Abstract
An emerging hypothesis, linking modulation of neurogenesis with the onset and subsequent treatment of depression, has received much attention recently as an attractive explanation for successful behavioral changes induced by antidepressant medication in both humans and animals. However, evidence for such a link remains elusive and inconsistent. This review discusses evidence for modulation of neurogenesis as a neurobiological substrate for depression within the context of heterogeneous animal models of depression. Examining the evidence currently available linking neurogenesis and depression is problematic for at least four reasons: 1) approaches to document ongoing neurogenesis and neuronal lineage commitment are varied, making cross-study comparison difficult; 2) as the functional contribution of adult neurogenesis has yet to be completely determined, it is speculative to state a functional significance to changes in neurogenesis; 3) there is diversity in animal models of depression with variable degrees of correlation with human depression; and 4) there remains insufficient knowledge of molecular factors and changes in gene expression that conclusively link neurogenesis modulation and depression. This review examines the current state of evidence regarding the following: 1) consistent data collection delineating the existence of neurogenesis, its stages of progression, and stage modulation; 2) the functional contribution of adult hippocampal neurogenesis and the use of stress-based animal models for its modulation, 3) possible molecular links between antidepressant medication and neurogenesis, specifically neurotrophins and trophic factors; and finally 4) specific suggestions for further investigations necessary to warrant full acceptance of a link between modulation of neurogenesis and depression.
Key words: Stress, Hippocampus, Dentate gyrus, BDNF, Antidepressants, BrdU
INTRODUCTION
Over the last decade, the persistence of adult neurogenesis (the generation of new neurons) has generated enthusiasm among both neuroscientists and the general public for its potential role in brain function and use in brain repair. Once dogmatically refuted (54), neurogenesis has been conclusively demonstrated in adult mammals, including primates and humans (10,34,40,42,43,64,70,87). One emerging reason for excitement today is the possibility that enhancing neurogenesis could offer a new treatment for psychiatric illness. An emerging hypothesis linking neurogenesis modulation with the onset and subsequent cure of depression has received much attention. This is partly due to the fact that chronic administration of most antidepressants leads to an increase in neurogenesis. Some researchers have even proposed that neurogenesis is a “requirement” for antidepressant behavioral effects (90), but it is still unknown if diminished neurogenesis could be a cause, consequence, or correlate of depression (38,100). In fact, despite a plethora of discovery-driven research, the functional contribution of adult neurogenesis remains elusive. As a result, it continues to be difficult to draw conclusions regarding the possibility of an evidence-based link between neurogenesis and depression.
This mixed evidence for a link between the modulation of neurogenesis and depression can be attributed to four factors. First, there exist multiple approaches to document the existence of neurogenesis and its progressive phases, making cross-study comparison difficult (65). Additionally, little is known about the mechanisms responsible for the initiation of neurogenesis and the continuing progression of new cells through subsequent phases (Fig. 1). Secondly, though research has established a role in learning and memory and emotion for the hippocampus, the functional contribution of adult neurogenesis in this region has yet to be completely determined. This has led to hypotheses that still need to be fully tested. Thirdly, there is great diversity and variability in animal models of depression and these models also exhibit variable degrees of correlation with human depression. Finally, there is still insufficient knowledge of molecular factors and changes in gene expression that may drive neurogenesis modulation and depression. The purpose of this review is to discuss the evidence for modulation of neurogenesis as a neurobiological substrate for depression within the context of various animal models with their heterogeneous natures and behavioral manifestation of depression.
Figure 1.
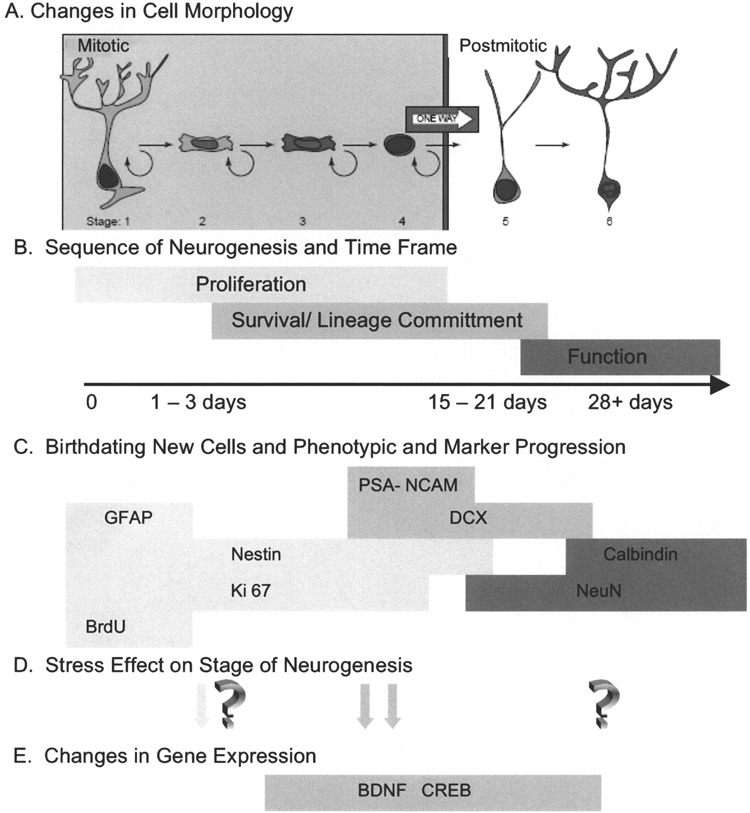
Progression of adult hippocampal neurogenesis. From their initial generation until their maturation as a functioning neuron, emerging evidence suggests that the process of neurogenesis consists of sequential progression of the new cell through distinct stages that are identified by a variety of detection methods. (A) Changes in cell morphology and location relative to the granule cell layer formed the original observations that newly generated cells underwent a specific maturation process en route to expressing the morphology of mature neurons. In conjunction with the phenotypic markers discussed below, Kempermann et al. (56) have proposed six specific stages of cell maturation. (B) While it was initially envisioned that proliferating cells terminally exited cell cycle before beginning lineage commitment, it is now recognized that cells expressing early markers of lineage commitment can continue to proliferate. Likewise, there is a transition between expression of lineage commitment markers and the initiation of functional neuronal properties. The population of new cells in the hippocampus is not synchronized, but it appears that any individual cell would progress from mitosis to full maturation in