Abstract
Background
Because of improvements in cancer treatment, more than 80% of all children with cancer now survive at least five years from the time of diagnosis. As a result, late sequelae of cancer and its treatment have become more common, particularly second malignancies. We studied the current incidence of second malignancies among childhood cancer survivors in Germany.
Methods
This study is based on the cohort of the German Childhood Cancer Registry (Deutsches Kinderkrebsregister, DKKR). Persons given the diagnosis of a first malignancy at any time in the years 1980–2014 who were no more than 14 years old at the time of diagnosis and survived at least six months thereafter were included in the study. Cumulative incidences and hazard ratios were calculated, and comparisons with the general population were made with the aid of standardized incidence ratios (SIR).
Results
Among the 47 650 survivors included in the study, there were 1262 cases of second malignancies. After a follow-up interval of up to 35 years, the cumulative incidence of second malignancies was 8.27% (95% confidence interval [7.51; 9.03]). Second malignancies were more common in female patients (hazard ratio 1.29, [1.16; 1.44]) and in those who had had a systemic cancer as their initial malignancy (hazard ratio 1.22 [1.09; 1.36]). The SIR compared to the general population for the period 1955–2014 was 7.08 [6.42; 7.9] for female patients and 5.83 [5.27; 6.42] for male patients.
Conclusion
The cumulative incidence of second malignancies is 5.4% at 25 years and 8.3% at 35 years; these figures may be slight underestimates. The DKKR is an epidemiologic registry containing no data about treatment, so the effect of treatment on the risk of second malignancies could not be studied. The acquisition and evaluation of treatment data for the overall cohort is currently one of the main tasks for research on the late sequelae of childhood cancer. This may enable conclusions to be drawn about whether treatment strategies that have been introduced to lessen the risk of a second malignancy actually have the desired effect.
Nowadays in Germany, 85% of children who had cancer before the age of 15 survive for at least 5 years; 83% survive for 10 years (1). Current research is therefore focusing increasingly on the occurrence and prevention of late sequelae. One of the most life-threatening late sequelae is the development of a second malignancy. The International Agency for Research on Cancer (IARC) describes such malignancies as a second neoplasm of different topology and/or morphology in a patient (2). Recurrences, metastases, infiltrations, or transformations are not classed as second malignancies (2). Different publications have shown an increased risk of second malignancy after childhood cancers (3). Potential risk factors are genetic predisposition, chemotherapy, radiotherapy, stem cell transplantation, and effects related to time and age (4). The US Childhood Cancer Survivor Study (CCSS) reported a cumulative incidence after 30 years of between 7.9% (5) and 9.3% (3), if only second malignancies are included that developed more than five years after the initial malignancy. In the UK, a cumulative incidence of 1.6% in those who reached age 20 and 13.6% in those who reached age 60 was published (6). In 2009 the German Childhood Cancer Registry (GCCR) reported a cumulative incidence over 25 years of 3.3% (7) Previously a standardized incidence ratio (SIR) for second malignancies of 12.5 was observed compared with the general population up to age 15 (8).
We used the GCCR data with follow-up to 2014 to study the cumulative incidence of second malignancies in children who had a first malignancy when younger than 15. We particularly focused on second malignancies of the breast and thyroid, and on leukemias.
Methods
Since 1980 the GCCR has collected all cases of childhood malignancy in those younger than 15 in the former West Germany, and since 1991 the new federal states have been included (1). Within the initial five years, follow-up care was delivered by oncologic pediatric hospitals (9), which reported to the respective clinical study (therapy optimization studies). Subsequently, patients still receiving aftercare (at least up to the age of 18) were actively followed up by the hospitals or, alternatively, in a 5-year cycle by the GCCR (1). The second malignancies reported by patients were validated by contacting the treating doctors or hospitals. A very small proportion of second malignancies reported could not be validated by a hospital, but some of these were registered if they were plausible.
Study population
We included in the analysis all patients who developed a first malignancy when younger than 15 years of age, as defined by the ICCC-3 (10), who had been diagnosed between 1 January 1980 and 31 December 2014 and who were resident in Germany at the time of diagnosis. The patients had to have survived at least six months without developing a second malignancy. Patients were included in the analysis only if information on sex and date of birth was complete. In total, 1262 patients with a second malignancy were observed (table 1). For the purpose of comparison with the German general population we used the categories of the ICD-10. Malignant non-melanoma skin cancer (ICD-10 C44) and non-malignant tumors of the central nervous system were not included in the comparison with the general population because there were no relevant comparison data for Germany.
