Abstract
Background: The outcome of patients with intermediate stage hepatocellular carcinoma (HCC) treated by transarterial chemoembolization (TACE) remains poor. Search for a more effective therapy is still necessary. Objective: This study aimed to investigate the effect of combining TACE with Kang’ai (KA) injection for treating patients with intermediate stage HCC. Methods: A total of 89 patients with intermediate stage HCC were enrolled and divided into TACE +KA group (n = 48) receiving repeated TACE plus KA injection, and TACE group (n = 41) receiving repeated TACE alone. All patients were prospectively studied. Primary endpoints were overall survival (OS) and time to radiologic progression (TTP). Results: The TACE + KA group had significantly longer median OS (27.0 vs 21.0 months, P = .038) and TTP (12.0 vs 10.0 months, P = .028) than TACE group. The 1-, 2-, and 3-year OS rates in the TACE + KA group were markedly higher than in TACE group (88.5%, 58.8%, and 20.8% vs 81.3%, 44.9%, and 6.7%, respectively, P = .038), while the 1- and 2-year TTP rates in the TACE + KA group were significantly lower than in TACE group (49.3% and 86.9% vs 75.3% and 100%, P = .028). TACE + KA group displayed significantly lower incidences of intrahepatic and extrahepatic metastases, as well as postembolization syndrome than TACE group (P < .05). Multivariate analyses revealed group (P = .023), maximum tumor size (P = .019), and tumor number (P = .034) as significant predictors for OS, and group (P = .046), maximum tumor size (P = .002) and α-fetoprotein level (P = .020) as significant predictors for TTP. Both TACE and KA injection were well tolerated. Conclusion: TACE plus KA injection is more effective than TACE alone for treating patients with intermediate stage HCC in this nonrandomized study. Further research is warranted.
Keywords: hepatocellular carcinoma (HCC), transarterial chemoembolization (TACE), Kang’ai (KA) injection
Introduction
Liver cancer is a major cause of cancer-related mortality worldwide. In 2013, primary liver cancer, as reported by the World Health Organization, caused 745 517 deaths over the world, and hepatocellular carcinoma (HCC) represented the majority of these liver cancers.1 In China, HCC ranks the second most common cause of cancer-related mortality with an annual mortality rate of 24.15 per 100 000 persons in 2009.2 Moreover, approximately 383 203 persons die from liver cancer annually in China, which accounts for 51% of the deaths from liver cancer worldwide.2
The Barcelona Clinic Liver Cancer (BCLC) classification system is widely adopted as the treatment algorithm for HCC, which has been endorsed by the European Association for the Study of the Liver (EASL) and the American Association for the Study of the Liver Disease (AASLD).3-5 It provides guidance on both the recommended therapy and corresponding prognosis at the presenting stages. Most HCCs are diagnosed at an intermediate to advanced stage with few effective therapeutic options available.6 Transarterial chemoembolization (TACE) is recommended as the first-line therapy for patients with intermediate stage HCC in BCLC stage classification (single nodule >5 cm or multinodular tumors >3 cm; Okuda stage I-II; Child-Pugh A-B; Eastern Cooperative Oncology Group (ECOG) performance status test 0).4,5,7,8 However, the prognosis of patients with intermediate stage HCC remains poor, with a reported median survival of 19 to 34 months, and 3-year survival rate of 29% to 47%, respectively.9-11 Therefore, search for a more effective therapy for this cohort of patients is still imperative.
Recently, traditional Chinese medicines (TCMs, including plants, animal parts, and minerals) have aroused a great deal of interest for their potential therapeutic effects on HCC. Previous studies demonstrated that TCMs were able to retard HCC progression through multiple actions, such as tumor growth inhibition, anti-metastatic, anti-inflammation, anti–liver cancer stem cells activities and other activities.12 Kang’ai (KA) injection is a composite anti-tumor formula for injection that mainly consists of five active molecules, namely ginsenosides Rg1, Re, Rf, Rb1, and astragaloside at concentrations of 118.33 µg/mL, 75.06 µg/mL, 12.56 µg/mL, 34.06 µg/mL, and 56.50 µg/mL, respectively.13 The formula was reported to be able to improve immune function and raise white blood cell (WBC) count; inhibit tumor cell cloning; arrest cell cycle, induce apoptosis, and suppress invasion and metastasis of cancer cells from lung cancer, intestinal carcinoma and HCC.14 To the best of our knowledge, there is no study that has investigated the effect of TACE in combination with KA injection on patients with intermediate stage HCC in the literature.
So, we conducted this study to evaluate the efficacy and safety of combining KA injection with TACE for treating patients with HCC at intermediate stage.