an interval of 3–4 weeks. It is important to note that not all cells will progress to maturity. Some will remain proliferative and constitute a pool of amplifying neural progenitor cells. Other cells will fail to survive the process of lineage commitment and this interval also appears to be critical for regulating the extent of neurogenesis. (C) Identification of progression through the stages of neurogenesis has been facilitated by the characterization of markers that have restricted expression. In the absence of a definitive neural stem cell marker, these cells are identified by the coexpression of markers such as glial fibrillary acidic protein (GFAP), which has been shown to be present in neural stem cells (4). Detection of proliferating cells can be done with exogenous administration of thymidine analogs such as BrdU, labeling a cohort of dividing cells, or by marking the presence of endogenous cell cycle proteins such as Ki-67. Multiple immunohistochemical labeling makes it possible to stage a cell’s progress through neurogenesis by the combinatorial expression of early lineage commitment markers such as nestin, polysialylated neural cell adhesion molecule (PSA-NCAM), and doublecortin (DCX), and to distinguish these from more mature markers of neurons, such as NeuN and calbindin. (D) The transition through stages of neurogenesis provides an opportunity to determine at what point in the process an experimental manipulation exerts its effect. In the case of animal models of stress, early studies suggested that proliferation was altered under stress conditions, while more recent studies have identified the period of lineage commitment and survival as vulnerable to stress. While there is evidence of plasticity of mature hippocampal neurons in response to stress, it is yet to be determined if these alterations are shared by adult-generated neurons. (E) Identifying the stage of neurogenesis that is altered provides an opportunity to examine specific regulatory mechanisms that may participate in the response to stress. As discussed in the text, evidence is emerging that BDNF and CREB may be important mechanisms of regulating the response to stress. The onset and duration of expression of BDNF and CREB is still under investigation. This figure was modified with permission from Kempermann et al. (56).
DOCUMENTING NEUROGENESIS: A WEALTH OF CHOICES
After extensive investigation, adult neurogenesis has been shown conclusively to exist under normal conditions in only two adult brain regions, the sub-ventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus. As there is no evidence for participation of SVZ neurogenesis in animal models of depression, this review will focus on hippocampal neurogenesis. In the adult hippocampus, cell division (proliferation) occurs in the SGZ, a thin layer of cells on the hilar border of the dentate gyrus granular cell layer. These cells migrate a short distance into the granule layer and differentiate into neurons that eventually become phenotypically mature and are indistinguishable from other granule cells (38,53,99,104). Not all of these newly generated cells survive; in fact, it is estimated that less than half survive to complete lineage commitment (10). The cellular or molecular events that distinguish between cell survival and death remain to be discovered.
To unravel the regulation of neurogenesis, it is helpful to characterize the progression of newly generated cells from initial cell division through maturation. This is schematically represented in Figure 1A. The ongoing elucidation of definitive stages of neurogenesis and appropriate markers to delineate each stage has been problematic. As shown in Figure 1B, the most commonly used staging parameters are cell proliferation, cell survival with eventual lineage commitment, and neuronal differentiation. However, even such a simple, straightforward definition has been met with controversy and conflicting results as many studies inadequately discriminate the stage under investigation. Insight into the molecular factors driving stage transition is lacking, and it is not yet clear why some cells progress along lineage commitment to become a functional neuron, some die, and some continue to remain in an undifferentiated, proliferative state. Although it is now known that environmental factors, particularly stress, can alter a newly generated cell’s fate, it is not known what cellular or molecular mechanisms regulate this. Analysis is made more difficult in that, unlike developmental neurogenesis, adult neurogenesis is an individual cell process without synchrony in the population. Thus, neurons of all developmental stages are present at any given time point in the adult hippocampus (56).
Determining the existence of adult-generated neurons requires proof that the cell is both new and that it is a neuron. The most prevalent method used to birth date neurons is administration of 5-bromo-2′-deoxyuridine (BrdU), a thymidine analog incorporated into replicated DNA during the S phase of the cell cycle (17). The use of BrdU birth dating is simple, rapidly processed, and has established concurrent validity with the older, but cumbersome, method of using tritiated thymidine (80). BrdU has two important advantages over tritiated thymidine, in that it is compatible with multiple immunohistochemical staining for multiple cell class-specific markers to determine cell phenotype (87) and it permits stereological quantification of cell number. Recent studies have utilized multiple thymidine analogs to examine temporal events of cell cycling (8,105). This has allowed further elucidation of factors influencing immediate survival of a newly generated cell separate from the event of the initial, labeled cell division.
