Abstract
Background
Hepatocellular carcinoma (HCC) is a complication of chronic hepatitis B and C virus (HBV and HCV) infection. New York City (NYC) has a high prevalence of HBV and HCV, and infected persons likely face increased mortality from HCC and other causes. We describe the mortality profile of NYC residents with HBV or HCV, emphasizing the contributions of HCC and HIV coinfection.
Methods
Two existing data sets were combined to examine all individuals diagnosed with HBV or HCV in NYC first reported to the Health Department during 2001–2012 and their HCC, HIV, and vital status. Logistic regression was used to calculate the odds of HCC diagnosis by viral hepatitis status, whereas Cox proportional hazard regression was used to estimate the hazard of death by HCC/HIV status.
Results
In total, 120 952 and 127 933 individuals were diagnosed with HBV or HCV, respectively. HCV-infected individuals had 17% higher odds of HCC diagnosis than HBV-infected individuals and 3.2 times higher odds of HIV coinfection. Those with HCV were twice as likely to die during the study period (adjusted hazard ratio, 2.04; 95% confidence interval, 1.96–2.12). The risk of death increased for those with HIV or HCC and was highest for those with both conditions.
Conclusions
HCC and HIV represent substantial risks to survival for both HBV- and HCV-infected individuals. Individuals with HBV need close monitoring and treatment, when indicated, and routine HCC screening. Those with HCV need increased, timely access to curative medications before developing liver disease.
Keywords: hepatocellular carcinoma, HIV coinfection, survival, viral hepatitis
New York City (NYC) has a high burden of chronic hepatitis B (HBV) and hepatitis C virus (HCV) infection relative to the rest of the United States [1–4]. HBV and HCV infection increase the risk of liver disease, including cirrhosis, liver cancer, and liver failure, which in turn increases the risk of death for infected individuals [5]. By 2013, deaths attributable to HCV surpassed deaths due to all nationally notifiable diseases combined, including HIV [6], highlighting the severity of the epidemic and its influence on survival.
In addition, HIV coinfection decreases survival among those with HBV or HCV, even for individuals successfully on HIV therapy [7–11]. In particular, coinfected individuals appear at greater risk of liver-related mortality, and there is evidence that HIV coinfection accelerates the progression of liver disease in those with viral hepatitis infection [10, 12, 13]. Approximately 6% of those with HBV and 15% of those with HCV in NYC are coinfected with HIV [7].
Another important influence on survival is hepatocellular carcinoma (HCC), the leading form of liver cancer. Infection with HBV or HCV is found in up to 80% of HCC cases [14]. HCC has a high fatality rate, and the rate of HCC diagnoses has been increasing in NYC [15, 16].
A previous NYC Health Department analysis examined the epidemiology of HBV and HCV infection and survival among individuals with HCC. Among individuals diagnosed with HCC during 2001–2012, almost 60% of cases also had evidence of HBV or HCV infection [17].
Although that study was informative for understanding the interplay of viral hepatitis infection, HCC, and survival, it did not allow for estimating the incidence of HCC, studying risk factors for HCC among those with viral hepatitis infection, or evaluating the added impact of HIV on survival among those with HCC. This follow-up analysis was designed to examine the incidence, risk factors, and influence of HCC on mortality across all individuals diagnosed with chronic viral hepatitis in NYC over a 12-year period.
METHODS
Analytic Data Set Generation
Two previously generated data sources were matched for this analysis: (1) a combined surveillance data set of hepatitis B and C cases reported to the Health Department during 2000–2013, all HIV diagnoses through 2013, and deaths during 2000–2013, matched as part of an initiative to integrate surveillance data across Health Department programs (Program Collaboration and Service Integration [PCSI]), and (2) a data set of HCC diagnoses during 2001–2012 provided by the New York State Cancer Registry matched to hepatitis B and C cases reported to the Health Department during 1999–2012. Match methods and data set details for each component data set are described elsewhere [17, 18].
These two data sets were used to create a master analytic data set of all HBV and HCV cases first reported to the Health Department during 2001–2012, with HCC diagnoses, HIV diagnoses, or death occurring during the same period. Cases were matched using unique Health Department identification numbers present in each data set, leading to 99.3% of HCC cases being successfully matched to the PCSI data set. Individuals whose hepatitis infection was diagnosed before their date of birth (n = 27) and those diagnosed with HCC (n = 3) or viral hepatitis (n = 222) after death were excluded. Additionally, individuals coinfected with HCV and HBV (n = 5134) were not included because of their small number relative to monoinfected individuals.
Statistical Analysis
Descriptive analyses examined patient gender, year of birth, HIV, HCC, and vital status, and age at viral hepatitis diagnosis, HCC diagnosis, and HIV diagnosis stratified by HBV or HCV infection. Socioeconomic status was estimated by calculating the ZIP code–based neighborhood poverty level for each individual based on their ZIP code reported at hepatitis diagnosis. Neighborhood poverty level was defined as the percentage of individuals in a given ZIP code with incomes <100% of the federal poverty level, which was determined by US Census 2000 data for cases diagnosed before 2004, by the American Community Survey (ACS) 2007–2011 for cases diagnosed in 2005–2009, by ACS 2008–2012 for cases diagnosed in 2010, and by ACS 2009–2013 for cases diagnosed in 2011–2012. Statistical differences between groups were assessed using chi-square tests for categorical variables and t tests (or Wilcoxon tests if not normally distributed) for continuous variables.
The incidence of HCC was calculated separately for those with HBV and HCV, using the number of cases of HCC from 2001–2012 for each infection group as the numerator and the person-years contributed from the time of viral hepatitis diagnosis to HCC diagnosis, death, or December 31, 2012, as the denominator. Cases where hepatitis infection was diagnosed after HCC (n = 608) were excluded from the incidence calculations. The incidence was age-adjusted using the US 2000 Standard Population [19].