Patients and Methods
Patients and Study Design
Between November 2013 and March 2017, a total of 134 consecutive HCC patients were diagnosed and treated in our unit. Inclusion criteria were as follows: (1) ages between 18 and 75 years, (2) intermediate HCC in BCLC stage classification,4,5,7,8 and (3) TACE with or without KA therapy. Exclusion criteria included (1) human immunodeficiency (HIV) infection or active tuberculosis; (2) extrahepatic malignancy; (3) chronic cardiovascular, respiratory, or renal diseases; (4) previous history of organ transplantation; (5) pregnant or breastfeeding women; (6) contraindications to an arterial procedure such as impaired clotting tests (platelet count <50 × 109/L or prothrombin activity <50%); (7) using other herbal remedies outside of medical treatment. As shown in Figure 1, we excluded 45 patients: 14 with early HCC in BCLC stage classification and good hepatic function who were transferred to receive hepatic resection, 9 undergoing multimodality therapy (including TACE followed by radiofrequency ablation or hepatic resection), 8 denying any invasive therapy (either hepatic resection, TACE or radiofrequency ablation), 6 with major vascular invasion and/or distant metastasis, 5 receiving other herbal remedies outside of medical treatment and 3 lost to follow-up. The remaining 89 patients with intermediate stage HCC were enrolled in this study and divided into concurrent group receiving both repeated TACE plus KA injection (n = 48) and monotherapy group receiving only repeated TACE (n = 41). KA injection was only applied to patients who consented to use it after providing detailed information regarding the safety, efficacy, and cost to patients and their family members, and after discussion with their physicians. All study procedures were in accordance with the ethical standards of the ethics committee of the Second Affiliated Hospital of Kunming Medical University on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and signed informed consents were obtained from all patients.
Figure 1.
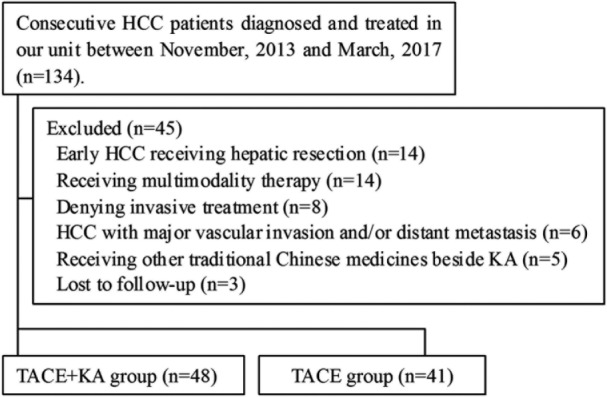
Disposition of patients in this study.
Diagnostic Criteria
All HCC diagnoses were made according to the diagnostic criteria recommended by the EASL and the AASLD guidelines on noninvasive diagnosis.4,15 Radiologic imaging techniques, including ultrasound (US), triphasic computed tomography (CT), and contrast-enhanced magnetic resonance imaging (MRI), were applied to characterize the tumors. In principle, a unique dynamic radiological behavior (contrast uptake in the arterial phase by CT, MRI, angiography, or US) represented the radiologic hallmark of HCC. When showing the radiological hallmark of HCC, noninvasive diagnosis can be established by one imaging technique in nodules above 2 cm and 2 coincidental techniques (CT, MRI, and US contrast) in nodules of 1 to 2 cm in diameter.
TACE Procedure
TACE was performed in a sterile operating room by two highly experienced interventional radiologists in our institution according to the method described by Chung et al.16 Briefly, after local anesthesia, the right superficial femoral artery was punctured with Seldinger technique. A 5-F French catheter was introduced into the abdominal aorta under fluoroscopy guidance. Selective angiography via the superior mesenteric artery or the common hepatic artery was performed to identify the exact site of HCC and its feeding arteries. After catheterization, a 2.7-F French catheter was advanced through the 5-F catheter into the feeding arteries. An emulsion of 10 to 15 mL lipiodol (Andre Guerbet, Aulnay-sous-Bois, France) and 3 anticancer agents (ie, 100-150 mg oxaliplatin, 500-750 mg 5-fluorouracil and 10-20 mg epirubicin) followed by 1- to 2-mm diameter gelatin sponge particles (Cutanplast; MasciaBrunelli, Milan, Italy) was slowly injected into the feeding arteries. The dose of emulsion of lipiodol and anticancer agents, and the pieces of embolic materials used for TACE were determined by the tumor size and extent of the lesions.
Follow-up and Treatment Protocol
After TACE, patients were prospectively followed up every 1 to 3 months during the first year and at 3- to 6-month intervals thereafter in our unit till death or till the censor time of the study (March 2017). At each visit, medical interviews (concerning adherence to antiviral therapy, treatment-related adverse events, and cirrhosis complications), physical examination, laboratory tests (liver and kidney functions, coagulation, lipids, glucose and electrolytes profiles, α-fetoprotein [AFP], and hepatitis B virus (HBV)-DNA, etc), radiologic imaging tests (abdominal US, chest X-ray, dynamic abdominal CT, or MRI) were performed in all patients. Chest CT or bone scans were performed when distant metastasis was suspected. Data were documented in our electronic medical record system. All tumor responses were independently evaluated by 2 expert radiologists (Profs Zhu and Pu, both with more than 20 years of experience) who were blinded to our study protocol. And all tumors were evaluated for response using dynamic radiologic modalities (CT or MRI) according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST).17 For patients who showed viable tumor lesions (contrast uptake in arterial phase) or intrahepatic metastasis or new lesions, if clinically feasible, repeated TACE was performed by respective physicians, who made the decisions based on extrahepatic disease, hepatic function, general health, patient willingness, and economic conditions. For patients in concurrent group, KA injection (Changbai Mountain Pharmaceutical co, Ltd) was intravenously given at a standard dose of 40 mL (ie, ginsenosides Rg1 4.73 mg, Re 3.00 mg, Rf 0.50 mg, Rb1 1.36 mg, and astragaloside 2.26 mg) in 250 mL normal saline per day for a median of 15 days (range 11-20 days) during in-hospital time at each episode of TACE. Vigorous oral nucleos(t)ides including tenofuvir, entecavir, and lamivudine were administered to patients with detectable HBV-DNA. Ascitic patients received oral diuretics and/or albumin infusion. All patients received hepatic cytoprotective and regenerative agents (ie, glycyrrhizin, reduced glutathione, polyene phosphatidylcholine, and ademetionine).
