Abstract
Introduction: Conventional cancer treatment, including surgery, chemotherapy, and radiotherapy, may not be sufficient to eradicate all malignant cells and prevent recurrence. Intensive treatment often leads to a depressed immune system, drug resistance, and toxicity, hampering the treatment outcomes. BioBran/MGN-3 Arabinoxylan is a standardized arabinoxylan concentrate which has been proposed as a plant-based immunomodulator that can restore the tumor-induced disturbance of the natural immune system, including natural killer cell activity to fight cancer, complementing conventional therapies. Objectives: To comprehensively review the available evidence on the effects and efficacies of MGN-3 as a complementary therapy for conventional cancer treatment. Methods: Systematic search of journal databases and gray literature for primary studies reporting the effects of MGN-3 on cancer and cancer treatment. Results: Thirty full-text articles and 2 conference abstracts were included in this review. MGN-3 has been shown to possess immunomodulating anticancer effects and can work synergistically with chemotherapeutic agents, in vitro. In murine models, MGN-3 has been shown to act against carcinogenic agents, and inhibit tumor growth, either by itself or in combination with other anticancer compounds. Fourteen successful MGN-3 treated clinical cases were found. Eleven clinical studies, including 5 nonrandomized, pre-post intervention studies and 6 randomized controlled trials (RCTs) were located. Reported effects include enhanced immunoprofile, reduced side effects, improved treatment outcomes; one RCT established significantly increased survival rates. There are no reports on adverse events on MGN-3. Most of the clinical trials are small studies with short duration. Conclusion: There is sufficient evidence suggesting MGN-3 to be an effective immunomodulator that can complement conventional cancer treatment. However, more well-designed RCTs on MGN-3 are needed to strengthen the evidence base.
Keywords: MGN-3, BioBran, Arabinoxylan, Ribraxx, systematic review, cancer, complementary medicine, chemotherapy, chemoprotective, immunomodulation, immunotherapy, adjunct therapy, rice bran, adenocarcinoma, carcinogenesis
Introduction
Cancer is a leading cause of death worldwide with an estimated 14.1 million new cases and 8.2 million cancer deaths occurring worldwide in 2012.1 Conventional cancer treatment, including surgery, chemotherapy, and radiotherapy, which focuses on eliminating cancer cells, can be effective in the short term.2 However, such approaches are insufficient to eradicate all malignant cells, especially in advanced cancer, resulting in recurrence. Repeated cycles of such intensive treatment can suppress the immune system, promote chemotherapy- and radiotherapy-resistant tumors, as well as local and systemic toxicity.3 Immunotherapy, which uses nonchemical, biological substances called biological response modifiers (BRMs) or immunomodulators to induce, boost, or restore the body’s natural defense capability to fight cancer,4 is now being acknowledged as an important cancer treatment strategy that can work in combination with conventional therapies.5,6
BioBran/MGN-3 Arabinoxylan (MGN-3) is a natural blend of hemicelluloses derived from partially hydrolyzed rice bran with shiitake mushroom enzymes (Lentinus edodes mycelia extract). It is developed and manufactured in Japan by Daiwa Pharmaceutical Co, Ltd, and marketed worldwide as a nontoxic food supplement under different brand names such as BioBran (Globally), Lentin Plus (Japan/Asia), Ribraxx (Australia/New Zealand), BRM4 (United States), and others.7 The main chemical structure of MGN-3 is an arabinoxylan with a xylose in its main chain and an arabinose polymer in its side chain. The result of a methylation analysis suggests that a complex structure of heteropolysaccharide (arabinogalactan, arabinoxylan, arabinan, β-1,3:1,4-glucan) is behind the immunomodulatory mechanisms and antitumor activity of MGN-3.8 MGN-3 has been promoted as a plant-based BRM for enhancing the depleted immune system during and after conventional cancer treatment, claiming support from research findings and good clinical results.9
Objectives
To perform a comprehensive literature review of the available evidence on the effects and efficacies of MGN-3 as a complementary therapy to support conventional cancer treatment.
Methods
Systematic searches were conducted using research databases including PubMed, ProQuest, MEDLINE, EBSCOhost (All), Cochrane CENTRAL, Embase, and EBM Review (All) without any restriction in year of publications. Keywords used include “MGN-3,” “BioBran,” “arabinoxylan,” “rice bran,” and “plant immunomodulators” in combination with “cancer” or “chemotherapy.” Additionally, the collected papers on MGN-3 published by BioBran Research Foundation,10 the official website of MGN-3 (https://biobran.org), the English language website of Daiwa Pharmaceutical (http://www.daiwa-pharm.com/english), and the references of included articles were searched manually. The criteria for inclusion were (1) primary research reports published in English inclusive of in vitro, in vivo, clinical study, and case reports and (2) research studies focused on the effects of MGN-3 on any form of cancer alone or as a combination therapy, or the combined effects of MGN-3 with one or more chemotherapeutic agents, or the effects of MGN-3 on cancer patients.
Results
Search Results
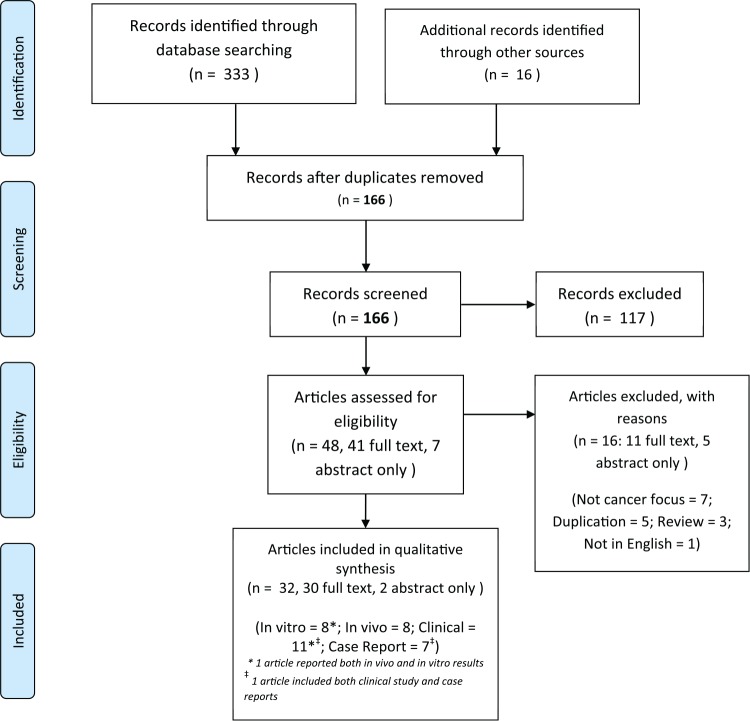
Systematic searches were conducted by 2 authors (DM and SLO) independently between March to May 2017. The searches yielded 166 unique records after duplications were removed. After initial screening, 48 articles were assessed for eligibility. Sixteen articles were excluded with reasons as follows: duplication (5),11-15 not cancer focused (7),16-22 not primary study (3),23-25 and 1 case report article published in Japanese was also excluded.26 Thirty full-text articles and 2 conference abstracts were included in this review, consisting of 7 in vitro studies;27-33 1 article reporting results from both in vitro and in vivo studies;34 7 in vivo studies;35-41 6 clinical case reports;42-47 and 11 clinical studies,48-58 with one of them also including case reports of selected follow-up patients.48 The literature search flow is summarized in Figure 1.
Figure 1.
Literature search flow diagram (based on PRISMA [Preferred Reporting Items for Systematic Reviews and Meta-Analyses] 2009).
In Vitro Studies
The characteristics of the in vitro studies are listed in Table 1. In these studies, both the immunomodulating and synergistic anticancer effects of MGN-3 were demonstrated.
Table 1.