As shown schematically in Figure 1C, proliferating cells also can be positively identified with Ki-67, an endogenous cell cycle protein marker. This enables broader identification of proliferating cells than exogenous thymidine analog administration (i.e., BrdU), which is only incorporated during cell cycle S phase. Concurrent validity studies have been conducted with Ki-67 colocalized with BrdU-positive cells (49). However, Ki-67 labeling is only detected in cycling cells (i.e., those cells in S through M phases of cell division) and does not serve to birth date cells through subsequent maturation. As a result, the ultimate fate of cells generated at a specific time period cannot be examined without exogenous thymidine analog administration.
Beyond the initial labeling of proliferating cells, there is a progressive expression of markers delineating immature to mature neuronal development. Differential expression of specific markers along with morphological analysis now allows detailed identification of the specific neurogenic phases once cell division has occurred (32,56). This is detailed schematically in Figure 1C. For example, immature progenitor or neural stem cells in the hippocampus have been identified on the basis of the presence of glial fibrillary acidic protein (GFAP) when coexpressed with vimentin and nestin (4). Nestin, an intermediate filament found in immature cells, has also been used to identify progenitor cells of neuroectodermal commitment. Nestin cannot be used as a definitive marker, however, as it has also been detected in vascular-related structures in the mature brain. Polysialylated neural cell adhesion molecules (PSA-NCAM), a cell surface molecule expressed by migrating neuroblasts, has been used to identify neurogenic cells in an early stage of lineage commitment in both the dentate gy-rus and the rostral migratory stream. Doublecortin, a cytoskeletal protein present in migrating or early differentiating neurons, has been extensively used recently to identify immature neurons (18,19).
While NeuN has classically been used as a marker of mature neurons, calbindin, a Ca2+ binding protein, is also found in all mature granular cells in both the dentate gyrus and the olfactory bulb glomerular layer (76). Recently, the generation of transgenic mice expressing fluorescent reporter genes under the control of tissue-specific promoters has proven to be an efficient way to visualize progenitor cells and their progeny (13,32).
While a wealth of cell phenotype markers exists, there has been a lack of consensus in study designs to identify newly generated cells and to correlate new cells with their phenotypic identity. In addition, the need to specify the particular stage of neurogenic progression in interpreting results is only now being recognized by investigators. Finally, not all studies have used rigorous stereological quantification of cell number, hindering the ability to compare study outcomes. With increasing awareness of these issues, one hopes that future studies will be designed in such a way as to address these concerns and provide a standardized body of knowledge that allows for better comparison of published studies.
THE FUNCTIONAL CONTRIBUTION OF ADULT HIPPOCAMPAL NEUROGENESIS
Concurrent with efforts to delineate stages of neurogenesis, considerable effort has gone into determining what these new neurons in the hippocampus are doing. The function of newly generated neurons in the adult hippocampus is still a matter of debate and may be a reflection of our incomplete understanding of hippocampal function (60,98). Although integration of new granule cells has been shown to occur into the trisynaptic circuitry of the hippocampus (104), the contribution of this activity-dependent flux in individual new neurons is difficult to address experimentally (26). While there is evidence that individual newly generated neurons become functional, it is still unclear how a population of new neurons modifies hippocampal circuitry function. Furthermore, it is unclear whether hippocampal-dependent tasks such as spatial learning tasks result in enhanced neurogenesis or if enhanced neurogenesis leads to improved hippocampal function (5). It is reasonable to think that both may be true.
Neurogenesis appears to be a remarkably plastic process, modifiable by numerous macro- and micro-environmental influences (68). Recent research has clearly established that a variety of stimuli may positively or negatively alter neurogenesis (44,57,58,66, 83,84,103). In fact, it appears that nearly every factor investigated has some ability to modulate the process. If neurogenesis is so responsive to environmental changes, it seems probable that its modulation normally reflects the organism’s ability to adapt to novel or changing conditions. Likewise, it is possible that extreme environmental events such as stress may lead to adverse adaptive neurogenesis modification. It may be significant that neurogenesis occurs at the convergence of input into the three-synaptic circuitry of the hippocampus: the dentate gyrus. It is well known that the hippocampus is extensively involved in emotional processing, and in the processing of learning and memory. Therefore, the addition of granular neurons at this location could be strategic for hippocampal functioning, as even small changes in granular cells could have far-reaching effects by adapting the existing network for future function.
The key to elucidating a possible role of neurogenesis in the onset and treatment of depression likely lies in further investigation into the function of these new cells in hippocampal circuitry. It is plausible that new hippocampal neurons in the dentate gyrus adjust the strength of connections within the hippocampal circuitry in an activity and experience-dependent manner (59). For example, interindividual differences in behavioral reactivity to novelty among rats has been found to correlate with neurogenesis levels in the dentate gyrus with “well-adjusted” rats (low reactivity to novelty) exhibiting higher basal levels of neurogenesis (67). Interestingly, this rate of neurogenesis was established before exposure to a novel stimulus, demonstrating that differences in neurogenesis can affect future function.
