study, the bone cancer pain model was established by implanting Walker 256 mammary carcinoma cells into the cavity of the tibia, which had been reported to be one of the most frequently used models of bone cancer pain.27 Consistent with a previous report,28 the results in this study showed that an intra-tibial injection of Walker 256 mammary carcinoma cells obviously decreased PWTs from day 6 until day 20 postinoculation. The present study also demonstrated that either EA stimulation or a morphine injection significantly increased the pain thresholds of tumor-bearing rats. This is supported by reports of Zhang et al15,16 that EA attenuated the hyperalgesia induced by an intratibial injection of AT-3.1 prostate cancer cells. We further compared the difference in the analgesic effect between EA and strong opioid analgesics using morphine. The data from this study demonstrated that morphine has a strong analgesic effect and that EA has a comparatively weaker analgesic potency. Moreover, our previous study had focused on the relationship between the analgesic effect and EA’s parameters, and revealed that EA’s analgesic potency on bone cancer pain might not related to the current frequency (2 Hz, 100 Hz or 2/100 Hz) or to the treatment frequency (once a day or once every other day).29 Han has reported that 2 Hz EA accelerates the release of enkephalin, β-endorphin and endomorphin, while 100 Hz EA selectively increases the release of dynorphin. A combination of the two frequencies produces a simultaneous release of all four opioid peptides, resulting in a maximal therapeutic effect.20 Because of these reasons, in this study, we choose a 2 Hz and 100 Hz alternative EA stimulation that was performed once every other day.
In addition to its analgesic properties, morphine is known to have immunosuppressive effects, which increases the risk of opportunistic infections.30 In the present study, the immunosuppressive effect of morphine has also observed. Our data showed that morphine inhibited Con A–induced T cell proliferation and reduced plasma IL-2 levels in BCP rats, especially compared with the Control or Sham BCP rats. Moreover, morphine treatment reduced the percentages of the T lymphocyte subpopulations in the spleen. These results were similar to previous reports that demonstrated that morphine significantly decreased the production of IL-2,31,32 impaired mitogen-stimulated lymphocyte proliferation32,33 and induced the depletion of CD4+ and CD8+ T cells in the spleen.34 It had been further reported that morphine depleted all the major peripheral T cell subsets to the same extent, including the naive, central memory, and effector memory subsets.34 Besides this, we also observed that some cellular immune functions, such as the IL-2 content and the percentage of CD3+CD8+ T cells, are also slightly suppressed by modeling bone cancer pain. A previous study reported that pain had an immunosuppressive effect,35 and surgery was widely known to cause immune suppression.36 Although morphine-induced immunosuppression had been illustrated by many previous in vivo and in vitro experiments,4,31-33 no significant difference in immune function was found between the BCP group and the BCP + Mor group in the present study. This paradox may result from different therapeutic frequency and diverse administration of morphine. Morphine was injected intraperitoneally once every other day in this study; however, previous data showed subcutaneous injection of morphine for 7 consecutive days led to morphine-induced immunosuppression.4
EA is generally known for its immunomodulatory effects aside from its analgesic effect.37 Cabioglu and Cetin37 summarized the immunomodulatory effects of EA into 3 categories: local, neuronal, and neurohumoral immunomodulation. Moreover, immune molecules and immune cells are the main components participate in the neurohumoral immunomodulation of EA.37 Previous evidence demonstrated that EA increased the induction of IL-2 production of spleen lymphocytes from injured trauma rats and could improve trauma stress–induced immunosuppression.38 Lai et al39 reported that EA at Zusanli (ST36), Hegu (LI4), and Sanyinjiao (SP6) significantly increased the content of CD4+ and CD4+/CD8+ in liver cancer, gastric cancer, and the hypodermic tumor rat model with implantation of the Walker 256 cell strain. Gao et al40 reported that the CD4+/CD8+ T cell ratio as well as plasma IL-2, IL-1β, interferon-γ concentrations were markedly downregulated due to chronic constriction injury–induced pathogenic pain and returned to normal level 12 days following EA. In the current study, EA enhanced plasma IL-2 content and the percentage of CD3+CD8+ T cells which were decreased by BCP model. Furthermore, compared with morphine, EA stimulation enhanced Con A–induced T cell proliferation and resulted in higher CD3+CD4+ and CD3+CD8+ T cell populations in the spleen on day 20 postinoculation (equivalent to day 14 following EA). However, there was no significant increase in the percentage of CD3+ T cells or in the CD4+/CD8+ ratio between EA stimulation and morphine injection. This discrepancy may result from not only different pathological status of the pain but also different observed timepoints.