Endpoints
Primary endpoints were overall survival (OS) and time to radiologic progression (TTP). OS was defined as the time period from the initial TACE to the date of death of any cause. TTP was calculated as the time between the initial TACE and the objective tumor progression, which was evaluated according to the mRECIST criteria.17 Objective tumor progression referred to ≥20% increment in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started or intrahepatic metastasis or macrovascular invasion or extrahepatic metastasis.17
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median and ranges, and compared using Student’s t test or the Mann-Whitney U test, as appropriate. Categorical variables were presented as the number of events and percentage, and compared by the χ2 test. Survival analyses were performed using the Kaplan-Meier method and compared by the log-rank test. Baseline factors associated with survival were identified by univariate and multivariate analyses using the Cox proportional hazards regression model. Multivariate analysis was conducted using variables that showed association in univariate analysis with a P value <.1. All statistical analyses were executed with SPSS 17.0 software (SPSS Inc, Chicago, IL).
Results
Patient Characteristics
As shown in Table 1, the baseline characteristics of patients in the 2 groups were statistically comparable (P > .05). Male patients were predominant in both groups, accounting for 87.5% and 78.0% in the TACE + KA and TACE groups, respectively. The majority of patients in both groups were with Child-Pugh class B, HBV infection, maximum tumor size between 5.0 and 10.0 cm, 2 to 3 tumors, AFP levels <200 ng/mL, and no ascites. The 2 groups had similar ages and serum levels of albumin, alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), creatinine, prothrombin time (PT), white blood cell (WBC), hemoglobin (HGB), platelet count (PLT), and HBV-DNA. The average follow-up periods were 18.7 and 16.3 months in the TACE + KA and TACE groups, respectively.
Table 1.
Baseline Demographic and Disease Characteristics of Patients in TACE + KA and TACE Groups.
| Background Factors | TACE + KA Group (n = 48) | TACE Group (n = 41) | P |
|---|---|---|---|
| Age, y, mean ± SD | 53.9 ± 10.3 | 55.7 ± 8.8 | .385 |
| Male, n (%) | 42 (87.5) | 32 (78.0) | .235 |
| Child-Pugh class, n (%) | |||
| A | 22 (45.8) | 20 (48.8) | .781 |
| B | 26 (54.2) | 21 (51.2) | |
| Etiology, n (%) | |||
| HBV | 33 (68.8) | 32 (78.0) | .728 |
| HCV | 7 (14.6) | 5 (12.2) | |
| Alcohol | 3 (6.3) | 1 (2.4) | |
| Other | 5 (10.4) | 3 (7.3) | |
| Maximum tumor size (cm), n (%) | |||
| 3.1-5.0 | 17 (35.4) | 7 (17.1) | .151 |
| 5.1-10.0 | 21 (43.8) | 23 (56.1) | |
| ≥10.1 | 10 (20.8) | 11 (26.8) | |
| No. of lesions, n (%) | |||
| 1 | 12 (25.0) | 18 (43.9) | .115 |
| 2-3 | 32 (66.7) | 22 (53.7) | |