THE LINK BETWEEN STRESS, DEPRESSION, AND HIPPOCAMPAL NEUROGENESIS
As neurogenesis is detected by histological analysis, studies seeking linkage of neurogenesis with depression have been investigated in animal models. Animal models of depression have been elusive in their ability to define the more cognitive manifestations of depression, instead quantifying various behavioral outcome measures in response to various stressors. These behaviors closely resemble human depressive symptoms such as lethargy, decreased interaction, and anhedonia, and animals exposed to natural and laboratory stress exhibit diminished appetite, exploration, grooming, and social behavior. These animal responses to stress appear to be the equivalent of human stress responses. In fact, antidepressant efficacy is defined by the medication’s ability to diminish such behavioral effects in preclinical (animal) studies.
Experimental procedures producing stress have been used widely in rodent models to elucidate neural mechanisms underlying fear, anxiety, and depression. A wide spectrum of paradigms has been used: foot shock, forced swimming, restraint, and cold exposure, among others (25,73,85). These methods are easy to quantify and control, but are intrusive and not particularly relevant to natural events. Additionally, such paradigms may produce physical effects that activate central pain pathways and confound the interpretation of results. The ability of these stressors to produce direct systemic effects may be the reason that experimental results differ so widely. Furthermore, these laboratory stressors are artificial as they are not encountered in the wild and consequently may have limited relevance (27). Nevertheless, stress is a consistent, potent inhibitor of neurogenesis in nonhuman mammals (21,35, 41,46,77–79,101).
Further insight is available from studies using more natural stressors. These include natural stressful events such as exposure to predator odor or psycho-social stress, in contrast to laboratory stressors such as restraint, and exogenously elevated cortisol levels (9,46,48,92). Chronic psychosocial stress accompanied by constantly elevated endogenous glucocorticoid levels has been shown to lead to structural changes of hippocampal pyramidal neurons and a reduction of dentate gyrus neurogenesis in adult tree shrews, monkeys, and rodents (9,35,43,46,48,92).
The literature is mixed in regard to which stage of neurogenesis is primarily affected by stress. As indicated in Figure 1D, early experiments implicated proliferation as diminished with stress (41,45). Recent reports have further specified the stages of neurogenesis and have implicated a change in the microenvironment as a result of stress that does not change the rate of cell division but diminishes that newly generated cell’s ability to survive (69,89,102).
The hippocampus is an important brain structure involved in the mediation of stress responses. It has been implicated in the direct and indirect integration of neurochemical, neurohormonal, and cognitive responses to stress. As part of the limbic system, the hippocampus has a high concentration of type 2 glucocorticoid receptors and modulates glucocorticoid hormone (corticosterone in rodents, cortisol in primates including humans) release via a negative feedback loop inhibiting the hypothalamus–pituitary–adrenal (HPA) axis. The hippocampus is also sensitive to environmental- and experience-dependent structural changes and thus is vulnerable to stress-related adverse adaptive modification. Stress-related changes in the hippocampus in animals include dendrite atrophy, decreased synaptic plasticity, and cell death as well as diminished neurogenesis (28,31,41,51,71,86). Due to the hippocampus’ role in emotional processing and learning and memory, it is likely that the hippocampus plays a role in stress-related mood disorders such as depression; however, this remains to be demonstrated conclusively.
Stress has been implicated as the primary causal agent of depression in humans (6,37,52,91,93), and evidence for depression-associated structural changes in the hippocampus of adult humans appears similar to that found in animals. Thus, hippocampal volume loss of up to 20% has been reported in depressed humans using high resolution magnetic resonance imaging (96). These patients additionally displayed abnormalities of declarative and verbal memory, suggesting an association between depression-associated cognitive deficits and structural changes in the hippocampus. Given the relevance of the stress-based hypothesis of depression, hippocampal changes in animal models may provide insights into altered human hippocampal structure and function in depression. Because stress is a potent downregulator of adult neurogenesis in animal models, it is plausible that this stress-induced reduction in neurogenesis may be one aspect of the pathophysiology of depression and warrants further study (20,35,39,42,43,46).
ANTIDEPRESSANT MODULATION OF NEUROGENESIS
The pathophysiology of depression was not at all understood when antidepressant medication first became available. The medical management of depression was largely ineffective until a serendipitous discovery 50 years ago that depletion of monamines in the CNS during pharmacological management of hypertension led to severe depression. Since the late 1950s, the theory regarding the chemical foundation of depression became known as the monoamine hypothesis. This theory postulates that the debilitating symptoms of depression result from CNS deficiencies in serotonin (5-HT), norepinephrine (NE), and/or dopamine (DA) transmission (95). Indeed, most antidepressants do modulate the reuptake (select serotonin or norepinephrine reuptake inhibitors and tricyclics), metabolism (monoamine oxidase inhibitors), or availability of one or more of these neurotransmitters. The monoamine hypothesis reflects the state of research in the last half of the 20th century prior to investigation of molecular changes with chronic antidepressant administration.
Despite much investigation since the advent of antidepressants, there is still no consensus as to the cellular mechanism of action of these medications. Although recent drug development studies have suggested that 5-HT autoreceptor downregulation via antagonists or postsynaptic receptor agonist may augment and/or accelerate the antidepressant-like effects of SSRIs (22,61,81), recent research has moved beyond theories based on pharmacology of the synapse to downstream molecular events. The time lag for therapeutic action of antidepressant treatment suggests that depression is more than neurotransmitter disruption and that long-term regulation of pathways activated by drug treatment is required (72). Hence, studies over the past 10 years have moved beyond the monoamine hypothesis to investigate downstream adaptations of receptor-coupled signal transduction proteins and their corresponding genes as agents for antidepressant medication effectiveness (30).
