Abstract
Background
Magnolia tree bark has been widely used in traditional Asian medicine. However, to our knowledge, no studies have been reported investigating the effects of dietary supplementation with magnolia bark extract in chickens.
Objective
We tested the hypothesis that dietary supplementation of chickens with a Magnolia officinalis bark extract would increase growth performance in uninfected and Eimeria maxima/Clostridium perfringens co-infected chickens.
Methods
A total of 168 chickens were fed from hatch either a standard diet or a diet supplemented with 0.33 mg or 0.56 mg M. officinalis bark extract/kg (M/H low or M/H high, respectively) from days 1 to 35. At day 14, half of the chickens were orally infected with E. maxima, followed by C. perfringens infection at day 18 to induce experimental avian necrotic enteritis. Daily feed intake, feed conversion ratio, body weight gain, and final body weight were measured as indicators of growth performance. Serum α1-acid glycoprotein (AGP) concentrations were measured as an indicator of systemic inflammation, and intestinal lesion scores were determined as a marker of disease progression. Transcript levels for catalase, heme oxygenase 1, and superoxide dismutase in the intestine, liver, spleen, and skeletal muscle were measured as indicators of antioxidant status.
Results
Growth performance increased between days 1 and 35 in uninfected and E. maxima/C. perfringens co-infected chickens fed M/H-low or M/H-high diets compared with unsupplemented controls. Gut lesion scores were decreased, whereas AGP concentrations were unchanged, in co-infected chickens fed magnolia-supplemented diets compared with unsupplemented controls. In general, transcripts for antioxidant enzymes increased in chickens fed magnolia-supplemented diets compared with unsupplemented controls, and significant interactions between dietary supplementation and co-infection were observed for all antioxidant enzyme transcript levels.
Conclusion
Magnolia bark extract might be useful for future development of dietary strategies to improve poultry health, disease resistance, and productivity without the use of antibiotic growth promoters.
Keywords: chicken, Eimeria maxima, Clostridium perfringens, necrotic enteritis, magnolia
Introduction
The bark of Magnolia officinalis and Magnolia obovata have been used in traditional Chinese, Japanese, and Korean medicine (1). The Chinese name houpu refers to the thick (hou) bark and unadorned (pu) part of the plant (2). Historically, houpu has been used in humans to treat anxiety and mood disorders (3) and as an analgesic (1). The major active components of houpu include magnolol, honokiol, 4-O-methylhonokiol, and obovatol (1). Both magnolol and its isomer honokiol selectively interact with γ-aminobutyric acid receptors (4). These biphenolic lignans possess in vitro and in vivo anxiolytic, nootropic, antidepressant, anti-inflammatory, antitumorigenic, and antioxidant activities (5–11). However, relatively few studies, if any, have investigated the medicinal effects of magnolia bark extracts, or its active compounds, in veterinary medicine.
Among the most important and economically devastating infectious diseases for the global poultry industry are avian coccidiosis and necrotic enteritis, with estimated annual losses exceeding $6 billion (12–14). These related diseases are caused by 2 distinct enteric pathogens, Eimeria (coccidiosis) and Clostridium perfringens (necrotic enteritis). Field outbreaks of coccidiosis typically occur simultaneously with necrotic enteritis. Over the past 40 y, prophylactic use of therapeutic antibiotic growth promoters and anticoccidial drugs has been used to control coccidiosis and necrotic enteritis. However, coccidiosis and necrotic enteritis have re-emerged as serious threats facing the commercial poultry industry as a consequence of the international movement to ban antibiotics use in food animal production (15, 16).
A large, diverse array of alternatives to antibiotics for control of avian coccidiosis and necrotic enteritis have been reported (12, 17). Among these are plant-based phytochemicals (18). Phytochemicals that have been shown to enhance protective immunity to coccidiosis and necrotic enteritis include anethole, artemisinin, capsaicin, carvacrol, cinnamaldehyde, curcumin, propyl thiosulfinate, and propyl thiosulfinate oxide (19–24). Plant-based chemical extracts that increase disease resistance in which the active components have not been identified include those from Aloe vera (aloe), Artemisia annua (sweet wormwood), Azadirachta indica (neem), Bidens pilosa (Spanish needle), Capsicum spp. (pepper), Carthamus tinctorius (safflower), Curcuma longa (turmeric), Echinacea purpurea (purple cone flower), Gyamopsis tetragonoloba (guar), Khaya senegalensis (African mahogany), Prunus salicina (oriental plum), Punica granatum (pomegranate), and the mushrooms Lentinus edodes (shiitake), Ganoderma lucidum (reishi), and Fomitella fraxinea (20, 25–38). The current study was undertaken to investigate whether dietary supplementation with magnolia bark extract might promote the growth performance, antioxidant responses, or both in broiler chickens that were either uninfected or co-infected with E. maxima and C. perfringens in an experimental model of avian necrotic enteritis.
Methods
Preparation of magnolia extract
An extract of M. officinalis bark was obtained from Pancosma (Geneva, Switzerland). Briefly, the crude material was washed, dried at 50°C to a dry matter content of ≥91%, and comminuted. The dried material was extracted with supercritical carbon dioxide at a flow rate of 1200–1400 L/h for 3.5 h at a pressure of 25–30 MPa and a temperature of 35–40°C, and the extract was taken up in ethanol (39).
Chickens and experimental design
All of the experiments were approved by the Beltsville Agricultural Research Center Institutional Animal Care and Use Committee. A total of 168 day-old Ross 708 male broiler chickens (28 chickens/group, 7 chickens/experimental unit) were obtained from Longenecker's Hatchery (Elizabethtown, PA), randomly housed in Petersime starter brooder cage units, and provided with feed and water ad libitum. The experimental design consisted of a completely randomized 2 × 3 factorial arrangement with uninfected or E. maxima/C. perfringens co-infected chickens, each provided with an unsupplemented diet or with diets supplemented with 0.33 mg (M/H low) or 0.56 mg (M/H high) M. officinalis bark extract/kg.
Experimental necrotic enteritis disease model
Chickens were fed an antibiotic-free starter diet containing 18% dry matter protein (USDA-Feed Mill) from days 1 to 18 of age and a standard grower diet containing 24% dry matter protein from days 19 to 35 (Table 1, Figure 1). Both diets were either unsupplemented or supplemented with low or high amounts of magnolia bark extract. Chickens were infected by oral gavage on day 14 with 1.0 × 104 oocysts/bird of E. maxima Beltsville strain 41A, as described (40). At day 18, E. maxima–infected chickens were infected by oral gavage with 1.0 × 109 CFUs/bird of C. perfringens strain Del-1 (40). Uninfected chickens received an equal volume of PBS by oral gavage.
TABLE 1.