Characteristics of the Included In Vitro Studies.
| No. | Study | Target Cell | MGN-3 (μg/mL) | Combined Agent | Duration | Outcomes |
|---|---|---|---|---|---|---|
| 1 | Ghoneum et al (2000)27–Abstract only | Squamous cell carcinoma (SCC13) | Not specified | Nil | 72 h | 30% decrease in cell number after 48 h and 50% decrease at 72 h of culture as compared with control. Increases in intracellular levels of IL-10 and 12, but not INF-γ |
| 2 | Ghoneum and Gollapudi (2003)28 | Human leukemic cells (HUT 78) | 100, 300, 1000 | Agonistic anti-CD95 antibody | 3-24 h | 200% increase in apoptotic cells pretreated with MGN-3 compared with control |
| 3 | Ghoneum and Gollapudi (2005)29 | Human breast cancer cells (BCC) MCF-7, ZR-75, HCC70 | 100, 500, 1000 | Saccharomyces cerevisiae at a cell to yeast ratio of 1:10 | 2 h | Dose-dependent effects of accelerated yeast phagocytosis by MCF-7 cells and enhanced apoptosis of cancer cells MCF-7 (2-fold), ZR-75 (2.5-fold), and HCC70 (1.8-fold) by MGN-3, compared with control |
| 4 | Ghoneum and Gollapudi (2005)30 | Monolayer BCC MCF-7 and nontumorigenic breast epithelial (MCF-10A) | 100 | S cerevisiae at a cell to yeast ratio of 1:10 | 1-4 h | MGN-3 increased the yeast-phagocytosis of MCF-7 cells by 2-fold, and enhanced yeast-induced apoptosis compared with control. No phagocytosis of yeast by MCF-10A cells observed |
| 5 | Gollapudi and Ghoneum, (2008)31 | Human BCC MCF-7 and HCC70 | 100, 500, 1000 | Daunorubicin (1 × 10−9 to 1 × 10−6 M) | 3 days | MGN-3 increased susceptibility of MCF-7 (5.5-fold) and HCC70 (2.5-fold) to daunorubicin. Increased accumulation of daunorubicin in the cancer cells were observed |
| 6 | Ghoneum and Gollapudi (2011)32 | Human multiple myeloma (U266) cells | 50 or 100 | Curcumin (2.5-10 μM) | 3 days | 87% decrease in cell number and a 2.6-fold increase in the percentage of apoptotic U266 cells by 100 μg/mL MGN-3 plus 10 μM curcumin. The effect was dose dependent |
| 7 | Ghoneum et al (2014)33 | Nonmetastatic human BCC (MCF-7) and metastatic murine BCC (4T1) | 100, 250, 500, 600, 750, 1000 | Paclitaxel (1 × 10−1 to 1 × 10−6 M) | 24, 48 h | MGN-3 increased the susceptibility of both types of cancer cells to paclitaxel by over 100-fold; achieved through DNA damage, enhanced apoptosis, and inhibition of cell proliferation in 4T1 cells |
| 8 | Pérez-Martínez et al (2015)34 | Erythroleukemia (K562); Jurkat T lymphoid leukemia; Ewing sarcoma (A673); neuroblastoma (NB1691) | 100 | NK cells | Overnight | MGN-3 stimulated NK cells induced a higher expression of the activation associated receptors CD25 and CD69; increased NK cell cytotoxic activity against all cell lines tested; and promoted NK cell expansion |
Immunomodulating Anticancer Effect
The growth of squamous cell carcinoma (SCC13) was arrested after incubating in MGN-3 for up to 72 hours.27 Tumor cell growth was arrested with the increase in apoptosis through the CD95 death receptor pathway. An increase of more than 200% in the rate of apoptosis of MGN-3 pretreated tumor cells (human T cell leukemic HUT 78) to the agonistic anti-CD95 antibody was observed. The effect was shown to be dose dependent.28 Compared with resting NK cells, MGN-3 stimulated NK cells induced a higher expression of the activation associated receptors CD25 and CD69. Statistically significant increases in cytotoxicity of MGN-3 stimulated NK cells against several cancer cells were consistently observed, but cytotoxicity was absent against normal cells.34
Synergistic Anticancer Effect
MGN-3 works synergistically with natural anticancer substances such as Saccharomyces cerevisiae (Baker’s yeast) and curcumin, as well as chemotherapy drugs (daunorubicin and paclitaxel) in a dose-dependent manner.
Ghoneum and Gollapudi29,30 confirmed the ability of MGN-3 to accelerate the phagocytosis of S cerevisiae in breast cancer and enhance the yeast-induced apoptosis of breast cancer cells (BCCs).29,30 After incubating different BCCs (MCF-7, ZR-75, HCC70) with heat-killed S cerevisiae and MGN-3, a 2-fold increase was observed in the attachment and uptake of yeast by the treated MCF-7 cells, compared with untreated cells, in a time-dependent manner.30 Treatment with MGN-3 also resulted in a 1.8- to 2.5-fold increase in the percentage of apoptosis in different BCC cell lines.29 The yeast-phagocytosis and apoptosis effect was not present in nontumorgenic breast cells.30
Culturing human multiple myeloma (MM) cell line U266 with MGN-3 (50 or 100 μg/mL) and curcumin (2.5-10 μM) for 3 days, Ghoneum and Gollapudi32 also showed that the proliferation of U266 cells was inhibited by MGN-3 alone or curcumin alone.32 However, an optimal synergistic effect was observed with a combination of 100 μg/mL MGN-3 plus 10 μM curcumin, characterized by an 87% decrease in U266 cell numbers and a 2.6-fold increase in the percentage of apoptotic U266 cells.32
The survival rates of BCCs (MCF-7 and HCC70 cells) cultured for 3 days with different concentrations of daunorubicin (1 × 10−9 to 1 × 10−6 M) with or without MGN-3 (100-1000 μg/mL) were measured.31 MGN-3 increased the accumulation of daunorubicin in the cancer cells and significantly decreased the cell survival of MCF-7 (by 5.5-fold) and HCC70 cells (by 2.5-fold) as compared to BCCs treated with daunorubicin alone.31 Through a similar experimental design in a later study, MGN-3 was demonstrated to sensitize non-metastatic human BCC MCF-7 and metastatic murine BCC 4TI to paclitaxel and to increase their susceptibility to the chemotherapeutic agent by over 100-fold. The synergistic effects include causing DNA damage, enhancing apoptosis, and inhibiting cell proliferation of BCCs.33
In Vivo Studies
The characteristics of the in vivo studies are listed in Table 2. Through various murine models, these studies showed that MGN-3 could potentially prevent cancer, inhibit its growth, and work synergistically with other chemotherapeutic agents.
Table 2.
Characteristics of the Included In Vivo Studies.