The odds of being diagnosed with HCC or HIV for those with HBV vs HCV were calculated using a logistic regression model, with HCC status or HIV status as a binary outcome. Models were adjusted for gender, age at and year of hepatitis diagnosis, neighborhood poverty level, and, in the case of HCC as the outcome, HIV status. Year of viral hepatitis diagnosis was included to account for the duration of known infection during the study period, whereas age at viral hepatitis diagnosis was included to account for the length of time an individual likely lived with undiagnosed infection, both of which could influence opportunities for liver health screening, changes in health behaviors, or other factors that could influence the outcomes of interest.
In addition to vital status, data were available on the underlying cause of death for each deceased individual, recorded using International Classification of Diseases, 10th Revision (ICD-10), codes. The most common causes of death were examined, categorized, and presented stratified by viral hepatitis type (see Supplementary Table 1 for ICD-10 categorizations).
Kaplan-Meier analysis was used to examine the median survival time and survival probability since viral hepatitis diagnosis. Survival time was calculated from the date of viral hepatitis diagnosis to the date of death or censored at December 31, 2012. The crude relative hazard of death for those with HCV vs HBV was calculated using Cox proportional hazard regression, as was the hazard adjusted for HCC status, HIV status, gender, year and age at hepatitis diagnosis, and neighborhood poverty level. Additionally, proportional hazards regression was used to model the hazard of death separately for those with HBV or HCV, stratified by HCC and/or HIV status and adjusted for gender, age and year of hepatitis diagnosis, and neighborhood poverty level. Violations of the proportional hazards assumption were identified in both the models for HCV and HBV among those with HCC and HIV; an interaction term with time was included for these groups, and hazard ratios are presented for specific time points (6 months, 1 year, and 5 years after viral hepatitis diagnosis). To avoid the possibility that having HCC could influence the timing of viral hepatitis diagnosis relative to those without HCC, all survival analyses excluded those with HCC diagnoses within 6 months of viral hepatitis diagnosis (n = 358 for HBV; n = 450 for HCV).
Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC). The Institutional Review Boards of the NYC Health Department and the NYC Department of Health approved the study.
RESULTS
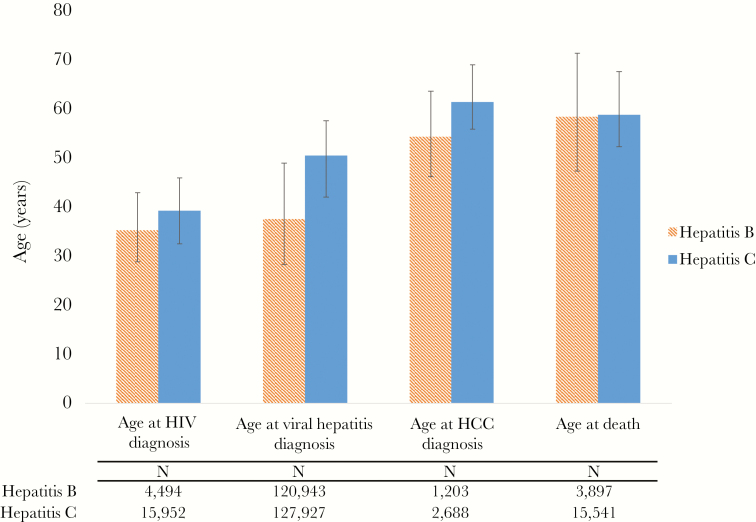
During 2001–2012, 120 952 individuals with HBV and 127933 individuals with HCV were reported to the Health Department. During this time, 3897 deaths were recorded in NYC among those with HBV (3.2%), compared with 15 541 deaths among those with HCV (12.2%, P < .01). Although more than 60% of those with HCV were born during 1945–1965 (the “baby boomer” generation), the plurality (49.0%) of those with HBV were born during 1966–1985. Tables 1 and 2 present the demographic breakdown for HBV and HCV cases, respectively, by HCC and vital status. Those with HBV were diagnosed at a younger age than those with HCV (median, 37.4 years; interquartile range [IQR], 28.2–48.8; vs median, 50.3 years; IQR, 41.9–57.4; P < .01); however, the median age at death was very similar for both groups (Figure 1), and well below the cutoff for premature death (ie, age 65 years) [20].
Table 1.