Consistent with the neurogenic theory of stress-based depression, many studies have found a concurrent increase or restoration of neurogenesis and amelioration of behavioral symptoms of depression with chronic antidepressant treatment. For example, recent research has shown that chronic administration of antidepressants, particularly SSRIs, results in an increase in hippocampal cell proliferation and survival after stress-induced diminished neurogenesis (55, 73,75). Though these studies provide preliminary evidence for a causal link between diminished neurogenesis, depression, and the amelioration of both with antidepressants, the results of a wide range of studies have been mixed, as shown below, and the field still awaits conclusive evidence.
MOLECULAR LINKS BETWEEN ANTIDEPRESSANTS AND NEUROGENESIS: INVOLVEMENT OF NEUROTROPHINS AND TROPHIC FACTORS
In searching for a unifying hypothesis for depression and neurogenesis, it has been suggested that a mechanistic relationship may exist between behaviors, neurotrophin expression, and newly generated neuronal survival (69) (Fig. 1E). In particular, brain-derived neurotrophic factor (BDNF) and its receptor trk B (89) have been implicated in the mechanism of action of antidepressant drugs (14,15,29,62,74,97), and may be partly responsible for the effect of enhanced neurogenesis observed with many antidepressant medications (23,73). It is also possible that stress may reduce expression of endogenous growth factors, suggesting a link between stress, neurogenesis, and factors that regulate neuronal survival and growth during development and throughout life (2).
There is mounting evidence that a major downstream mediator of the therapeutic effect of antidepressants is increased expression of BDNF. Growth factors, including BDNF, play an important role in nervous system development but also serve a role in the maintenance and survival of neurons in the adult, thus contributing to ongoing neural adaptation and plasticity throughout the life span. A number of studies have shown that trophic factor modulation enhances adult neurogenesis (11,12). In a carefully quantified study of long-term survival, Scharfman et al. (94) found continuous infusion of BDNF into the hippocampus of adult rats increased neurogenesis. Aberg et al. (1) reported that peripheral infusion of insulin-like growth factor-I (IGF-I) increased progenitor cell proliferation in the dentate gyrus of adult rats. Other reports have found antidepressant-like behavioral effects with central administration of IGF-I and BDNF (50,97,106), leading Hashimoto et al. (47) to suggest that BDNF plays a role in the pathophysiology of mood disorders.
Not all studies have confirmed such conclusions. Some results have found no change in BDNF expression after stress and antidepressant intervention (3,16, 24). Another recent study found an increase in hippocampal BDNF expression, but not an increase in IGF-I, with chronic administration of fluoxetine, an SSRI (33). Evidence for antidepressant and stress influence on BDNF remains mixed, and firm conclusions cannot yet be stated regarding the interplay of neurotrophins and growth factors, stress, antidepressants, and neurogenesis (30). It is probable that these molecules themselves do not control mood, but act as necessary tools in the activity-dependent modulation of hippocampal neural networks involved in depression and neurogenesis (14).
Recently, there has been increasing interest in examining intracellular signal transduction pathways that may regulate neurogenesis. For example, the cAMP cascade and the cAMP response element-binding protein (CREB) have recently been implicated as processes that enhance survival of newborn neurons in the adult hippocampus (36). Because activation of the cAMP-CREB pathway increases the expression of BDNF in the hippocampus (82), which in turn increases neurogenesis in the adult brain, it is plausible that this may be a pathway involved in neurogenesis modulation seen with depression and its treatment with antidepressants. It has indeed been suggested that CREB may serve as a convergence point for multiple classes of antidepressants drugs (7); however, to date very few studies have addressed this hypothesis.
SUMMARY
There appears to be sufficient evidence to justify continued investigation into a molecular link between adult hippocampal neurogenesis and depression. While it is probable that modulation of hippocampal neurogenesis contributes to the onset and alleviation of depression, much investigation is still needed into the mechanisms that may regulate this process. The articulation of precise and consistent definitions of study variables should lead to improved focus for research directions, as well as contributing to outcomes that can be meaningfully compared across studies.
We propose some suggestions to improve consistency between studies of the link between neurogenesis and depression. The full use of recent, improved definitions for staging neurogenesis (Fig. 1) and the use of appropriate markers for each stage will provide a clear picture of which stages of neurogenesis are modulated by stress and which are unaffected. Along with this, investigating the molecular regulation of neurogenesis stage progression under basal and stressful conditions will be essential to further substantiate the existence of a cause/effect relationship. Once these molecular pathways are elucidated, it will then be necessary to define clear hippocampal circuitry-mediated behavioral changes that correlate with changes in stress-modulated molecular pathways. Along with this, studies will need to be designed to establish a functional contribution by newly generated granular cells at both basal and stress diminished states.