| ≥4 | 4 (8.3) | 1 (2.4) | |
| α-Fetoprotein (0-10 ng/mL), n (%) | |||
| <200 ng/mL | 34 (70.8) | 22 (53.7) | .247 |
| 200-1000 ng/mL | 6 (12.5) | 8 (19.5) | |
| >1000 ng/mL | 8 (16.7) | 11 (26.8) | |
| Albumin (35-50 g/L), mean ± SD | 34.4 ± 6.2 | 32.5 ± 4.5 | .113 |
| ALT (5-40 U/L), mean ± SD | 53.0 ± 32.2 | 51.0 ± 33.2 | .769 |
| AST (8-40 U/L), mean ± SD | 75.4 ± 40.4 | 78.1 ± 37.2 | .746 |
| Total bilirubin (3.4-17.1 µmol/L), mean ± SD | 20.4 ± 7.5 | 22.7 ± 9.6 | .200 |
| Direct bilirubin (0-5.1 µmol/L), mean ± SD | 9.0 ± 4.0 | 10.6 ± 5.9 | .114 |
| Creatine (62-115 µmol/L), mean ± SD | 72.7 ± 15.6 | 73.1 ± 22.7 | .912 |
| Prothrombin time (11.0-15.0 s), mean ± SD | 15.0 ± 2.2 | 15.7 ± 2.2 | .192 |
| White blood cell count (3.50-9.50 ×109/L), mean ± SD | 5.14 ± 2.08 | 4.52 ± 1.71 | .132 |
| Hemoglobin (130-175 g/L), mean ± SD | 125.5 ± 29.4 | 122.3 ± 27.6 | .602 |
| Platelet count (125-350 ×109/L), mean ± SD | 119.3 ± 60.1 | 135.1 ± 81.7 | .299 |
| HBV-DNA (log10 copies/mL), mean ± SD | 3.96 ± 1.46 (n = 33) | 4.09 ± 1.43 (n = 32) | .732 |
| Ascites, no/mild/moderate to severe, n (%) | 27 (56.3%)/12 (25.0%)/9 (18.8%) | 24 (58.5%)/14 (34.1%)/3 (7.3%) | .247 |
| No. of TACE sessions, mean ± SD | 3.1 ± 1.6 | 3.0 ± 1.5 | .723 |
| Follow-up period (mo), mean ± SD | 18.7 ± 8.3 | 16.3 ± 8.1 | .180 |
Abbreviations: TACE, transarterial chemoembolization; KA, Kang’ai injection; HBV, hepatitis B virus; HCV, hepatitis C virus; ALT, alanine transaminase; AST, aspartate transaminase.
Clinical Efficacy
As shown in Figure 2, the TACE + KA group had a median OS period of 27.0 months (95% confidence interval [CI] 21.7-32.3), compared with 21.0 months (95% CI 15.6-26.4) in the TACE group (hazard ratio [HR] 0.658, 95% CI 0.377-0.982; P = .038). The 1-, 2-, and 3-year OS rates were 88.5%, 58.8%, and 20.8% in the TACE + KA group, compared with 81.3%, 44.9%, and 6.7% in the TACE group (P = .038).
Figure 2.
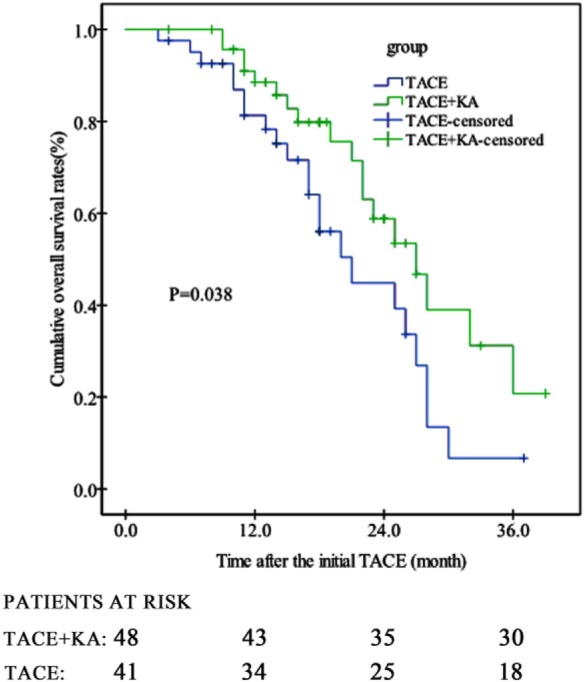
Cumulative overall survival rates of patients in TACE + KA and TACE groups (P = .038 by log-rank test). TACE, transarterial chemoembolization; KA, Kang’ai injection.
In Figure 3, the TACE+KA group showed a significantly longer median TTP period (12.0 months, 95% CI 12.0-14.0) than the TACE group (10.0 months, 95% CI 8.2-11.8) (HR 0.694, 95% CI 0.411-0.965; P = .028). The 1- and 2-year TTP rates were, respectively, 49.3% and 86.9% in the TACE + KA group, compared with 75.3% and 100% in the TACE group (P = .028). We performed a further analysis on tumor progression (Table 2), which revealed that patients receiving TACE + KA had significantly lower frequencies of intrahepatic and extrahepatic metastases than those receiving TACE alone (P < .05), while frequencies of increased viable tumor tissue of TACE-targeted lesions and major vascular invasion were comparable (P > .05).
Figure 3.