Diet composition
| Low protein, | High protein, | |
|---|---|---|
| % | % | |
| Ingredient | ||
| Corn | 69.01 | 55.78 |
| Soybean meal | 23.99 | 37.03 |
| Soybean oil | 2.75 | 2.97 |
| Dicalcium phosphate | 2.00 | 1.80 |
| Calcium carbonate | 1.40 | 1.51 |
| Salt | 0.35 | 0.38 |
| Poultry vitamin mix1 | 0.20 | 0.22 |
| Poultry mineral mix2 | 0.15 | 0.15 |
| dl-Methionine | 0.10 | 0.10 |
| Choline chloride (60%) | 0.05 | 0.06 |
| Total | 100 | 100 |
| Calculated values (dry matter basis) | ||
| Crude protein | 18.00 | 24.00 |
| Calcium | 1.19 | 1.20 |
| Available phosphorus | 0.54 | 0.51 |
| Lysine | 1.00 | 1.40 |
| Methionine | 0.42 | 0.49 |
| Cysteine + methionine | 0.65 | 0.80 |
| True metabolizable energy, kcal/kg | 3585 | 3450 |
The vitamin mixture provided the following nutrients per kilogram of diet: vitamin A, 2000 IU; vitamin D3, 22 IU; vitamin E, 16 mg; vitamin K, 0.1 mg; thiamin, 3.4 mg; riboflavin, 1.8 mg; vitamin B-6, 6.4 mg; vitamin B-12, 0.013 mg; biotin, 0.17 mg; pantothenic acid, 8.7 mg; folic acid, 0.8 mg; and niacin, 23.8 mg.
The mineral mixture provided the following nutrients per kilogram of diet: Fe, 0.4 mg; Zn, 0.2 mg; Mn, 0.18 mg; Co, 0.0013 mg; Cu, 0.021 mg; and Se, 0.0002 mg.
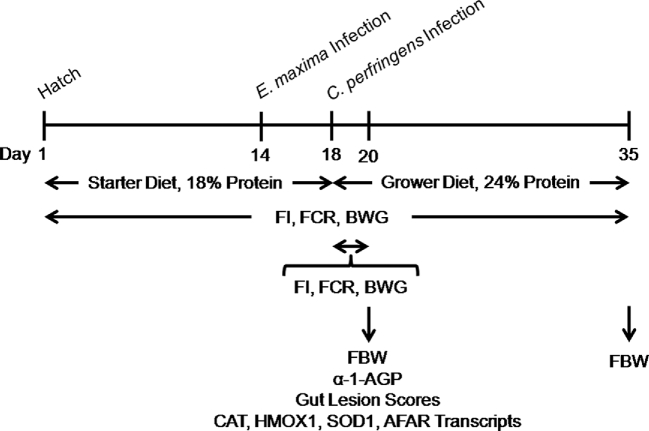
FIGURE 1.
Schematic outline of the experimental design. AFAR, aflatoxin B1 aldehyde reductase; BWG, body weight gain; CAT, catalase; FBW, final body weight; FCR, feed conversion ratio; FI, average daily feed intake; HMOX1, heme oxygenase 1, SOD1, superoxide dismutase 1; α-1-AGP, α1-acid glycoprotein.
Measurement of chicken growth performance, serum α1-acid glycoprotein, and intestinal lesion scores
Average daily feed intake, feed conversion ratio, body weight gain, and final body weight were measured as variables of growth performance, as described (40). Each variable was measured during 2 time periods: the entire experimental period (days 1–35) and in the 2-d period immediately after C. perfringens infection in co-infected chickens (days 18–20) when intestinal lesions are at maximum size (12). Serum samples were collected at day 20 and α1-acid glycoprotein (AGP) concentrations were determined by ELISA, as described (41). Intestinal lesion scores were determined at day 20 on a scale from 0 (none) to 4 (high) by 5 independent observers in a blinded fashion, as described (42).
Antioxidant and detoxifying gene transcript level measurements
Chickens were killed on day 20 by cervical dislocation; the intestinal jejunum, liver, spleen, and breast muscle were collected and homogenized by using a hand-held rotor-stator homogenizer (TissueRuptor; Qiagen); and tissue homogenates processed by qRT-PCR for levels of transcripts encoding the antioxidant enzymes catalase (CAT), heme oxygenase 1 (HMOX1), and superoxide dismutase 1 (SOD) 1, and the detoxifying enzyme aflatoxin B1 aldehyde reductase (AFAR), as described (43). Briefly, total RNA was extracted by using TRIzol (Invitrogen) and purified with the RNeasy Mini RNA purification kit (Qiagen), and 5.0 μg total RNA were treated with 1.0 U DNase I (Sigma) for 15 min at 22°C followed by 10 min at 70°C. The RNA was reverse-transcribed by using the StrataScript first-strand synthesis system (Stratagene) according to the manufacturer's recommendations. cDNA was amplified by using the Mx3000P QPCR system (Agilent Technologies) and Brilliant SYBR Green qPCR master mix (Stratagene) for 40 cycles at 72°C for 1 min with oligonucleotide primers for chicken CAT, HMOX1, SOD1, AFAR, and GAPDH (Table 2). The levels of individual transcripts were normalized to those of GAPDH by the Q-gene program (44). To normalize individual replicates, the logarithmic-scaled threshold cycle (Ct) values were transformed to linear units of normalized expression before calculating the means and SEMs for the references and individual targets, followed by determination of mean normalized expression (44).
TABLE 2.
Primers used for real-time PCR1
| Target gene | Primer sequence | Annealing temperature | GenBank accession no. |
|---|---|---|---|
| CAT | F 5′-ACTGCAAGGCGAAAGTGTTT-3′ | 58°C | NM001031215.1 |
| R 5′-GGCTATGGATGAAGGATGGA-3′ | |||
| HMOX1 | F 5′-CTGGAGAAGGGTTGGCTTTCT-3′ | 60°C | NM205344 |
| R 5′-GAAGCTCTGCCTTTGGCTGTA-3′ | |||
| SOD1 | F 5′-ATTACCGGCTTGTCTGATGG-3′ | 58°C | NM205064.1 |
| R 5′-CCTCCCTTTGCAGTCACATT-3′ | |||
| AFAR | F 5′-CAAACTGCAGGGTTCTCTTGG-3 | 60°C | XM417628.2 |
| R 5′-GAAGTAGTTGGGGCAGTCGTG-3′ | |||
| GAPDH | F 5′-GGTGGTGCTAAGCGTGTTAT-3′ | 60°C | K01458 |
| R 5′-ACCTCTGTCATCTCTCCACA-3′ |
AFAR, aflatoxin B1 aldehyde reductase; CAT, catalase; F, forward primer; HMOX1, heme oxygenase 1; R, reverse primer; SOD1, superoxide dismutase 1.
Statistical analysis
Each cage was considered as an experimental unit. All of the treatments were conducted in quadruplicate and data were expressed as mean ± SEM values for each treatment group. Mean separations were carried out by using Duncan's multiple range test. Statistical analysis was carried out by 2-factor ANOVA by using SAS software (version 9.4; SAS Institute) with magnolia extract supplementation and variables of necrotic enteritis as main factors and interaction of main effects. P values ≤0.05 were considered significant.