Understanding the relationship between neurogenesis and antidepressants is of high clinical significance. Additional studies using various animal models of depression must be completed to substantiate claims that neurogenesis is required for behavioral effects of antidepressant medications. Increased investigation of signaling pathways involved with the effect of antidepressants on neurogenesis will not only suggest new means for effective modulation, but close the numerous gaps that still exist in our understanding of this process. Progress in understanding the biological linkage between neurogenesis and depression may likewise lead to modulation of gene expression as treatment for depression. Although there is insufficient evidence to date to substantiate such potential therapeutic approaches, elucidating the molecular pathways common to neurogenesis and depression would allow us to explore the modulation of neurogenesis as an effective intervention for depression.
REFERENCES
- 1. Aberg M. A.; Aberg N. D.; Hedbacker H.; Oscarsson J.; Eriksson P. S. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 20(8):2896–2903; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aloe L.; Alleva E.; Fiore M. Stress and nerve growth factor. Findings in animal models and humans. Pharmacol. Biochem. Behav. 73(1):159–166; 2002. [DOI] [PubMed] [Google Scholar]
- 3. Altar C. A.; Whitehead R. E.; Chen R.; Wortwein G.; Madsen T. M. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol. Psychiatry 54(7):703–709; 2003. [DOI] [PubMed] [Google Scholar]
- 4. Alvarez-Buylla A.; Lim D. A. For the long run: maintaining germinal niches in the adult brain. Neuron 41(5):683–686; 2004. [DOI] [PubMed] [Google Scholar]
- 5. Ambrogini P.; Cuppini R.; Cuppini C.; Ciaroni S.; Cecchini T.; Ferri P.; Sartini S.; Del Grande P. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci. Lett. 286(1):21–24; 2000. [DOI] [PubMed] [Google Scholar]
- 6. Anderson M. F.; Aberg M. A.; Nilsson M.; Eriksson P. S. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Dev. Brain Res. 134(1–2):115–122; 2002. [DOI] [PubMed] [Google Scholar]
- 7. Blendy J. A. The role of CREB in depression and antidepressant treatment. Biol. Psychiatry 59(12):1144–1150; 2006. [DOI] [PubMed] [Google Scholar]
- 8. Burns K. A.; Kuan C. Y. Low doses of bromo- and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur. J. Neurosci. 21(3):803–807; 2005. [DOI] [PubMed] [Google Scholar]
- 9. Cameron H. A.; Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61(2):203–209; 1994. [DOI] [PubMed] [Google Scholar]
- 10. Cameron H. A.; McKay R. D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435(4):406–417; 2001. [DOI] [PubMed] [Google Scholar]
- 11. Cameron H. A.; Hazel T. G.; McKay R. D. Regulation of neurogenesis by growth factors and neurotransmitters. J. Neurobiol. 36(2):287–306; 1998. [PubMed] [Google Scholar]
- 12. Cao L.; Jiao X.; Zuzga D. S.; Liu Y.; Fong D. M.; Young D.; During M. J. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 36(8):827–835; 2004. [DOI] [PubMed] [Google Scholar]
- 13. Carlen M.; Meletis K.; Bambabe-Heider F.; Frisen J. Genetic visualization of neurogenesis. Exp. Cell Res. 312(15):2851–2859; 2006. [DOI] [PubMed] [Google Scholar]
- 14. Castren E.; Voikar V.; Rantamaki T. Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 7(1):18–21; 2007. [DOI] [PubMed] [Google Scholar]
- 15. Castren E. Neurotrophic effects of antidepressant drugs. Curr. Opin. Pharmacol. 4(1):58–64; 2004. [DOI] [PubMed] [Google Scholar]
- 16. Conti A. C.; Cryan J. F.; Dalvi A.; Lucki I.; Blendy J. A. cAMP response element-binding protein is essential for the upregualtion of brain-derived neruotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J. Neurosci. 22(8):3262–3268; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper-Kuhn C. M.; Kuhn H. G. Is it all DNA repair?. Methodological considerations for detecting neurogenesis in the adult brain Dev. Brain Res. 134(1–2):13–21; 2002. [DOI] [PubMed] [Google Scholar]
- 18. Couillard-Despres S.; Winner B.; Schaubeck S.; Aigner R.; Vroemen M.; Weidner N.; Bogdahn U.; Winkler J.; Kuhn H. G.; Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 21(1):1–14; 2005. [DOI] [PubMed] [Google Scholar]
- 19. Couillard-Despres S.; Winner B.; Karl C.; Lindemann G.; Schmid P.; Aigner R.; Laemke J.; Bogdahn U.; Winkler J.; Bischofberger J.; Aigner L. Targeted transgene expression in neuronal precursors: Watching young neurons in the old brain. Eur. J. Neurosci. 24:1535–1545; 2006. [DOI] [PubMed] [Google Scholar]
- 20. Czeh B.; Michaelis T.; Watanabe T.; Frahm J.; de Biurrun G.; van Kampen M.; Bartolomucci A.; Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA 98(22):12796–12801; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Czeh B.; Welt T.; Fischer A. K.; Erhardt A.; Schmitt W.; Muller M. B.; Toschi N.; Fuchs E.; Keck M. E. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol. Psychol. 52(11):1057–1065; 2002. [DOI] [PubMed] [Google Scholar]