| No. | Study | Animal Model | MGN-3 Dosage | Combined Agent | Duration | Outcomes |
|---|---|---|---|---|---|---|
| 1 | Jacoby et al (2001)35 | Sprague-Dawley derived albino rats | Daily oral dosing of 5 or 50 mg/kg | 1 × intraperitoneal (IP) dose of cisplatin or doxorubicin on day 3 | 11 days | Rats receiving MGN-3 were healthier; gained weight and had a lower incidence of diarrhea and gross intestinal pathology compared with control |
| 2 | Endo and Kanbayashi (2003)36 | BALB/c female mice | 0.1 mL at 10 mg/mL in water or by IP daily 1 wk before cisplatin | Single shot of cisplatin (0.1 mL at the concentration of 15 mg/kg) | 28 days | MGN-3 (both orally and IP) showed accelerated protection against severe loss of body weight of mice due to cisplatin. The result was statistically significant |
| 3 | Badr El-Din et al (2008)37 | Female Swiss albino mice inoculated with Ehrlich ascites carcinoma (EAC) cells, bearing solid tumors | MGN-3 dissolved in 0.9% saline given via IP or intratumoral injections at 40 mg/kg body weight (BW) daily for 5 wk | Nil | 5 wk | MGN-3 significantly delayed growth in both tumor volume (63.27%) and tumor weight (45.2%) compared with control, through increased apoptosis of EAC cells (1.8-fold), influenced plasma cytokine production, downregulated immune suppressing cytokine IL-10, and increased NK cells activity. No adverse side effects due to MGN-3 treatment were observed |
| 4 | Noaman et al (2008)38 | Female Swiss albino mice inoculated with EAC cells, bearing solid tumors | MGN-3 dissolved in 0.9% saline given via IP at 25 mg/kg BW, 6 times a week from day 4 or day 11 after inoculation and end at day 25 | Nil | 25 days | MGN-3 suppressed the growth of tumors; normalized lipid peroxidation level, augmented glutathione contents, and enhanced antioxidant enzymes activity in blood, liver, and tumor tissue. More pronounced effects were observed when treated by MGN-3 as early as day 4 |
| 5 | Pérez-Martínez et al (2015)34 | NOD-scidIL-2Rgnull mice injected intravenously (IV) with NB-1691luc 2 × 105 neuroblastoma cells | 100 μg/mL overnight for activation of NK cells | NK cells (fresh or MGN-3 activated). NK cellular IV therapy began 7 days after tumor cells inoculation, twice a week for 4 wk | 4 wk | MGN-3 stimulated NK cells inhibited neuroblastoma growth and increased survival compared with control. The results were statistically significant |
| 6 | Badr El-Din et al (2016)39 | Male Wistar rats | 1 dose of 40 mg/kg BW via IP injection every other day a total of 8 mo | Orally administered carcinogenic MNNG (N-methyl-N′-nitro-N-nitrosoguanidine) at 200 mg/kg BW daily for 2 weeks | 8 mo | MGN-3 significantly lowered incidence of dysplasia and gastric cancer when combined with MNNG; effects observed include suppression of Ki-67 tumor marker, upregulation of apoptotic gastric cancer cells via mitochondrial-dependent pathway, and protection against decrease in lymphocyte levels |
| 7 | Badr El-Din et al (2016)40 | Female Swiss albino mice inoculated with EAC cells, bearing solid tumors | 1 dose of 40 mg/kg BW via IP injection every other day starting from day 8 until day 30 | Paclitaxel at a dose of 2 mg/kg BW every other day starting from day 8 until day 30 | 30 days | MGN-3 plus paclitaxel significantly suppressed tumor volume (88%) compared with paclitaxel only (77%) or MGN-3 only (59%). Inhibition of tumor growth was associated with reduced cancer cell proliferation, increased DNA damage and apoptosis |
| 8 | Badr El-Din et al (2016)41–Abstract only | Male albino rats | 25 mg/kg BW 5 times/week IP for 2 wk prior to receiving carcinogens and continued for 20 wk | Carcinogenic NDEA (N-nitrosodiethylamine) (200 mg/kg BW) single dose IP, plus promoter CCl4 (3 mL/kg BW) weekly subcutaneously for 6 weeks | 20 wk | MGN-3 inhibited hepatocarcinogenesis induced by NDEA and CCI4 via induction of apoptosis and inhibition of cancer cell proliferation; maintained AST, ALT, ALP, and gamma-GT levels close to normal values |
Preventive Effect Against Carcinogenic Agents
Significantly lower incidences of dysplasia and gastric cancer were observed in male Wistar rats fed with carcinogen methylnitronitrosoguanidine (MNNG) plus MGN-3, compared with those administered MNNG alone.39 The preventive effects of MGN-3 observed include suppression of Ki-67 tumor marker, upregulation of apoptotic gastric cancer cells via the mitochondrial-dependent pathway, and protection against decrease in lymphocyte levels.39
Male albino rats pretreated with MGN-3 before receiving carcinogenic N-nitrosodiethylamine (NDEA) and carbon tetrachloride (CCl4) showed a significant reduction in liver tumor incidence, marked decrease in the percentage of preneoplastic foci in hepatic parenchyma, and inhibition in the development of hepatocellular carcinoma, compared with controls. MGN-3 treated rats were also able to maintain close to normal levels of hepatic diagnostic markers.41
Inhibition of Cancer Growth
Female Swiss albino mice bearing solid Ehrlich carcinoma (SEC) tumors were treated with intraperitoneal injections of MGN-3.37,38 Compared with controls, MGN-3-treated mice exhibited a significant delay in tumor growth measured by tumor volume (63.27%) and tumor weight (45.2%), without any observed adverse side effects due to the treatment.37 The anticancer mechanisms of MGN-3 were shown to be immunomodulating through upregulation of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), and downregulation of interleukin-10 (IL-10), as well as inducing apoptosis in the SEC cells.37 Elevated levels of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, catalase, and glutathione-S-transferase, in blood and liver of MGN-3-treated animals comparable to the normal levels were observed.38 The effects of MGN-3 treatment were more pronounced in mice that were treated earlier rather than later.
NOD-scidIL-2Rgnull mice inoculated with neuroblastoma cells were treated with intravenous NK cellular therapy using either fresh NK cells or NK cells activated with MGN-3 overnight. The mice treated with MGN-3 activated NK cells had upregulated NK cell activation markers, significant neuroblastoma growth inhibition, and higher survival rate compared to control groups.34
Synergistic Effects With Chemotherapeutic Agents
Female Swiss albino mice inoculated with Ehrlich ascites carcinoma cells were treated with paclitaxel only, MGN-3 only, or MGN-3 plus paclitaxel. Tumor volumes were significantly suppressed in MGN-3 plus paclitaxel group (88% smaller relative to controls), compared with paclitaxel only group (77%), and MGN-3 only group (59%).40
MGN-3 may also potentially protect against the adverse effects of chemotherapeutic agents. Sprague-Dawley derived albino rats fed with MGN-3 were healthier, gained weight, and had a lower incidence of diarrhea and gross intestinal pathology, after being administered a dose of either cisplatin or doxorubicin, compared with control.35 Similarly, MGN-3 given either orally or intraperitoneally to BALB/c female mice was shown to protect them from severe weight loss associated with the injection of cisplatin.36
Case Reports
The included case reports42-48 narrated a total of 14 successful MGN-3-treated clinical cases. The patient characteristics and outcomes are summarized in Table 3.
Table 3.
Patient Characteristics and Outcomes of the Included Clinical Case Reports.
| No. | Report | Patient, Age | Cancer | Conventional and Adjunct Treatment | MGN-3 Dosage | Outcomes |
|---|---|---|---|---|---|---|
| 1 | Ghoneum and Brown (1999)48 | Male, 39 y | Acute myelogenous leukemia | Chemotherapy | 3 g/d after chemotherapy | White blood cells (WBCs) count at 5.6 × 109/L after completion of chemotherapy and maintained. NK activity increased from 7.9 LUs to 113 LUs after 1 wk of MGN-3 and maintained at high level for 4 y at the point of reporting |
| Male, 52 y | Acute myelogenous leukemia | No conventional treatment | 3 g/d | WBCs count at 18.7 × 109/L pretreatments. Reduced to 11 × 109/L after 1 mo. Condition stable for 4 y at the point of reporting | ||
| Male, age unknown | Prostate cancer | Hormonal therapy | 3 g/d after hormonal therapy | Prostate-specific antigen (PSA) level at 0.1 after hormonal therapy. PSA level remained within normal range for 4 y at the point of reporting | ||
| Female, age unknown | Recurrence breast cancer | Surgery and chemotherapy | 3 g/d after completion of chemotherapy | NK cell activity increased 2-fold from baseline (16.4 LUs) after 1 wk. Further increased to 128 LUs and remained high 4 y at the point of reporting. No evidence of recurrence seen in computed tomography (CT) scans or biopsies | ||