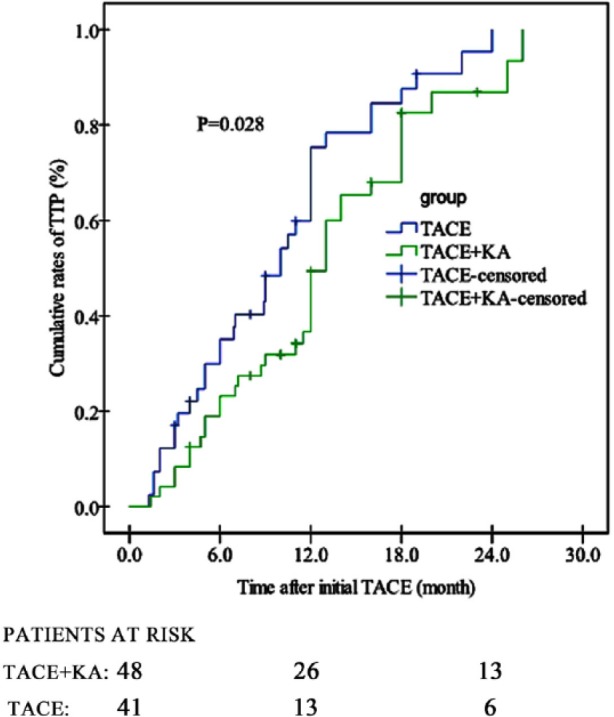
Cumulative TTP rates of patients in TACE + KA and TACE groups (P = .028 by log-rank test). TTP, time to radiologic progression; TACE, transarterial chemoembolization; KA, Kang’ai injection.
Table 2.
Tumoral Progression of Patients in the TACE + KA and TACE Groups.
| Group | Increased viable Tumoral Tissue | Intrahepatic Metastasis | Major Vascular Invasion | Extrahepatic Metastasis |
|---|---|---|---|---|
| TACE + KA, n (%) | 8 (16.7) | 22 (45.8) | 21 (43.8) | 12 (25.0) |
| TACE, n (%) | 9 (22.0) | 28 (68.3) | 24 (58.5) | 19 (46.3) |
| P a | .527 | .033 | .164 | .035 |
Abbreviations: TACE, transarterial chemoembolization; KA, Kang’ai injection.
P value for comparison between TACE + KA and TACE groups.
Baseline Factors Affecting the OS and TTP of Patients
Baseline laboratory variables were taken as the date when the initial session of TACE was performed. Univariate analysis revealed that the following 7 factors were independent prognostic variables for OS: group (HR 0.524, 95% CI 0.282-0.975, P = .031), maximum tumor size (HR 1.156, 95% CI 1.046-1.277, P = .004), tumor number (HR 0.632, 95% CI 0.401-0.998, P = .029), albumin (HR 0.937, 95% CI 0.883-0.995, P = .033), AST (HR 1.007, 95% CI 1.001-1.014, P = .029), TBIL (HR 1.048, 95% CI 1.008-1.089, P = .018), and DBIL (HR 1.101, 95% CI 1.040-1.166, P = .031) (Table 3). However, multivariate analysis showed that only the following 3 factors remained as independent prognostic variables: group (HR 0.658, 95% CI 0.377-0.982, P = .023), maximum tumor size (HR 1.152, 95% CI 1.020-1.302, P = .019), and tumor number (HR 0.629, 95% CI 0.386-0.987, P = .034) (Table 3).
Table 3.
Univariate and Multivariate Cox Analysis of Prognostic Factors for Overall Survival.
| Baseline Variables | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Groupa | 0.524 | 0.282-0.975 | .031 | 0.658 | 0.377-0.982 | .023 |
| Maximum tumor sizeb | 1.156 | 1.046-1.277 | .004 | 1.152 | 1.020-1.302 | .019 |
| Tumor numberc | 0.632 | 0.401-0.998 | .029 | 0.629 | 0.386-0.987 | .034 |
| Albumin | 0.937 | 0.883-0.995 | .033 | |||
| AST | 1.007 | 1.001-1.014 | .029 | |||
| TBIL | 1.048 | 1.008-1.089 | .018 | |||
| DBIL | 1.101 | 1.040-1.166 | .031 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; AST, aspartate transaminase; TBIL, total bilirubin; DBIL, direct bilirubin; AFP, α-fetorpotein; TACE, transarterial chemoembolization; KA, Kang’ai injection.
Group: TACE + KA = 2, TACE = 1.
Maximum tumor size: 3.1-5.0 cm=1, 5.1-10.0 cm = 2, ≥10.1 cm = 3.
Tumor number: 1 tumor = 1, 2-3 tumors = 2, ≥4 tumors = 3.
As for TTP, univariate analysis demonstrated the following 5 factors as independent prognostic variables: group (HR 0.646, 95% CI 0.407-0.946, P = .027), maximum tumor size (HR 1.176, 95% CI 1.102-1.255, P < .001), tumor number (HR 0.576, 95% CI 0.416-0.796, P = .001), DBIL (HR 1.060, 95% CI 1.010-1.112, P = .038), and AFP level (HR 1.894, 95% CI 1.390-2.580, P < .001) (Table 4). The multivariate analysis showed only the following 3 factors as independent prognostic factors: group (HR 0.694, 95% CI 0.411-0.965, P = .046), maximum tumor size (HR 1.139, 95% CI 1.049-1.237, P = .002), and AFP level (HR 1.481, 95% CI 1.064-2.060, P = .020) (Table 4).
Table 4.
Univariate and Multivariate Cox Analysis of Prognostic Factors for Time to Radiologic Progression.