Results
Growth performance
The effect of dietary supplementation with magnolia bark extract on chicken growth performance is presented in Table 3. Growth performance was measured over the entire experimental period (days 1–35) and in the 2-d period immediately after C. perfringens infection in co-infected chickens [days 18–20 when intestinal lesions are maximum (12)]. Between days 1 and 35, both uninfected and E. maxima/C. perfringens co-infected chickens fed the magnolia extract–supplemented diet showed increased daily feed intakes, body weight gains, and final body weights compared with birds given the unsupplemented diet. Between days 18 and 20, uninfected and co-infected chickens fed the magnolia-supplemented diet showed increased body weight gains and final body weights, but decreased feed conversion ratios, compared with unsupplemented controls. Between days 1 and 35, E. maxima/C. perfringens co-infected chickens fed both the unsupplemented and magnolia-supplemented diets showed increased daily feed intakes and feed conversion ratios compared with uninfected birds. Between days 18 and 20, co-infected chickens fed the unsupplemented and magnolia-supplemented diets showed increased daily feed intakes and feed conversion ratios, but decreased body weight gains and final body weights, compared with uninfected controls. No significant interactions between dietary magnolia supplementation and E. maxima/C. perfringens co-infection either at days 1–35 or days 18–20 were observed on daily feed intakes, feed conversion ratios, body weight gains, or final body weights.
TABLE 3.
Effect of magnolia extract dietary supplementation and E. maxima/C. perfringens co-infection on growth performance in broiler chickens1
| Days 1–35 | Days 18–20 | |||||||
|---|---|---|---|---|---|---|---|---|
| FI2 | FCR3 | BWG4 | FBW5 | FI6 | FCR7 | BWG8 | FBW9 | |
| Uninfected | ||||||||
| Basal diet | 79.3 | 1.56 | 50.9 | 1827.6 | 116.5 | 2.02 | 57.3 | 593.4 |
| M/H low | 85.8 | 1.53 | 56.3 | 2012.2 | 109.8 | 1.78 | 62.0 | 688.6 |
| M/H high | 84.0 | 1.49 | 56.5 | 2020.0 | 107.3 | 1.71 | 63.0 | 703.3 |
| Co-infected | ||||||||
| Basal diet | 85.5 | 1.67 | 51.4 | 1843.4 | 138.5 | 3.96 | 36.5 | 572.4 |
| M/H low | 87.5 | 1.55 | 56.2 | 2010.9 | 124.3 | 2.80 | 48.8 | 650.3 |
| M/H high | 91.0 | 1.67 | 54.9 | 1963.8 | 135.8 | 2.49 | 50.8 | 638.8 |
| Pooled SEM | 1.50 | 0.04 | 1.53 | 53.5 | 6.95 | 0.25 | 2.61 | 22.0 |
| Main effect means | ||||||||
| Feed | ||||||||
| Basal | 82.4 | 1.62 | 51.2 | 1835.3 | 127.5 | 2.99 | 46.9 | 582.9 |
| M/H low | 86.7 | 1.54 | 56.3 | 2011.6 | 117.1 | 2.29 | 55.4 | 669.5 |
| M/H high | 87.5 | 1.58 | 55.7 | 1991.9 | 121.6 | 2.10 | 56.9 | 671.1 |
| Pooled SEM | 1.06 | 0.03 | 1.08 | 37.79 | 4.91 | 0.18 | 1.85 | 15.54 |
| Necrotic enteritis | ||||||||
| Uninfected | 83.0 | 1.53 | 54.6 | 1953.3 | 111.2 | 1.84 | 60.8 | 661.8 |
| Co-infected | 88.0 | 1.63 | 54.2 | 1939.4 | 132.9 | 3.08 | 45.4 | 620.5 |
| Pooled SEM | 0.86 | 0.03 | 0.88 | 30.86 | 4.01 | 0.15 | 1.51 | 12.69 |
| Source of variation, P | ||||||||
| Feed | 0.0067 | 0.2946 | 0.0072 | 0.0074 | 0.3389 | 0.0065 | 0.0025 | 0.0335 |
| Necrotic enteritis | 0.0007 | 0.0088 | 0.7574 | 0.7538 | 0.0013 | <0.0001 | <0.0001 | 0.0009 |
| Feed × necrotic enteritis | 0.1943 | 0.2394 | 0.7824 | 0.7831 | 0.6098 | 0.0841 | 0.2329 | 0.6165 |
BWG, body weight gain; FBW, final body weight; FCR, feed conversion ratio; FI, feed intake; M/H high, 0.56 mg M. officinalis bark extract/kg; M/H low, 0.33 mg M. officinalis bark extract/kg.
Average daily FI between days 1 and 35 in g/chicken.
FCR between days 1 and 35 in g feed/g BWG.
BWG between days 1 and 35 in g/chicken.
FBW at day 35 in g/chicken.
Average daily FI between days 18 and 20 in g/chicken.
FCR between days 18 and 20 in g feed/g BWG.
BWG between days 18 and 20 in g/chicken.
FBW at day 20 in g/chicken.
Serum AGP concentrations
At day 20, serum AGP concentrations were unchanged in both uninfected and E. maxima/C. perfringens co-infected chickens fed the magnolia extract–supplemented diet compared with chickens fed the unsupplemented diet (Figure 2A). At the same time, AGP concentrations were increased in E. maxima/C. perfringens co-infected chickens fed both the unsupplemented and magnolia-supplemented diets compared with uninfected chickens (P = 0.0002). No significant interaction between dietary magnolia supplementation and E. maxima/C. perfringens co-infection was observed on AGP concentrations.
FIGURE 2.