| 2 | Kawai (2004)42 | Female, 64 y | Umbilical metastasis of recurrent colorectal cancer (Sister Mary Joseph’s Nodule [SMJN]) | 5-Fu 500 mg, Isovorin 250 mg (10A), Topotecin 40 mg administered once a week from 19th month onward | 3 g/d for first 18 mo; 6 g/d subsequently | Patient survived 2 y and 2 mo since detection (typical survival time: 2 wk to 11 mo) and was still alive at the time of reporting. MGN-3 helped to prolong life and improved quality of life |
| 3 | Kaketani (2004)43 | Male, 64 y | Terminal pancreatic cancer with distant metastasis | Oral anticancer drugs (Furtulon 1200 mg/time and Endoxan 200 mg/time) ACM π water (MRN-100A) 300 mL/d |
6 g/d | Initial prognosis was 3 mo of life. 5 mo after treatment, CT images showed pancreatic cancer to be unmeasurable and reduced liver metastasis similarly. Endoscopy 12 mo after treatment showed almost no abnormality. Patient survived 17 mo with a normal life and died of hematemesis not related to cancer |
| 4 | Okamura (2004) 44 | Male, 67 y | Liver cancer with intestinal metastasis | 1 y of treatment at another hospital previously Intravenous (IV) infusion of Bio-reproducing Protein (BRP) once every 4 wk |
3 g/d administered for 157 days | Initially pronounced to have 1 mo to live. The general condition, tumor markers, and immunocompetence improved after 2 y. Patient entered 7th year of treatment at the time of reporting living a normal working life and received treatment on outpatient basis |
| Male, 65 y | Liver cancer | Conventional treatment at 2 other medical institutions previously IV infusion of BRP once every 4 wk |
3 g/d for 72 days | Liver cancer marker and immunocompetence improved after 6 mo. Liver function markers raised initially and decreased at 1 y, with jaundice disappeared, appetite improved, no pain occurred through to 1 y 11 mo of treatment | ||
| Female, 71 y | Liver cancer | Initial treatment at another hospital with poor results IV infusion of BRP once every 4 wk |
3 g/d for 392 days | Hepatic tumor markers decreased after 6 mo of treatment. Clinical symptoms improved and jaundice disappeared | ||
| Female, 76 y | Lung cancer (adenocarcinoma spread through both lung fields) | Initial treatment at another hospital with no improvement IV infusion of BRP once every 4 wk |
3 g/d for 128 days | Lung tumor maker (tissue polypeptide antigeny [TPA]) decreased after 4 mo. Coughing decreased. Immunocompetence (TK activity) gradually improved | ||
| Male, 58 y | Colorectal cancer with liver metastasis | Underwent surgery at another hospital IV infusion of BRP once every 4 wk |
3 g/d for 77 days | Liver function (glutamic-pyruvic transaminase [GPT]) improved rapidly and immunocompetence (TK activity) stabilized after 1 mo of treatment. Patient continued to work with no subjective symptoms at the time of reporting | ||
| 5 | Markus et al (2006)45 | Male, 68 y | Metastatic hemangiopericytoma of the skin with multiple pulmonary nodules | Wide local excision for removal of the skin lesion Patient refused further conventional treatment for pulmonary nodules |
Self-administered with unknown dosage | Lung masses steadily decreased in size by serial imaging and became undetectable after 34 mo of MGN-3 therapy |
| 6 | Hajto et al (2015)46 | Female. 28 y | Left ovary sarcoma and endometrium sarcoma with a metastasis in the right ovary | Removal of left ovary followed with hysterectomy and adnexectomy Chemotherapy with a CYVADIC protocol commenced 4 wk after second surgery Started 0.75 ng/kg mistletoe lectin twice a week since 3rd cycle of chemotherapy |
45 mg/kg given twice a week started at 3rd cycle of chemotherapy | A rapid improvement of quality of life was observed after starting the immunotherapy (lectin + MGN-3). Patient was better able to tolerate the next 3 cycles of chemotherapy. At the time of reporting, the patient was tumor-free after 5 y |
| 7 | Hajto et al (2016)47 | Female, 74 y | Inoperable lung adenocarcinoma | 4 cycles carboplatin and paclitaxel. Second-line treatment with 75 mg/d erlotinib (Tarceva) given for 7 mo. 0.75 ng/kg mistletoe lectin given twice weekly | 45 mg/kg given twice a week | After a treatment for 7 mo, a nearly complete remission (CR) of the primary tumor and CR of all metastases was established. The quality of life was excellent and the patient was able to work 100% |
Most patients were older than 50 years with only 2 patients of younger age, and 2 patients with age unreported. Types of primary cancer in these cases include leukemia (2), prostate (1), breast (1), colorectal (2), pancreas (1), liver (3), lung (2), skin (1), and ovary (1). Most patients started taking MGN-3 either in conjunction with conventional cancer treatment, or after completion of conventional treatment. Dosages of MGN-3 reported in these cases were 3 g/d, 6 g/d, and 45 mg/kg per body weight (BW), administered orally. In some cases, MGN-3 was combined with other forms of complementary therapies, including ACM π water,43 Bio-reproducing Protein (BRP),44 and mistletoe lectin.46,47
In nonterminal cancer cases, reported results were: improvements in tumor markers, immunocompetence profile, and initial symptoms44; patient conditions were stable with no sign of cancer recurrence at follow-up48; and patients showed improved quality of life (QoL) reported as subjective improvements in sleep, appetite, digestion, physical activity and decrease in anxiety and pain; reduced adverse effects during chemotherapy, and cancer remission.46,47 In one self-treated case, the patient refused further conventional treatment for metastatic lung tumor after the initial removal of his skin lesion. The metastatic lung tumor became undetectable after 34 months of self-treatment with MGN-3.45 In 3 cases of terminal cancer with poor prognosis, the patients were reported to be able to survive much longer beyond the initial estimated lifespans with improved QoL, and even normal working lives.42-44
Clinical Studies
Eleven clinical studies were included in this review. Among the 11 clinical studies, 5 are nonrandomized, pre-post intervention studies,48,50,51,57,58 and 6 are randomized controlled trials (RCTs).49,52-56 The characteristics of these studies are summarized in Table 4 (nonrandomized studies) and Table 5 (RCTs).
Table 4.
Characteristics of the Included Nonrandomized Clinical Studies.
| No. | Study | N | Cancer Type | Duration | MGN-3 Dosage | Conventional and Adjunct Treatment | Outcome Measurement | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 1 | Ghoneum and Brown (1999)48 | 32 | Prostate, breast, multiple myeloma, leukemia | 2 wk Follow-up to 4 y |
Orally at 3 g/d | Most patients went through conventional treatment before study | NK cells activity; NK granularity; in vivo T and B cells proliferation; tumor-associated antigens (TAA) | Increased in NK cells activity (10×); NK cell granularity and binding capacity; T and B cells proliferation. Improved TAA measurements in selected patients. Follow-up results of 4 patients were reported |
| 2 | Tsunekawa (2004)50 | 16 | Various malignancies | 6 mo | Orally at 3 g/d | Patients went through surgery, irradiation therapy, and/or chemotherapy treatment before study | Body height and weight, leukocyte count and subsets, NK cell activity, tumor markers, adverse reactions, and interruptions | No subjective or objective adverse effects. Improvement in leukocyte count and subsets, increased and normalized NK cell activity after treatment |
| 3 | Lissoni et al (2008)51 | 22 | Various malignancies | 2 mo | Orally at 2 g/d 1st mo, and 1 g/d 2nd mo | Only supportive care for pain, vomit, nausea, and neoplastic cachexia | Total lymphocytes, T lymphocytes (CD3+, CD4+, CD8+), T helper (Th), T regulatory (Treg), NK cells | Non–statistically significant increase in the mean number of Th cells and decrease in Treg cell. Th/Treg mean ratio significantly enhanced |
| 4 | Golombick et al (2016)57 | 20 | Early B-cell lymphoid malignancies (monoclonal gammopathy of undetermined significance [MGUS], smoldering multiple myeloma [SMM], chronic lymphocytic leukemia [CLL]) | 6 mo | Orally at 2 g/d | 6 g/d of curcumin | FBC, paraprotein, free light chains/ratio, C-reactive protein (CRP) erythrocyte sedimentation rate (ESR) rate, B2 microglobulin, sIgGs, and surface leukocyte markers | Increased neutrophil count in 80% of MGUS/SMM patients. Reduction in raised ESR in 40% of the MGUS/SMM patients |
| 5 | Hajto et al (2016)58 | 35 | Various malignancies (mostly stage II-IV) | 6 mo | Orally between 12 and 45 mg/kg twice a week | Conventional oncologic therapy 0.5-1.0 ng/kg mistletoe lectin given twice a week |
Quality of life questionnaire (pain, anxiety, physical activity, appetite, sleep, digestion, side effect, self-perceived improvement) | Improvement of physical activity and decrease of side effects during conventional oncotherapy |
Table 5.