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| Baseline Variables | HR | 95%CI | P value | HR | 95% CI | P |
| Groupa | 0.648 | 0.407-0.946 | .027 | 0.694 | 0.411-0.965 | .046 |
| Maximum tumor sizeb | 1.176 | 1.102-1.255 | .000 | 1.139 | 1.049-1.237 | .002 |
| AFPc | 1.894 | 1.390-2.580 | .000 | 1.481 | 1.064-2.060 | .020 |
| Tumor numberd | 0.576 | 0.416-0.796 | .001 | |||
| DBIL | 1.060 | 1.010-1.112 | .038 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; AFP, α-fetoprotein; DBIL, direct bilirubin; TACE, transarterial chemoembolization; KA, Kang’ai injection.
Group: TACE + KA = 2, TACE = 1.
Maximum tumor size: 3.1-5.0 cm = 1, 5.1-10.0 cm = 2, ≥10.1 cm = 3.
AFP <200 ng/mL = 1, 200-1000 ng/mL = 2, >1000 ng/mL = 3.
Tumor number: 1 tumor = 1, 2-3 tumors = 2, ≥4 tumors = 3.
Adverse Events
After TACE treatment, postembolization syndrome, consisting of fever, abdominal pain, nausea, and a mild-to-moderate degree of ileus, is the most frequent complication, which is followed by infection. Infection should be considered when a patient presents with high fever (≥39.0 °C), or elevated WBC counts with increased neutrophil percentage, or positive blood culture for bacteria. Severe TACE-related complications may include oncolytic syndrome, tumor rupture, gastrointestinal bleeding, deep venous thrombosis, acute cholecystitis, femoral artery pseudoaneurysm, acute pancreatitis, acute hepatic failure, all of which contribute to TACE-related mortality (defined as the mortality occurring within 1 month post-TACE treatment). In our study, 14 patients in the TACE + KA group and 21 in the TACE group developed postembolization syndrome (29.2% vs 51.2%, P = .034). Ten patients in the TACE + KA group and 14 in the TACE group had infection (20.8% vs 34.1%, P = .158). No TACE-related mortality was recorded in both groups. As for KA treatment, 5 patients had mild allergic reaction (red, itchy and small rashes over the arms and body) after receiving KA infusion, which disappeared after stoppage of KA and receiving antihistamine therapy.
Discussion
Currently, the therapeutic approaches to intermediate stage HCC are still disputed. There is substantial evidence supporting that hepatic resection is both safe and effective for treating selected patients with intermediate stage HCC (eg, a single tumor larger than 5cm in diameter or multiple tumors regardless of size and number),18 and hepatic resection was shown to achieve a survival benefit over TACE.19,20 Therefore, Galle et al21 argued that the usage of the standard therapeutic approach (ie, TACE) for all intermediate stage HCC patients was probably an oversimplification and might not be appropriate for all patients, because intermediate stage HCC comprised the largest subgroup of patients with substantial disease heterogeneity, in which liver function impairment, tumor burden, serum α-fetoprotein level, microvascular invasion, and tumor differentiation contributed to the prognosis. Nevertheless, TACE was still recommended as the first-line therapy for intermediate stage HCC by the BCLC system due to the fact that HR is associated with substantial risks of liver failure and unacceptably high recurrence rates.18
Since 2002, TACE was established as the standard of care for intermediate stage HCC when 2 randomized studies showed a survival benefit.11,22 However, the outcomes of HCC treated by TACE remained unsatisfactory. Previous studies reported that the 1-, 2-, and 3-year survival rates ranged 57% to 82%, 31% to 63%, and 26% to 47%, respectively,11,22 the median survival periods between 17.4 and 34 months,9,10,23 and the median TTP periods from 4.9 to 7.9 months.23-25 In the present study, we clearly demonstrated that adding adjuvant therapy of KA injection to TACE improved the efficacy of TACE per se in the treatment of intermediate stage HCC in BCLC classification system. Patients receiving both TACE and KA injections had significantly prolonged OS and TTP, higher survival rates, and reduced incidence of postembolization syndrome as well as lower frequencies of intrahepatic and extrahepatic metastases, when compared with those receiving only TACE.
As we know, TACE procedure can led to ischemic or hypoxic change of HCC, which elicits angiogenic activity, resulting in recurrence, proliferation, and metastasis of residual tumors.26,27 In theory, a therapy that can compensate for the angiogenic and proliferative effects of TACE may improve the efficacy of TACE. As mentioned before, KA injection is a composite antitumor formula for injection that consists mainly of ginsenosides Rg1, Re, Rf, Rb1, and astragaloside.13 Ginsenosides were shown to have anticancer, antioxidant, antiproliferative and anti-inflammatory properties. Li et al28 reported that ginsenoside Rg1 is an effective anticancer and antioxidant constituent of total saponins of Panax ginseng, which has antitumor activity in TF-1/Epo cells by inhibiting TF-1/Epo cell proliferation and inducing cell apoptosis. Su et al29 showed that ginsenoside Rg1 could inhibit mRNA expression and production of pro-inflammatory mediators, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, cyclooxygenase (COX)-2, and inducible nitric oxide synthase (iNOS) from lipopolysaccharide (LPS)-stimulated macrophages. After heating, ginsenoside Re can convert into ginsenosides Rg2, Rg6, and F4, which have strong anticancer effects by causing S phase arrest, activating caspase-8, caspase-9, and caspase-3, and altering apoptotic factors such as Bcl-2 and Bax.30 Ginsenoside Re can also inhibit mRNA expression and production of pro-inflammatory mediators (TNF-α, IL-1β, IL-6, COX-2, and iNOS).29 Ginsenoside Rf can induce G2/M phase cell cycle arrest, cell apoptosis, upregulation of Bax and downregulation of Bcl-2, Cdk1, and cyclin B1, activation of caspase-3 and -9, and the release of cytochrome c in human osteosarcoma cell lines MG-63, OS732, U-2OS, HOS, and SAOS-2.31 Nevertheless, Okita et al32 argued that ginsenoside Rb1 had no anti-proliferative effects on human hepatoma cells.