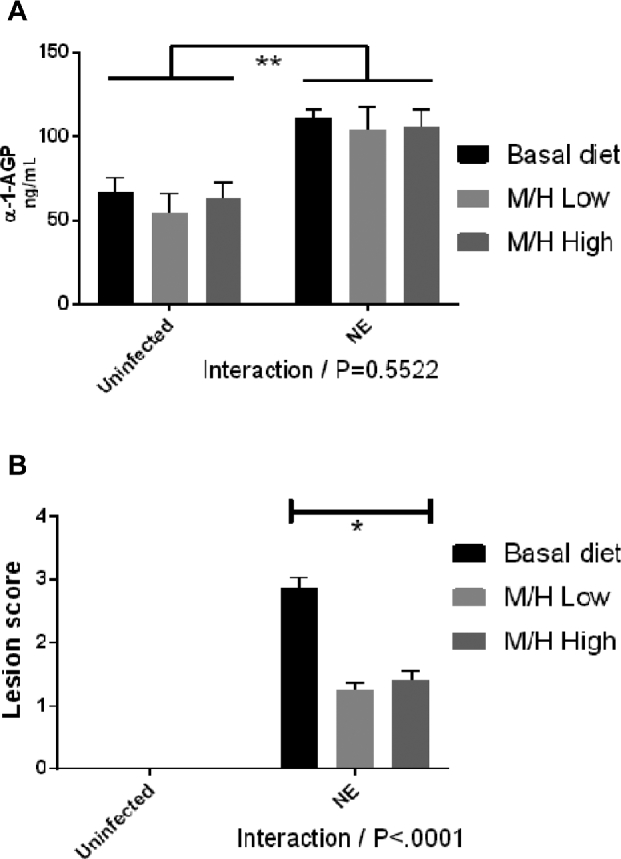
Effects of dietary supplementation with magnolia bark extract on serum α-1-AGP concentrations (A) and intestinal lesion scores (B). Chickens were fed unsupplemented or magnolia extract–supplemented diets and were uninfected or co-infected with E. maxima and C. perfringens. Serum α-1-AGP concentrations and intestinal lesion scores were measured on day 20. Values are means ± SEMs, n = 4. *Difference between chickens fed the magnolia extract–supplemented diet compared with unsupplemented controls, P < 0.05. E. maxima/C. perfringens co-infected chickens compared with uninfected controls, P < 0.05. P values for the interaction between dietary magnolia extract supplementation and E. maxima/C. perfringens co-infection are listed below each panel. M/H high, 0.56 mg M. officinalis bark extract/kg; M/H low, 0.33 mg M. officinalis bark extract/kg; NE, Necrotic enteritis; α-1-AGP, α1-acid glycoprotein.
Gut lesion scores
At day 20, gut lesion scores were decreased in E. maxima/C. perfringens co-infected chickens fed the magnolia extract–supplemented diets compared with co-infected chickens fed the unsupplemented diet (Figure 2B) (P < 0.0001).
Antioxidant and detoxifying transcript levels
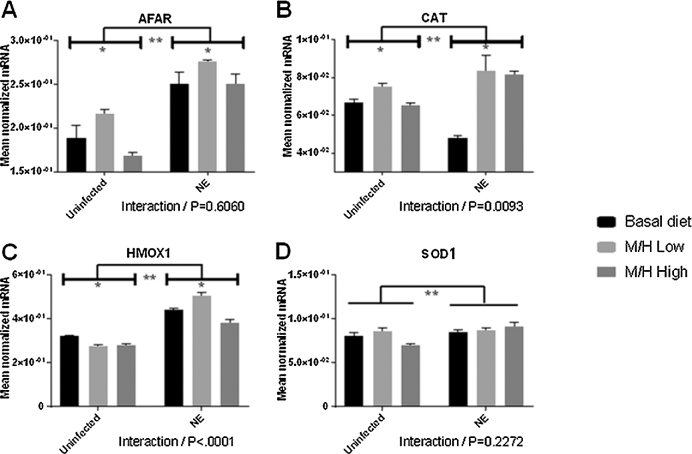
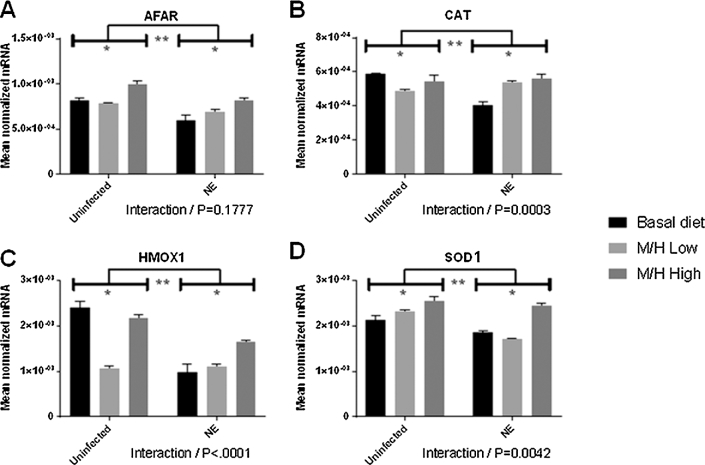
The expression levels of the antioxidant enzymes CAT, HMOX1, SOD1, and the detoxifying enzyme AFAR, in the intestinal jejunum (Figure 3), liver (Figure 4), spleen (Figure 5), and breast muscle (Figure 6) were measured at day 20 (2 d after C. perfringens infection). Previous studies have established that these 4 enzymes are transcriptionally regulated by phytonutrients through the nuclear factor E2–related factor 2 (Nrf2)–Kelch-like ECH-associated protein 1 (Keap1) pathway (45). In the jejunum, AFAR and CAT transcript levels generally increased in both uninfected and E. maxima/C. perfringens co-infected chickens fed the magnolia-supplemented diet compared with unsupplemented controls (Figure 3). HMOX1 transcript levels generally decreased in magnolia-supplemented chickens compared with unsupplemented controls, except for the M/H-low group in co-infected chickens in which HMOX1 transcripts were increased in the supplemented compared with the unsupplemented groups. All 4 transcript levels increased in the magnolia-supplemented, co-infected group compared with the magnolia-supplemented, uninfected group. Significant interactions between magnolia supplementation and E. maxima/C. perfringens co-infection were observed for CAT (P = 0.0093) and HMOX1 (P < 0.001) transcript levels.
FIGURE 3.
Effects of dietary supplementation with magnolia bark extract on antioxidant and detoxifying gene transcript levels in the intestine. Chickens were fed unsupplemented or magnolia extract–supplemented diets and were uninfected or co-infected with E. maxima and C. perfringens. The levels of transcripts for AFAR (A), CAT (B), HMOX1 (C), and SOD1 (D) in the intestinal jejunum were measured at day 20. Values are means ± SEMs, n = 4. *Difference between chickens fed the magnolia extract–supplemented diet compared with unsupplemented controls, P < 0.05. **Difference between E. maxima/C. perfringens co-infected chickens compared with uninfected controls, P < 0.05. P values for the interaction between dietary magnolia extract supplementation and E. maxima/C. perfringens co-infection are listed below each panel. AFAR, aflatoxin B1 aldehyde reductase; CAT, catalase; HMOX1, heme oxygenase 1; M/H high, 0.56 mg M. officinalis bark extract/kg; M/H low, 0.33 mg M. officinalis bark extract/kg; NE, Necrotic enteritis; SOD1, superoxide dismutase 1.
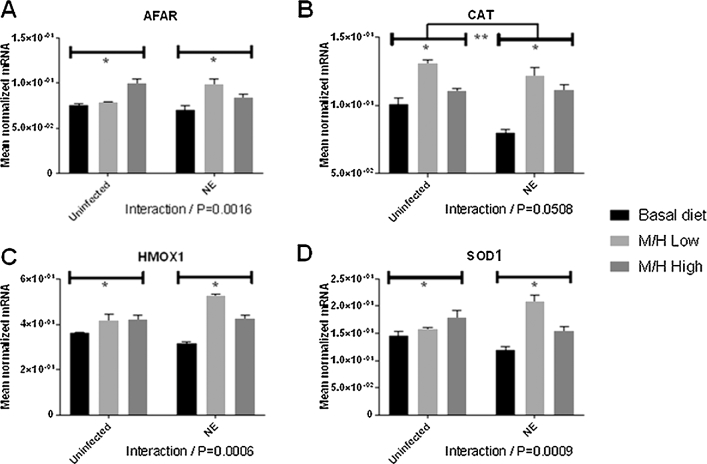
FIGURE 4.