The issue of the neurohumoral mechanism of EA on immune function is still obscure and complicated. It was reported that EA regulated the balance between Th1 and Th2 cytokines in splenic T cells in part through the ERK1/2, p38, NF-κB, and AP-1 pathways.36 Since endogenous opioid receptors had been found on immune cells, such as B lymphocytes, T lymphocytes, natural killer cells, granulocytes, and monocytes, it was regarded that the immunomodulatory effect of acupuncture or EA might be related with the opioid system.37 Accumulating evidence showed that EA increases the release of central and peripheral opioid peptides and also enhances the activity and expression of their receptor, which contribute primarily to EA analgesia.19-21,23 However, it was still not known whether EA modulated the immune system of bone cancer pain in rats via the opioid system. To clarify this relationship, we intervened with the opioid receptor using the antagonist naloxone to test its effect on the analgesia and immunomodulation of EA. Our data demonstrated that an intraperitoneal injection of naloxone (0.3 mg/kg) not only significantly reversed the analgesia of EA but also reduced the Con A–induced T cell proliferation and plasma IL-2 levels that had been enhanced by EA. Nevertheless, naloxone did not significantly change the percentages of the T cell subgroups, such as the CD3+, CD3+CD4+, and CD3+CD8+ T cell populations. These results suggested that the mechanism of immunomodulation by EA may in part involve the opioid system. We speculate that the differential effects of morphine and EA on cellular immunity may result from different sources of opioid peptides. The opioid peptide released by EA stimulation is homogenous, but the plant-extracted morphine is heterogenous. In pervious experiment, we had observed a similar phenomenon between recombinant rat β-endorphin (50 μg/kg, intraperitoneally) and morphine (10 mg/kg, intraperitoneally).41 Transplantation of in vitro–differentiated β-endorphin cells into the paraventricular nucleus improved natural killer cell cytolytic function in immune-deficient fetal alcohol–exposed rats.42 A review also reported that enhancement of endogenous levels of β-endorphin results in increased peripheral natural killer cell and macrophage activities, elevated levels of anti-inflammatory cytokines, and reduced levels of inflammatory cytokines.43
To summarize, the findings of this study demonstrated that mechanical allodynia of BCP rats were alleviated by both electroacupuncture and systematic morphine treatment, and the latter has a greater analgesic potency. However, EA increased Con A–induced T lymphocyte proliferation and plasma IL-2 levels as well as the percentages of CD3+CD4+ and CD3+CD8+ T cells in splenic monocytes which were suppressed by morphine or BCP surgery. This study also found that both the analgesic effect and the enhanced T lymphocyte function of EA were reversed by an intraperitoneal injection of naloxone, rather than T cell differentiation. These findings provided preliminary evidence that EA has dual effects of analgesia and immunomodulation in treating bone cancer pain. In addition, the peripheral opioid system may contribute not only to the analgesic effect of EA but also to its partial immunomodulatory effect. Nevertheless, several uncertainties should be investigated in the future; for instance, whether EA exerts its immunomodulatory effect by upregulating the expression of opioid receptors located on immune cells or by increasing the release of opioid peptide, how EA can modulate the percentage of splenic T cells and the different roles of opioid peptide played in EA’s analgesia and immunomodulation. Thus, further studies should be carried out to explore the underlying mechanism of EA’s immunomodulation in the future.
Conclusions
EA could significantly alleviated BCP-induced mechanical allodynia. Although the analgesic effect of EA was weaker than that of morphine, EA had a significant immunomodulation effect on cellular immunity. Both analgesic and immunomodulatory effect of EA might share the same mechanism via the opioid-mediated pathway, which need to be further investigated.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 81102643, 81674061), the Zhejiang Provincial Natural Science Found of China (No. LY14H270016), the China Postdoctoral Science Foundation (No. 2014M550334), and the Foundation of the Zhejiang Health Committee (No. 2014KYA162).