Table 1. Characteristics of the FM cohort and the SM cases of the German Childhood Cancer Registry (GCCR) (6-month survivors, 1980–2014).
| Characteristics | GCCR data (1980–2014) | Subgroup for comparison with the general population (1995–2014)* | ||
| Male | Female | Male | Female | |
| Cohort N | 26 672 | 20 978 | 24 329 | 19 287 |
| Mean age at FM diagnosis (years) | 6.6 | 6.4 | 6.0 | 5.9 |
| Mean follow-up (years) | 11.0 | 11.2 | 11.9 | 12.0 |
| Median follow-up (years) | 9.3 | 9.6 | 10.6 | 10.9 |
| Deceased (N) | 5666 (21.4%) | 4070 (19.4%) | 3579 (14.7%) | 2584 (13.4%) |
| Cases | ||||
| Second malignancy (N) | 632 | 630 | 379 | 411 |
| Mean age at FM diagnosis (years) | 6.7 | 7.1 | 6.8 | 7.4 |
| Mean age at SM diagnosis (years) | 17.6 | 19.9 | 16.8 | 19.5 |
| Mean length of time to SM (years) | 10.4 | 12.1 | 9.4 | 11.6 |
*Excluding non-malignant CNS tumors and non-melanoma skin cancer
FM?=?First malignancy; SM?=?second malignancy; N =?Total group size
Statistical analyses
The period at risk started six months after the diagnosis of the first malignancy and ended with the date of a diagnosis of a second malignancy, the date of death, the date of the latest vital status, or 31 December 2014, whichever came first.
Cumulative incidences with their respective 95% confidence intervals were calculated by using the Aalen–Johansen method, taking into account concurrent risks (11) for all years up to a maximum of 35 years after the initial malignancy. The cumulative incidence was calculated for all types of malignancies combined as well as separately for solid and systemic first malignancies. The event “death” was considered a concurrent risk in all evaluations. In analyzing second malignancies of the thyroid and breast, lymphocytic leukemia, or acute myeloid leukemia, we also included all other second malignancies as concurrent events.
We used the method developed by Fine and Gray (12) to calculate hazard ratios, using the covariables “systemic” versus “solid” first malignancies, age at diagnosis of the first malignancy, and sex, with death considered as a concurrent event.
To calculate the standardized incidence ratio we stratified the number of expected cases by the age distribution of the cohort and the cancer incidence rates for Germany from the Robert Koch Institute’s cancer registry data by sex, age, and calendar year (13). This analysis was restricted to the time period 1995–2014, because no information was available for earlier calendar years. The standardized incidence ratio was calculated for all second malignancies combined as well as separately for breast cancer, thyroid cancer, and leukemia.
Results
47 650 patients with childhood cancer were included. The average age at diagnosis of the first cancer was 6.4 years for girls and 6.6 years for boys (table 1). 55% of patients were male; 54% of first malignancies were solid tumors. The most common solid first malignancies were malignant neurological tumors (13.3% of all first malignancies), followed by non-malignant neurological tumors (27.4%) and neuroblastoma (7.8%). The most common systemic first malignancies were lymphocytic leukemias (27.4%) and non-Hodgkin’s lymphomas (6.7%).
As active follow-up in the GCCR was done only every five years, 19.3% of patients were censored in 2013, 8.3% in 2012, 4.0 in 2011, 2.6% in 2010, and 4.0% in 2009. Before 2009 the loss to follow-up was 0.3% per year.
In total we observed 1262 cases of second malignancy according to ICCC-3 in the cohort. 140 cases were excluded from the comparison with the general population (1995–2014), as they had been diagnosed before 1995. A further 314 cases of melanoma skin tumors and non-malignant neurological tumors were also excluded because they were not included in the data from the Robert Koch Institute.
The mean age at which a second malignancy was diagnosed was 19.9 years for female patients and 17.6 years for male patients (range 1.2–46.2 years).