Effects of dietary supplementation with magnolia bark extract on antioxidant and detoxifying gene transcript levels in the liver. Chickens were fed unsupplemented or magnolia extract–supplemented diets and were uninfected or co-infected with E. maxima and C. perfringens. The levels of transcripts for AFAR (A), CAT (B), HMOX1 (C), and SOD1 (D) in the liver were measured at day 20. Values are means ± SEMs, n = 4. *Difference between chickens fed the magnolia extract–supplemented diet compared with unsupplemented controls, P < 0.05. **Difference between E. maxima/C. perfringens co-infected chickens compared with uninfected controls, P < 0.05. P values for the interaction between dietary magnolia extract supplementation and E. maxima/C. perfringens co-infection are listed below each panel. AFAR, aflatoxin B1 aldehyde reductase; CAT, catalase; HMOX1, heme oxygenase 1; M/H high, 0.56 mg M. officinalis bark extract/kg; M/H low, 0.33 mg M. officinalis bark extract/kg; NE, Necrotic enteritis; SOD1, superoxide dismutase 1.
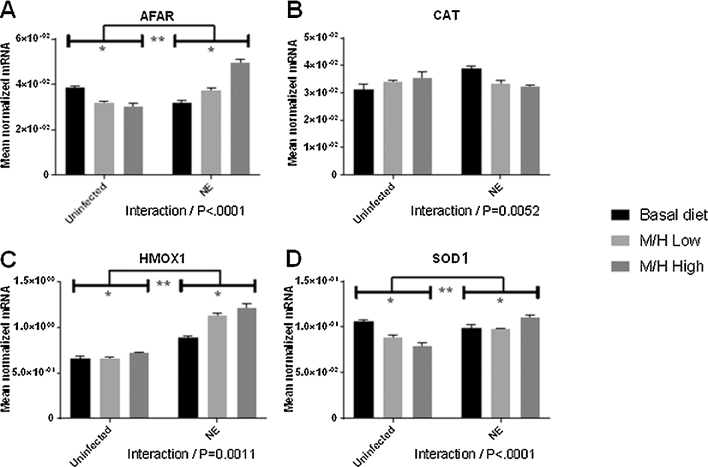
FIGURE 5.
Effects of dietary supplementation with magnolia bark extract on antioxidant and detoxifying gene transcript levels in the spleen. Chickens were fed unsupplemented or magnolia extract–supplemented diets and were uninfected or co-infected with E. maxima and C. perfringens. The levels of transcripts for AFAR (A), CAT (B), HMOX1 (C), and SOD1 (D) in the spleen were measured at day 20. Values are means ± SEMs, n = 4. *Difference between chickens fed the magnolia extract–supplemented diet compared with unsupplemented controls, P < 0.05. **Difference between E. maxima/C. perfringens co-infected chickens compared with uninfected controls, P < 0.05. P values for the interaction between dietary magnolia extract supplementation and E. maxima/C. perfringens co-infection are listed below each panel. AFAR, aflatoxin B1 aldehyde reductase; CAT, catalase; HMOX1, heme oxygenase 1; M/H high, 0.56 mg M. officinalis bark extract/kg; M/H low, 0.33 mg M. officinalis bark extract/kg; NE, Necrotic enteritis; SOD1, superoxide dismutase 1.
FIGURE 6.
Effects of dietary supplementation with magnolia bark extract on antioxidant and detoxifying gene transcript levels in breast muscle. Chickens were fed unsupplemented or magnolia extract–supplemented diets and were uninfected or co-infected with E. maxima and C. perfringens. The levels of transcripts for AFAR (A), CAT (B), HMOX1 (C), and SOD1 (D) in breast muscle were measured at day 20. Values are means ± SEMs, n = 4. *Difference between chickens fed the magnolia extract–supplemented diet compared with unsupplemented controls, P < 0.05. **Difference between E. maxima/C. perfringens co-infected chickens compared with uninfected controls, P < 0.05. P values for the interaction between dietary magnolia extract supplementation and E. maxima/C. perfringens co-infection are listed below each panel. AFAR, aflatoxin B1 aldehyde reductase; CAT, catalase; HMOX1, heme oxygenase 1; M/H high, 0.56 mg M. officinalis bark extract/kg; M/H low, 0.33 mg M. officinalis bark extract/kg; NE, Necrotic enteritis; SOD1, superoxide dismutase 1.
In the liver, the levels of all 4 antioxidant enzyme transcripts increased in both uninfected and E. maxima/C. perfringens co-infected chickens fed the magnolia-supplemented diet compared with unsupplemented controls (Figure 4). In general, all 4 transcript levels increased in co-infected chickens fed the M/H-low diet compared with the uninfected, M/H-low group, but decreased in chickens fed the M/H high diet compared with the uninfected, M/H-high group. Significant interactions between magnolia supplementation and E. maxima/C. perfringens co-infection were observed for AFAR (P = 0.0016), HMOX1 (P = 0.0006), and SOD1 (P = 0.0009).
In the spleen, the levels of AFAR, HMOX1, and SOD1 transcripts increased in the magnolia-supplemented, E. maxima/C. perfringens co-infected group compared with the unsupplemented, co-infected group (Figure 5). HMOX1 transcripts increased, whereas AFAR and SOD1 transcripts decreased, in the magnolia-supplemented, uninfected group compared with the unsupplemented, uninfected group. AFAR, MHOX1, and SOD1 transcripts increased in magnolia-supplemented, co-infected group compared with the magnolia-supplemented, uninfected group. Significant interactions between magnolia supplementation and E. maxima/C. perfringens co-infection were observed for AFAR (P < 0.0001), CAT (P = 0.0052), HMOX1 (P = 0.0011), and SOD1 (P < 0.0001).
In breast muscle, transcript levels for all 4 enzymes generally increased in the magnolia-supplemented, co-infected and supplemented, uninfected groups compared with the corresponding unsupplemented groups, with the exception of CAT and HMOX1, in which decreased transcript levels were seen (Figure 6). In this same tissue, all 4 transcript levels generally decreased in the unsupplemented or magnolia-supplemented, E. maxima/C. perfringens co-infected groups compared with the corresponding uninfected groups'. Significant interactions between magnolia supplementation and E. maxima/C. perfringens co-infection were observed for CAT (P = 0.0003), HMOX1 (P < 0.0001), and SOD1 (P = 0.0042).