- 22. Dawson L. A.; Nguyen H. Q.; Smith D. L.; Schechter L. E. Effect of chronic fluoxetine and WAY-100635 treatment on serotonergic neurotransmission in the frontal cortex. J. Psychopharmacol. 16(2):145–152; 2002. [DOI] [PubMed] [Google Scholar]
- 23. De Foubert G.; Carney S. L.; Robinson C. S.; Destexhe E. J.; Tomlinson R.; Hicks C. A.; Murray T. K.; Gaillard J. P.; Deville C.; Xhenseval V.; Thomas C. E.; O’Neill M. J.; Zetterstrom T. S. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience 128(3):597–604; 2004. [DOI] [PubMed] [Google Scholar]
- 24. Dias B. G.; Banerjee S. B.; Duman R. S.; Vaidya V. A. Differential regulation of brain derived neurotropic factor transcripts by antidepressant treatments in the rat brain. Neuropharmacology 45:553–563; 2003. [DOI] [PubMed] [Google Scholar]
- 25. Djordjevic J.; Cvijic G.; Davidovic V. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol. Res. 52(1):67–72; 2003. [PubMed] [Google Scholar]
- 26. Doetsch R.; Hen R. Young and excitable: The function of new neurons in the adult mammalian brain. Curr. Opin. Neurobiol. 15(1):121–128; 2005. [DOI] [PubMed] [Google Scholar]
- 27. Dranovsky A.; Hen R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol. Psychol. 59:1136–1143; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D’Sa C.; Duman R. S. Antidepressants and neuroplasticity. Bipolar Disord. 4(3):183–194; 2002. [DOI] [PubMed] [Google Scholar]
- 29. Duman R. S.; Heninger G. R.; Nestler E. J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 54(7):597–606; 1997. [DOI] [PubMed] [Google Scholar]
- 30. Duman R. S.; Monteggia L. M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59(12):1116–1127; 2006. [DOI] [PubMed] [Google Scholar]
- 31. Duman R. S.; Malberg J.; Nakagawa S.; D’Sa C. Neuronal plasticity and survival in mood disorders. Biol. Psychol. 48(8):732–739; 2000. [DOI] [PubMed] [Google Scholar]
- 32. Encinas J. M.; Vaahtokari A.; Enikolopov G. Fluxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA 103(21):8233–8238; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Engesser-Cesar C.; Anderson A. J.; Cotman C. W. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience 144(3):1033–1044; 2007. [DOI] [PubMed] [Google Scholar]
- 34. Eriksson P. S.; Perfilieva E.; Bjork-Eriksson T.; Alborn A. M.; Nordborg C.; Peterson D. A.; Gage F. H. Neurogenesis in the adult human hippocampus. Nat. Med. 4:1313–1317; 1998. [DOI] [PubMed] [Google Scholar]
- 35. Fuchs E.; Flugge G. Stress: Glucocorticoids and structural plasticity of the hippocampus. Neurosci. Biobehav. Rev. 23(2):295–300; 1998. [DOI] [PubMed] [Google Scholar]
- 36. Fujioka T.; Fujioka A.; Duman R. S. Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J. Neurosci. 24(2):319–328; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujita M.; Charney D. S.; Innis R. B. Imaging serotonergic neurotransmission in depression: Hippocampal pathophysiology may mirror global brain alterations. Biol. Psychol. 48(8):801–812; 2000. [DOI] [PubMed] [Google Scholar]
- 38. Gage F. H. Structural plasticity: Cause, result or correlate of depression. Biol. Psychol. 48:713–714; 2000. [DOI] [PubMed] [Google Scholar]
- 39. Gould E.; Gross C. G. Neurogenesis in adult mammals: Some progress and problems. J. Neurosci. 22(3):619–623; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gould E.; Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience 80(2):427–436; 1997. [DOI] [PubMed] [Google Scholar]
- 41. Gould E.; Tanapat P. Stress and hippocampal neurogenesis. Biol. Psychol. 46(11):1472–1479; 1999. [DOI] [PubMed] [Google Scholar]
- 42. Gould E.; Reeves A. J.; Fallah M.; Tanapat P.; Gross C. G.; Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc. Natl. Acad. Sci. USA 96(9):5263–5267; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gould E.; Reeves A. J.; Graziano M. S.; Gross C. G. Neurogenesis in the neocortex of adult primates. Science 286(5439):548–552; 1999. [DOI] [PubMed] [Google Scholar]
- 44. Gould E.; Tanapat P.; Rydel T.; Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol. Psychol. 48(8):715–720; 2000. [DOI] [PubMed] [Google Scholar]
- 45. Gould E.; Tanapat P.; McEwen B. S.; Flugge G.; Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. USA 95(6):318–371; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gould E.; McEwen B. S.; Tanapat P.; Galea L. A.; Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 17(7):2492–2498; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hashimoto K.; Shimizu E.; Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 45(2):104–114; 2004. [DOI] [PubMed] [Google Scholar]
- 48. Hayley S.; Borowski T.; Merali Z.; Anisman H. Central monoamine activity in genetically distinct strains of mice following a psychogenic stressor: Effects of predator exposure. Brain Res. 892(2):293–300; 2001. [DOI] [PubMed] [Google Scholar]
- 49. Heine V. M.; Maslam S.; Zareno J.; Joels M.; Lucassen P. J. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur. J. Neurosci. 19(1):131–144; 2004. [DOI] [PubMed] [Google Scholar]
- 50. Hoshaw B. A.; Malberg J. E.; Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 1037(1–2):204–208; 2005. [DOI] [PubMed] [Google Scholar]