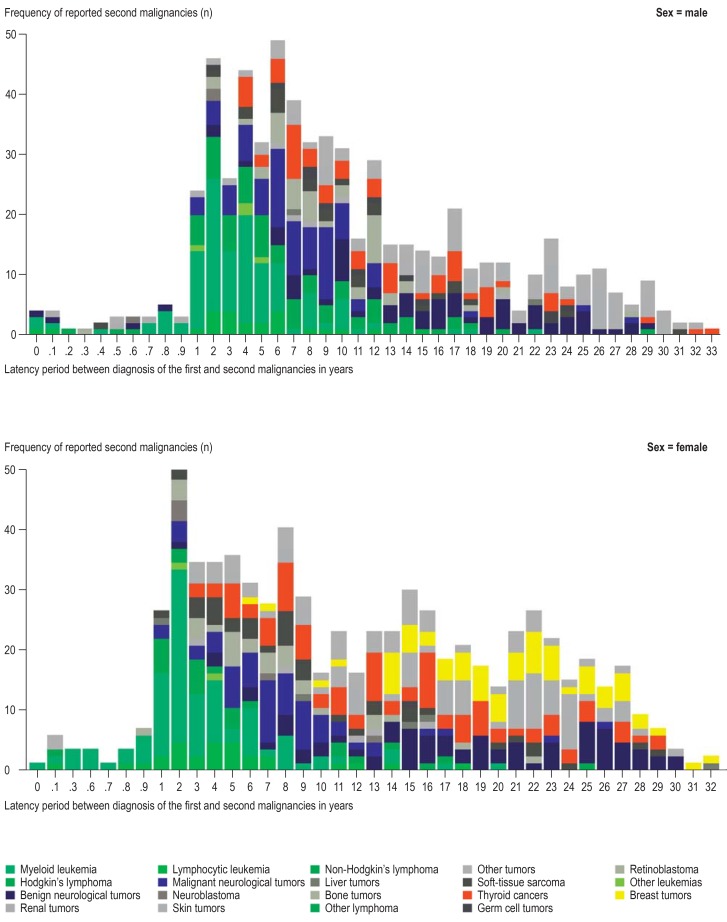
In the first 15 years after a first malignancy, 866 second malignancies were observed, 339 (39.1%) of these were systemic second malignancies, most of them acute myeloid leukemia (figure 1). Over the same time period, 396 solid second malignancies were recorded, of these 129 (32.6%) were neurological tumors, 88 (22.2%) were thyroid tumors, and 62 (15.7%) were bone tumors.
Figure 1.
Distribution of latency periods between first and second malignancies by type of second malignancy separated out for men and women, who survived for at least six months a childhood cancer in Germany between 1980 and 2014. The decrease in numbers with increasing follow-up time results from the small number of survivors with long-term follow-up and will change with continued follow-up
15–35 years after a first malignancy, 394 second malignancies were observed; of these, only 15 were systemic (3.8%). The most common solid second malignancies were non-malignant neurological tumors (n = 92, 23.4%), malignant skin tumors (n = 103, 26.1%), and thyroid tumors (n = 60, 15.2%). In women, 53 cases of breast cancer were observed (22.7% of all 233 second malignancies in women).
After lymphocytic leukemias as first malignancies, the most common second malignancies were acute myeloid leukemia, neurological tumors, thyroid tumors, and skin tumors (etable). Similar clusterings were also observed in children with a neurological first malignancy. After Hodgkin’s lymphoma, second malignancies of the thyroid and skin were observed most commonly; the diagnosis of breast cancer was recorded 27 times (37.0% of all 73 second malignancies in women after Hodgkin’s lymphoma).
eTable. Common combinations of FM und SM (classified according to ICCC-3) in 6-month survivors of childhood cancers in Germany, 1980–2014, separated by sex.