Demographic Characteristics of Individuals Diagnosed With Chronic Hepatitis B Reported to the New York City Department of Health and Mental Hygiene, 2001–2012, by HCC Diagnosis and Vital Status
| Overall | HCC Status | Vital Status | ||||||
|---|---|---|---|---|---|---|---|---|
| HCC | No HCC | Deceased | Alive | |||||
| No. | % | No. (Column %) |
No. (Column %) |
P | No. (Column %) |
No. (Column %) |
P | |
| Total | 120 952 | - | 1203 (1.0)a | 119 749 (99.0)a | <.001 | 3897 (3.2)a | 117 055 (96.8)a | <.001 |
| Genderb | ||||||||
| Male | 66 892 | 55.3 | 1020 (84.8) | 65 872 (55.0) | <.001 | 2704 (69.4) | 64 188 (54.8) | <.001 |
| Female | 52 874 | 43.7 | 177 (14.7) | 52 697 (44.0) | 1187 (30.5) | 51687 (44.2) | ||
| Transgender | 50 | 0.04 | 0 (0) | 50 (0.04) | 6 (0.1) | 44 (0.04) | ||
| Missing | 1136 | 0.9 | 6 (0.5) | 1130 (0.9) | 0 (0) | 1136 (1.0) | ||
| Year of birth | ||||||||
| Pre-1945 | 9501 | 7.9 | 323 (26.9) | 9178 (7.7) | <.001 | 1533 (39.3) | 7968 (6.8) | <.001 |
| 1945–1965 | 42 081 | 34.8 | 679 (56.4) | 41 402 (34.6) | 1850 (47.5) | 40 231 (34.4) | ||
| 1966–1985 | 59 284 | 49.0 | 190 (15.8) | 59 094 (49.4) | 487 (12.5) | 58 797 (50.2) | ||
| >1985 | 10 086 | 8.3 | 11 (0.9) | 10 075 (8.4) | 27 (0.7) | 10 059 (8.6) | ||
| Deceased | 3897 | 3.2 | 510 (42.4) | 3387 (2.8) | <.001 | - | - | - |
| HIV diagnosis | 4495 | 3.7 | 42 (3.5) | 4453 (3.7) | .678 | 875 (22.5) | 3620 (3.1) | <.001 |
| HCC diagnosis | 1203 | 1.0 | - | - | - | 510 (13.1) | 693 (0.6) | <.001 |
| Neighborhood poverty level,c % | ||||||||
| <10 | 11 879 | 9.8 | 150 (12.5) | 11 729 (9.8) | <.001 | 402 (10.3) | 11 477 (9.8) | <.001 |
| 10–<20 | 40 858 | 33.8 | 422 (35.1) | 40 436 (33.8) | 1273 (32.7) | 39 585 (33.8) | ||
| 20–<30 | 40 583 | 33.6 | 397 (33.0) | 40 186 (33.6) | 1079 (27.7) | 39 504 (33.8) | ||
| 30–100 | 16 228 | 13.4 | 183 (15.2) | 16 045 (13.4) | 795 (20.4) | 15 433 (13.2) | ||
| Missing | 11 404 | 9.4 | 51 (4.2) | 11 353 (9.5) | 348 (8.9) | 11 056 (9.5) | ||
Abbreviation: HCC, hepatocellular carcinoma.
aPercentage of total.
b“Gender” is classified according to available information about an individual’s gender identity and sex assigned at birth from laboratory reporting, medical records, and patient self-report. Most persons identified as transgender had their gender identity collected through HIV surveillance, so persons not co-infected with HIV tend to be classified as female or male, even if they are of transgender experience.
cNeighborhood poverty level was defined as the proportion of residents in the ZIP code at the time of the first hepatitis report with incomes below 100% of the federal poverty level.
Table 2.
Demographic Characteristics of Individuals Diagnosed With Chronic Hepatitis C Reported to the New York City Department of Health and Mental Hygiene, 2001–2012, by HCC Diagnosis and Vital Status
| Overall | HCC status | Vital Status | ||||||
|---|---|---|---|---|---|---|---|---|
| HCC | No HCC | Deceased | Alive | |||||
| No. | % | No. (Column %) |
No. (Column %) |
P | No. (Column %) |
No. (Column %) |
P | |
| Total | 127 933 | - | 2688 (2.1)a | 125 245 (97.9)a | <.001 | 15 541 (12.2)a | 112 392 (87.9)a | <.001 |
| Genderb | ||||||||
| Male | 80 518 | 62.9 | 2007 (74.7) | 78 511 (62.7) | <.001 | 10 260 (66.0) | 70 258 (62.5) | <.001 |
| Female | 46 484 | 36.3 | 672 (25.0) | 45 812 (36.6) | 5273 (33.9) | 41 211 (36.7) | ||
| Transgender | 101 | 0.1 | 2 (0.1) | 99 (0.1) | 8 (0.1) | 93 (0.1) | ||
| Missing | 830 | 0.7 | 7 (0.3) | 823 (0.7) | 0 (0) | 830 (0.7) | ||
| Year of birth | ||||||||
| Pre-1945 | 18 833 | 14.7 | 1075 (40.0) | 17 758 (14.2) | <.001 | 4903 (31.6) | 13 930 (12.4) | <.001 |
| 1945–1965 | 80 630 | 63.0 | 1578 (58.7) | 79 052 (63.1) | 9765 (62.8) | 70 865 (63.1) | ||
| 1966–1985 | 25 771 | 20.1 | 31 (1.2) | 25 740 (20.6) | 844 (5.4) | 24 927 (22.2) | ||
| >1985 | 2699 | 2.1 | 4 (0.2) | 2695 (2.2) | 29 (0.2) | 13 930 (12.4) | ||
| Deceased | 15 541 | 12.2 | 1518 (56.5) | 14 023 (11.2) | <.001 | - | - | - |
| HIV diagnosis | 15 958 | 12.5 | 251 (9.3) | 15 707 (12.5) | <.001 | 3752 (24.1) | 12 206 (10.8) | <.001 |
| HCC diagnosis | 2688 | 2.1 | - | - | - | 1518 (9.8) | 1170 (1.0) | <.001 |
| Neighborhood poverty level,c % | ||||||||
| <10 | 15 458 | 12.1 | 390 (14.5) | 15 068 (12.0) | <.001 | 1678 (10.8) | 13 780 (12.3) | <.001 |
| 10–<20 | 41 010 | 32.1 | 826 (30.7) | 40 184 (32.1) | 4387 (28.2) | 36 623 (32.6) | ||
| 20–<30 | 29 190 | 23.0 | 682 (25.4) | 28 508 (22.8) | 3798 (24.4) | 25 392 (22.6) | ||
| <30–100 | 31 662 | 24.8 | 649 (24.1) | 31 013 (24.8) | 4251 (27.4) | 27 411 (24.4) | ||
| Missing | 12 105 | 8.4 | 141 (5.3) | 10 472 (8.4) | 1427 (9.2) | 9186 (8.2) | ||
Column percentages may not sum to 100% due to rounding.