Moreover, astragalosides were shown to possess antimetastatic and antiangiogenic effects. Astragaloside II can sensitize human cancerous cells resistant to 5-FU-induced cell death by inhibiting autophagy through MAPK-mTOR pathway to interfere with Beclin-1 and LC3.33 Astragaloside IV when combined with curcumin displays synergistic effects in suppressing tumor growth and angiogenesis in an orthotopic nude-mouse model of human HCC.34 Astragaloside IV can also attenuate the invasive and migratory abilities of HCC cells through the inhibition of epithelial-mesenchymal transition by targeting the Akt/GSK-3β/β-catenin pathway.35
Our study showed that KA injection in combination with TACE was able to reduce the incidence of intrahepatic and extrahepatic metastases of HCC and postembolization syndrome compared with TACE alone, resulting in delayed TTP and improved survival benefits, which may be attributed to the anticancer, antiproliferative, antiangiogenic, antimetastatistic, and anti-inflammatory effects of the constituents in KA injection. These constituent molecules may work in a synergistic manner, which still needs to be clarified in future studies.
Our study had some discrepancies with previous studies in terms of median OS periods, survival rates and median TTP periods, which may be due to difference in patient selection, in terms of liver function status, etiology, maximum tumor size, number of tumor lesions, and other factors. Takayasu et al36 showed that patients with Child-Pugh class A had better 3-year survival than patients with Child-Pugh class B after TACE treatment. Cantarini et al37 demonstrated that patients with HBV-related HCC might have a worse prognosis than those with HCV-related HCC. Takayasu38 reported that the 3-year survival of patients with 2 or 3 tumors >3 cm or 4 or more tumors was 55% and 46% in Child-Pugh class A, respectively, and 30% and 22% in class B, respectively. In our study, male patients accounted for more than 75%, Child-Pugh class B more than 50%, HBV infection more than 65%, large tumor larger than 5 cm more than 60%, and multiple lesions more than 55%. Differences in these factors may account for the discrepant clinical outcomes between our study and others. Zhu et al39 reported that AFP ≥400 ng/mL, vascular invasion and TACE treatment were associated with patient survival after TACE. Takayasu et al9 reported that degree of liver damage, maximum tumor size, number of lesions, and portal vein invasion were independent predictors for patient survival after TACE. In our study, we found that group (or treatment modality), maximum tumor size, and tumor number were significant predictors for OS, while group (or treatment modality), maximum tumor size and AFP level were independent predictors for TTP, which is similar to these studies.
Our study had the following limitations. First, the sample sizes in the TACE + KA and TACE groups were limited. Second, the treatment allocation was not randomized. Third, the follow-up period was relatively short, for only 40 months.
Conclusions
In summary, our study showed that adding adjuvant therapy of KA injection to TACE improved the efficacy of TACE alone in the treatment of intermediate HCC with delayed tumor progression, improved patient survival, and reduced postembolization syndrome, which is probably associated with the anti-inflammatory, antitumor, and antimetastatic effects of KA constituents. More randomized, controlled trials with larger sample sizes and those recruiting patients with more diverse backgrounds are warranted to achieve more reliable results.
Acknowledgments
We thank Prof Jian-Ping Zhu and Jin-Min Pu for their excellent CT evaluation comments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. International Agency for Research on Cancer; World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed September 18, 2017.