| First malignancies | ||||||||||||||||||||||||
| Second malignancies | Lymphocytic leukemia | Acute myeloid leukemia | Hodgkin’s- lymphoma | Non-Hodgkin’s lymphoma | NMCNS | MCNS | Bone tumors | Soft tissue tumors | Thyroid tumors | Skin tumors | Othertumors | Total | ||||||||||||
| m | f | m | f | m | f | m | f | m | f | m | f | m | f | m | f | m | f | m | w | m | f | m | f | |
| Lymphocytic leukemia | 1 | 3 | 7 | 7 | 2 | 0 | 3 | 5 | 1 | 1 | 2 | 4 | 2 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 5 | 24 | 27 |
| Acute myeloid leukemia | 48 | 30 | 5 | 4 | 2 | 5 | 7 | 7 | 0 | 0 | 13 | 8 | 9 | 10 | 6 | 7 | 1 | 0 | 0 | 0 | 20 | 22 | 111 | 93 |
| Hodgkin’s lymphoma | 10 | 3 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 17 | 4 |
| Non-Hodgkin’s lymphoma | 21 | 5 | 4 | 1 | 7 | 2 | 8 | 4 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 46 | 18 |
| Non-malignant CNS tumors | 28 | 22 | 2 | 2 | 1 | 0 | 7 | 9 | 4 | 0 | 21 | 28 | 2 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 6 | 11 | 74 | 74 |
| Malignant CNS tumors | 26 | 25 | 1 | 1 | 1 | 0 | 5 | 2 | 2 | 5 | 23 | 14 | 3 | 1 | 5 | 5 | 0 | 0 | 0 | 0 | 14 | 3 | 80 | 56 |
| Bone tumors | 8 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 1 | 2 | 5 | 11 | 10 | 0 | 0 | 0 | 0 | 11 | 6 | 39 | 28 |
| Soft tissue tumors | 4 | 7 | 0 | 0 | 4 | 2 | 2 | 1 | 3 | 2 | 3 | 5 | 0 | 3 | 3 | 5 | 0 | 0 | 0 | 0 | 5 | 14 | 24 | 39 |
| Thyroid tumors | 16 | 25 | 2 | 7 | 21 | 16 | 3 | 1 | 2 | 3 | 11 | 9 | 0 | 1 | 2 | 8 | 0 | 0 | 0 | 0 | 6 | 15 | 63 | 85 |
| Skin tumors | 20 | 23 | 2 | 1 | 12 | 16 | 10 | 5 | 1 | 2 | 21 | 13 | 2 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 9 | 12 | 78 | 76 |
| Breast cancer | 0 | 10 | 0 | 3 | 0 | 27 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 7 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 65 |
| Other tumors | 23 | 15 | 3 | 3 | 8 | 5 | 7 | 8 | 2 | 4 | 5 | 3 | 4 | 4 | 7 | 5 | 0 | 0 | 0 | 1 | 17 | 17 | 76 | 65 |
| Total | 205 | 173 | 26 | 29 | 58 | 73 | 56 | 47 | 15 | 19 | 107 | 89 | 25 | 35 | 43 | 49 | 1 | 0 | 0 | 1 | 96 | 115 | 632 | 630 |
| FM without SM | 7479 | 5766 | 1462 | 1220 | 1366 | 876 | 2255 | 835 | 1969 | 1764 | 3344 | 2449 | 1161 | 1026 | 1623 | 1295 | 78 | 128 | 24 | 32 | 5279 | 4957 | 26 040 | 20 348 |
| N = 0 | N = 1–2 | N = 3–10 | N = 11–20 | N = 20–30 | N > 30 | |||||||||||||||||||
FM and SM can occur in the same entity, if these differ in terms of morphology and/or topology and it has been confirmed that the FM has not recurred.
FM = first malignancy; SM = second malignancy; ICCC-3 = International Classification of Childhood Cancer; NMCNS = non-malignant CNS tumor, m = male; MCNS = malignant CNS tumor, f= female; CNS = central nervous system
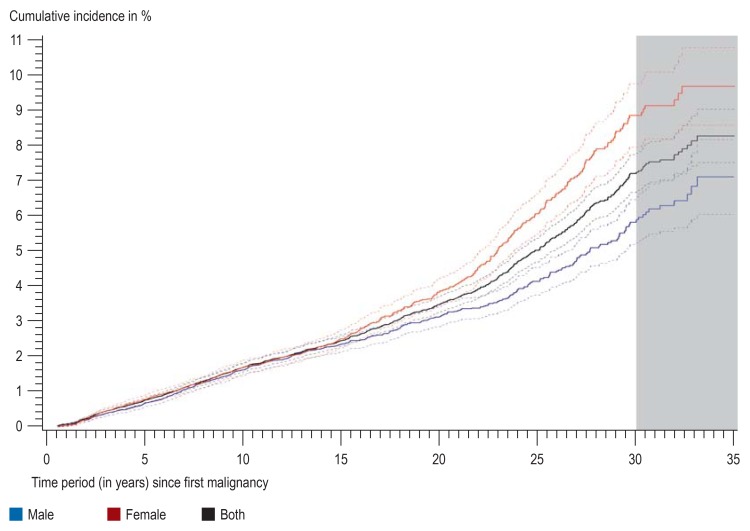
After 25 years of follow-up the cumulative incidence was 5.40%, and after 35 years, it was 8.27% (95% confidence interval [CI]: [7.51; 9.03]) (figure 2). In the first 20 years no difference was seen between men and women, but afterwards the cumulative incidence was higher in women as breast cancer became more common after this point in time. The cumulative incidence after 35 years in women was 9.72% [8.61; 10.82] and in men, 7.14% [7.07; 8.20]. The cumulative incidence after systemic first malignancies was higher than after solid first tumors (9.3% versus 7.2% [Table 2]). After 35 years the cumulative incidence for breast tumors was 1.97%; for thyroid tumors it was 1.21%; for lymphocytic leukemia, 0.14%; and for acute myeloid leukemia, 0.36% (table 3).