Abbreviation: HCC, hepatocellular carcinoma.
aPercentage of total.
b“Gender” is classified according to available information about an individual’s gender identity and sex assigned at birth from laboratory reporting, medical records, and patient self-report. Most persons identified as transgender had their gender identity collected through HIV surveillance, so persons not co-infected with HIV tend to be classified as female or male, even if they are of transgender experience.
cNeighborhood poverty level was defined as the proportion of residents in the ZIP code at the time of the first hepatitis report with incomes below 100% of the federal poverty level.
Figure 1.
Median age (interquartile range) at various stages for individuals diagnosed with chronic hepatitis B or C reported to the New York City Department of Health and Mental Hygiene, 2001–2012. Abbreviation: HCC, hepatocellular carcinoma.
HIV Coinfection
HIV coinfection was more common among those with HCV than HBV (12.5% vs 3.7%, respectively; P < .01). The adjusted odds of HIV coinfection were 3.2 times larger for those with HCV than those with HBV (adjusted odds ratio [aOR], 3.18; 95% confidence interval [CI], 3.06–3.30). A greater proportion of deceased individuals had HIV coinfection compared with those alive at the end of the study period (Tables 1 and 2). Among all individuals with HIV, 23.5% of those with HCV coinfection and 19.5% of those with HBV coinfection were deceased by the end of 2012.
HCC Diagnoses
There were 1203 cases of HCC diagnosed among those with HBV (1.0%) and 2688 among those with HCV (2.1%) during 2001–2012. The age-adjusted incidence of HCC was 156.1 cases per 100 000 person-years for those with HBV and 226.8/100 000 person-years for those with HCV. For individuals with HBV, the median age at viral hepatitis diagnosis (IQR) was 51.8 (43.5–61.6) years for those with HCC vs 37.3 (28.1–48.6) years (P < .01) for those without HCC. Individuals with HCV and HCC were diagnosed with HCV at a median age (IQR) of 58.2 (52.6–66.2) years vs at age 50.6 (41.8–57.1) years (P < .01) if they did not have HCC.
The adjusted odds of an HCC diagnosis were 17% higher among those with HCV than those with HBV (aOR, 1.17; 95% CI, 1.09–1.26). Using separate models for individuals with HBV and HCV, factors associated with HCC diagnosis were examined. For both those with HBV and HCV, male gender and increasing age at viral hepatitis diagnosis were associated with increased odds of HCC diagnosis, whereas HIV coinfection and increasing year of hepatitis diagnosis were associated with decreased odds (Table 3).
Table 3.
Adjusted Odds of Hepatocellular Carcinoma Diagnosis by Various Characteristics Among Individuals With Chronic Hepatitis B or C, Reported to the New York City Department of Health and Mental Hygiene During 2001–2012
| Hepatitis B (n = 108 760) | Hepatitis C (n = 116 787) | |||
|---|---|---|---|---|
| Adjusted OR | 95% CI | Adjusted OR | 95% CI | |
| Genderb | ||||
| Female | Reference | - | Reference | - |
| Male | 4.88 | 4.14–5.77 | 2.20 | 2.00–2.41 |
| Transgender | -A | - | 4.57 | 1.11–18.82 |
| HIV diagnosis | 0.66 | 0.48–0.90 | 0.78 | 0.68–0.90 |
| Year of hepatitis diagnosis | ||||
| 2001–2004 | Reference | - | Reference | - |
| 2005–2008 | 0.51 | 0.45–0.59 | 0.54 | 0.49–0.59 |
| 2009–2012 | 0.31 | 0.26–0.36 | 0.31 | 0.28–0.35 |
| Age at hepatitis diagnosis, y | ||||
| <30 | Reference | - | Reference | - |
| 30–39 | 3.08 | 2.26–4.20 | 0.97 | 0.51–1.86 |
| 40–49 | 7.05 | 5.27–9.43 | 5.73 | 3.35–9.78 |
| 50–59 | 12.48 | 9.33–16.68 | 16.51 | 9.72–27.99 |
| 60 or older | 19.46 | 14.55–26.02 | 34.80 | 20.53–58.98 |
| Neighborhood poverty level,c % | ||||
| <10 | Reference | - | Reference | - |
| 10–<20 | 0.93 | 0.77–1.12 | 0.93 | 0.82–1.05 |
| 20–<30 | 1.00 | 0.83–1.21 | 1.05 | 0.93–1.20 |
| <30–100 | 1.13 | 0.90–1.40 | 1.04 | 0.91–1.18 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aOdds ratio undefined because no individuals with hepatitis B and hepatocellular carcinoma were identified as transgender.
b“Gender” is classified according to available information about an individual’s gender identity and sex assigned at birth from laboratory reporting, medical records, and patient self-report. Most persons identified as transgender had their gender identity collected through HIV surveillance, so persons not co-infected with HIV tend to be classified as female or male, even if they are of transgender experience.
cNeighborhood poverty level was defined as the proportion of residents in the ZIP code at the time of the first hepatitis report with incomes below 100% of the federal poverty level.
Mortality by HCC and HIV Status
The 10-year survival rate after HBV diagnosis was 52% among those with HCC compared with 96% among those without HCC. Among those with HCV, the 10-year survival rate was 35% for those diagnosed with HCC compared with 83% for those without HCC. The unadjusted relative hazard of death was 4 times higher for those with HCV than those with HBV (hazard ratio [HR], 4.08; 95% CI, 3.94–4.23). After adjustment, those with HCV were 2 times more likely to die after hepatitis diagnosis than those with HBV (adjusted HR, 2.04; 95% CI, 1.96–2.12).