Discussion
The present study was conducted to investigate the effects of dietary supplementation of newly hatched broiler chickens with magnolia bark extract on growth performance, antioxidant status, and resistance to necrotic enteritis. During the normal growth period (days 1–35), feed intake, body weight gain, and final body weight increased in uninfected and E. maxima/C. perfringens co-infected chickens fed magnolia-supplemented diets compared with unsupplemented controls. During the time of maximum gut pathology in E. maxima/C. perfringens co-infected chickens (days 18–20; 2 d after C. perfringens infection), body weight gain and final body weight increased, and the feed conversion ratio decreased, in uninfected and co-infected chickens fed magnolia-supplemented diets compared with unsupplemented controls. At day 20, gut lesion scores decreased, whereas AGP concentrations were unchanged, in co-infected chickens fed magnolia-supplemented diets compared with unsupplemented controls. Also at day 20, transcripts for antioxidant enzymes generally increased in chickens fed magnolia-supplemented diets compared with unsupplemented controls, and significant interactions between dietary supplementation and co-infection were observed for all antioxidant enzyme transcript levels.
Growth performance is among the most important outcomes for commercial broiler production. Currently, a typical US commercial broiler consumes a total of ∼9 pounds (∼4082 g) of feed and reaches 5 pounds (∼2268 g) of body weight in 35–40 d, with a live-weight feed conversion ratio of 1.6 kg feed/kg body weight gain (46). Over the past 50 y, US broiler growth rates have doubled and corresponding feed conversion rates have been reduced by ∼50%, primarily as a consequence of improved breeding strategies (47). A number of factors, however, threaten the continuing trend toward more-efficient poultry production, including discontinuation of the use of antibiotic growth promoters and, relatedly, the re-emergence of infectious diseases (12, 14, 17). Dietary supplementation with phytochemicals and micronutrients has recently emerged as an attractive alternative to antibiotic growth promoters to increase chicken growth and enhance resistance to infectious pathogens (48–50). To the best of our knowledge, the current study is the first to describe the beneficial effects of M. officinalis extract on broiler growth performance and resistance to necrotic enteritis.
To begin to elucidate the molecular mechanisms through which magnolia bark extract might promote resistance to E. maxima/C. perfringens infection, we evaluated antioxidant enzymes whose catalytic activities, expression levels, or both are altered in experimental necrotic enteritis. Georgieva et al. (51) reported that serum concentrations of malondialdehyde (MDA), a lipid peroxidation product and in vivo biomarker of oxidative stress, increased in chickens infected with Eimeria tenella compared with uninfected controls. Lee et al. (52, 53) and Xu et al. (54) reported that serum MDA concentrations, CAT and SOD1 activities, and glutathione peroxidase 7 (GPX7) transcript levels increased in broiler chickens co-infected with E. maxima and C. perfringens compared with uninfected controls. In contrast, transcripts encoding peroxiredoxin 6 (PRDX6), a thiol-specific antioxidant enzyme involved in intracellular redox regulation, decreased in co-infected birds compared with uninfected controls. In further studies, providing chickens with the micronutrient selenium, either through dietary supplementation or in ovo injection, reversed the effects of experimental necrotic enteritis (i.e., MDA concentrations, CAT and SOD1 activities, and GPX7 transcript levels decreased, whereas PRDX transcript levels increased, compared with unsupplemented controls) (52–54).
Many dietary phytochemicals induce the expression of enzymes involved in cellular antioxidant response (e.g., CAT, HMOX1, SOD1) and the detoxification of carcinogens (e.g., AFAR). The antioxidant defense system is composed of endogenous enzymatic, endogenous nonenzymatic, and exogenous mediators (55). SOD enzymes (SOD1, SOD2, and SOD3) catalyze the conversion of superoxide anion (O2−) to hydrogen peroxide (H2O2) and molecular oxygen (O2) (56). SOD1, or Cu,Zn-SOD, utilizes copper and zinc ions for both structural and catalytic roles. CAT catalyzes the reaction of hydrogen peroxide (H2O2) to H2O and O2 (57). HMOX1 is a rate-limiting enzyme in the catabolism of heme to biliverdin, free iron, and carbon monoxide (58). AFAR catalyzes the NAD(P)H-dependent reduction and detoxification of a dialdehydic metabolite of the hepatocarcinogen aflatoxin B1 (59). Nrf2 is a transcription factor that regulates the induction of these and other detoxifying and antioxidant genes through the antioxidant response elements, or electrophile response elements, located in their promoter regions (45). Normally, Nrf2 is localized in the cytoplasm in association with its repressor protein, Keap1, where it silences Nrf2 activity through its ubiquitination and proteolysis. Oxidation of cysteine residues within Keap1 by phytochemicals, or phytochemical-induced Nrf2 phosphorylation, have been proposed to dissociate Keap1 from Nrf2, allowing the transcription factor to enter the nuclease and bind to antioxidant response elements of antioxidant and detoxifying enzyme genes, thereby driving their expression (45). On the basis of the results of the current study, a similar mechanism may be operative in the case of magnolia bark extract–induced upregulation of AFAR, CAT, HMOX1, and SOD1 genes. Of interest, Pang et al. (2) reported that CAT and SOD1 activities in plasma and liver increased after in vivo treatment of mice with magnolol and honokiol, the major active components of M. officinalis, whereas Rajgopal et al. (60) showed that magnolia bark extract activated Nrf2-dependent HMOX1 gene expression in vitro in murine hepatocytes.
In summary, this study shows that dietary supplementation of broiler chickens experimentally co-infected with E. maxima and C. perfringens with a magnolia bark extract between days 1 and 35 of age improves growth performance and reduces intestinal lesions compared with unsupplemented controls. In general, transcripts for the antioxidant enzymes CAT, HMOX1, and SOD1, and the detoxifying enzyme AFAR, increased in uninfected and E. maxima/C. perfringens co-infected chickens fed magnolia-supplemented diets compared with unsupplemented controls, and significant interactions between dietary supplementation and co-infection were observed for all enzyme transcript levels depending on the tissue of origin. These findings might be of benefit for the future development of dietary strategies to improve poultry health, disease resistance, and growth productivity without the use of in-feed antibiotic growth promoters.
Acknowledgments
The authors’ responsibilities were as follows—SO, UDG, and HSL: conducted the research; SO: analyzed the data and performed the statistical analysis; SO and EPL: wrote the manuscript; HSL: had responsibility for the content; and all authors: designed the research, and read and approved the final manuscript.
Notes
Supported in part by a trust agreement established between the Agricultural Research Service, USDA, and Pancosma (Geneva, Switzerland).
Author disclosures: SO, UDG, DB, EPL, and HSL, no conflicts of interest.