- 2. Chen W, Zheng R, Zhang S, et al. The incidences and mortalities of major cancers in China, 2009. Chin J Cancer. 2013;32:106-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752-1763. [DOI] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [DOI] [PubMed] [Google Scholar]
- 7. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [DOI] [PubMed] [Google Scholar]
- 8. Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127(suppl 1):S179-S188. [DOI] [PubMed] [Google Scholar]
- 9. Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469. [DOI] [PubMed] [Google Scholar]
- 10. Llovet JM, Di Bisceglie AM, Bruix J, et al. ; Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [DOI] [PubMed] [Google Scholar]
- 11. Llovet JM, Real MI, Montaña X, et al. ; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [DOI] [PubMed] [Google Scholar]
- 12. Wang X, Wang N, Cheung F, Lao L, Li C, Feng Y. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integr Med. 2015;13:142-164. [DOI] [PubMed] [Google Scholar]
- 13. Yang Z, Zhang YA, Zhu YJ, Sun ZP, Li XR. UPLC-MS/MS determination of five main components in kang’ai injection. Chin Pharm J. 2011;46:297-299. [Google Scholar]
- 14. He XR, Han SY, Li PP. Injectable Chinese herbal formula Kang’ai for nonsmall cell lung cancer: trial sequential analysis of 2,259 participants from 31 randomized controlled trials. J Cancer Res Ther. 2016;12:735-743. [DOI] [PubMed] [Google Scholar]
- 15. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [DOI] [PubMed] [Google Scholar]
- 16. Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627-634. [DOI] [PubMed] [Google Scholar]
- 17. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhong JH, Rodríguez AC, Ke Y, Wang YY, Wang L, Li LQ. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine (Baltimore). 2015;94:e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pecorelli A, Lenzi B, Gramenzi A, et al. ; Italian Liver Cancer (ITA.LI.CA) group. Curative therapies are superior to standard of care (transarterial chemoembolization) for intermediate stage hepatocellular carcinoma. Liver Int. 2017;37:423-433. [DOI] [PubMed] [Google Scholar]
- 20. Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [DOI] [PubMed] [Google Scholar]
- 21. Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumour beyond TACE: from surgery to systemic therapy. J Hepatol. 2017;67:173-183. [DOI] [PubMed] [Google Scholar]
- 22. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [DOI] [PubMed] [Google Scholar]
- 23. Lewandowski RJ, Mulcahy MF, Kulik LM, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255:955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hao MZ, Lin HL, Chen QZ, et al. Safety and efficacy of transcatheter arterial chemoembolization with embospheres in treatment of hepatocellular carcinoma. J Dig Dis. 2017;18:31-39. [DOI] [PubMed] [Google Scholar]
- 25. Dong J, Zhai X, Chen Z, et al. Treatment of huge hepatocellular carcinoma using cinobufacini injection in transarterial chemoembolization: a retrospective study. Evid Based Complement Alternat Med. 2016;2016:2754542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Wei Q, Zuo GW, et al. Ginsenoside Rg1 induces apoptosis through inhibition of the EpoR-mediated JAK2/STAT5 signalling pathway in the TF-1/ Epo human leukemia cell line. Asian Pac J Cancer Prev. 2014;15:2453-2459. [DOI] [PubMed] [Google Scholar]
- 29. Su F, Xue Y, Wang Y, Zhang L, Chen W, Hu S. Protective effect of ginsenosides Rg1 and Re on lipopolysaccharide-induced sepsis by competitive binding to toll-like receptor 4. Antimicrob Agents Chemother. 2015;59:5654-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jang HJ, Han IH, Kim YJ, et al. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J Agric Food Chem. 2014;62:2830-2836. [DOI] [PubMed] [Google Scholar]
- 31. Shangguan WJ, Li H, Zhang YH. Induction of G2/M phase cell cycle arrest and apoptosis by ginsenoside Rf in human osteosarcoma MG-63 cells through the mitochondrial pathway. Oncol Rep. 2014;31:305-313. [DOI] [PubMed] [Google Scholar]
- 32. Okita K, Li Q, Murakamio T, Takahashi M. Anti-growth effects with components of Sho-saiko-to (TJ-9) on cultured human hepatoma cells. Eur J Cancer Prev. 1993;2:169-175. [DOI] [PubMed] [Google Scholar]
- 33. Wang M, Huang C, Su Y, Yang C, Xia Q, Xu DJ. Astragaloside II sensitizes human hepatocellular carcinoma cells to 5-fluorouracil via suppression of autophagy. J Pharm Pharmacol. 2017;69:743-752. [DOI] [PubMed] [Google Scholar]
- 34. Zhang S, Tang D, Zang W, et al. Synergistic inhibitory effect of traditional chinese medicine astragaloside IV and curcumin on tumor growth and angiogenesis in an orthotopic nude-mouse model of human hepatocellular carcinoma. Anticancer Res. 2017;37:465-473. [DOI] [PubMed] [Google Scholar]
- 35. Qin CD, Ma DN, Ren ZG, et al. Astragaloside IV inhibits metastasis in hepatoma cells through the suppression of epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin pathway. Oncol Rep. 2017;37:1725-1735. [DOI] [PubMed] [Google Scholar]
- 36. Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56:886-892. [DOI] [PubMed] [Google Scholar]
- 37. Cantarini MC, Trevisani F, Morselli-Labate AM, et al. ; Italian Liver Cancer (ITA.LI.CA) group. Effect of the etiology of viral cirrhosis on the survival of patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101:91-98. [DOI] [PubMed] [Google Scholar]
- 38. Takayasu K. Transarterial chemoembolization for hepatocellular carcinoma over three decades: current progress and perspective. Jpn J Clin Oncol. 2012;42:247-255. [DOI] [PubMed] [Google Scholar]
- 39. Zhu SL, Zhong JH, Ke Y, Ma L, You XM, Li LQ. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol. 2015;21:9630-9637. [DOI] [PMC free article] [PubMed] [Google Scholar]