Figure 2A and B displays the survival curves for individuals with HBV and HCV, respectively, stratified by both HCC and HIV status. Hazard ratios comparing the risk of death for those with HIV, HCC, or both vs neither condition indicated that for both the HBV- and HCV-infected groups, the risk of death increased for those with either HCC or HIV compared with those without these conditions and was highest for individuals with both HCC and HIV (and increased over time) (Table 4).
Figure 2.
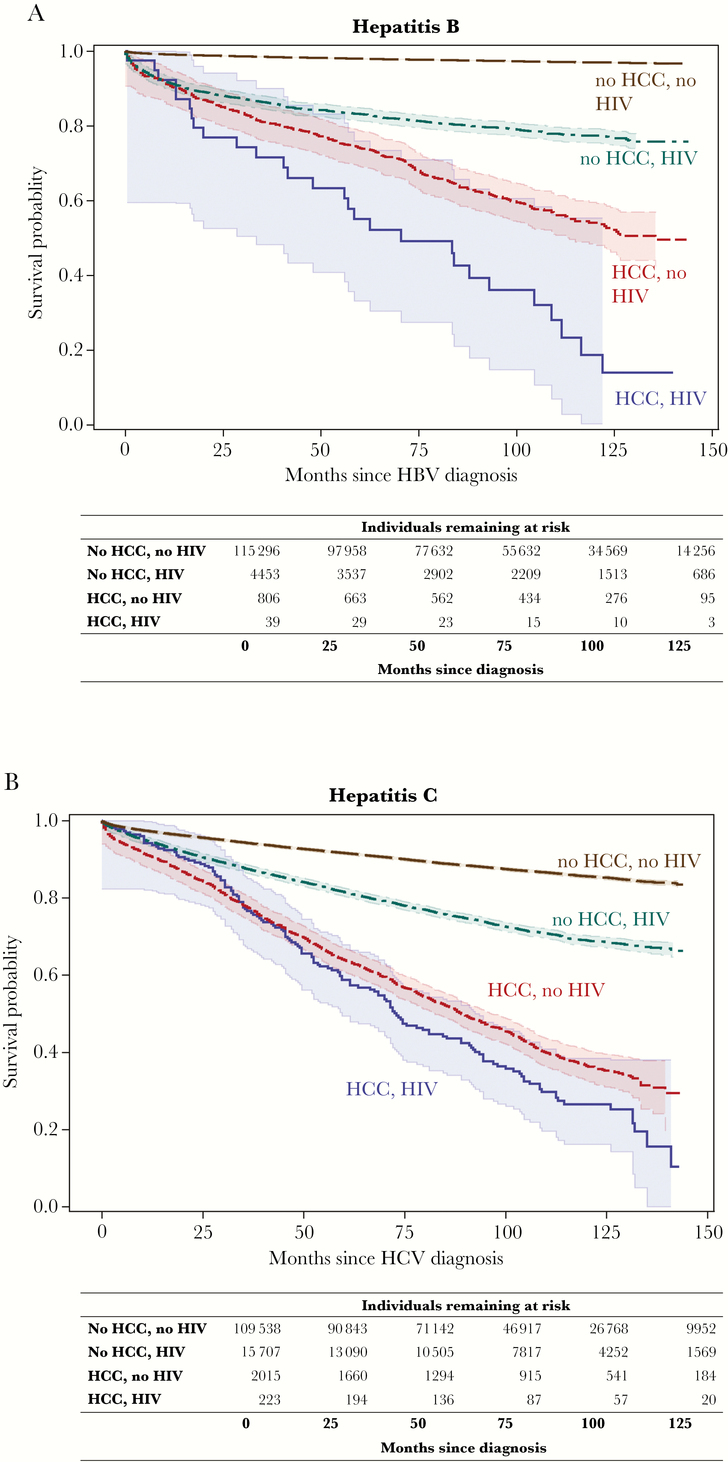
Kaplan-Meier survival curves for time since viral hepatitis diagnosis for individuals with a) hepatitis B or b) hepatitis C, stratified by hepatocellular carcinoma and HIV status, in New York City, 2001–2012. Survival curves include 95% Hall-Wellner confidence bands. Abbreviations- HCC: hepatocellular carcinoma; HBV: hepatitis B virus; HCV: hepatitis C virus
Table 4.
Adjusted Hazard Ratios for the Risk of Death Among Those With Chronic Hepatitis B or C Reported to the New York City Department of Health and Mental Hygiene During 2001–2012 by Hepatocellular Carcinoma and HIV Status
| Hepatitis B | Hepatitis C | |||
|---|---|---|---|---|
| Adjusted HRa | 95% CI | Adjusted HRa | 95% CI | |
| No HCC, no HIV | Reference | - | Reference | - |
| No HCC, HIV | 7.86 | 7.21–8.58 | 2.61 | 2.50–2.72 |
| HCC, no HIV | 6.95 | 6.14–7.87 | 3.79 | 3.55–4.05 |
| HCC and HIVb | ||||
| 6 mo | 10.65 | 4.96–22.89 | 2.89 | 1.96–4.25 |
| 12 mo | 17.00 | 9.76–29.62 | 4.00 | 3.02–5.59 |
| 60 mo | 50.32 | 33.02–76.66 | 8.52 | 7.13–10.18 |
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio.
aHazard ratios adjusted for gender, year of hepatitis diagnosis, age at hepatitis diagnosis, and neighborhood poverty level.
bDue to an observed violation in the proportional hazards assumption, hazard ratios for individuals with HCC and HIV compared with those with neither are presented separately at different time points after hepatitis diagnosis.