Figure 2.
Cumulative incidence of second malignancies with a maximum follow-up period of 35 years in 6-month survivors of childhood cancers in Germany between 1980 and 2014, separated out for both sexes as well as combined. Dotted lines: 95% confidence interval. Grey area: For the last 5 years, uncertainty is greater because of incomplete follow-up
Table 2. Cumulative incidence rates of second malignancy with maximum follow-up of 35 years in 6-month survivors of childhood cancers in Germany*.
| First malignancy | Second malignancy | Sex | FM N | SM N | Cum. inc. | 95% confidence interval | |
| All FM | All second malignancies | Both | 47 650 | 1262 | 8.27% | 7.51 | 9.03 |
| All second malignancies | Male | 26 672 | 632 | 7.14% | 7.07 | 8.20 | |
| All second malignancies | Female | 20 978 | 630 | 9.72% | 8.61 | 10.82 | |
| Lymphocytic leukemia | Both | 47 650 | 51 | 0.14% | 0.10 | 0.18 | |
| Acute myeloid leukemia | Both | 47 650 | 139 | 0.36% | 0.29 | 0.42 | |
| Thyroid cancer | Both | 47 650 | 148 | 1.21% | 0.79 | 1.64 | |
| Breast cancer | Female | 20 978 | 65 | 1.97% | 1.27 | 2.66 | |
| Solid FM | All second malignancies | Both | 25 276 | 581 | 7.26% | 6.18 | 8.34 |
| Systemic FM | All second malignancies | Both | 22 374 | 681 | 9.31% | 8.24 | 10.39 |
*1981–2014 FM and SM defined according to ICCC-3
FM = first malignancy; SM = second malignancy; ICCC-3 = International Classification of Childhood Cancer; Cum. inc. = cumulative incidence; N = group size total
Table 3. SIRs for second malignancy in 6-month survivors of childhood cancers compared with cancer incidence in the general population of Germany*.
| First malignancy | Second malignancy | Sex | O | E | SIR | 95% confidence interval | |
|
All first malignancies |
All second malignancies |
Male | 397 | 68.2 | 5.83 | 5.27 | 6.42 |
| Female | 411 | 58.0 | 7.08 | 6.42 | 7.79 | ||
| Acute lymphocytic leukemia |
Male | 113 | 8.96 | 12.61 | 10.44 | 15.10 | |
| Female | 102 | 5.74 | 17.78 | 14.57 | 21.49 | ||
| Breast cancer | Male | 0 | 0.05 | 0.00 | 0.00 | ||
| Female | 63 | 9.05 | 6.96 | 5.39 | 8.85 | ||
| Thyroid cancer | Male | 59 | 1.88 | 31.35 | 24.08 | 40.15 | |
| Female | 80 | 5.12 | 15.62 | 12.46 | 19.33 | ||
|
Solid first malignancies |
All second malignancies |
Male | 178 | 29.7 | 5.99 | 5.15 | 6.92 |
| Female | 199 | 28.3 | 7.04 | 6.11 | 8.07 | ||
|
Systemic first malignancies |
All second malignancies |
Male | 220 | 38.4 | 5.72 | 5.00 | 6.52 |
| Female | 212 | 29.8 | 7.11 | 6.20 | 8.12 | ||
| Age at first malignancy | |||||||
| 0–4 | All second malignancies |
Male | 168 | 24.3 | 6.92 | 5.93 | 8.02 |
| Female | 151 | 19.7 | 7.67 | 6.52 | 8.97 | ||
| 5–9 | All second malignancies |
Male | 117 | 18.4 | 6.35 | 5.28 | 7.58 |
| Female | 124 | 14.2 | 8.73 | 7.30 | 10.38 | ||
| 10–14 | All second malignancies |
Male | 113 | 25.5 | 4.44 | 3.68 | 5.32 |
| Female | 136 | 24.2 | 5.62 | 4.74 | 6.63 | ||
*Period 1995–2014, all second malignancies comprise all malignant neoplasms except C44.
O = observed cases; E = expected cases; FM = first malignancy; SM = second malignancy; ICCC-3 = International Classification of Childhood Cancer;
SIR = standardized incidence ratio
The results were confirmed by the regression models that had been adjusted for sex, age reached, and year of diagnosis. The adjusted hazard ratios showed an increased risk for developing a second malignancy after a systemic first malignancy (HR = 1.22 [1.09; 1.36]) and for female patients (1.29 [1.16; 1.44]).
The standardized incidence ratio for all second malignancies was 7.08 [6.42; 7.79] in women and 5.83 [5.27; 6.42] in men. The standardized incidence ratios for the individual second malignancies, different age groups, and by solid and systemic first malignancies are shown in Table 3.
Discussion
Of 47 650 children who survived a cancer diagnosis for at least six months since 1980, 1262 developed a second malignancy within 35 years.
Systemic second malignancies were found most often within the first 15 years after a first malignancy, with solid tumors being more common afterwards. The cumulative incidence of second malignancies was higher after systemic first malignancies. The higher incidence in women can be explained primarily by breast cancer as a second malignancy.
Compared with the German general population, women had 7.08 times the risk of developing a second malignancy and men 5.83 times the risk. The reason for the lower standardized incidence ratio in the age group 10–14 years at diagnosis of the first malignancy seems to be primarily the shorter follow-up period in this group.
Previous publications using the German data have reported 276 second malignancies after 18 years of follow-up (14) and 659 second malignancies after 28 years, with a cumulative incidence over 25 years of 3.3%. The now established cumulative incidence after 25 years of 5.4% showed the positive effect of the measures undertaken to obtain greater completeness of the data collection on second malignancies. The cumulative incidence over 35 years was 8.3%.
Our results are consistent with the international literature, although direct comparisons were difficult because of different study designs. Without non-melanoma skin cancer, the US CCSS Study reported a cumulative incidence between the 6th and 30th year of 7.9% (5). This is similar to our findings, although the studies used different methods. We included all second malignancies that occurred at least six months after a first malignancy, whereas the CCSS Study included only second malignancies that developed after five years or longer. In our evaluations we observed a large number of systemic second malignancies in the first five years. Furthermore, our data were based in a mean follow-up period of 10 years, whereas the data of the CCSS Study reflected results after a mean follow-up period of 23 years.
The Anglo–French CCSS reported a cumulative incidence of breast cancer after 30 years of 2.8% (15) in 4400 cancer patients with at least three years’ survival. This is slightly higher than in our study. A German study of breast cancer after Hodgkin’s lymphoma reported a cumulative incidence of 16% after 30 years (16). 96% of patients had received radiotherapy to the chest. Another German study of second malignancies after Hodgkin’s lymphoma with 30 years of follow-up found a cumulative incidence of 19% for all types of second malignancies and 4.4% for second malignancies of the thyroid (17).
A French survivors’ cohort of 3254 children with childhood cancer that included those who had survived for two years showed a cumulative incidence for thyroid adenoma of 4.3% after 40 years; this is twice the rate we found in our analysis (18).
In 1998, Westermeier et al. reported a standardized incidence ratio of 12.5 for second malignancies in children younger than 15 years of age when the first malignancy was diagnosed, compared with a general incidence of first malignancy in children younger than 15 years in Germany (8), on the basis of 127 second malignancies and a maximum follow-up period of 15 years.
The SEER (Surveillance, Epidemiology and End-Results) cohort established a standardized incidence ratio for all types of second malignancy up to the age of 47 and for the time period 1973–2002 of 5.9 for women and 6.0 for men (19). In our study, the value for men was of a similar order of magnitude whereas the standardized incidence ratio in women was higher.
Strengths and limitations
One of the strengths of our study was the size of the cohort, which included all childhood cancer cases in Germany, as well as the long follow-up period. Thanks to passive and active follow-up, we achieved a high degree of completeness for the second malignancy reports. Furthermore, all reports from patients were medically validated. Another strength was the option of including second malignancies that appeared as early as six months after the first malignancy, thereby reaching an exact estimate of the cumulative incidence in the first years.
The number of second malignancies was slightly underestimated, especially in the last follow-up periods. The small number of observed cases with 35 years of follow-up can be explained by the small number of patients with long term follow-up.
In the early years of the GCCR, no standardized notification systems existed for second malignancies. Although different methods were used in order to capture as many second malignancies as possible, we cannot rule out slight under-reporting, especially as we did not link up with adult registries.
Under-reporting across all person-years is also possible, since in some patients, follow-up was incomplete. This, however, did not affect cumulative incidence rates since these patients were censored at the date of their latest/last vital status.
The standardized incidence ratio was calculated only for a subgroup of the cohort, because population-based incidence rates for cases older than 15 years were not available for the entire period and all diagnoses. This is regrettable since non-malignant neurological tumors—especially meningiomas—and malignant non-melanoma skin tumors—especially basal cell carcinomas, which both had to be excluded for the SIR calculation, represented 8.9% (n = 125) of all second malignancies in our database.
A further source of possible bias is the fact that the expected case numbers were based on data from the general population and included cases of second malignancies. In the young age groups under study, second malignancies may account for a substantial proportion of the comparison rates, which may result in an underestimate of the standardized incidence ratio. Second malignancies after childhood cancer account for 1% of all cancers occurring before age 45 in Germany.
Many studies have shown an association between the treatment of the first malignancy and the risk for a second malignancy (3– 5, 14, 16, 17). However, the GCCR does not collect therapeutic information, although a case–control study is currently in progress in order to reconstruct this information.
Second malignancies represent a heavy burden on the survivors of childhood cancers and currently affect more than 8% of survivors in Germany. In order to reduce the risk of second malignancies, more research into therapeutic associations or genetic risk factors is needed. Since in Germany, more than 95% of children are treated within the framework of a therapy optimization study, it is possible to tailor therapeutic strategies quickly to newly identified long-term risks.
The clinical perspective.
Almost one in 10 survivors of a childhood cancer will develop a second malignancy within 35 years, with tumors of the central nervous system, the thyroid, and the female breast, as well as leukemias being particularly common. For this reason it is particularly important in this group of patients to consider cancer even if they have very non-specific symptoms. Fatigue, exhaustion, headache, impaired vision, or nausea and vomiting can be the initial symptoms of a neurological tumor. Abnormal pallor, anemia, loss of appetite with weight loss or frequent gingival or nose bleeds can be the first symptoms of leukemia. Since thyroid and breast tumors are almost symptomless in the very early stages, regular monitoring using imaging techniques is required.
Box. Important methods for analyzing late sequelae.
• Cumulative incidence
The cumulative incidence describes the new cases of a disease in a group of persons at risk for developing this disease. It can be interpreted as a person’s risk of developing the disease under study within this time period.
• Standardized incidence ratio
The standardized incidence ratio (SIR) compares the number of cases observed in a cohort with the number of expected cases. This is calculated on the basis of the age distribution in the cohort and the corresponding age specific incidence rates in the normal populations.
• Aalen–Johansen estimator
The Aalen–Johansen estimator is an extension of the Kaplan–Meier method. It can be used to estimate the probability of the occurrence of an event of interest (for example, a second malignancy) while taking into account a concurrent event (for example, death).
• Fine and Gray regression
The Fine and Gray regression is an extension of the Cox regression and is used to analyze survival times. Similar to the Aalen–Johansen estimator, this method takes into account concurrent events.
Key Messages.
47 650 patients who had had childhood cancer were followed up for up to 35 years and during this period developed 1262 second malignancies. The cumulative incidence for second malignancies was 8.27% and is set to rise with increasing survival times.
Compared with Germany’s general population, the age-adjusted risk for a second malignancy was 6.5 times higher. More women and patients with a systematic first malignancy were affected.
Survivors of childhood cancer should be under constant observation with regard to late sequelae. In this setting it is of particular importance to be attentive to possible second malignancies.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Acknowledgment
The authors thank the German Society for Paediatric Oncology and Haematology (GPOH) and the associated therapy optimization and registry studies, as well as the reporting hospitals for making their data available. We also thank the patients and their parents for their permission to collect their data in the GCCR. The GCCR is funded in equal parts (a third each) by the German Federal Ministry of Health, the Ministry for Social Affairs, Labor, Health, and Demography of the federal state of Rhineland–Palatinate, and the health ministries of all 16 federal states. This publication forms part of the dissertation of Peter Scholz-Kreisel, who is funded by an initiative of the Federal Ministry of Education and Research, funding reference No 02NUK042A.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Kaatsch P, Grabow D, Spix C. German Childhood Cancer Registry—annunal report 2016 (1980-2015) Institute for Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz 2016. www.kinderkrebsregister.de/dkkr/ergebnisse/jahresberichte.html. (last accessed on 13 April 2018) [Google Scholar]
- 2.World Health Organization. World Health Organization. Geneva: 2013. International Classification of Diseases for Oncology (ICD-O) (3rd edition, 1st revision), 3rd ed. [Google Scholar]
- 3.Meadows A, Friedman D, Neglia J, et al. Second neoplasms in survivors of childhood cancer: findings from the childhood cancer survivor study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi D, Helenowski I, Hijiya N. Secondary malignancies in pediatric cancer survivors: perspectives and review of the literature. Int J Cancer. 2014;135:1764–1773. doi: 10.1002/ijc.28991. [DOI] [PubMed] [Google Scholar]
- 5.Friedman D, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the childhood cancer survivor study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reulen R, Frobisher C, Winter D, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305:2311–2319. doi: 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 7.Kaatsch P, Debling D, Blettner M, Spix C. Second malignant neoplasms after childhood cancer in Germany—results from the long-term follow-up of the German childhood cancer registry. Strahlenther Onkol. 2009 185;(Suppl 2):8–10. doi: 10.1007/s00066-009-1005-0. [DOI] [PubMed] [Google Scholar]
- 8.Westermeier T, Kaatsch P, Schoetzau A, Michaelis J. Multiple primary neoplasms in childhood: data from the German children’s cancer registry. Eur J Cancer. 1998;34:687–693. doi: 10.1016/s0959-8049(97)00326-2. [DOI] [PubMed] [Google Scholar]
- 9.Grabow D, Spix C, Blettner M, Kaatsch P. Strategy for long-term surveillance at the German Childhood Cancer Registry—an update. Klin Padiatr. 2011;223:159–164. doi: 10.1055/s-0031-1275352. [DOI] [PubMed] [Google Scholar]
- 10.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer 3rd edition. Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 11.Aalen O, Johansen S. An empirical transition matrix for non-homogeneous markov chain based on cencored observations. Scand J Stat. 1978;5:141–150. [Google Scholar]
- 12.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Krebs in Deutschland 2011/2012, 10. Robert Koch-Institut (ed.) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. Berlin: 2015. [Google Scholar]
- 14.Klein G, Michaelis J, Spix C, et al. Second malignant neoplasms after treatment of childhood cancer. Eur J Cancer. 2003;39:808–817. doi: 10.1016/s0959-8049(02)00875-4. [DOI] [PubMed] [Google Scholar]
- 15.Guibout C, Adjadj E, Rubino C, et al. Malignant breast tumors after radiotherapy for a first cancer during childhood. J Clin Oncol. 2005;23:197–204. doi: 10.1200/JCO.2005.06.225. [DOI] [PubMed] [Google Scholar]
- 16.Schellong G, Riepenhausen M, Ehlert K, et al. Breast cancer in young women after treatment for hodgkin’s disease during childhood or adolescence—an observational study with up to 33-year follow-up. Dtsch Arztebl Int. 2014;111:3–9. doi: 10.3238/arztebl.2014.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dörffel W, Riepenhausen M, Lüders H, Brämswig J, Schellong G. Secondary malignancies following treatment for hodgkin’s lymphoma in childhood and adolescence. Dtsch Arztebl Int. 2015;112:320–327. doi: 10.3238/arztebl.2015.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddy N, El-Fayech C, Guibout C, et al. Thyroid adenomas after solid cancer in childhood. Int J Radiat Oncol Biol Phys. 2012;84:e209–e215. doi: 10.1016/j.ijrobp.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Inskip P, Curtis R. New malignancies following childhood cancer in the United States, 1973-2002. Int J Cancer. 2007;121:2233–2240. doi: 10.1002/ijc.22827. [DOI] [PubMed] [Google Scholar]