Characteristics of the Included Randomized Controlled Clinical Studies.
| No. | Study | No. of Patients | Cancer Type | Duration | MGN-3 Dosage | Conventional and Adjunct Treatment | Outcome Measurement | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 1 | Takahara and Sano (2004) 49 | 205 (MGN-3: 96, Control: 109) | Various malignancies | 18 mo | Orally at 3 g/d | Complementary and alternative therapy and anticancer drugs | NK cells activity, survival rate; custom quality of life scoring on pain, malaise, nausea, and appetite | MGN-3 group achieved higher survival rate and better appetite than control group |
| 2 | Bang et al (2010)52 | 68 (MGN-3: 38, Control: 30) | Hepatocellular carcinoma (stages I and II) | 3 y | Orally at 1 g/d for 12 mo during treatment | Transarterial oily chemoembolization (TOCE) or TOCE and percutaneous ethanol injection treatment (PEIT) | Overall response to treatment, α-fetoprotein (AFP) levels, tumor volume, recurrence, and survival | MGN-3 group showed lower recurrence, higher survival after 2nd year, and significantly lower AFP change compared with control |
| 3 | Cholujova et al (2013)53 | 48 (MGN-3: 32, Placebo: 16) | Multiple myeloma | 3 mo | Orally at 2 g/d | Alternating courses of chemotherapy: alkylating agents, anthracyclines, and glucocorticoids | Immunophenotypic analysis; NK cells activity; and cytokine profiles | Increased NK activity, myeloid DCs level, and augmented concentrations of Th cell type 1-related cytokines in MGN-3 group compared with placebo group |
| 4 | Masood et al (2013)54 | 50 (MGN-3: 25, Control: 25) | Breast cancer | 6 mo | Orally at 3 g/d 1 wk before and 1 wk after each chemotherapy cycle | 6 cycles of chemotherapy | Chemotherapy induced side effects (tiredness, anorexia, vomiting, and hair loss) | MGN-3 group experienced significant reduction in tiredness; increased appetite; no antiemetic needs; and less hair fall compared with control group |
| 5 | Itoh et al (2015)55 | 20 (MGN-3: 10, Placebo: 10) | Cervical cancer | 3 wk | Orally at 3 g/d during radiation therapy | Radiation therapy: combination of external-beam radiation therapy and brachytherapy Chemotherapy: cisplatin and 5-fluorouracil |
Gastrointestinal side effects of chemoradiotherapy (diarrhea, nausea, vomiting, loss of appetite, safety); WBC count; NK cell activity | MGN-3 group showed less diarrhea, less decrease in WBC count & less adverse events, but results were not significant due to lack of statistical power |
| 6 | Petrovics et al (2016)56 | 50 (MGN-3: 25, Control: 25) | Different malignancies with chronic fatigue syndrome | 6 mo | Orally at 3 g/d for 24 wk | Oncothermia for MGN-3 group only Chemo- or radiotherapy as routine care |
Quality of life score (EORTC QLQ-C3), pain score (visual analogue scale [VAS]), body pH level, blood abnormalities, ECG, blood test, fatigue scale (Chalder Fatigue Questionnaire [CFQ]) | MGN-3 group showed changes in body pH level to be less acidic. Average of CFQ score was significantly reduced in MGN-3 group compared with no change in control group |
Nonrandomized, Pre-post Intervention Studies
The immunomodulating effects of MGN-3 were examined by Ghoneum and Brown.48 Thirty-two cancer patients (various malignancies) with depressed NK cell activity post conventional cancer treatment were treated with MGN-3 for 2 weeks. A significant increase in NK cell activity up to tenfold was observed. Increase in NK cell granularity and binding capacity, improvement in T and B cell proliferation in vivo, and improvement in tumor-associated antigens were observed in a selected number of patients.48 Similarly, Tsunekawa50 reported normalization of NK cell activity and improvement in white blood cell (WBC) count in a small study of 16 cancer patients. These were patients with various malignancies who went through conventional cancer treatment before starting the MGN-3 therapy for 6 months. No subjective or objective adverse effects were recorded.
In another study, however, no substantial change in NK cell counts was observed in 22 cancer patients after taking MGN-3 for 2 months.51 Instead, an increase in the T helper (Th) lymphocytes to T regulatory (Treg) lymphocytes (CD4+ CD25+) ratio (Th:Treg) was recorded, demonstrating the effect of anticancer immunity restoration of the MGN-3 therapy. Nonetheless, due to the small number of patients, the results were not statistically significant.51 More recently, Golombick et al57 reported that the combination therapy of MGN-3 and curcumin helped 20 patients with early B-cell lymphoid malignancies to delay disease progression by increasing neutrophil count and reducing the raised erythrocyte sedimentation rate (ESR). No significant change in NK cell counts was detected. Again, results from this study lack statistical power.
The QoL improvement effects of MGN-3 were studied by Hajto et al.58 A total of 35 patients with various malignancies were administered a combination of mistletoe lectin and MGN-3 as a complementary therapy for a minimum of 6 months during or after conventional cancer treatment. Patients responded to a custom binary-response questionnaire with 8 questions regarding various aspects of their QoL after treatment, including pain, anxiety, physical activity, appetite, and sleep, reporting a subjective improvement of physical activity and decrease in side effects as the most important benefits of the complementary therapy.58
Randomized Controlled Studies
The QoL improvement effects of MGN-3 were previously studied in a large RCT with 205 progressive cancer patients in late stages (III-IV) of various malignancies.49 Checking patient QoL by observation and enquiry during the study using a custom grading scale, Takahara and Sano49 reported that, patients who received MGN-3 plus standard complementary and alternative therapy (CAT) achieved a higher survival rate (54.2% vs 35.8%) and a better appetite than the control group who received only standard CAT. There was no clear difference between groups in terms of percentage changes in increasing and decreasing NK activity, but the MGN-3 + CAT group had a higher percentage of patients with unchanged NK activity compared with the CAT only group.49 It was unclear whether this study was sufficiently blinded.
The RCT by Bang et al52 can be regarded as the first clinical evidence for the antitumor efficacy of MGN-3. The participants of this 3-year RCT consisted of 68 patients with liver cancers of stage I or II. A higher survival rate after the second year (35% vs 6.7%) and lower recurrence of the disease (31.6% vs 46.7%) for liver cancer patients taking MGN-3, compared with the controls, were the results of MGN-3 applied in conjunction with conventional cancer treatment in 38 patients.52 Thirty patients receiving only conventional therapies were assigned as controls. Significant decreases in the tumor marker level (α-fetoprotein [AFP]), and tumor volumes, as compared with baseline, were also observed in the MGN-3 group whereas the control group showed no significant changes in either AFP or tumor volume.52
MGN-3 was shown to reduce chemotherapy-induced side effects among breast cancer patients who were undergoing 6 cycles of chemotherapy in another RCT with 50 patients.54 MGN-3 was given to 25 patients 1 week before and 1 week after each cycle of chemotherapy, with the control group receiving only chemotherapy. Side effects were assessed by a custom questionnaire given to patients at the start of each cycle. Significant reductions in tiredness, increased appetite, no anti-emetic needs, and reduced hair loss compared with the control group were reported.54
In cervical cancer patients undergoing chemoradiotherapy, MGN-3 was shown to be better than placebo in reducing the diarrheal side effect in a clinical trial reported by Itoh et al., using a custom symptom scoring system.55 Adverse events were graded by the National Cancer Institute scale (Common Terminology Criteria for Adverse Events [CTCAE] v3.0). Only 2 patients in the MGN-3 group developed grade 2 (moderate) events during chemotherapy, with the control group tending to develop adverse events of higher grades than did the MGN-3 group.55 Patients taking MGN-3 also showed less decrease in WBC count. However, the results from this pilot trial with only 20 patients were not significant due to the lack of statistical power.55
For patients with chronic fatigue syndrome (CFS) due to cancer or cancer treatment, MGN-3 plus Oncothermia was shown to be able to reduce the CFS symptoms in an RCT with 50 CFS patients diagnosed with various malignancies.56 The 25 patients who received 3 g/d of MGN-3 and Oncothermia once a week had significantly lower mean scores of Chalder Fatigue Questionnaire (CFQ) compared with baseline after 6 months of treatment. No significant change in mean CFQ scores was recorded in the control group who received only conventional chemo or radiotherapy treatment.56
The effects of MGN-3 on innate immunity were studied by Cholujova et al53 in an RCT with 48 multiple myeloma patients (MGN-3 group, 32; placebo, 16). Detailed blood analyses were performed before and during the 3 months of treatment. Significant increase in NK activity, myeloid dendritic cell level, and augmented concentrations of Th cell type 1-related cytokines were observed in the MGN-3 group. No significant change in the placebo group was observed. Hence, MGN-3 clearly improved the innate immunity profile of the patients compared with placebo.53
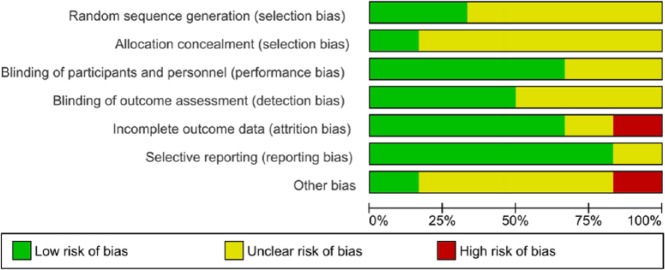
Risk of Bias Assessment
We have assessed the risk of bias of the included RCTs using the Cochrane Risk of Bias tool.59 The results of the assessment are summarized in Figures 2 and 3. Because of insufficient information provided on randomization and blinding, most included RCTs have unclear risk in at least one or more items in selection bias, performance bias, and detection bias. Only the study by Itoh et al.55 is considered low risk in these items. Notwithstanding, this study has a high risk of attrition bias due to a high percentage of participants being excluded from assessment (30%).55 The risks of attrition bias and reporting bias are low in most other studies except the study by Masood el al,54 which has unclear risk of bias across all items of assessment due to insufficient detail in published information. We assess the study by Takahara and Sano49 to have a high risk in other bias. It was conducted by a commercial hospital specialized in CAT for cancer patients, thus the risk of bias cannot be excluded due to conflict of interest. With industry influence being a potential source of other bias, only the study by Petrovics el al56 provided disclosure on funding and clarified the roles of industry partners in the study, which the other studies did not.