References
- 1. Colson J, Koyyalagunta D, Falco FJ, Manchikanti L. A systematic review of observational studies on the effectiveness of opioid therapy for cancer pain. Pain Physician. 2011;14:E85-E102. [PubMed] [Google Scholar]
- 2. Wei G, Moss J, Yuan CS. Opioid-induced immunosuppression: is it centrally mediated or peripherally mediated? Biochem Pharmacol. 2003;65:1761-1766. [DOI] [PubMed] [Google Scholar]
- 3. Bosshart H. Morphine-mediated suppression of phagocytosis. Int Immunopharmacol. 2010;10:264-265. [DOI] [PubMed] [Google Scholar]
- 4. Tsai YC, Won SJ, Lin MT. Effects of morphine on immune response in rats with sciatic constriction injury. Pain. 2000;88:155-160. [DOI] [PubMed] [Google Scholar]
- 5. Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20(suppl 1):s9-s15. [PubMed] [Google Scholar]
- 6. Zou W, Guo Q, Wang E, Cai J, Cheng Z. Intrathecal morphine suppresses immune function in rats with inflammatory-induced pain. J Int Med Res. 2007;35:626-636. [DOI] [PubMed] [Google Scholar]
- 7. Alonzo NC, Bayer BM. Opioids, immunology, and host defenses of intravenous drug abusers. Infect Dis Clin North Am. 2002;16:553-569. [DOI] [PubMed] [Google Scholar]
- 8. Fang JQ, Du JY, Liang Y, Fang JF. Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats. Mol Pain. 2013;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du JY, Fang JQ, Liang Y, Fang JF. Electroacupuncture attenuates mechanical allodynia by suppressing the spinal JNK1/2 pathway in a rat model of inflammatory pain. Brain Res Bull. 2014;108:27-36. [DOI] [PubMed] [Google Scholar]
- 10. Liang Y, Fang JQ, Du JY, Fang JF. Effect of electroacupuncture on activation of p38MAPK in spinal dorsal horn in rats with complete Freund’s adjuvant-induced inflammatory pain. Evid Based Complement Alternat Med. 2012;2012:568273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang YL, Yin XH, Shen YF, He XF, Fang JQ. Low frequency electroacupuncture alleviated spinal nerve ligation induced mechanical allodynia by inhibiting TRPV1 upregulation in ipsilateral undamaged dorsal root ganglia in rats. Evid Based Complement Alternat Med. 2013;2013:170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang Y, Du JY, Qiu YJ, Fang JF, Liu J, Fang JQ. Electroacupuncture attenuates spinal nerve ligation-induced microglial activation mediated by p38 mitogen-activated protein kinase. Chin J Integr Med. 2016;22:704-713. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Z, Wang C, Gu G, et al. The effects of electroacupuncture at the ST36 (Zusanli) acupoint on cancer pain and transient receptor potential vanilloid subfamily 1 expression in Walker 256 tumor-bearing rats. Anesth Analg. 2012;114:879-885. [DOI] [PubMed] [Google Scholar]
- 14. Mao-Ying QL, Cui KM, Liu Q, et al. Stage-dependent analgesia of electro-acupuncture in a mouse model of cutaneous cancer pain. Eur J Pain. 2006;10:689-694. [DOI] [PubMed] [Google Scholar]
- 15. Zhang RX, Li A, Liu B, et al. Electroacupuncture attenuates bone-cancer-induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur J Pain. 2008;12:870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang RX, Li A, Liu B, et al. Electroacupuncture attenuates bone cancer pain and inhibits spinal interleukin-1β expression in a rat model. Anesth Analg. 2007;105:1482-1488. [DOI] [PubMed] [Google Scholar]
- 17. Choi TY, Lee MS, Kim TH, Zaslawski C, Ernst E. Acupuncture for the treatment of cancer pain: a systematic review of randomised clinical trials. Support Care Cancer. 2012;20:1147-1158. [DOI] [PubMed] [Google Scholar]
- 18. Ninkovic J, Roy S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids. 2013;45:9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang S-M, Kain ZN, White P. Acupuncture analgesia: I. The scientific basis. Anesth Analg. 2008;106:602-610. [DOI] [PubMed] [Google Scholar]
- 20. Han J-S. Acupuncture and endorphins. Neurosci Lett. 2004;361:258-261. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Hackel D, Peng F, Rittner HL. Long-term antinociception by electroacupuncture is mediated via peripheral opioid receptors in free-moving rats with inflammatory hyperalgesia. Eur J Pain. 2013;17:1447-1457. [DOI] [PubMed] [Google Scholar]