To further characterize mortality, the underlying cause of death was examined for deceased individuals. Death due to HCC was the fourth leading cause of death for those with HBV (12.7% of deaths) and the seventh leading cause for those with HCV (7.4% of deaths) (Table 5). HIV/AIDS-associated causes of death were the third most common for both HBV- and HCV-infected individuals. Unsurprisingly, the large majority of deaths among those with HCC was due to HCC, though that percentage was greater for those with HBV than those with HCV (74.3% of deaths vs 65.9%) (data not shown).
Table 5.
Underlying Cause of Death Distribution, Rankings, and Age at Death for Individuals With Chronic Hepatitis B or C Reported to the New York City Department of Health and Mental Hygiene During 2001–2012
| Hepatitis B (n = 3897) |
Hepatitis C (n = 15 541) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Cause of Death Category | No. | % | Median Age at Death (IQR), y | Cause of Death Category | No. | % | Median Age at Death (IQR), y |
| 1 | Nonliver cancers | 833 | 21.4 | 59.0 (48.7–69.2) | Cardiovascular disease | 3458 | 22.2 | 60.0 (52.5–72.6) |
| 2 | Cardiovascular disease | 803 | 20.6 | 65.6 (53.7–77.4) | Nonliver cancers | 2312 | 14.9 | 57.8 (51.9–66.4) |
| 3 | HIV/AIDS-associated | 594 | 15.2 | 42.8 (37.7–49.6) | HIV/AIDS-associated | 1939 | 12.5 | 48.8 (43.6–53.7) |
| 4 | Hepatocellular carcinoma | 493 | 12.7 | 52.7 (45.3–61.5) | Other causes | 1723 | 11.1 | 54.3 (47.1–64.6) |
| 5 | Other causes | 421 | 10.8 | 54.1 (42.6–71.4) | Hepatitis C-associated | 1536 | 9.9 | 54.7 (49.5–61.4) |
| 6 | Hepatitis B-associated | 184 | 4.7 | 53.9 (46.2–64.8) | Drug/alcohol-associated | 1357 | 8.7 | 47.2 (40.9–52.4) |
| 7 | Respiratory disease | 188 | 4.8 | 68.4 (55.6–77.9) | Hepatocellular carcinoma | 1152 | 7.4 | 59.2 (53.1–67.6) |
| 8 | Liver disease (noncancer) | 133 | 3.4 | 53.8 (47.3–63.7) | Respiratory disease | 804 | 5.2 | 58.6 (51.5–70.0) |
| 9 | Diabetes-associated | 115 | 3.0 | 62.8 (54.6–71.1) | Liver disease (noncancer) | 746 | 4.8 | 54.6 (48.9–61.4) |
| 10 | Drug/alcohol-associated | 95 | 2.4 | 41.2 (35.9–48.7) | Diabetes-associated | 450 | 2.9 | 58.0 (51.6–68.3) |
For International Classification of Disease, 10th Revision, codes used for each underlying cause of death category, see Supplementary Table 1.
Abbreviation: IQR, interquartile range.
DISCUSSION
This study examined the relationship between viral hepatitis infection, HIV infection, HCC, and survival among NYC residents over a 12-year period. We found that overall survival was worse for those with HCV than those with HBV; 12.2% of the cohort with HCV died vs 3.2% of those with HBV. The crude risk of death was 4 times higher for those with HCV than HBV, and 2 times higher after adjustment, consistent with findings from other studies [21] and with our previous findings on survival specifically among individuals with HCC [17]. Individuals with HCV were also 3.2 times more likely to be diagnosed with HIV and 17% more likely to be diagnosed with HCC than those with HBV, even after adjusting for other factors that might have affected these diagnoses. Those with HCV were on average 10 years older at the time of viral hepatitis diagnosis and at HCC diagnosis than those with HBV, which is consistent with the most common risk factors for HBV and HCV infection in NYC; among those with HCV, injection and intranasal drug use are some of the most commonly reported risk factors [22], whereas most individuals with HBV in NYC were born in HBV-endemic countries and likely acquired their infections at birth or as children [23]. The older age at diagnosis for individuals with HCV implies that many of these individuals lived for many years with undetected infection, delaying the receipt of appropriate medical care, screening opportunities, chances for treatment initiation, and other measures that could have limited negative health consequences associated with delayed diagnosis, which include hospitalization (all cause), advanced liver disease, and death (all cause) [24]. Our finding that the odds of an HCC diagnosis increases with increasing age at viral hepatitis diagnosis reinforces this point.
As found in other studies, HIV coinfection was much more common among those with HCV than those with HBV [7, 25]. However, HIV/AIDS-related conditions caused a relatively equal proportion of deaths across both groups, and a roughly equal proportion of HIV/HCV-coinfected vs HIV/HBV-coinfected individuals were deceased by the end of the study period, consistent with other findings that the death rates among individuals with HIV are similar between those with HBV or HCV coinfection [7]. In examining the factors associated with HCC diagnosis, HIV infection appeared to be a significant protective factor for those with HCV and those with HBV. However, it might only appear protective because many of those with HIV coinfection died before they could develop HCC, especially for those diagnosed earlier in the study period when HIV-related and all-cause mortality among individuals with HIV was higher [26]. In another study of HCV and mortality in NYC, individuals with HIV coinfection died at a median age of 52.0 years [8], which is more than 10 years before the median age of HCC diagnosis among HCV-infected individuals in our study. Alternatively, there might be underdiagnosis of HCC among individuals with HIV infection, as providers might be too occupied with their patients’ HIV-related care to consider recommendations for HCC screening.