Figure 2.
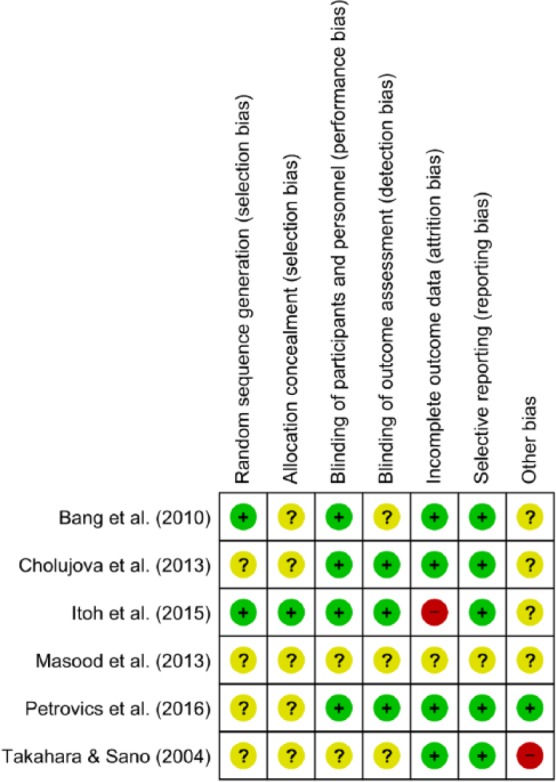
Risk of bias summary: Review authors’ judgements about each risk of bias item for each included study.
Figure 3.
Risk of bias graph: Review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Discussion
Current evidence supports MGN-3 to be a BRM. MGN-3 is an evidence based and standardized arabinoxylan concentrate from plant origin which, similar to bacterial pathogen associated molecular pattern molecules, can stimulate the body’s natural immune system to protect against cancer growth.46 The effect of MGN-3 in upregulating the cytotoxic activities of NK cells, by multiple-fold, has been most prominently demonstrated.34,48-50,53 The anticancer effect of NK cells is the subject of intense current research and NK cell immunotherapy is being touted as the future of cancer immunotherapy.4 Effectiveness of NK activity has been associated with good prognosis in patients with metastatic cancers.60,61 This may explain the life-prolongation effect of MGN-3 as reported in a number of clinical studies49,52 and case reports.42-44 However, MGN-3 treatment does not increase the absolute NK cell counts as noted in clinical studies,51,57 which is a limitation. Cancer patients with low NK cell counts (<0.15 × 109/L) tend to have lower survival rates,62,63 indicating that lower basic level of natural antitumor activity can reduce the efficacy of a BRM. While they may benefit from the upregulated NK activity with MGN-3 treatment to a certain extent,49 combining NK cellular therapy64 with MGN-3 may further improve prognosis in these patients; an approach that warrant future clinical investigation.34
Other immunomodulatory effects of MGN-3 include the following: increase susceptibility of cancer cells to undergo apoptosis mediated by CD95 death receptors28; influence plasma cytokine production (upregulated TNF-α and IL-12, while downregulating IL-10)37; enhance the activity of antioxidant enzymes38; improve T and B cell proliferation48; improve Th cell concentration,51,53 downregulate Treg cells51; and activate dendritic cells.13,22,53 These effects have been presented in depth by Ghoneum65 in a recent review, supporting the use of MGN-3 as an effective BRM in cancer therapy.
MGN-3 has been tested in clinical trials as an adjunct therapy during conventional chemo- and radiotherapy with effects that include (1) improvement of immunoprofile,53,55 (2) reduction of side effects (diarrhea, nausea, pain, tiredness, anorexia, and vomiting),52,54-56,58 and (3) improvement in treatment outcomes.52 Outcome improvement and reduction of side effects have been mostly attributed to an improved balance of the innate immune system. However, MGN-3 has also been shown to work synergistically with daunorubin31 and paclitaxel33,40 in preclinical studies, as well as transarterial oily chemoembolization (TOCE) and percutaneous ethanol injection treatment (PEIT) for liver cancer treatment in a clinical trial,52 by sensitizing the cancer cells to these agents. More research is needed to explore such synergistic effects with other chemotherapeutic agents and radiation therapy to fully explore the potential of MGN-3 as a combination therapy in conventional cancer treatment.
As a follow-up therapy after conventional cancer treatment, MGN-3 has demonstrated effects in restoring weakened immunoprofile,48-51 improving QoL and survival rate of late-stage cancer patients,49 as well as reducing recurrence.52 Such results are consistent with the clinical experiences reported in the published case reports.42-47 As such, MGN-3 may also be considered as part of the follow-up care after conventional cancer treatment.
No adverse reaction was reported in studies that explicitly monitored potential MGN-3 induced adverse effects in vivo37 and in a clinical study.50 No adverse event due to MGN-3 was reported in any of the included clinical trials or clinical case reports. Furthermore, the safety MGN-3 has been studied in a series of animal studies. The median lethal dose (LD50) of MGN-3 is more than 36 g/kg, and the “no observed adverse effect level (NOAEL)" is 200 mg/kg/d or higher.66 Therefore, typical MGN-3 dosages of 3 to 6 g/d or 45 mg/kg BW/d are extremely safe.
At present, there is no study on the pharmacokinetics of MGN-3 and the achievable level of MGN-3 in serum is unknown (personal email communication with Dr Mamdooh Ghoneum at Charles Drew University of Medicine and Science on August 4, 2017). Since concentrations of MGN-3 used in some of in vitro studies were very high (500-1000 μg/mL),28,29,31,33 it is unclear whether these concentrations can be of clinical relevance. Although the MGN-3 dosages (5-50 mg/kg/BW) used for in vivo studies34,35,37-41 were more closely matched to the typical dosage used in human studies, without understanding of the pharmacokinetics of MGN-3, questions remain whether the higher bioavailability via intraperitoneal injection in some animal studies36-41 can be applicable to humans.
The clinical research on MGN-3 is still at its early stage with only a small number of RCTs available in the literature. Most of them are small pilot trials with limited participants (N < 100) and short duration (<=6 months). At the moment only one RCT is available to support clinical evidence.52 The other RCT that has adequate size (N = 205) and duration (18 months)49 suffers from methodological limitations including inadequate blinding, no placebo control, and potential risk of bias due to conflict of interest. Hence, large, well-designed, long-term placebo-controlled RCTs are needed to further evaluate the effects of MGN-3 as a complementary therapy to support conventional cancer treatment.