- 51. Jacobs B. L.; van Praag H.; Gage F. H. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol. Psychiatry 5(3):262–269; 2000. [DOI] [PubMed] [Google Scholar]
- 52. Jacobs B. L.; van Praag H.; Gage F. H. Depression and the birth and death of brain cells. Am. Sci. 88:340–345; 2000. [Google Scholar]
- 53. Johansson C. B.; Momma S.; Clarke D. L.; Risling M.; Lendahl U.; Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96(1):25–34; 1999. [DOI] [PubMed] [Google Scholar]
- 54. Kaplan M. S. Environment complexity stimulates visual cortex neurogenesis: Death of a dogma and a research career. Trends Neurosci. 24(10):617–620; 2001. [DOI] [PubMed] [Google Scholar]
- 55. Katoh-Semba R.; Asano T.; Ueda H.; Morishita R.; Takeuchi I. K.; Inaguma Y.; Kato K. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 16(10):1328–1330; 2002. [DOI] [PubMed] [Google Scholar]
- 56. Kempermann G.; Jessberger S.; Steiner B.; Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27(8):447–452; 2004. [DOI] [PubMed] [Google Scholar]
- 57. Kempermann G.; Gage F. H. Experience-dependent regulation of adult hippocampal neurogenesis: Effects of long-term stimulation and stimulus withdrawal. Hippocampus 9(3):321–332; 1999. [DOI] [PubMed] [Google Scholar]
- 58. Kempermann G.; Gage F. H. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res. Dev. Brain Res. 134(1–2):1–12; 2002. [DOI] [PubMed] [Google Scholar]
- 59. Kempermann G.; Kronenberg G. Depressed new neurons—adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol. Psychol. 54(5):499–503; 2003. [DOI] [PubMed] [Google Scholar]
- 60. Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis J. Neurosci. 22(3):635–638; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kimura T.; Ishihara K.; Ozawa K.; Sasa M. Augmentation of serotonin-induced inhibition of neuronal activity in the hippocampus following repeated treatment with methamphetamine. Ann. NY Acad. Sci. 1025:242–247; 2004. [DOI] [PubMed] [Google Scholar]
- 62. Kodama M.; Fujioka T.; Duman R. S. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol. Psychol. 56(8):570–580; 2004. [DOI] [PubMed] [Google Scholar]
- 63. Kornack D. R.; Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science 294(5549):2127–2130; 2001. [DOI] [PubMed] [Google Scholar]
- 64. Kornack D. R.; Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. USA 96(10):5768–5773; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kuhn H. G.; Peterson D. A. Detection and phenotypic characterization of adult neurogenesis. In: Gage F. H.; Kempermann G.; Song H., eds. Adult neurogenesis, 2nd ed. Cold Spring Harbor: Cold Spring Harbor Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee J.; Duan W.; Long J. M.; Ingram D. K.; Mattson M. P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 15(2):99–108; 2000. [DOI] [PubMed] [Google Scholar]
- 67. Lemaire V.; Aurousseau C.; Le Moal M.; Abrous D. N. Behavioral trait of reactivity to novelty is related to hippocampal neurogenesis. Eur. J. Neurosci. 11(11):4006–4014; 1999. [DOI] [PubMed] [Google Scholar]
- 68. Lennington J. B.; Yang Z.; Conover J. C. Neural stem cells and the regulation of adult neurogenesis. Reprod. Biol. Endocrinol. 1(1):99; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li X. C.; Jarvis E. D.; Alvarez-Borda B.; Lim D. A.; Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc. Natl. Acad. Sci. USA 97(15):8584–8589; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Luskin M. B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11:173–189; 1993. [DOI] [PubMed] [Google Scholar]
- 71. Magarinos A. M.; McEwen B. S.; Flugge G.; Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 16(10):3534–3540; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Malberg J. E.; Blendy J. A. Antidepressant action: To the nucleus and beyond. Trends Pharmacol. Sci. 26(12):631–638; 2005. [DOI] [PubMed] [Google Scholar]
- 73. Malberg J. E.; Duman R. S. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology 28(9):1562–1571; 2003. [DOI] [PubMed] [Google Scholar]
- 74. Malberg J. E.; Schechter L. E. Increasing hippocampal neurogenesis: A novel mechanism for antidepressant drugs. Curr. Pharm. Des. 11(2):145–155; 2005. [DOI] [PubMed] [Google Scholar]
- 75. Malberg J. E.; Eisch A. J.; Nestler E. J.; Duman R. S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20(24):9104–9110; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McDonald H. Y.; Wojtowicz J. M. Dynamics of neurogenesis in the dentate gyrus of rats. Neurosci. Lett. 385(1):70–75; 2005. [DOI] [PubMed] [Google Scholar]
- 77. McEwen B. S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22:105–122; 1999. [DOI] [PubMed] [Google Scholar]
- 78. McEwen B. S. The neurobiology of stress: From serendipity to clinical relevance. Brain Res. 886(1–2):172–189; 2000. [DOI] [PubMed] [Google Scholar]
- 79. McEwen B. S. Effects of adverse experiences for brain structure and function. Biol. Psychol. 48(8):721–731; 2000. [DOI] [PubMed] [Google Scholar]
- 80. Miller M. W.; Nowakowski R. S. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 457(1):44–52; 1988. [DOI] [PubMed] [Google Scholar]
- 81. Mitchell P. J.; