Abstract
Long-term memory (LTM) helps to efficiently direct and deploy the scarce resources of the attentional system; however, the neural substrates that support LTM-guidance of visual attention are not well understood. Here, we present results from fMRI experiments that demonstrate that cortical and subcortical regions of a network defined by resting-state functional connectivity are selectively recruited for LTM-guided attention, relative to a similarly demanding stimulus-guided attention paradigm that lacks memory retrieval and relative to a memory retrieval paradigm that lacks covert deployment of attention. Memory-guided visuospatial attention recruited posterior callosal sulcus, posterior precuneus, and lateral intraparietal sulcus bilaterally. Additionally, 3 subcortical regions defined by intrinsic functional connectivity were recruited: the caudate head, mediodorsal thalamus, and cerebellar lobule VI/Crus I. Although the broad resting-state network to which these nodes belong has been referred to as a cognitive control network, the posterior cortical regions activated in the present study are not typically identified with supporting standard cognitive control tasks. We propose that these regions form a Memory-Attention Network that is recruited for processes that integrate mnemonic and stimulus-based representations to guide attention. These findings may have important implications for understanding the mechanisms by which memory retrieval influences attentional deployment.
Keywords: cingulate, functional MRI, lateral IPS, posterior precuneus
Introduction
Human attentional and short-term memory capacity is extremely limited while real-world human visual performance is remarkably robust, especially in familiar environments. This apparent discrepancy between superior visual performance and limited attentional capacity can be reconciled by taking into account the role of long-term memory (LTM), which can guide attention to the most relevant information in an environment (Chun 2000; Summerfield et al. 2006; Hutchinson et al. 2014; Rosen et al. 2014, 2015a, 2015b). A rich literature has highlighted the behavioral advantage of memory in guiding attention (Chun and Jiang 1998, 2003; Henderson and Hollingworth 1999; Moores et al. 2003; Hollingworth 2004, 2005; Olivers 2011); however, the neural mechanisms underlying this cooperation is a more recent topic of investigation (Hutchinson and Turk-Browne 2012; Dixon et al. 2014; Goldfarb et al. 2016).
Prior investigations agree that memory-guided visuospatial attention (relative to baseline control measurements) recruits the dorsal attention network; however, it does not appear that this network is recruited more strongly relative to an equally demanding stimulus-guided visuospatial task (Summerfield et al. 2006; Stokes et al. 2012; Rosen et al. 2015a). Therefore, the unique contributions of memory-guidance of attention likely are processed elsewhere in the brain. One previous study (Summerfield et al. 2006) directly contrasted memory-guided and stimulus-guided attention and found that the left hippocampus was recruited for memory-guided attention. Importantly, this study did not observe any cortical activation differences between LTM-guided and stimulus-guided attention.
Recent work from our laboratory examined LTM-guidance of visuospatial attention using a change-detection paradigm with natural scenes in which subjects learned the location of changes in the scene images and later used this LTM to guide their attention. We compared subjects’ ability in detecting scene changes when relying on LTM to guide visuospatial attention to their ability in detecting changes when an explicit visuospatial cue guided their attention (Rosen et al. 2015a). We found that the cognitive control network (CCN), as defined by resting-state functional connectivity (Yeo et al. 2011), was recruited for LTM-guided attention compared to stimulus-guided attention. In particular, the posterior portion of the CCN, including the posterior precuneus (PrC-p), posterior callosal sulcus (CaS-p), and lateral intraparietal sulcus (latIPS), were most strongly recruited. This was the first study to show distinct recruitment of these cortical regions for LTM-guided spatial attention. However, in that study, LTM-guided attention and LTM retrieval, per se, could not be disentangled from one another.
Recently, it has been proposed that these 3 regions in the left hemisphere make up a Parietal Memory Network (PMN), which is characterized by deactivation during encoding of novel items and increasing activation with increasing familiarity (encoding-retrieval flip) (Gilmore et al. 2015). We propose an alternative (but not mutually exclusive) hypothesis, the Memory-Attention Network hypothesis, which posits that the 3 bilateral cortical regions form a Memory-Attention Network that is most strongly recruited to integrate information from mnemonic and external sources.
To examine these issues, we designed a novel target detection task with a “LTM-guided attention condition”, a “stimulus-guided attention condition” in which the visuospatial attentional demands were matched, and “LTM retrieval condition” in which the memory retrieval demands were matched. Because our prior findings differed from those of an earlier study (Summerfield et al. 2006), our first goal was to seek to replicate the findings of our previous study (Rosen et al. 2015a). This earlier study demonstrated that 3 regions of the posterior CCN, the posterior precuneus (PrC-p), posterior callosal sulcus (CaS-p), and lateral intraparietal sulcus (latIPS), are recruited more strongly for LTM-guided attention than for stimulus-guided attention.
The second goal was to determine if activation of the posterior CCN is specific to LTM-guided attention or whether it is more general to memory retrieval. A similar pattern of greater recruitment during LTM retrieval compared to stimulus-guided attention would suggest that these regions are recruited for LTM retrieval in general. If however, these regions are specific to LTM-guided attention, we should not see stronger recruitment of these regions during LTM retrieval compared to stimulus-guided attention. Importantly, for assessing compatibility of our results with the Memory-Attention Network hypothesis and the Parietal Memory Network hypothesis, all stimuli in all conditions were matched on familiarity.
A third goal of our study was to examine subcortical contributions to LTM-guided visual attention. Recent functional parcellations of subcortical structures including the cerebellum, striatum, and thalamus (Buckner et al. 2011; Choi et al. 2012, and Randy Buckner and Thomas Yeo, personal communication) allowed us to investigate the whole brain contributions to LTM-guided attention (Goldfarb et al. 2016).
Our present findings demonstrate that latIPS, PrC-p, and CaS-p, along with the CCN portions of the cerebellum, thalamus, and striatum, are recruited for processes that support the integration of information from memory and the external environment.
Methods
Participants
Twenty-five healthy human participants (13 male) with normal or corrected-to-normal vision were recruited from Boston University and the greater Boston community. All participants were compensated and gave written informed consent to participate in the study, which was approved by the Institutional Review Board of Boston University. All participants were right handed, between the ages of 22 and 34, and participated in 2 sessions (training and test) across 2 days. One female participant was excluded from all analyses due to persistent sleepiness during the scan session and below-chance performance.
Visual Stimuli and Experimental Paradigm
Experiments were conducted over 2 sessions on consecutive days, with the training session on Day 1 and the fMRI scanning session on Day 2. The primary conditions of interest were the LTM-guided attention condition and 2 contrasting experimental conditions: a stimulus-guided attention condition and an LTM retrieval condition. The stimulus-guided attention condition was designed to be well matched with the LTM-guided attention condition in its attentional demands, but place minimal demands on memory retrieval. Conversely, the LTM retrieval condition was designed to be well matched with the LTM-guided attention condition in its mnemonic demands but place minimal demands on covert visuospatial attention. A visual-motor control was also included as a baseline for the region of interest analyses. In studies of memory-attention interactions in vision, it is unlikely that the control conditions will completely devoid all aspects of either LTM retrieval or visuospatial attention; nevertheless, each aspect is clearly much less prevalent in the relevant control condition than in the LTM-guided attention condition.
Stimuli: 48 object image categories (e.g., lamps, phones, trophies) were used in this experiment. Each object category contained 4 exemplars (i.e., 4 pictures of different trophies) for a total of 192 images. This list of object categories was divided into List A and List B each containing 24 object categories. Half of the subjects (Group A) were presented with objects from List A as target objects for the experimental conditions and objects from List B as distractor images and images for the visual-motor control condition. The other half of subjects (Group B) was presented with List B as targets, and List A as distractors and for visual-motor control. For each group (Group A or Group B) the list of target categories was further divided into 3 lists of 8 object categories to be used in 1 of the 3 experimental conditions (LTM-guided attention, stimulus-guided attention, and LTM retrieval). Assignment of each of these lists of 8 object categories was counterbalanced across subjects within the group.
Day 1: 3 separate training paradigms were conducted for each subject for stimuli that were used in the 3 different experimental conditions, an LTM-guided spatial attention condition, an LTM retrieval condition, and a stimulus-guided attention condition. Training was conducted separately for each condition and the order of study was counterbalanced across subjects. Within each condition, subjects studied all 4 exemplars of each of the 8 stimuli categories in a random order. Each exemplar was studied 4 times. Exposure to stimuli was matched across all study conditions because previous work has shown familiarity impacts which brain regions are recruited for memory (Schon et al. 2008, 2013) and to allow us to test the Parietal Memory Network (Gilmore et al. 2015) and Memory-Attention Network hypotheses.
LTM-Guided Attention Training
In the first (“instructional”) phase of training, subjects were presented with Word A above and below fixation and with a red arrow pointing to 1 of 8 peripheral locations and a picture of Object A at the cued location (e.g., the word “lamp” appeared with picture of a lamp). In a second (“probe”) phase of training, subjects were presented with only Word A (and no explicit directional cue) followed by a blank screen. After a brief delay Object A appeared at the studied location. No responses were taken during this study phase, but subjects were instructed that during the delay they should try to anticipate where the image would appear. Subjects studied 8 word-location pairings with 1 object presented at a time. The 8 possible peripheral locations were equally distributed around circle centered at fixation, and positioned off of the canonical (up-down-left-right) axes in order to reduce verbalization of position information and encourage spatial encoding. Subjects viewed each image exemplar twice per training phase for total of 4 viewings of each object.
LTM Retrieval Training
Subjects studied a set of 8 arbitrary category associations. In each pairing 1 category was presented as a word and the other was presented as images, with 4 exemplars per category. Word categories and image categories were chosen from distinctly different lists. We had a large number of image categories from which to select and all distractor and control condition images were drawn for categories not used for target images in any condition for that subject. In the first (“instructional”) phase of training, subjects viewed Word B above and below fixation (e.g., “hourglass”) with Object C presented (e.g., picture of a telephone) at fixation. The category pairings (e.g., hourglass-telephone) were arbitrary, but consistent in that the object images were exemplars of shared category (telephone). In the second (“probe”) phase of training subjects were presented with only Word B followed by a blank screen. After a brief delay the paired object would appear at the center of the screen. No responses were taken during this study phase, but subjects were instructed that during the delay they should try to anticipate which object would appear. Subjects studied 8 word-object paired associations. Subjects viewed each image exemplar twice per training phase for a total of 4 viewings of each object.
Explicit Memory Test
At the end of training on Day 1, we tested that subjects had encoded all word-location and word-object pairings. Subjects were asked to perform an explicit memory test in which they used a mouse cursor to drag object images onto their associated location (LTM-guided attention) or word (LTM retrieval). All subjects were 100% accurate on the LTM retrieval explicit test. 22 out of 24 subjects were 100% accurate on the LTM-guided attention task. The 2 subjects who did not perform perfectly were retrained on a shortened version of LTM-guided attention training and retested. After 1 round of retraining, they reached 100% accuracy as well.
Stimulus-Guided Attention, Visual-Motor Control and Distractor Images Training
In order to equate stimulus familiarity across all conditions, subjects also studied images that would be used in the visuospatial attention condition (8 objects with 4 exemplars each, for a total of 32 objects), in the visual-motor control condition (9 additional exemplars taken from the distractor categories) and as distractors. Each stimulus appeared on screen for 1 s (same duration as the LTM-guided attention and LTM retrieval training) and were asked to make a judgment about each object (e.g., “Will it fit in a shoebox?”). Subjects saw each stimulus exemplar 4 times.
Day 2: The tasks were presented during fMRI scanning in a blocked design. At the beginning of each block, a cue indicated to the subject which task to perform (stimulus-guided attention, LTM retrieval, LTM-guided attention, or visual-motor control). Each block contained 8 trials.
Stimulus-Guided Attention Condition
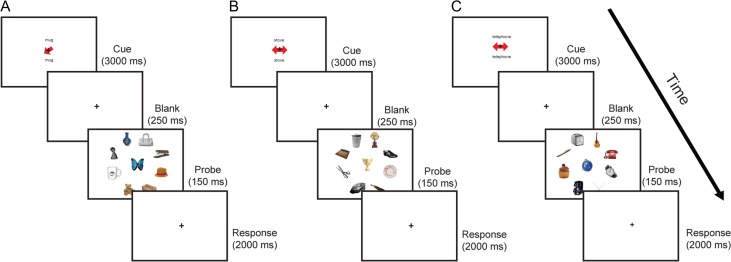
A category word that had not been studied (i.e., not associated with an object or location) was presented on the screen (1.85 s) above and below a fixation cross along with an arrow pointing to 1 of the 8 locations (Fig. 1A), followed by a blank screen for 1 s. Subjects were instructed to use the direction of the arrow to covertly guide their attention to the cued location to determine if the object that appeared at that location was a match or a non-match to the category word (i.e., if the word “telephone” appeared, subjects should try to detect a picture of a telephone at the cued location). On 50% of trials the object appeared at the cued location, and on 50% of trials it appeared at a different peripheral location. After the 1 s blank period, 9 objects (8 equally spaced in the periphery and 1 at fixation) flashed up on the screen for 150 ms, and then participants gave their response within a 2 s window.
Figure 1.
Task paradigms for (A) Stimulus-guided attention, (B) LTM retrieval, (C) LTM-guided attention. On each trial subjects were first cued with a category word above and below fixation and were then asked to detect the presence/absence of an image exemplar in a briefly presented probe array. Additionally, a red arrow was placed at fixation. (A) In the stimulus-guided attention condition, the red cue arrow indicated the location in the image array where the subjects were asked to report the presence or absence of an image exemplar of the named category. (B) In the LTM retrieval condition, subjects were asked to retrieve from LTM the paired-associate category matched to the cue word and report whether the central image was an exemplar of the paired-associate category; the red double-headed cue arrow was irrelevant in this condition as subjects always directed attention to the central location. (C) In the LTM-guided attention condition, subjects were asked to retrieve the location paired with the category word and report whether or not an image exemplar of the named category appeared at that location. Red arrows were uninformative in the LTM-guided attention condition.
LTM Retrieval Condition
A word that had been studied with an associated object was presented on the screen (1.85 s) above and below a fixation cross along with an uninformative and task-irrelevant double-headed arrow (Fig. 1B). This uninformative arrow was presented to match the visual drive across conditions. Subjects were asked to retrieve the object associated with the word and then respond whether the object that appeared at the center of the screen was a match (50% of trials) or a non-match. After a blank screen for 1 s, an array of 9 objects appeared on the screen 150 ms, followed by a 2 s response window.
LTM-Guided Attention Condition
A word that had been studied with an associated location was presented on the screen (1.85 s) above and below a fixation cross along with an uninformative double-headed arrow (Fig. 1C). Subjects were instructed to retrieve the associated location and deploy their attention covertly to that location. They then responded whether the object that appeared at that location was a match (50% of trials) or a non-match to the word. On non-match trials, the target appeared at 1 of the other 7 peripheral locations to ensure that subjects were not diffusely attending to the entire periphery, but rather were attending to only 1 location. After a blank screen for 1 s, and an array of 9 objects appeared on the screen 150 ms, followed by a 2 s response window.
Visual-Motor Control Condition
In the visual-motor control or baseline condition, subjects saw the word “passive” above and below fixation. After a blank, 9 objects appeared and subjects made a random button press. The same 9 object images (taken from the distractor categories) appeared in different configurations (among the 9 positions) for every visual-motor control trial.
Eye Tracking
Subjects were instructed to maintain fixation throughout the scans. Eye position was monitored using an EyeLink 1000 (SR Research, Mississiauga, Ontario, Canada) eye tracker that captured eye gaze location through the mirror while subjects were in the scanner. Calibration was performed before every other run using a 3 × 3 grid of equally spaced points on the screen. A square region of interest (3.5° × 3.5° of visual angle) was defined around the central fixation point. The number of saccades outside of the fixation region of interest was tallied.
MR Data Acquisition
Magnetic resonance imaging (MRI) data were acquired using a 3 Tesla Siemens TIM Trio MR imager located at the Center for Brain Science at Harvard University in Cambridge, MA. All data were acquired using a 32-channel head coil. T2*-weighted functional echo planar images (EPI) were acquired using a slice-accelerated EPI sequence that permits simultaneous multi-slice acquisitions using the blipped-CAIPI technique (Setsompop et al. 2012). Sixty-nine slices were acquired with a slice acceleration factor of 3, at an angle parallel to a line between the AC-PC (oblique axial) using AutoAlign Scout. Images were acquired at a nominal 2 mm isotropic spatial resolution (matrix size = 108 × 108 × 69). About 6/8 partial-fourier acquisition was employed to keep TE at a feasible value (TE/TR/flip-angle/bandwidth = 30 ms/2 s/80 deg/1596 Hz/px). Each participant participated in 9–12 functional scans (each 191 TRs; 6 min 22 s duration) in 1 scan session with each run containing 8 blocks, 2 of each type (LTM-guided attention, LTM retrieval, Stimulus-guided attention, and visual-motor control). Functional data were aligned with high-res T1-weighted images.
MR Data Analysis
Functional data were aligned with high-resolution (1.0 × 1.0 × 1.3 mm) T1-weighted images. For 17 participants the high-resolution structural images were acquired at the same facility; for 7 participants they were acquired on an identical scanner and coil at the Martinos Center for Biomedical Imaging at Massachusetts General Hospital in Charlestown, MA. These high-resolution structural images were used to create a computerized reconstruction of each cerebral cortical hemisphere (Fischl et al. 1999).
Functional MRI data were preprocessed using FreeSurfer 5.3.0 (Charlestown, MA, Fischl 2012). First, slice time correction was performed to account for the multi-slice acquisition, followed by motion correction, intensity normalization, and spatial smoothing (3 mm FWHM). Single participant fMRI data were then registered to an average cortical surface space (Freesurfer “fsaverage” brain) using surface spherical registration (Greve and Fischl 2009). B0 field maps were not collected with functional scans and therefore corrections for B0 distortions could not be made; this could reduce the accuracy of co-registration between functional data and structural data used to model the cortical surface, but is unlikely to bias the findings of this within-subject analysis.
Surface-based cortical analyses were performed separately in each hemisphere on the average cortical surface, while data for volume-based analyses used for subcortical structures were registered to an average 3D brain. For both volume and surface-based analyses, data were analyzed for each voxel or vertex, respectively, using a general linear model (GLM) with each condition as a predictor (i.e., 1 for LTM-guided, stimulus-guided attention, LTM retrieval, visual-motor control), separately for each run. Singular value decomposition reduced the 6 motion correction vectors to 3 eigenvectors which were included as nuisance regressors in the model. The blood-oxygen-level dependent (BOLD) signal was modeled as a linear, time-invariant system with γ response function assumed for each condition with a delay δ = 2.25 and a delay time constant τ = 1.25 (Boynton et al. 1996). An estimated response was generated by convolving the response function with the block length (i.e., the time in each condition) and minimizing the residual error (FS-FAST, Cortech). For cortical analyses, random effects group analyses were performed using surface-based averaging techniques (Fischl et al. 1999). For volume-based analyses random effects group analyses were performed after registration to an average 3D brain and a subcortical mask was applied. A t-test was performed for each vertex or voxel to compare differences in activation between conditions. The significance of these activation differences is displayed on the fsaverage brain (Fig. 2A and B).
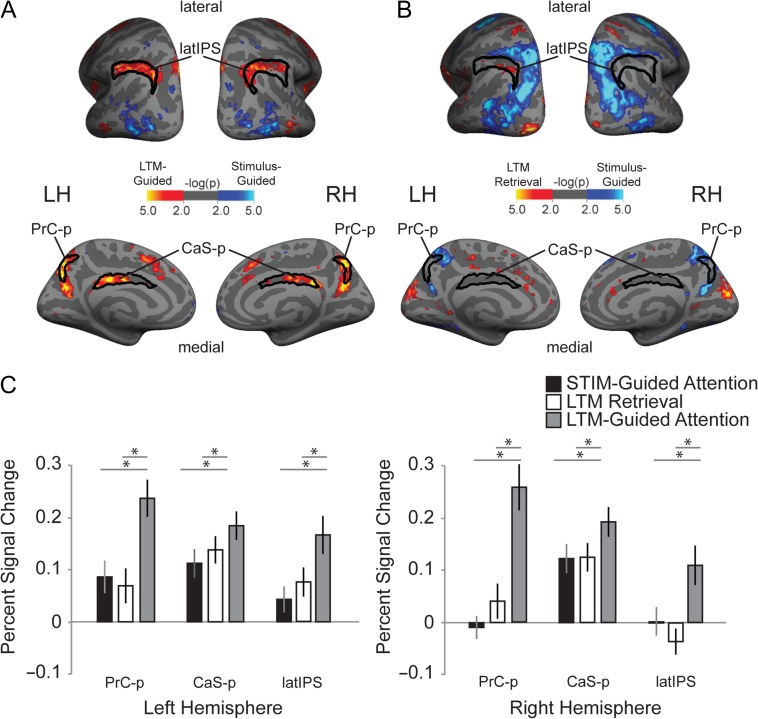
Figure 2.
Group-averaged cortical task activation maps. Posterior-lateral and medial views of right and left hemispheres are displayed for 2 task contrasts, LTM-guided attention vs. Stimulus-guided attention (A) and LTM retrieval vs. Stimulus-guided attention (B). ROI overlays were defined by Yeo et al. 2011 from resting-state functional connectivity. (C) displays percent signal change contrast for all 3 task conditions vs. the visual-motor control condition. LTM-guided attention yields stronger bilateral activation than Stimulus-guided attention or LTM retrieval conditions in 3 previously identified cortical cognitive control regions, posterior precuneus (PrC-p), posterior callosal sulcus (CaS-p) and lateral intraparietal sulcus (latIPS). * indicates P < 0.05, Holm–Bonferroni corrected.
To correct for multiple comparisons, we employed FS-FAST to perform Monte Carlo simulations of smoothed white noise to establish cluster-wise thresholds for the population maps (Forman et al. 1995). This nonparametric procedure protects against inflated false positives that are often seen in fMRI analyses (Eklund et al. 2016). The Monte Carlo simulation generated random volumes of normally distributed values that were then smoothed by a 6 mm smoothing kernel. Clusters were defined as areas of contiguous vertices or voxels for surface and volume analyses, respectively, with significance values below a threshold of P < 0.01 followed by cluster thresholding at a corrected α of P < 0.05. Surface results are presented in Table 1 and volume results are presented in Table 2.
Table 1.
Significant areas of cortical activation in the task contrasts
| Anatomical Region | Hemisphere | x | y | z | Size (mm2) | t-value |
|---|---|---|---|---|---|---|
| LTM-Guided > Stimulus-Guided Attention | ||||||
| Posterior Precuneus/Retrosplenial Cortex | LH | −13.6 | −67.4 | 39.6 | 1590.01 | 7.886 |
| RH | 6.9 | −65.2 | 40.9 | 1278.48 | 7.908 | |
| Mid-Cingulate | LH | −7.9 | −22.4 | 29.3 | 389.93 | 6.869 |
| RH | 8.4 | −36.1 | 27.9 | 365.23 | 7.140 | |
| Dorsolateral PFC | LH | −38.5 | 44.5 | 1.4 | 886.94 | 5.993 |
| RH | 38.8 | 47.6 | 6.5 | 1053.39 | 6.212 | |
| LH | −40.8 | 19.5 | 31.5 | 146.80 | 3.883 | |
| RH | 44.6 | 27.0 | 27.8 | 676.11 | 4.188 | |
| LH | −30.3 | 11.1 | 49.3 | 528.70 | 4.373 | |
| RH | 22.7 | 13.6 | 41.9 | 390.09 | 3.766 | |
| Lateral IPS/SMG | LH | −53.7 | −40.6 | 43.0 | 1825.02 | 5.159 |
| RH | 35.5 | −68.1 | 42.4 | 1519.16 | 5.101 | |
| Anterior Insula/Lateral OFC | LH | −26.6 | 23.5 | −8.2 | 451.75 | 5.573 |
| RH | 32.6 | 16.9 | 1.1 | 257.68 | 4.505 | |
| Middle Temporal Gyrus | LH | −55.1 | −42.7 | −9.9 | 221.62 | 3.973 |
| RH | 55.0 | −46.0 | −3.7 | 255.76 | 4.263 | |
| Dorsal Anterior Cingulate | LH | −8.9 | 11.8 | 42.8 | 327.99 | 4.270 |
| RH | 7.7 | 23.5 | 39.3 | 218.84 | 4.251 | |
| RH | 12.2 | 20.5 | 26.9 | 128.99 | 3.461 | |
| Stimulus-Guided > LTM-Guided Attention | ||||||
| Lateral Occipital | LH | −44.1 | −70.3 | −1.4 | 1115.68 | 6.585 |
| RH | 41.4 | −67.3 | −0.9 | 873.47 | 6.055 | |
| RH | 37.4 | −81.1 | 4.8 | 230.43 | 5.538 | |
| Superior Parietal Lobule | RH | 24.6 | −74.6 | 28.3 | 117.22 | 3.454 |
| LTM Retrieval > Stimulus-Guided Attention | ||||||
| Pars triangularis | LH | −39.1 | 25.9 | 4.4 | 969.97 | 6.758 |
| Occipital pole | LH | −22.0 | −96.0 | 0.9 | 623.82 | 6.439 |
| RH | 23.1 | −95.5 | 4.9 | 177.19 | 4.315 | |
| Posterior Insula | LH | −36.1 | −14.5 | 4.4 | 182.47 | 4.821 |
| Angular Gyrus | LH | −44.5 | −55.4 | 40.4 | 293.90 | 4.789 |
| Cuneus | LH | −4.8 | −80.4 | 23.8 | 627.85 | 4.432 |
| RH | 6.9 | −78.8 | 20.1 | 721.06 | 5.444 | |
| Supramarginal Gyrus | LH | −39.8 | −22.3 | 20.2 | 268.72 | 4.324 |
| Postcentral Sulcus | RH | 21.9 | −39.1 | 56.1 | 130.96 | 3.875 |
| Postcentral Gyrus | LH | −21.7 | −34.3 | 54.9 | 219.00 | 3.741 |
| RH | 15.7 | −29.0 | 61.5 | 152.30 | 3.571 | |
| Lateral Orbitofrontal Cortex | LH | −29.9 | 36.0 | −8.1 | 154.78 | 3.608 |
| Stimulus-Guided Attention > LTM Retrieval | ||||||
| Superior Precentral sulcus | LH | −20.7 | −1.9 | 44.9 | 856.83 | 8.669 |
| RH | 26.4 | −6.8 | 42.3 | 1886.39 | 6.269 | |
| Intraparietal Sulcus/Lateral Occipital Cortex | LH | −28.8 | −71.3 | 18.9 | 6018.21 | 8.193 |
| RH | 15.9 | −58.3 | 57.4 | 5738.27 | 8.004 | |
| Fusiform Gyrus | LH | −28.7 | −75.6 | −3.8 | 285.63 | 5.685 |
| Retrosplenial Cortex | LH | −16.7 | −57.8 | 23.9 | 148.26 | 5.302 |
| RH | 20.0 | −54.3 | 22.8 | 353.08 | 7.361 | |
| LTM-Guided Attention > LTM Retrieval | ||||||
| latIPS/IPS/PrC-p/Retrosplenial Cortex | LH | −10.8 | −67.8 | 48.9 | 5576.52 | 8.438 |
| LH | −16.0 | −58.1 | 25.1 | 464.00 | 6.932 | |
| RH | 17.4 | −57.1 | 27.2 | 7774.63 | 9.701 | |
| Superior Precentral Sulcus Dorsolateral PFC | LH | −20.4 | −0.7 | 45.2 | 1276.67 | 7.608 |
| RH | 33.8 | 9.8 | 50.9 | 4898.01 | 7.276 | |
| Anterior Insula/Lateral OFC | LH | −30.1 | 19.3 | −4.2 | 299.22 | 6.678 |
| RH | 30.1 | 21.8 | −0.8 | 432.03 | 7.354 | |
| Inferior Precentral Sulcus | LH | −48.7 | 4.1 | 26.2 | 329.81 | 4.926 |
| Fusiform Gyrus | LH | −28.7 | 74.8 | −4.4 | 579.17 | 4.265 |
| Mid-Cingulate/Posterior Callosal Sulcus | LH | −5.1 | −42.4 | 18.4 | 206.77 | 3.978 |
| RH | 8.2 | −44.2 | 16.2 | 368.70 | 6.455 | |
| Dorsal Anterior Cingulate | RH | 8.0 | 25.9 | 37.8 | 762.54 | 5.936 |
| Middle Temporal Gyrus | RH | 51.9 | −52.3 | −1.2 | 614.78 | 6.278 |
| LTM Retrieval > LTM-Guided Attention | ||||||
| Supramarginal Gyrus | LH | −48.0 | −26.1 | 19.6 | 528.92 | 6.807 |
| RH | 47.0 | −23.2 | 20.4 | 205.84 | 4.276 | |
| Cuneus | LH | −7.3 | −84.4 | 32.3 | 567.60 | 5.628 |
| RH | 6.5 | −81.5 | 30.2 | 693.77 | 5.144 | |
| Pars Triangularis | LH | −37.4 | 28.0 | 2.4 | 337.05 | 5.363 |
| Postcentral Sulcus | LH | −25.8 | −41.9 | 56.2 | 232.06 | 4.544 |
| RH | 20.4 | −37.9 | 56.0 | 222.00 | 6.309 | |
| Medial Orbitofrontal Cortex | LH | −7.9 | 54.4 | −10.2 | 161.80 | 4.650 |
| Posterior Insula | LH | −34.6 | −16.3 | −4.6 | 229.31 | 4.322 |
| dMPFC | RH | 9.2 | −3.3 | 48.3 | 131.16 | 5.771 |
| Postcentral Gyrus | LH | −20.6 | −27.6 | 64.2 | 331.11 | 3.458 |
| RH | 20.5 | −28.4 | 55.9 | 467.66 | 5.162 | |
| Occipital Pole | LH | −21.3 | −95.7 | 6.5 | 254.54 | 4.166 |
| RH | 25.5 | −95.0 | 3.0 | 261.25 | 4.654 | |
| Superior Temporal Sulcus | LH | −46.6 | −34.1 | −0.4 | 148.87 | 3.749 |
Table 2.
Significant areas of subcortical activation in thalamus, cerebellum, and striatum in the task contrasts
| Anatomical Region | x | y | z | Size (mm3) | t-value |
|---|---|---|---|---|---|
| LTM-Guided > STIM-Guided Attention | |||||
| Right Cerebellum | 26 | −69 | −25 | 1520 | 5.558 |
| Right Thalamus/Striatum | 8 | −7 | 11 | 5104 | 5.044 |
| Left Striatum/Thalamus | −16 | −5 | 9 | 5240 | 4.410 |
| Left Cerebellum | −30 | −55 | −35 | 2264 | 4.794 |
| −6 | −77 | −31 | 864 | 3.124 | |
| STIM-Guided > LTM-Guided Attention | |||||
| None | |||||
| LTM Retrieval > STIM-Guided Attention | |||||
| Left Caudate | −20 | 27 | 1 | 896.0 | 4.851 |
| STIM-Guided Attention > LTM Retrieval | |||||
| Left Cerebellum | −4 | −75 | −19 | 15 488 | 6.553 |
| Right Cerebellum | 20 | −35 | −47 | 2128 | 4.302 |
| Right Thalamus | 16 | −29 | 9 | 1600 | 6.000 |
| LTM-Guided Attention > LTM Retrieval | |||||
| Left Thalamus | −16 | −29 | 11 | 7648 | 7.126 |
| Right Thalamus | 12 | −23 | 13 | 14 400 | 6.453 |
| Left Cerebellum | −18 | −73 | −27 | 39 216 | 7.025 |
| LTM Retrieval > LTM-Guided Attention | |||||
| None | |||||
Cortical ROIs
Three bilateral cortical ROIs were defined from the Yeo and colleagues (2011) 7-network parcellation definition of the CCN. Our previous study showed that 3 regions within the CCN, including lateral intraparietal sulcus (latIPS), posterior callosal sulcus (CaS-p) and posterior precuneus (PrC-p) were more strongly activated during LTM-guided attention than stimulus-guided attention (Rosen et al. 2015a). Each region was mapped from a pre-defined label on the Freesurfer “fsaverage” brain onto the appropriate cortical hemisphere of each participant to define each ROI. Percent signal change was calculated using the regressor beta weights for each condition (LTM-guided visuospatial attention, LTM retrieval, and stimulus-guided attention, visual-motor control), with the visual-motor control acting as our baseline condition and averaged across blocks and runs within each of the 3 cortical ROIs per hemisphere for each individual subject (Fig. 2C).
Subcortical ROIs
Three subcortical regions, striatum, cerebellum, and thalamus, were investigated using parcellations from the same group (Buckner et al. 2011; Choi et al. 2012, and personal communication with Randy Buckner and Thomas Yeo). These parcellations were defined using resting-state functional connectivity. For a given structure (e.g., striatum, Choi et al. 2012), each voxel was assigned in a winner-take-all fashion to 1 of the 7 networks defined by Yeo and colleagues (2011) based on the strength of its connectivity to the cortical networks. Analysis is presented for the subcortical ROIs that were assigned to the CCN; these ROIs include the central head of the caudate, cerebellar lobule VI/Crus I, and mediodorsal thalamus, respectively.
Results
Behavioral Results
Subjects performed all tasks with high accuracy (≥94% on all conditions). The 2 guided attention tasks were designed to be equally demanding. There was no significant difference in accuracy between the LTM-guided attention (Mean = 94.0% ± 1.23) and stimulus-guided attention conditions (Mean = 95.0 ± 0.62; t(23) = 1.02, P = 0.32). Although subjects were not instructed to respond as rapidly as possible, we did observe a modest difference in response time between the stimulus-guided condition and the LTM-guided conditions (MeanStim-Guided = 898 ± 46 ms, MeanLTM-Guided = 921 ± 42 ms, P = 0.03) and a significant difference between response time for the LTM retrieval condition (MeanLTM retrieval = 826 ± 42 ms) and the other conditions (P’s < 0.0001). For this reason, in the subsequent fMRI analysis, RT was included as a separate nuisance regressor by trial. The length of our trials (5 s) was sufficient to examine time-on-task effects (Grinband et al. 2008; Yarkoni et al. 2009). The use of trial-based RT regressors and block-based condition regressors addresses orthogonality concerns that can plague RT regression (Mumford et al. 2015). The LTM retrieval task, which was intended to match memory retrieval demands of the LTM-guided attention condition, but not to match attentional task demands, had higher accuracy (Mean = 97.19% ± 0.48) than either guided attention condition (all P < 0.01).
Eye Movements
Participants were instructed to maintain central fixation throughout the experiment during fMRI scanning. Eye position was monitored via video camera for all subjects and eye movements in excess of 2° of visual angle were recorded. Overall, participants maintained fixation on 90.8% of trials (Stimulus-guided: 91.2%, LTM-guided: 91.7%; LTM Retrieval: 92.6%; Visual-Motor Control: 87.5%). A 1-way ANOVA revealed no effect of condition amongst the 3 task conditions (F(1,21) = 0.437, P = 0.516) or amongst all 4 conditions including the visual-motor control (F(1,21) = 1.509, P = 0.233; eye movement statistics are lower-bound corrected because Mauchly’s test for sphericity was not met).
Cortical ROI Analysis
Based on the findings of our prior fMRI study (Rosen et al. 2015a) of LTM-guided attention using a change-detection paradigm with real-world scenes, we approached this study with 3 a priori selected regions of interest per hemisphere: “posterior precuneus” (PrC-p), lateral IPS (latIPS), “and posterior callosal sulcus/mid-cingulate” (CaS-p) (Fig. 2). The ROIs used in our analysis were defined from a prior resting-state functional connectivity analysis of cortical networks performed in 500 human subjects (Yeo et al. 2011; 7-Network, CCN). The results replicate, using a new paradigm, our prior finding that each of these 3 parietal lobe regions is significantly more activated in the LTM-guided attention condition than in the stimulus-guided attention condition. The group-average of the contrast between LTM-guided attention and stimulus-guided attention produced activation patterns that were well captured by the Yeo resting-state ROIs (Fig. 2A). Activation was restricted to the posterior 2/3rds of the Yeo CaS-p ROI in each hemisphere and did not extend beyond the ROIs. The activation pattern was nearly a perfect fit to the Yeo latIPS ROI in the left hemisphere; this pattern was largely mirrored in the right hemisphere, with somewhat sparser activation. Activation completely filled the crescent-shaped Yeo PrC-p ROI in each hemisphere, extending into the inferior portion of the parieto-occipital sulcus.
In contrast, the LTM retrieval condition compared with the same contrast condition (stimulus-guided attention) yielded no group-level activation in 5 of the 6 ROIs and only a modest spot of activation in the sixth ROI, LH latIPS (Fig. 2B). This result was confirmed by ROI analysis (Fig. 2C). A 2-way (Hemisphere × Condition) ANOVA was performed in each ROI. There was a significant main effect of Hemisphere in latIPS (F(1,23) = 18.864, P < 0.0001), but not in the PrC-p or CaS-p: (PrC-p: F(1,23) = 2.091, P = 0.162; CaS-p: F(1,23) = 0.025, P = 0.877). All 3 regions showed a significant main effect of Condition (PrC-p: F(2,46) = 41.656, P < 0.0001; CaS-p: F(2,46) = 12.203, P < 0.0001; latIPS: F(2,46) = 20.947, P < 0.0001). Each ROI also showed a significant Condition × Hemisphere interaction (PrC-p: F(2,46) = 7.522, P = 0.001; CaS-p: F(2,46) = 3.915, P = 0.027; latIPS: F(2,46) = 13.304, P < 0.0001). The hemispheric asymmetry in latIPS is largely driven by the LTM retrieval condition, which exhibits activation in LH but deactivation in RH. Post-hoc t-tests revealed that in each hemisphere, all 3 ROIs (PrC-p, CaS-p, latIPS) showed the greatest activation during LTM-guided attention compared to both STIM-guided attention and LTM retrieval (all P < 0.05, Holm–Bonferroni corrected, Fig. 2C).
Additionally, as in our previous study (Rosen et al. 2015a), we examined these results using MNI coordinates from 1 alternative cortical network parcellation (Power et al. 2011; Supplementary Materials, Supplementary Table 1) and ROIs from another alternative cortical parcellation (17 Network from Yeo et al. 2011, Supplementary Materials, Supplementary Table 2, Supplementary Fig. 1). All findings were replicated for the 3 ROIs in both alternate frameworks. In our analysis of the Yeo-17 we also examined the angular gyrus ROI (maroon) that abuts the lateral IPS regions. Although this region exhibited greater activation in the LTM-guided attention condition than the other 2 conditions, LTM-guided attention (vs. baseline) did not differ from zero (Supplementary Table 2) and therefore reflects a difference in deactivation rather than activation. Together, these findings demonstrate that these 3 parietal regions, posterior precuneus (PrC-p), lateral IPS (latIPS), and posterior callosal sulcus/mid-cingulate (CaS-p), are recruited more strongly during tasks that involve the integration of mnemonic and attentional processes as compared to memory or attention alone.
We examined activation contrasts across the rest of the cortex using a surface-based cluster analysis (see Table 1, Fig. 2, and Supplementary Figs 1 and 2). Not surprisingly, the cluster analysis confirmed that the 3 a priori parietal ROIs, lateral IPS, posterior precuneus and posterior callosal sulcus/mid-cingulate were more strongly activated in the LTM-guided attention condition than in both of the other conditions (stimulus-guided attention and LTM retrieval). In addition, significant clusters of activation were observed in bilateral anterior insula and right dorsal anterior cingulate for the contrasts of LTM-guided attention versus each task condition and versus the control condition; these regions are well-established as fronto-opercular cognitive control or “salience network” regions (Dosenbach et al. 2007; Seeley et al. 2007; Power et al. 2011; Yeo et al. 2011; Power and Petersen 2013) that are recruited across a very broad range of cognitive tasks. Significant clusters were observed in right middle temporal gyrus and right dorsolateral PFC in the 2 task contrasts (LTM-guided > Stimulus-guided; LTM-guided > LTM-retrieval), but these observations reflected deactivation during the stimulus-guided and LTM retrieval conditions rather than activation (vs. control) for LTM-guided attention. We confirmed these findings with ROIs from the Yeo 7-network (see Supplementary Table 3). The dorsal attention network regions of the intraparietal sulcus and superior precentral sulcus (Frontal Eye Fields) were strongly activated by both the LTM-guided attention and stimulus-guided attention conditions, relative to the LTM retrieval condition; however, the 2 attention conditions exhibited little difference in these regions. The lateral occipital cortex was strongly activated by the stimulus-guided attention condition, relative to the other 2 conditions (Table 1).
The LTM retrieval condition exhibited greater activation than the 2 attention tasks in left pars triangularis, left posterior insula, and left supramarginal gyrus perhaps reflecting verbal/semantic retrieval processes in the LTM retrieval condition. Greater LTM retrieval activation was observed in bilateral postcentral gyrus and right postcentral sulcus. Curiously, the LTM retrieval condition exhibited greater activation than the 2 covert attention conditions in 2 occipital lobe regions. Greater occipital pole activation is consistent with greater spatial attention directed to the fovea in the LTM retrieval condition (Somers et al. 1999). Activation in bilateral cuneus was not present in the LTM retrieval versus control contrast, and thus the activation observed in the other contrasts likely reflects a negative bold effect for the covert attention conditions that has been observed with peripheral visual stimuli and covert attention (Tootell et al. 1998; Shmuel et al. 2002).
Subcortical Results
In order to examine subcortical contributions to LTM-guided attention, we performed both volume-based cluster analyses (Table 2) and ROI analyses (Fig. 3). Both the contrast of LTM-guided compared to stimulus-guided attention and LTM-guided attention compared to LTM retrieval revealed activation spanning the mediodorsal nucleus of the thalamus and the head of the caudate bilaterally, as well as a large bilateral swath of activation in the cerebellum, including bilateral VI lobule of the cerebellum, extending into Crus I (Fig. 3). The contrast of stimulus-guided attention compared to LTM retrieval revealed bilateral activation within the posterior thalamus and bilaterally within the cerebellum. The reverse contrast revealed a small cluster of activation within the left striatum.
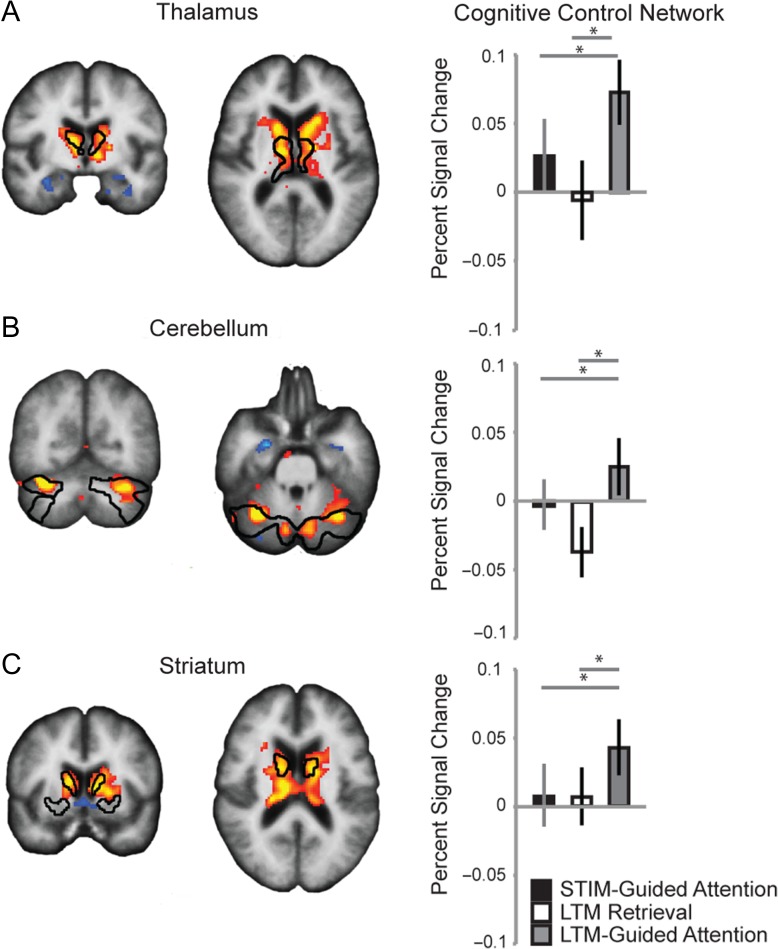
Figure 3.
Group-averaged subcortical activation within CCN subregions. CCN subdivisions of (A) Thalamus, (B) Cerebellum, and (C) Striatum were more strongly activated by LTM-guided attention than by stimulus-guided attention or LTM retrieval conditions. Subcortical ROIs (black outlines) were previously defined in a group analysis performed by the Buckner lab ((Buckner et al. 2011; Choi et al. 2012) and personal communication with Buckner and Yeo) based on resting-state functional connectivity with the cortical CCN. Left plots depict the contrast of LTM-guided attention > Stimulus-guided attention. Note that thalamic activation and caudate activation abut in the axial slices of (A) and (C).
The striatum, cerebellum, and thalamus (Buckner et al. 2011; Choi et al. 2012) have been parcellated using functional connectivity with the cortical networks defined in (Yeo et al. 2011). Since our cortical areas of interest were well-localized by regions in Yeo’s CCN, we hypothesized that the striatum, cerebellum and thalamus parcels associated with the cortical CCN would also exhibit selective activation for the LTM-guided attention condition relative to both of the other conditions. We performed ROI analyses within the CCN portion of each of these structures. These results demonstrate that the subcortical ROI definitions, which were defined via resting-state functional connectivity with cortical networks, share task activation properties with the cortical networks. A 1-way ANOVA was conducted for each structure within the CCN using the 3 behavioral conditions as factors. A main effect of condition was found in all 3 structures (all P < 0.05, corrected). Post-hoc t-tests revealed that in all 3 structures, the CCN was significantly more strongly recruited for LTM-guided attention than for both LTM retrieval and stimulus-guided attention (all P < 0.05, corrected, Fig. 3). Taken together, these findings highlight the important contribution of subcortical CCN structures to LTM-guided attention.
In addition, we examined activation within hippocampus head and tail, but evidence fell short of supporting a role for either the head or the tail of either hemisphere of the hippocampus in memory-guided attention. No contrasts showed significant activation relative to baseline. None of the t-tests between any pair of conditions in any of the 4 ROIs were significant after correcting for multiple comparisons (See Supplementary Table 4).
Discussion
We investigated the neural mechanisms underlying the cooperation of LTM and attention and observed greater activation in 3 bilateral parietal cortical areas, lateral intraparietal sulcus (latIPS), posterior precuneus (PrC-p), and posterior callosal sulcus (CaS-p), for LTM-guided visuospatial attention than for either LTM retrieval (without covert visual attention) or stimulus-guided attention (without LTM retrieval). These observations replicate our prior work (Rosen et al. 2015a) on LTM-guided attention (vs. stimulus-guided attention) and extend this to the contrast with LTM retrieval. Importantly, the stimuli used across all conditions were counterbalanced across subjects and each subject had equal amounts of prior exposure to the stimuli for each condition. Prior resting-state functional connectivity analyses have demonstrated that these 3 parietal cortical regions form a network (Power et al. 2011; Yeo et al. 2011; Doucet et al. 2012; Shirer et al. 2012; Rosen et al. 2015a). Within frontal cortex, both LTM-guided attention contrasts exhibited greater activation within bilateral anterior insula and right dorsal anterior cingulate. The role of these areas deserves further investigation; however, since it is well-established that these “salience network” regions support cognitive control across a very broad range of tasks (Dosenbach et al. 2007; Seeley et al. 2007), we do not suggest that these regions play a role specific to memory-guided attention. In addition, these LTM-guided attention results were also observed in 3 subcortical ROIs, the head of the caudate nucleus in dorsal striatum, lobule VI/Crus I of cerebellum, and the mediodorsal nucleus of the thalamus, defined by resting-state functional connectivity with the CCN in cortex (Buckner et al. 2011; Choi et al. 2012; R. Buckner & T. Yeo, pers comm). Based on our task findings and prior results, we conclude that these 3 bilateral parietal cortical regions and 3 bilateral subcortical regions serve to integrate information retrieved from LTM to support the effective deployment of attention. Therefore, we hypothesize that these regions may form a Memory-Attention Network (MAN). Future studies using targeted connectivity analyses should be conducted in order to determine the nature and dynamics of this proposed network and other regions exhibiting similar task-evoked effects.
A recent review paper has proposed that 3 left hemisphere cortical regions—posterior inferior parietal lobule/dorsal angular gyrus, precuneus, and mid-cingulate cortex—form a “Parietal Memory Network” (PMN) that supports LTM retrieval and suggested that these regions are initially deactivated during encoding and show increasing activation as an item becomes more familiar with repeated study (Gilmore et al. 2015). Resting-state functional connectivity analyses (Doucet et al. 2012; Power et al. 2011; Rosen et al. 2015a; Shirer et al. 2012; Yeo et al. 2011) confirm that these 3 regions correspond to the cortical regions identified here as latIPS, PrC-p, and CaS-p, respectively. The Memory-Attention Network hypothesis and the Parietal Memory Network hypothesis both emphasize a key role for LTM retrieval, but differ in that the MAN hypothesis also emphasizes integration with attentional control leading to greater activation. The PMN hypothesis suggests attention can have either positive or negative effects on activation in these regions, with enhanced activation for presentation of highly familiar items and stronger deactivation for highly novel items and middling effects for moderately familiar or moderately novel stimuli (Gilmore et al. 2015). Since stimulus familiarity is equivalent across our 3 task conditions, this PMN hypothesis does not account for the present findings. The current findings demonstrated greater activation in these 3 cortical areas for LTM-guided attention than for LTM retrieval and thus support the MAN hypothesis. Another distinction is that task-based fMRI evidence only supports the Parietal Memory Network hypothesis for the left hemisphere, not for the right hemisphere (Nelson et al. 2010, 2013; Gilmore et al. 2015). However, recent studies have also suggested bilateral memory-related recruitment in the PrC-p and latIPS regions of the proposed PMN (Brodt et al. 2016; Chen et al. 2017; McDermott et al. 2017). Our results support the Memory-Attention Network hypothesis for all 3 regions (PrC-p, latIPS, and CaS-p) for both hemispheres, as well as for 3 subcortical structures. A closer look at our data (see Fig. 2C, Supplementary Table 5) reveals that the LTM-retrieval condition does in fact activate all 3 cortical ROIs in the left hemisphere relative to the sensory-motor baseline; however, in the right hemisphere this contrast produced no activation in PrC-p and latIPS. Thus, the left laterality of LTM-retrieval effects in our data mirror the data marshaled to support the PMN hypothesis (Nelson et al. 2013; Gilmore et al. 2015) as well as similar left parietal retrieval effects observed in prior studies (Wagner et al. 2005; Cabeza 2008; Nelson et al. 2010; Sestieri et al. 2010; Hutchinson et al. 2014). That is, our findings are consistent with both a LH PMN and a bilateral MAN.
Our data also indicate that the visuospatial attention condition, without explicit long-memory retrieval demands, activates LH PrC-p and bilateral CaS-p, but not the other areas, relative to the sensory-motor baseline (see Fig. 2C, Supplementary Table 5). Given the efficacy of both visuospatial attention and LTM retrieval in activating some of these cortical regions, can the stronger activation we observed here for LTM-guided attention be explained as simply the summed activation to 2 independent component processes, memory retrieval and attentional deployment? Our results in bilateral CaS-p, left PrC-p, and left latIPS are not inconsistent with this suggestion; however, our findings in right PrC-p, right latIPS, and the subcortical structures do not support this interpretation and thus imply that activation in these regions reflects additional processing that reflects integration of LTM information into attentional mechanisms.
Broadly speaking, LTM-guidance of visuospatial attention must be considered a form of cognitive control. Indeed, the ROIs used in our analysis were originally attributed as components of a large-scale CCN, as defined by resting-state functional connectivity (Yeo et al. 2011). On the other hand, these 3 parietal lobe regions are not included in most functional definitions of CCNs (Cole and Schneider 2007; Braver 2012; Sestieri et al. 2014; but see Dosenbach et al. 2007). This omission may reflect a bias toward assigning cognitive control functions primarily to the frontal lobes. It is also possible that, because latIPS, PrC-p, and CaS-p each lie adjacent to prominent “task-negative” or “default mode network” regions (Fox et al. 2005), these anatomically thin regions were obscured by their neighbors in previous studies. However, we suggest that the critical distinction may be that these parietal areas are preferentially recruited for a specific form of cognitive control, LTM-guidance of attention. Our current and prior results (Rosen et al. 2015a) demonstrate this for 2 different LTM-guided visuospatial attention paradigms; other forms of LTM-guided attention (e.g., LTM-guided object- or feature-based attention) warrant deeper investigation.
We extended our previous findings by including analysis of 3 subcortical structures, striatum, cerebellum and thalamus. Our cortical ROIs were originally defined as nodes of a CCN identified in a cortical parcellation based on resting-state functional connectivity (Yeo et al. 2011). Similarly, we examined ROIs defined as the CCN subregions of striatum, cerebellum, and thalamus from parcellations based on resting-state connectivity with the cortical networks from Yeo and colleagues (Buckner et al. 2011; Choi et al. 2012). These ROIs include bilateral head of the caudate, bilateral lobule VI/Crus I of the cerebellum, and bilateral mediodorsal thalamus, respectively. Each of these ROIs exhibited significantly greater activation for the LTM-guided attention condition than for LTM retrieval or stimulus-guided attention conditions. Our suggestion that the Memory-Attention Network includes striatal, cerebellar, and thalamic subregions is consistent with prior investigations of these subcortical subdivisions in humans and non-human primates. Recent neuroimaging work indicates a key role for striatum in the guidance of visuospatial attention by stimulus-response associations (Goldfarb et al. 2016), and primate studies have demonstrated that neurons in anterior caudate represent stimulus value and guide visuospatial attention (Kim and Hikosaka 2013; Yanike and Ferrera 2014). Human neuroimaging studies have revealed cerebellar recruitment for working memory and other cognitive tasks that is independent of motor responses (Chen and Desmond 2005; Stoodley et al. 2012; Brissenden et al., 2016) and cerebellar lobule VI/Crus I specifically has been activated in some working memory tasks (Chen and Desmond 2005; Stoodley et al. 2012; Peterburs et al. 2015). Mediodorsal thalamus has been implicated in spatial working memory in monkeys (Funahashi 2013) and lesions of mediodorsal thalamus have been reported to result in spatial memory loss (Isseroff et al. 1982) and recollection deficiencies (Zoppelt et al. 2003).
A prior study of LTM-guided visuospatial attention reported differential recruitment of left hippocampus relative to a stimulus-guided condition (Summerfield et al. 2006). Importantly, this study did not observe any cortical activation differences between LTM-guided and stimulus-guided attention. However, it is possible that volume-based group averaging techniques obscured cortical differences between these 2 attentional states. A follow-up study from the same laboratory (Stokes et al. 2012) reported left hippocampal activation during the cueing phase of LTM-guided attention, but deactivation during the target search phase. Here, as in our prior fMRI study that employed a different task paradigm (Rosen et al. 2015a), we failed to observe differential activation for LTM-guided attention relative to stimulus-guided attention within either the head or tail of the hippocampus in either hemisphere. However, our results were obtained in a block-design format, which combines cue and target phase responses and thus are not inconsistent with the findings of Stokes et al. (2012). Moreover, since it is well-established that there are multiple memory systems (Squire 1992) and multiple attention networks in the brain (Corbetta and Shulman 2002; Michalka et al. 2015), there may be multiple forms of memory-attention interactions (Hutchinson and Turk-Browne 2012; Patai et al. 2012; Goldfarb et al. 2016). A recent study has contrasted visuospatial attention guided by non-declarative, probabilistic stimulus-response associations with visuospatial attention guided by implicit contextual cueing and observed a dissociation between striatal and hippocampal contributions to the 2 forms of memory-guided attention (Goldfarb et al. 2016). In the present work, memory retrieval may have dominated more than in either of those paradigms, which may account for the differences in the pattern of activations observed.
Although our findings support a putative Memory-Attention Network, this hypothesis requires further investigation on multiple points. While the 6 regions lie within a broad CCN as defined by resting-state functional connectivity and thus in that sense belong to the same network, it is not yet apparent that these regions form a subnetwork dedicated only to memory-attention interactions. Some of these regions are significantly recruited by memory alone in previous studies (Nelson et al. 2013; Chen et al. 2017; McDermott et al. 2017) and in the present study for both memory and attention alone compared to baseline. Therefore, these regions may be most strongly recruited to support memory-attention interactions, but not exclusively the interactions. Efforts to demonstrate finer scale resting-state networks are equivocal on this point. For example, in the 17 network of the Yeo-Buckner parcellation and in the Power parcellation, while PrC-p and CaS-p form a tight subnetwork, lateral IPS is typically assigned to a different subnetwork. A closer analysis indicates that lateral IPS exhibits strong functional connectivity multiple CCN regions. Thus, lateral IPS may serve as a network hub that is recruited under strong memory-attention demands, but may not primarily belong to a memory-attention network. Alternatively, since prior work demonstrates that lateral IPS contains multiple functional subdivisions that differ on their roles in memory retrieval processes (Hutchinson et al. 2014), there might be a sub-region within lateral IPS that is largely exclusive to memory-attention interactions while other subregions participate in other cognitive control subnetworks. Similar concerns could be raised regarding whether the identified subcortical regions are primarily members of a memory-attention network or serve a broader range of cognitive control functions.
Taken together, these findings provide evidence that the cooperation between LTM and attention is supported by a network spanning cortical, cerebellar, thalamic and striatal regions. This Memory-Attention Network can be identified by resting-state functional connectivity as a subnetwork of a broader CCN (Buckner et al. 2011; Yeo et al. 2011; Choi et al. 2012). It remains possible that some regions, perhaps the posterior callosal sulcus region, simply support LTM retrieval and visuospatial attention as independent functions, while true integration of LTM retrieval and visuospatial attention depends on only a subset of the hypothesized Memory-Attention Network. Future investigations will be required to determine the specific mechanistic roles of each of these 6 brain regions. Furthermore, the issue of what, if any, specific contributions MAN regions make to working memory needs further investigation.
Supplementary Material
Notes
Conflict of Interest: None declared.
Supplementary Material
Funding
This work was supported by the National Eye Institute at the National Institutes of Health (grant number R01-EY022229) and by the National Institute of Child Health and Human Development (grant number F32 HD089514).