Abbreviations used:
- AFAR
aflatoxin B1 aldehyde reductase
- AGP
α1-acid glycoprotein
- CAT
catalase
- HMOX1
heme oxygenase 1
- Keap1
Kelch-like ECH-associated protein 1
- MDA
malondialdehyde
- M/H high
0.56 mg M. officinalis bark extract/kg
- M/H low
0.33 mg M. officinalis bark extract/kg
- Nrf2
nuclear factor E2–related factor 2
- SOD
superoxide dismutase
References
- 1. Lee Y-J, Lee YM, Lee C-K, Jung JK, Han SB, Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol Therapeut 2011;130(2):157–76. [DOI] [PubMed] [Google Scholar]
- 2. Pang YL, Han XF, Bamikole MA, Gong ZH, Tang SX, Tan ZL, Xiao WJ, Zhou CS, Wang M, Deng YL. Anti-diarrhea and anti-oxidant properties of magnolol. Trop J Pharm Res 2013;12(1):85–91. [Google Scholar]
- 3. Sarris J, McIntyre E, Camfield DA. Plant-based medicines for anxiety disorders, part 1. CNS Drugs 2013;27(3):207–19. [DOI] [PubMed] [Google Scholar]
- 4. Ai JL, Wang XM, Nielsen M. Honokiol and magnolol selectively interact with GARA(A) receptor subtypes in vitro. Pharmacology 2001;63(1):34–41. [DOI] [PubMed] [Google Scholar]
- 5. Lo YC, Teng CM, Chen CF, Chen CC, Hong CY. Magnolol and honokiol isolated from magnolia-officinalis protect rat-heart mitochondria against lipid-peroxidation. Biochem Pharmacol 1994;47(3):549–53. [DOI] [PubMed] [Google Scholar]
- 6. Li HB, Gao JM, Ying XX, Wang SP, Li JC. Protective effect of magnolol on TBHP-induced injury in H460 cells partially via a p53 dependent mechanism. Arch Pharm Res 2007;30(7):850–7. [DOI] [PubMed] [Google Scholar]
- 7. Xu Q, Yi LT, Pan Y, Wang X, Li YC, Li JM, Wang CP, Kong LD. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog Neuropsychopharmacol 2008;32(3):715–25. [DOI] [PubMed] [Google Scholar]
- 8. Chen YH, Lin FY, Liu PL, Huang YT, Chiu JH, Chang YC, Man KM, Hong CY, Ho YY, Lai MT. Antioxidative and hepatoprotective effects of magnolol on acetaminophen-induced liver damage in rats. Arch Pharm Res 2009;32(2):221–8. [DOI] [PubMed] [Google Scholar]
- 9. Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal 2009;11(5):1139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen JL, Man KM, Huang PH, Chen WC, Chen DC, Cheng YW, Liu PL, Chou MC, Chen YH. Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules 2010;15(9):6452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woodbury A, Yu SP, Wei L, García P. Neuro-modulating effects of honokiol: a review. Front Neurol 2013;4:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee KW, Lillehoj H, Jeong W, Jeoung H-Y, An D-J. Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poultry Sci 2011;90(7):1381–90. [DOI] [PubMed] [Google Scholar]
- 13. Wade B, Keyburn A. The true cost of necrotic enteritis. World Poultry 2015;31:16–7. [updated 2015 Oct 9]. Available from: http://www.poultryworld.net/Meat/Articles/2015/10/The-true-cost-of-necrotic-enteritis-2699819W/. [Google Scholar]
- 14. Oh ST, Lillehoj HS. The role of host genetic factors and host immunity in necrotic enteritis. Avian Pathol 2016;45(3):313–6. [DOI] [PubMed] [Google Scholar]
- 15. Van Immerseel F, Buck JD, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol 2004;33(6):537–49. [DOI] [PubMed] [Google Scholar]
- 16. Castanon J. History of the use of antibiotic as growth promoters in European poultry feeds. Poultry Sci 2007;86(11):2466–71. [DOI] [PubMed] [Google Scholar]
- 17. Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines 2006;5(1):143–63. [DOI] [PubMed] [Google Scholar]
- 18. Lillehoj H, Jang S, Lee S, Lillehoj E. Avian coccidiosis as a prototype intestinal disease: recent advances in host protective immunity and novel disease control strategies. Niewold TE. (ed). Intestinal health: key to optimise production. Wageningen Academic Publishers, 2015;71–116. Available from: https://doi.org/10.3920/978-90-8686-792-9_4. [Google Scholar]
- 19. del Cacho E, Gallego M, Francesch M, Quílez J, Sánchez-Acedo C. Effect of artemisinin on oocyst wall formation and sporulation during Eimeria tenella infection. Parasitol Int 2010;59(4):506–11. [DOI] [PubMed] [Google Scholar]
- 20. Lee SH, Lillehoj HS, Jang SI, Kim DK, Ionescu C, Bravo D. Effect of dietary curcuma, capsicum, and lentinus, on enhancing local immunity against Eimeria acervulina infection. J Poult Sci 2010;47(1):89–95. [Google Scholar]
- 21. Lee SH, Lillehoj HS, Jang SI, Lee KW, Bravo D, Lillehoj EP. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Vet Parasitol 2011;181(2):97–105. [DOI] [PubMed] [Google Scholar]
- 22. Lee SH, Lillehoj HS, Jang SI, Lee KW, Park MS, Bravo D, Lillehoj EP. Cinnamaldehyde enhances in vitro parameters of immunity and reduces in vivo infection against avian coccidiosis. Br J Nutr 2011;106(06):862–9. [DOI] [PubMed] [Google Scholar]
- 23. Kim DK, Lillehoj HS, Lee SH, Lillehoj EP, Bravo D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br J Nutr 2013;109(01):76–88. [DOI] [PubMed] [Google Scholar]
- 24. Kim DK, Lillehoj HS, Lee SH, Jang SI, Park MS, Min W, Lillehoj EP, Bravo D. Immune effects of dietary anethole on Eimeria acervulina infection. Poultry Sci 2013;92(10):2625–34. [DOI] [PubMed] [Google Scholar]
- 25. Allen PC, Lydon J, Danforth HD. Effects of components of Artemisia annua on coccidia infections in chickens. Poultry Sci 1997;76(8):1156–63. [DOI] [PubMed] [Google Scholar]
- 26. Allen PC. Dietary supplementation with Echinacea and development of immunity to challenge infection with coccidia. Parasitol Res 2003;91(1):74–8. [DOI] [PubMed] [Google Scholar]
- 27. Dalloul R, Lillehoj H, Lee J, Lee S, Chung K. Immunopotentiating effect of a Fomitella fraxinea-derived lectin on chicken immunity and resistance to coccidiosis. Poultry Sci 2006;85(3):446–51. [DOI] [PubMed] [Google Scholar]
- 28. Hassan SM, El-Gayar AK, Cadwell DJ, Bailey CA, Cartwright AL. Guar meal ameliorates Eimeria tenella infection in broiler chicks. Vet Parasitol 2008;157(1):133–8. [DOI] [PubMed] [Google Scholar]
- 29. Lee S-H, Lillehoj HS, Lillehoj EP, Cho S-M, Park D-W, Hong Y-H, Chun H-K, Park H-J. Immunomodulatory properties of dietary plum on coccidiosis. Comp Immunol Microbiol Infect Dis 2008;31(5):389–402. [DOI] [PubMed] [Google Scholar]