- 22. Taguchi R, Taguchi T, Kitakoji H. Involvement of peripheral opioid receptors in electroacupuncture analgesia for carrageenan-induced hyperalgesia. Brain Res. 2010;1355:97-103. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Gehringer R, Mousa SA, Hackel D, Brack A, Rittner HL. CXCL10 controls inflammatory pain via opioid peptide-containing macrophages in electroacupuncture. PLoS One. 2014;9:e94696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang XW, Hu S, Mao-Ying QL, et al. Activation of c-jun N-terminal kinase in spinal cord contributes to breast cancer induced bone pain in rats. Mol Brain. 2012;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paley CA, Johnson MI, Tashani OA, Bagnall AM. Acupuncture for cancer pain in adults. Cochrane Database Syst Rev. 2015;10:CD007753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao-Ying QL, Ren DH, Mi WL, Liu Q, Wang YQ. Analgesic effects of electroacupuncture combined with Celebrex on rats with tibial cancer pain. J Chin Integr Med. 2008;6:830-835. [DOI] [PubMed] [Google Scholar]
- 27. Ryu HK, Baek YH, Park YC, Seo BK. Current studies of acupuncture in cancer-induced bone pain animal models. Evid Based Complement Alternat Med. 2014;2014:191347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mao-Ying Q-L, Zhao J, Dong Z-Q, et al. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun. 2006;345:1292-1298. [DOI] [PubMed] [Google Scholar]
- 29. Du J, Fang J, Chen Y, Wu S, Liang Y, Fang J. Parametric optimization of electroacupuncture against bone-cancer pain in rats and its intervention on mRNA expression of opioid receptor and precursor [in Chinese]. Zhongguo Zhen Jiu. 2015;35:161-168. [PubMed] [Google Scholar]
- 30. Nguyen T, Kramer J, Vallejo R, et al. Citalopram enhances B cell numbers in a murine model of morphine-induced immunosuppression. Pain Pract. 2009;9:195-205. [DOI] [PubMed] [Google Scholar]
- 31. Pacifici R, di Carlo S, Bacosi A, Pichini S, Zuccaro P. Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int J Immunopharmacol. 2000;22:603-614. [DOI] [PubMed] [Google Scholar]
- 32. Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. Morphine modulates lymph node-derived T lymphocyte function: role of caspase-3, -8, and nitric oxide. J Leukoc Biol. 2001;70:527-536. [PubMed] [Google Scholar]
- 33. Roy S, Chapin RB, Cain KJ, Charboneau RG, Ramakrishnan S, Barke RA. Morphine inhibits transcriptional activation of IL-2 in mouse thymocytes. Cell Immunol. 1997;179:1-9. [DOI] [PubMed] [Google Scholar]
- 34. Zhang EY, Xiong J, Parker BL, et al. Depletion and recovery of lymphoid subsets following morphine administration. Br J Pharmacol. 2011;164:1829-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Page GG. The immune-suppressive effects of pain. Adv Exp Med Biol. 2003;521:117-122. [PubMed] [Google Scholar]
- 36. Wang K, Wu H, Wang G, Li M, Zhang Z, Gu G. The effects of electroacupuncture on TH1/TH2 cytokine mRNA expression and mitogen-activated protein kinase signaling pathways in the splenic T cells of traumatized rats. Anesth Analg. 2009;109:1666-1673. [DOI] [PubMed] [Google Scholar]
- 37. Cabioglu MT, Cetin BE. Acupuncture and immunomodulation. Am J Chin Med. 2008;36:25-36. [DOI] [PubMed] [Google Scholar]
- 38. Cheng XD, Wu GC, He QZ, Cao XD. Effect of continued electroacupuncture on induction of interleukin-2 production of spleen lymphocytes from the injured rats. Acupunct Electrother Res. 1997;22:1-8. [DOI] [PubMed] [Google Scholar]
- 39. Lai M, Wang SM, Zhang WL, et al. Effects of electroacupuncture on tumor growth and immune function in the Walker-256 model rat [in Chinese]. Zhongguo Zhen Jiu. 2008;28(8):607-609. [PubMed] [Google Scholar]
- 40. Gao YH, Wang JY, Qiao LN, et al. NK cells mediate the cumulative analgesic effect of electroacupuncture in a rat model of neuropathic pain. BMC Complement Altern Med. 2014;14:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Du JY, Liang Y, Fang JF, et al. Effect of systemic injection of heterogenous and homogenous opioids on peripheral cellular immune response in rats with bone cancer pain: a comparative study. Exp Ther Med. 2016;12:2568-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boyadjieva NI, Ortiguela M, Arjona A, Cheng X, Sarkar DK. β-Endorphin neuronal cell transplant reduces corticotropin releasing hormone hyperresponse to lipopolysaccharide and eliminates natural killer cell functional deficiencies in fetal alcohol exposed rats. Alcohol Clin Exp Res. 2009;33:931-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarkar DK, Murugan S, Zhang C, Boyadjieva N. Regulation of cancer progression by β-endorphin neuron. Cancer Res. 2012;72:836-840. [DOI] [PMC free article] [PubMed] [Google Scholar]