Although there was an elevated risk of death associated with either an HIV or HCC diagnosis, both conditions together appeared to act synergistically to further worsen survival, with the risk of death progressively increasing over time. The magnitude of the association between each of these conditions with death was much larger for those with HBV than those with HCV; at the same time, those with HCV had an increased risk of death compared with those with HBV regardless of HCC/HIV status. This suggests that HIV and HCC might be more substantial drivers of mortality for HBV-infected individuals who experience these conditions, whereas HCV-infected individuals in this study might have more competing sources of mortality, especially given the older age of this cohort [24]. Supporting this finding is that among HBV-infected individuals with HCC, 74.3% of those who died during the study period died of HCC, compared with 65.9% of those with HCV and HCC, consistent with studies showing that those with HBV have a higher risk of liver-related mortality than those with HCV, and with our previous investigation into this population [8, 18, 27]. However, given the higher likelihood of both HIV and HCC diagnoses and the greater number of deaths overall among individuals with HCV, HCC and HIV still represent substantial risks to survival for these persons.
This study has several limitations. Demographic information is limited to gender, date of birth, and patient address, as the majority of the data were from HBV and HCV laboratory reports collected through passive, routine surveillance; factors such as race/ethnicity or clinical details like comorbidities could not be accounted for in the analysis. However, we have previously examined the distributions of race/ethnicity among individuals with HCC and either HBV or HCV in NYC and found that those distributions aligned with what we had observed during periodic random surveys of our general HBV and HCV surveillance cohorts [18, 22, 28]. Additionally, without information on outmigration, our incidence calculations are likely underestimated, as the number of HCC cases would be undercounted and the person-time would be overestimated. In addition, deaths occurring outside NYC among NYC residents were not captured; however, it is likely that missing death data are nondifferential by viral hepatitis status. On the other hand, misclassification in cause of death data, which is often found to be a problem [29], might be differential between those with and without HCC or between those with HCV and HBV. Another limitation is that we do not have the date of viral hepatitis infection but only the date of first positive hepatitis test in NYC. Not only might this not be the actual date of diagnosis if an individual first tested positive outside of NYC, but it also might have little relation to the actual date of infection, as most new HBV and HCV infections are asymptomatic and do not prompt testing. Diagnosis date in NYC therefore more likely reflects general screening practices or clinical factors that might prompt testing, such as symptoms related to the development of HCC. Exclusion of HBV and HCV cases diagnosed in the 6 months before HCC diagnosis in our survival analyses was an attempt to lessen this bias.
A final limitation of this study is that matched data were only available through 2012. Matches of this extent are rarely available, and this study took advantage of already existing match efforts to create a combined data set to investigate unexamined aspects of survival among individuals with HBV or HCV in NYC. Indeed, a strength of this study is the successful effort to integrate many different data sources to make a large, comprehensive data set with a large sample size. Though the data are less recent, we believe the conclusions are still relevant and illuminating. In particular, this study demonstrates what might continue to happen to individuals with HCV if access to new curative therapies is not extensive. During the period of this study, treatments for HCV were much less effective, and treatment uptake and cure were low [30]; treatment likely played a limited role in influencing survival in the cohort examined here. However, going forward, the improved treatments are expected to reduce overall mortality and HCC incidence, especially if individuals are treated before they have a chance to develop cirrhosis [31, 32]. We hope to be able to more directly measure the impact of these new medications on survival in the future.
This study found that HCC is a substantial burden and risk factor for NYC residents with HBV or HCV and significantly increased the risk of death in this population. As many of those in the HCV-infected cohort are baby boomers, the elevated risk of HCC and its increasing risk with age are particularly concerning [33]. Additionally, as in the United States generally, NYC is experiencing an increase in HCV infections among younger individuals [34–36]. Our study contributes to the concern for this population, as these younger individuals face a lifetime of increased risk of morbidity and mortality related to their infection unless promptly treated.
These findings emphasize the need for prevention activities, including preventing HIV coinfection and the provision of harm reduction services. For HCV, the urgency to treat and cure as many individuals as possible before they develop cirrhosis is even more tangible, as this will be an important way to reduce many of the drivers of morbidity and mortality, including liver disease and liver cancer [28, 37]. For HBV, the emphasis needs to be on appropriate monitoring of infection and control through treatment, as indicated, and appropriate screening for liver disease and HCC [38], given its outsize impact on mortality for these individuals.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Financial support. This work was supported by New York City tax levies and a by Centers for Disease Control and Prevention Cooperative Agreement (6NU58DP006309) awarded to the New York State Department of Health for B.Q.