- 30. Lee S-H, Lillehoj HS, Cho S-M, Park D-W, Hong Y-H, Lillehoj EP, Heckert RA, Park H-J, Chun H-K. Protective effects of dietary safflower (Carthamus tinctorius) on experimental coccidiosis. J Poult Sci 2009;46(2):155–62. [Google Scholar]
- 31. Lillehoj HS, Kim DK, Bravo DM, Lee SH. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. BMC Proc 2011:5(Suppl 4):S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akhtar M, Hai A, Awais MM, Iqbal Z, Muhammad F, ul Haq A, Anwar MI. Immunostimulatory and protective effects of Aloe vera against coccidiosis in industrial broiler chickens. Vet Parasitol 2012;186(3):170–7. [DOI] [PubMed] [Google Scholar]
- 33. Dkhil MA. Anti-coccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol Res 2013;112(7):2639–46. [DOI] [PubMed] [Google Scholar]
- 34. Kim DK, Lillehoj HS, Lee SH, Jang SI, Lillehoj EP, Bravo D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poultry Sci 2013;92(10):2635–43. [DOI] [PubMed] [Google Scholar]
- 35. Lee SH, Lillehoj HS, Jang SI, Lillehoj EP, Min W, Bravo DM. Dietary supplementation of young broiler chickens with Capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br J Nutr 2013;110(05):840–7. [DOI] [PubMed] [Google Scholar]
- 36. Kim JE, Lillehoj HS, Hong YH, Kim GB, Lee SH, Lillehoj EP, Bravo DM. Dietary Capsicum and Curcuma longa oleoresins increase intestinal microbiome and necrotic enteritis in three commercial broiler breeds. Res Vet Sci 2015;102:150–8. [DOI] [PubMed] [Google Scholar]
- 37. Yang W, Tien Y, Chung C, Chen Y, Chiou W, Hsu S, Liu H, Liang C, Chang C. Effect of Bidens pilosa on infection and drug resistance of Eimeria in chickens. Res Vet Sci 2015;98:74–81. [DOI] [PubMed] [Google Scholar]
- 38. Gotep J, Tanko J, Forcados G, Muraina I, Ozele N, Dogonyaro B, Oladipo O, Makoshi M, Akanbi O, Kinjir H. Therapeutic and safety evaluation of combined aqueous extracts of Azadirachta indica and Khaya senegalensis in chickens experimentally infected with Eimeria oocysts. J Parasitol Res 2016;4692424. doi: 10.1155/2016/4692424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neufeld K. Animal feed additive having an antimicrobial and growth-promoting effect. World intellectual property organization/Patent cooperation treaty patent WO 2011127496 A1, October 20, 2011.
- 40. Lillehoj HS, Lee SH, Park SS, Jeong M, Lim Y, Mathis GF, Lumpkins B, Chi F, Ching C, Cravens RL. Calcium Montmorillonite-based dietary supplement attenuates necrotic enteritis induced by Eimeria maxima and Clostridium perfringens in broilers. J Poult Sci 2016;53(4):329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li G, Lillehoj HS, Lee KW, Lee SH, Park MS, Jang SI, Bauchan GR, Gay CG, Ritter GD, Bautista DA. Immunopathology and cytokine responses in commercial broiler chickens with gangrenous dermatitis. Avian Pathol 2010;39(4):255–64. [DOI] [PubMed] [Google Scholar]
- 42. Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 1970;28(1):30–6. [DOI] [PubMed] [Google Scholar]
- 43. Rengaraj D, Truong AD, Lee S-H, Lillehoj HS, Hong YH. Expression analysis of cytosolic DNA-sensing pathway genes in the intestinal mucosal layer of necrotic enteritis-induced chicken. Vet Immunol Immunopathol 2016;170:1–12. [DOI] [PubMed] [Google Scholar]
- 44. Muller P, Janovjak H, Miserez A, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT PCR (vol 32, pg 1378, 2002). BioTechniques 2002;33(3):514. [PubMed] [Google Scholar]
- 45. Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 2006;38(4):769–89. [DOI] [PubMed] [Google Scholar]
- 46. Best P. Poultry performance improves over past decades. Article WATT Executive Guide to World Poultry Trends 2011. [updated 2011 Nov 24]. Available from: https://www.wattagnet.com/articles/10427-poultry-performance-improves-over-past-decades. [Google Scholar]
- 47. Thiruvenkadan A, Prabakaran R, Panneerselvam S. Broiler breeding strategies over the decades: an overview. Worlds Poult Sci J 2011;67(2):309. [Google Scholar]
- 48. Allen PC, Danforth HD, Augustine PC. Dietary modulation of avian coccidiosis. Int J Parasitol 1998;28(7):1131–40. [DOI] [PubMed] [Google Scholar]
- 49. Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res 2014;113(10):3547–56. [DOI] [PubMed] [Google Scholar]
- 50. Quiroz-Castañeda RE, Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. Biomed Res Int 2015;430610. doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Georgieva N, Koinarski V, Gadjeva V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet J 2006;172(3):488–92. [DOI] [PubMed] [Google Scholar]
- 52. Lee S, Lillehoj H, Jang S, Jeong M, Xu S, Kim J, Park H, Kim H, Lillehoj E, Bravo D. Effects of in ovo injection with selenium on immune and antioxidant responses during experimental necrotic enteritis in broiler chickens. Poultry Sci 2014;93(5):1113–21. [DOI] [PubMed] [Google Scholar]
- 53. Lee SH, Lillehoj HS, Jang SI, Jeong M, Kim DK, Xu S, Lee SK, Kim JB, Park HJ, Kim HR. Immune and anti-oxidant effects of in ovo selenium proteinate on post-hatch experimental avian necrotic enteritis. Vet Parasitol 2014;206(3):115–22. [DOI] [PubMed] [Google Scholar]
- 54. Xu S, Lee S-H, Lillehoj HS, Hong YH, Bravo D. Effects of dietary selenium on host response to necrotic enteritis in young broilers. Res Vet Sci 2015;98:66–73. [DOI] [PubMed] [Google Scholar]
- 55. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94(2):329–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mondola P, Damiano S, Sasso A, Santillo M. The Cu, Zn superoxide dismutase: not only a dismutase enzyme. Front Physiol 2016;7:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zamocky M, Furtmüller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal 2008;10(9):1527–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 2000;279(6):L1029–37. [DOI] [PubMed] [Google Scholar]
- 59. Ellis EM, Judah DJ, Neal GE, Hayes JD. An ethoxyquin-inducible aldehyde reductase from rat liver that metabolizes aflatoxin B1 defines a subfamily of aldo-keto reductases. Proc Natl Acad Sci USA 1993;90(21):10350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rajgopal A, Missler SR, Scholten JD. Magnolia officinalis (Hou Po) bark extract stimulates the Nrf2-pathway in hepatocytes and protects against oxidative stress. J Ethnopharmacol 2016;193:657–62. [DOI] [PubMed] [Google Scholar]