Conclusion
Current evidence from preclinical studies, clinical case reports, and small clinical trials suggests that MGN-3 can be an effective BRM to complement the conventional cancer treatment through upregulating the patient’s immune system, especially in boosting the NK cell activity. MGN-3 appears safe in its application with no adverse event reported to date. It may be used as a complementary immune therapy to reduce side effects, improve treatment outcomes, and enhance long-term survival rate. The combination of MGN-3 with new biological targeting treatments may open new perspectives in the tumor therapy. Nevertheless, we call for additional study into the pharmacokinetics of MGN-3, as well as more well-designed RCTs to confirm its efficacies and strengthen the evidence to support its clinical application.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [DOI] [PubMed] [Google Scholar]
- 2. Sudhakar A. History of cancer, ancient and modern treatment methods. J Cancer Sci Ther. 2010;1:1-4. [DOI] [PubMed] [Google Scholar]
- 3. Qiao J, Liu Z, Fu YX. Adapting conventional cancer treatment for immunotherapy. J Mol Med (Berl). 2016;94:489-495. [DOI] [PubMed] [Google Scholar]
- 4. Kuroki M, Miyamoto S, Morisaki T, et al. Biological response modifiers used in cancer biotherapy. Anticancer Res. 2012;32:2229-2233. [PubMed] [Google Scholar]
- 5. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38:1551-1566. [DOI] [PubMed] [Google Scholar]
- 6. Biemar F, Foti M. Global progress against cancer-challenges and opportunities. Cancer Biol Med. 2013;10:183-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. BioBran.org. Biobran MGN-3—an overview. https://biobran.org/overview. Accessed May 18, 2017.
- 8. Miura T, Chiba M, Miyazaki Y, Kato Y, Maeda H. Chemical structure of the component involved in immunoregulation. In: BioBran/MGN-3 (Rice Bran Arabinoxylan Coumpound): Basic and Clinical Application to Integrative Medicine. 2nd ed. Tokyo, Japan: BioBran Research Foundation; 2013:14-22. [Google Scholar]
- 9. Paterson A. Therapeutic properties of biobran MGN-3. http://www.positivehealth.com/article/nutraceuticals/therapeutic-properties-of-biobran-mgn-3. Posit Heal; Published September 2002. Accessed September 19, 2017. [Google Scholar]
- 10. Tazawa K. BioBran/MGN-3 (Rice Bran Arabinoxylan Compound): Basic and Clinical Application to Integrative Medicine. 2nd ed. Tokyo, Japan: BioBran Research Foundation; 2013. [Google Scholar]
- 11. Ghoneum M, Hamilton J, Gollapudi S. Modified arabinoxylan rice bran (MGN-3/biobran), potentiates chemotherapy-induced apoptosis in human breast cancer cells. J Nutr. 2004;134:3541S. [Google Scholar]
- 12. Badr El-Din N, Noaman E, Ghoneum A, Ghoneum M. Rice bran supplement (MGN-3/Biobran) suppresses tumor growth via modulating cytokine production and increasing apoptotic level in ehrlich carcinoma-bearing mice. Clin Immunol. 2007;123(suppl):S117. [Google Scholar]
- 13. Cholujova D, Jakubikova J, Sulikova M, et al. The effect of MGN-3 arabinoxylan on natural killer and dendritic cells in multiple myeloma patients. Haematologica. 2011;96(suppl 1):S117-S118. [Google Scholar]
- 14. Badr El-Din NK, Fattah SMA, Pan D, Tolentino L, Ghoneum M. Chemopreventive activity of BioBran/MGN-3, an arabinoxylan from rice bran, against chemical induction of gastric dysplasia and adenocarcinoma in rats. Cancer Res. 2014;74(19 Suppl):Abstract 234. [Google Scholar]
- 15. Badr El-Din NK, Ali DA, Alaa El-Dein M, Ghoneum M. Biobran/MGN-3, arabinoxylan from rice bran, sensitizes breast adenocarcinoma tumor cells to paclitaxol in mice. Cancer Res. 2015;75(15 Suppl):Abstract 5312. [Google Scholar]
- 16. Giese S, Sabell GR, Coussons-Read M. Impact of ingestion of rice bran and shitake mushroom extract on lymphocyte function and cytokine production in healthy rats. J Diet Suppl. 2008;5:47-61. [DOI] [PubMed] [Google Scholar]
- 17. Ghoneum M, Badr El-Din NK, Fattah SMA, Tolentino L. Arabinoxylan rice bran (MGN-3/Biobran) provides protection against whole-body γ-irradiation in mice via restoration of hematopoietic tissues. J Radiat Res. 2013;54:419-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghoneum M, Jewett A. Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro. Cancer Detect Prev. 2000;24:314-324. [PubMed] [Google Scholar]
- 19. Ghoneum M, Agrawal S. MGN-3/Biobran enhances generation of cytotoxic CD8+ T cells via upregulation of DEC-205 expression on dendritic cells. Int J Immunopathol Pharmacol. 2014;27:523-530. [DOI] [PubMed] [Google Scholar]
- 20. Ghoneum M, Agrawal S. Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran). Int J Immunopathol Pharmacol. 2011;24:941-948. [DOI] [PubMed] [Google Scholar]
- 21. Ghoneum M, Abedi S. Enhancement of natural killer cell activity of aged mice by modified arabinoxylan rice bran (MGN-3/Biobran). J Pharm Pharmacol. 2004;56:1581-1588. [DOI] [PubMed] [Google Scholar]
- 22. Cholujova D, Jakubikova J, Sedlak J. BioBran-augmented maturation of human monocyte-derived dendritic cells. Neoplasma. 2009;56:89-95. [DOI] [PubMed] [Google Scholar]
- 23. Hajtó T, Adámy A, Langmár Z, et al. Enhanced effectiveness of conventional oncotherapy with plant immunomodulators: overview of recent advances. Adv Med Plant Res. 2013;1:56-65. [Google Scholar]
- 24. Tan BL, Norhaizan ME. Scientific evidence of rice by-products for cancer prevention: chemopreventive properties of waste products from rice milling on carcinogenesis in vitro and in vivo. Biomed Res Int. 2017;2017:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang S, Li W, Smith CJ, Musa H. Cereal-derived arabinoxylans as biological response modifiers: extraction, molecular features, and immune-stimulating properties. Crit Rev Food Sci Nutr. 2015;55:1035-1052. [DOI] [PubMed] [Google Scholar]
- 26. Meshitsuka K. A case of stage IV hepatocellular carcinoma treated by KM900, Biobran, and psychotherapy has presented significant good results. Pers Med Universe (Japanese Ed.) 2016;1:46-48. [Google Scholar]
- 27. Ghoneum M, Tachiki K, Ueyama K, Makinodan T, Makhijani N, Yamaguchi D. Natural biological response modifier (MGN-3) shown to be effective against tumor cell growth. Paper presented at the 8th International Congress on Anti-Aging & Biomedical Technologies; December 14, 2000; Las Vegas, NV. [Google Scholar]
- 28. Ghoneum M, Gollapudi S. Modified arabinoxylan rice bran (MGN-3/Biobran) sensitizes human T cell leukemia cells to death receptor (CD95)-induced apoptosis. Cancer Lett. 2003;201:41-49. [DOI] [PubMed] [Google Scholar]
- 29. Ghoneum M, Gollapudi S. Modified arabinoxylan rice bran (MGN-3/Biobran) enhances yeast-induced apoptosis in human breast cancer cells in vitro. Anticancer Res. 2005;25:859-870. [PubMed] [Google Scholar]
- 30. Ghoneum M, Gollapudi S. Synergistic role of arabinoxylan rice bran (MGN-3/Biobran) in S. cerevisiae-induced apoptosis of monolayer breast cancer MCF-7 cells. Anticancer Res. 2005;25:4187-4196. [PubMed] [Google Scholar]
- 31. Gollapudi S, Ghoneum M. MGN-3/Biobran, modified arabinoxylan from rice bran, sensitizes human breast cancer cells to chemotherapeutic agent, daunorubicin. Cancer Detect Prev. 2008;32:1-6. [DOI] [PubMed] [Google Scholar]
- 32. Ghoneum M, Gollapudi S. Synergistic apoptotic effect of arabinoxylan rice bran (MGN-3/Biobran) and curcumin (turmeric) on human multiple myeloma cell line U266 in vitro. Neoplasma. 2011;58:118-123. [DOI] [PubMed] [Google Scholar]
- 33. Ghoneum M, Badr El-Din NK, Ali DA, El-Dein MA. Modified arabinoxylan from rice bran, MGN-3/Biobran, sensitizes metastatic breast cancer cells to paclitaxel in Vitro. Anticancer Res. 2014;34:81-88. [PubMed] [Google Scholar]