References
- Boynton GM, Engel SA, Glover GH, Heeger DJ. 1996. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 16(13):4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS. 2012. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 16(2):106–113. http://doi.org/10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissenden JA, Levin EJ, Osher DE, Halko MA, Somers DC. 2016. Functional evidence for a cerebellar node of the dorsal attention network. J Neurosci. 36(22):6083–6096. http://doi.org/10.1523/JNEUROSCI.0344-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodt S, Pöhlchen D, Flanagin VL, Glasauer S, Gais S, Schönauer M. 2016. Rapid and independent memory formation in the parietal cortex. Pro Natl Acad Sci. 113(46):13251–13256. 10.1073/pnas.1605719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 106(5):2322–2345. http://doi.org/10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. 2008. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 46(7):1813–1827. http://doi.org/10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. 2005. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 24(2):332–338. http://doi.org/10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen HY, Gilmore AW, Nelson SM, McDermott KB. 2017. Are their multiple kinds of episodic memory? An fMRI investigation comparing autobiographical and recognition memory tasks. J Neurosci. 37(10):2764–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL. 2012. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 108(8):2242–2263. http://doi.org/10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun M. 2000. Contextual cueing of visual attention. Trends Cogn Sci. 4(5):170–178. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. 1998. Contextual cueing: implicit learning and memory of visual context guides spatial attention. Cognit Psychol. 36(1):28–71. http://doi.org/10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. 2003. Implicit, long-term spatial contextual memory. J Exp Psychol Learn Mem Cogn. 29(2):224–234. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. 2007. The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage. 37(1):343–360. http://doi.org/10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3(3):201–215. http://doi.org/10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Fox KCR, Christoff K. 2014. A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia. 62:321–330. http://doi.org/10.1016/j.neuropsychologia.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 104(26):11073–11078. http://doi.org/10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Zago L, Crivello F, Jobard G, Delcroix N, Mellet E, Tzourio-Mazoyer N, Mazoyer B, et al. 2012. Patterns of hemodynamic low-frequency oscillations in the brain are modulated by the nature of free thought during rest. NeuroImage. 59(4):3194–3200. http://doi.org/10.1016/j.neuroimage.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 113(28):7900–7905. http://doi.org/10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. 2012. FreeSurfer. NeuroImage. 62(2):774–781. http://doi.org/10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 9(2):195–207. http://doi.org/10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 33(5):636–647. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102(27):9673–9678. http://doi.org/10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. 2013. Space representation in the prefrontal cortex. Prog Neurobiol. 103:131–155. http://doi.org/10.1016/j.pneurobio.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, McDermott KB. 2015. A parietal memory network revealed by multiple MRI methods. Trends Cogn Sci. 19(9):534–543. http://doi.org/10.1016/j.tics.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Goldfarb EV, Chun MM, Phelps EA. 2016. Memory-guided attention: independent contributions of the hippocampus and striatum. Neuron. 89(2):317–324. http://doi.org/10.1016/j.neuron.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. 2009. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 48(1):63–72. http://doi.org/10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Wager TD, Lindquist M, Ferrera VP, Hirsch J. 2008. Detection of time-varying signals in event-related fMRI designs. NeuroImage. 43(3):509–520. http://doi.org/10.1016/j.neuroimage.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Hollingworth A. 1999. High-level scene perception. Annu Rev Psychol. 50(1):243–271. http://doi.org/10.1146/annurev.psych.50.1.243. [DOI] [PubMed] [Google Scholar]
- Hollingworth A. 2004. Constructing visual representations of natural scenes: the roles of short- and long-term visual memory. J Exp Psychol Hum Percept Perform. 30(3):519–537. http://doi.org/10.1037/0096-1523.30.3.519. [DOI] [PubMed] [Google Scholar]
- Hollingworth A. 2005. The relationship between online visual representation of a scene and long-term scene memory. J Exp Psychol Learn Mem Cogn. 31(3):396–411. http://doi.org/10.1037/0278-7393.31.3.396. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Turk-Browne NB. 2012. Memory-guided attention: control from multiple memory systems. Trends Cogn Sci. 16(12):576–579. http://doi.org/10.1016/j.tics.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Weiner KS, Bressler DW, Silver MA, Preston AR, Wagner AD. 2014. Functional heterogeneity in posterior parietal cortex across attention and episodic memory retrieval. Cereb Cortex 24:49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isseroff A, Rosvold HE, Galkin TW, Goldman-Rakic PS. 1982. Spatial memory impairments following damage to the mediodorsal nucleus of the thalamus in rhesus monkeys. Brain Res. 232(1):97–113. [DOI] [PubMed] [Google Scholar]
- Kim HF, Hikosaka O. 2013. Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values. Neuron. 79(5):1001–1010. http://doi.org/10.1016/j.neuron.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Gilmore AW, Nelson SM, Watson JM, Ojemann JG. 2017. The parietal memory network activates similarly for true and associative false recognition elicited via the DRM procedure. Cortex. 87:96–107. http://doi.org/10.1016/j.cortex.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Michalka SW, Kong L, Rosen ML, Shinn-Cunningham BG, Somers DC. 2015. Short-term memory for space and time flexibly recruit complementary sensory-biased frontal lobe attention networks. Neuron. 87(4):882–892. http://doi.org/10.1016/j.neuron.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores E, Laiti L, Chelazzi L. 2003. Associative knowledge controls deployment of visual selective attention. Nat Neurosci. 6(2):182–189. http://doi.org/10.1038/nn996. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Poline JB, Poldrack RA. 2015. Orthogonalization of regressors in fMRI models. PLoS ONE. 10(4):e0126255 http://doi.org/10.1371/journal.pone.0126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Arnold KM, Gilmore AW, McDermott KB. 2013. Neural signatures of test-potentiated learning in parietal cortex. J Neurosci. 33(29):11754–11762. http://doi.org/10.1523/JNEUROSCI.0960-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, et al. 2010. A parcellation scheme for human left lateral parietal cortex. Neuron. 67(1):156–170. http://doi.org/10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivers CNL. 2011. Long-term visual associations affect attentional guidance. Acta Psychol (Amst). 137(2):243–247. http://doi.org/10.1016/j.actpsy.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Patai EZ, Doallo S, Nobre AC. 2012. Long-term memories bias sensitivity and target selection in complex scenes. J Cogn Neurosci. 24(12):2281–2291. http://doi.org/10.1162/jocn_a_00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterburs J, Cheng DT, Desmond JE. 2015. The association between eye movements and cerebellar activation in a verbal working memory task. Cereb Cortex 26:3802–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Petersen SE. 2013. Control-related systems in the human brain. Curr Opin Neurobiol. 23(2):223–228. http://doi.org/10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. 2011. Functional network organization of the human brain. Neuron. 72(4):665–678. http://doi.org/10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Stern CE, Somers DC. 2014. Long-term memory guidance of visuospatial attention in a change-detection paradigm. Front Psychol. 5:266 http://doi.org/10.3389/fpsyg.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Stern CE, Michalka SW, Devaney KJ, Somers DC. 2015. a. Cognitive control network contributions to memory-guided visual attention. Cereb Cortex. 26:2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Stern CE, Michalka SW, Devaney KJ, Somers DC. 2015. b. Influences of long-term memory-guided attention and stimulus-guided attention on visuospatial representations within human intraparietal sulcus. J Neurosci. 35(32):11358–11363. http://doi.org/10.1523/JNEUROSCI.1055-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Ross RS, Hasselmo ME, Stern CE. 2013. Complementary roles of medial temporal lobes and mid-dorsolateral prefrontal cortex for working memory for novel and familiar trial-unique visual stimuli. Eur J Neurosci. 37(4):668–678. http://doi.org/10.1111/ejn.12062. [DOI] [PubMed] [Google Scholar]
- Schon K, Tinaz S, Somers DC, Stern CE. 2008. Delayed match to object or place: an event-related fMRI study of short-term stimulus maintenance and the role of stimulus pre-exposure. NeuroImage. 39(2):857–872. http://doi.org/10.1016/j.neuroimage.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27(9):2349–2356. http://doi.org/10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Spadone S, Romani GL, Shulman GL. 2014. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J Cogn Neurosci. 26(3):551–568. http://doi.org/10.1162/jocn_a_00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. 2010. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J Neurosci. 30(25):8445–8456. http://doi.org/10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. 2012. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 67(5):1210–1224. http://doi.org/10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. 2012. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. 2002. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 36(6):1195–1210. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. 1999. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA. 96(4):1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. 1992. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J Cogn Neurosci. 4(3):232–243. http://doi.org/10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- Stokes MG, Atherton K, Patai EZ, Nobre AC. 2012. Long-term memory prepares neural activity for perception. Proc Natl Acad Sci USA. 109(6):E360–E367. http://doi.org/10.1073/pnas.1108555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. 2012. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 59(2):1560–1570. http://doi.org/10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Lepsien J, Gitelman DR, Mesulam MM, Nobre AC. 2006. Orienting attention based on long-term memory experience. Neuron. 49(6):905–916. http://doi.org/10.1016/j.neuron.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. 1998. The retinotopy of visual spatial attention. Neuron. 21(6):1409–1422. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. 2005. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 9(9):445–453. http://doi.org/10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Yanike M, Ferrera VP. 2014. Representation of outcome risk and action in the anterior caudate nucleus. J Neurosci. 34(9):3279–3290. http://doi.org/10.1523/JNEUROSCI.3818-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. 2009. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: a multi-study fMRI analysis. PLoS ONE. 4(1):e4257 http://doi.org/10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106(3):1125–1165. http://doi.org/10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppelt D, Koch B, Schwarz M, Daum I. 2003. Involvement of the mediodorsal thalamic nucleus in mediating recollection and familiarity. Neuropsychologia. 41(9):1160–1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.