References
- 1. France AM, Bornschlegel K, Lazaroff J, et al. . Estimating the prevalence of chronic hepatitis B virus infection—New York City, 2008. J Urban Health 2012; 89:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts H, Kruszon-Moran D, Ly KN, et al. . Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology 2016; 63:388–97. [DOI] [PubMed] [Google Scholar]
- 3. Edlin BR, Eckhardt BJ, Shu MA, et al. . Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015; 62:1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balter S, Stark JH, Kennedy J, et al. . Estimating the prevalence of hepatitis C infection in New York City using surveillance data. Epidemiol Infect 2014; 142:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ly KN, Xing J, Klevens RM, et al. . Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis 2014; 58:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C Virus in the United States, 2003–2013. Clin Infect Dis 2016; 62:1287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prussing C, Chan C, Pinchoff J, et al. . HIV and viral hepatitis co-infection in New York City, 2000–2010: prevalence and case characteristics. Epidemiol Infect 2015; 143:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinchoff J, Drobnik A, Bornschlegel K, et al. . Deaths among people with hepatitis C in New York City, 2000–2011. Clin Infect Dis 2014; 58:1047–54. [DOI] [PubMed] [Google Scholar]
- 9. Chun HM, Mesner O, Thio CL, et al. ; Infectious Disease Clinical Research Program HIV Working Group HIV outcomes in Hepatitis B virus coinfected individuals on HAART. J Acquir Immune Defic Syndr 2014; 66:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuster D, Cheng DM, Quinn EK, et al. . Chronic hepatitis C virus infection is associated with all-cause and liver-related mortality in a cohort of HIV-infected patients with alcohol problems. Addiction 2014; 109:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep 2011; 8:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García-Samaniego J, Rodríguez M, Berenguer J, et al. . Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol 2001; 96:179–83. [DOI] [PubMed] [Google Scholar]
- 13. Mastroianni CM, Lichtner M, Mascia C, et al. . Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. Int J Mol Sci 2014; 15:9184–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–73.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017. Accessed 21 November 2017 Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. [Google Scholar]
- 16. New York State Cancer Registry. Cancer incidence and mortality in New York State, 1976–2014 Available at: http://www.health.ny.gov/statistics/cancer/registry/. Accessed 21 November 2017.
- 17. Drobnik A, Pinchoff J, Bushnell G, et al. . Matching HIV, tuberculosis, viral hepatitis, and sexually transmitted diseases surveillance data, 2000–2010: identification of infectious disease syndemics in New York City. J Public Health Manag Pract 2014; 20:506–12. [DOI] [PubMed] [Google Scholar]
- 18. Moore MS, Ivanina E, Bornschlegel K, et al. . Hepatocellular carcinoma and viral hepatitis in New York City. Clin Infect Dis 2016; 63:1577–83. [DOI] [PubMed] [Google Scholar]
- 19. Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes 2001; (20)1–10. [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. Premature mortality in the United States: public health issues in the use of years of potential life lost. MMWR Morb Mortal Wkly Rep 1986; 35:1S–11S. [PubMed] [Google Scholar]
- 21. Perazzo H, Pacheco AG, Luz PM, et al. . Age-standardized mortality rates related to viral hepatitis in Brazil. BMC Infect Dis 2017; 17(1):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drezner K, Bornschlegel K, McGibbon E, Balter S. Enhanced chronic hepatitis C surveillance in New York City, April 2009-January 2011. Public Health Rep 2013; 128:510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Surveillance for chronic hepatitis B virus infection - New York City, June 2008-November 2009. MMWR Morb Mortal Wkly Rep 2012; 61:6–9. [PubMed] [Google Scholar]
- 24. Moorman AC, Rupp LB, Gordon SC, et al. ; CHeCS Investigators Long-term liver disease, treatment, and mortality outcomes among 17,000 persons diagnosed with chronic hepatitis C virus infection: current chronic hepatitis cohort study status and review of findings. Infect Dis Clin North Am 2018; 32:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchez MA, Scheer S, Shallow S, et al. . Epidemiology of the viral hepatitis-HIV syndemic in San Francisco: a collaborative surveillance approach. Public Health Rep 2014; 129 (Suppl 1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith CJ, Ryom L, Weber R, et al. ; D:A:D Study Group Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 27. Falade-Nwulia O, Seaberg EC, Rinaldo CR, et al. . Comparative risk of liver-related mortality from chronic hepatitis B versus chronic hepatitis C virus infection. Clin Infect Dis 2012; 55:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ong P, Gambatese M, Begier E, et al. . Effect of cause-of-death training on agreement between hospital discharge diagnoses and cause of death reported, inpatient hospital deaths, New York City, 2008–2010. Prev Chronic Dis 2015; 12:E04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention. Surveillance for chronic hepatitis B virus infection - New York City, June 2008-November 2009. MMWR Morb Mortal Wkly Rep 2012; 61:6–9. [PubMed] [Google Scholar]
- 30. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V III. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butt AA, Yan P, Simon TG, Abou-Samra AB. Effect of paritaprevir/ritonavir/ombitasvir/dasabuvir and ledipasvir/sofosbuvir regimens on survival compared with untreated hepatitis C virus-infected persons: results from ERCHIVES. Clin Infect Dis 2017; 65:1006–11. [DOI] [PubMed] [Google Scholar]
- 32. Robert SB. The possible association between DAA treatment for HCV infection and HCC recurrence. Gastroenterol Hepatol (N Y) 2016; 12:776–9. [PMC free article] [PubMed] [Google Scholar]
- 33. Davis GL, Alter MJ, El-Serag H, et al. . Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010; 138:513–21, 521.e1–6. [DOI] [PubMed] [Google Scholar]
- 34. Zibbell JE, Iqbal K, Patel RC, et al. ; Centers for Disease Control and Prevention Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep 2015; 64:453–8. [PMC free article] [PubMed] [Google Scholar]
- 35. Zibbell JE, Asher AK, Patel RC, et al. . Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2017; 21:e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. New York City Department of Health and Mental Hygiene. Annual report: hepatitis B and C in New York City 2016 Available at: https://www1.nyc.gov/assets/doh/downloads/pdf/cd/hepatitis-b-and-c-annual-report-2016.pdf. Accessed 22 November 2017.
- 37. van der Meer AJ, Veldt BJ, Feld JJ, et al. . Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308:2584–93. [DOI] [PubMed] [Google Scholar]
- 38. Bruix J, Sherman M. AASLD practice guideline, management of hepatocellular carcinoma: an update Available at: www.aasld.org/sites/default/files/guideline_documents/HCCUpdate2010.pdf. Accessed 8 December 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.