- 34. Pérez-Martínez A, Valentín J, Fernández L, et al. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma invitro and invivo. Cytotherapy. 2015;17:601-612. [DOI] [PubMed] [Google Scholar]
- 35. Jacoby HI, Wnorowski G, Sakata K, Maeda H. The effect of MGN-3 on cisplatin and doxorubicin induced toxicity in the rat. J Nutraceut Funct Med Foods. 2001;3:3-11. [Google Scholar]
- 36. Endo Y, Kanbayashi H. Modified rice bran beneficial for weight loss of mice as a major and acute adverse effect of cisplatin. Pharmacol Toxicol. 2003;92:300-303. [DOI] [PubMed] [Google Scholar]
- 37. Badr El-Din NK, Noaman E, Ghoneum M. In vivo tumor inhibitory effects of nutritional rice bran supplement MGN-3/Biobran on ehrlich carcinoma-bearing mice. Nutr Cancer. 2008;60:235-244. [DOI] [PubMed] [Google Scholar]
- 38. Noaman E, Badr El-Din NK, Bibars MA, Mossallam AAA, Ghoneum M. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett. 2008;268:348-359. [DOI] [PubMed] [Google Scholar]
- 39. Badr El-Din NK, Fattah SMA, Pan D, Tolentino L, Ghoneum M. Chemopreventive activity of MGN-3/Biobran against chemical induction of glandular stomach carcinogenesis in rats and its apoptotic effect in gastric cancer cells. Integr Cancer Ther. 2016;15:NP26-NP34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Badr El-Din NK, Ali DA, Alaa El-Dein M, Ghoneum M. Enhancing the apoptotic effect of a low dose of paclitaxel on tumor cells in mice by arabinoxylan rice bran (MGN-3/Biobran). Nutr Cancer. 2016;68:1010-1020. [DOI] [PubMed] [Google Scholar]
- 41. Badr El-Din NK, Ali DA, Othman R, Ghoneum M. Prevention of hepatocarcinogenesis in rats by arabinoxylan rice bran, MGN-3/Biobran. Cancer Res. 2016;76(14 Suppl):Abstract 5259. [Google Scholar]
- 42. Kawai T. One case of a patient with umbilical metastasis of recurrent cancer (Sister Mary Joseph’s Nodule, SMJN) who has survived for a long time under immunomodulatory supplement therapy. Clin Pharmacol Ther. 2004;14:281-288. [Google Scholar]
- 43. Kaketani K. A case where an immunomodulatory food was effective in conservative therapy for progressive terminal pancreatic cancer. Clin Pharmacol Ther. 2004;14:273-279. [Google Scholar]
- 44. Okamura Y. The clinical significance of modified arabinoxylan from rice bran (BioBran/MGN-3) in immunotherapy for cancer. Clin Pharmacol Ther. 2004;14:289-294. [Google Scholar]
- 45. Markus J, Miller A, Smith M, Orengo I. Metastatic hemangiopericytoma of the skin treated with wide local excision and MGN-3. Dermatol Surg. 2006;32:145-147. [DOI] [PubMed] [Google Scholar]
- 46. Hajto T, Baranyai L, Kirsch A, Kuzma M, Perjési P. Can a synergistic activation of pattern recognition receptors by plant immunomodulators enhance the effect of oncologic therapy? Case Report of a patient with uterus and ovary sarcoma. Clin Case Reports Rev. 2015;1(10):235-238. [Google Scholar]
- 47. Hajto T, Horváth A, Baranyai L, Kuzma M, Perjési P. Can the EGFR inhibitors increase the immunomodulatory effects of standardized plant extracts (mistletoe lectin and arabonoxylan) with clinical benefit? Case report of a patient with lung adenocarcinoma. Clin Case Rep Rev. 2016;2:456-459. [Google Scholar]
- 48. Ghoneum M, Brown J. NK Immunorestoration and cancer patients by BioBran/MGN-3, a modified aracbynoxylan rice bran (study of 32 patients followed for up to 4 years). In: Klatz RM, Goldman R, eds. Anti-Aging Medical Therapeutics, Vol III Marina del Rey, CA: Health Quest; 1999:217-226. [Google Scholar]
- 49. Takahara K, Sano K. Life prolongation and QOL improvement effect of modified arabinoxylan from rice bran (BioBran/MGN-3) for progressive cancer. Clin Pharmacol Ther. 2004;14:267-271. [Google Scholar]
- 50. Tsunekawa H. Effect of long-term administration of immunomodulatory food on cancer patients completing conventional treatments. Clin Pharmacol Ther. 2004;14:295-302. [Google Scholar]
- 51. Lissoni P, Messina G, Brivio F, et al. Modulation of the anticancer immunity by natural agents: inhibition of T regulatory lymphocyte generation by arabinoxylan in patients with locally limited or metastatic solid tumors. Cancer Ther. 2008;6:1011-1016. [Google Scholar]
- 52. Bang MH, Van Riep T, Thinh NT, et al. Arabinoxylan rice bran (MGN-3) enhances the effects of interventional therapies for the treatment of hepatocellular carcinoma: a three-year randomized clinical trial. Anticancer Res. 2010;30:5145-5151. [PubMed] [Google Scholar]
- 53. Cholujova D, Jakubikova J, Czako B, et al. MGN-3 arabinoxylan rice bran modulates innate immunity in multiple myeloma patients. Cancer Immunol Immunother. 2013;62:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Masood AI, Sheikh R, Anwer RA. “Biobran MGN-3”; effect of reducing side effects of chemotherapy in breast cancer patients. Prof Med J. 2013;20:13-16. [Google Scholar]
- 55. Itoh Y, Mizuno M, Ikeda M, et al. A randomized, double-blind pilot trial of hydrolyzed rice bran versus placebo for radioprotective effect on acute gastroenteritis secondary to chemoradiotherapy in patients with cervical cancer. Evid Based Complement Alternat Med. 2015;2015:947390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petrovics G, Szigeti G, Hamvas S, Máté Á, Betlehem J, Hegyi G. Controlled pilot study for cancer patients suffering from chronic fatigue syndrome due to chemotherapy treated with BioBran (MGN-3-Arabinoxylan) and targeted radiofrequency heat therapy. Eur J Integr Med. 2016;8:29-35. [Google Scholar]
- 57. Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Addition of rice bran arabinoxylan to curcumin therapy may be of benefit to patients with early-stage B-cell lymphoid malignancies (monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, or stage 0/1 chronic lymphocytic leukemia). Integr Cancer Ther. 2016;15:183-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hajto T, Horvath A. Improvement of quality of life in tumor patients after an immunomodulatory treatment with standardized mistletoe lectin and arabinoxylan plant extracts. Int J Neurorehabil. 2016;3(2):2-4. [Google Scholar]
- 59. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Takeuchi H, Maehara Y, Tokunaga E, Koga T, Kakeji Y, Sugimachi K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am J Gastroenterol. 2001;96:574-578. [DOI] [PubMed] [Google Scholar]
- 61. Pasero C, Gravis G, Granjeaud S, et al. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget. 2015;6:14360-14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim JK, Chung JS, Shin HJ, et al. Influence of NK cell count on the survival of patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood Res. 2014;49:162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. He L, Zhu H-Y, Qin S-C, et al. Low natural killer (NK) cell counts in peripheral blood adversely affect clinical outcome of patients with follicular lymphoma. Blood Cancer J. 2016;6:e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Berrien-Elliott MM, Romee R, Fehniger TA. Improving natural killer cell cancer immunotherapy. Curr Opin Organ Transplant. 2015;20(6):671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ghoneum M. From bench to bedside: The growing use of arabinoxylan rice bran (MGN-3/Biobran) in cancer immunotherapy. Austin Immunol. 2016;1(2). [Google Scholar]
- 66. The safety of Biobran/MGN-3. In: BioBran/MGN-3 (Rice Bran Arabinoxylan Coumpound): Basic and Clinical Application to Integrative Medicine. 2nd ed. Tokyo, Japan: BioBran Research Foundation; 2013:9-13. [Google Scholar]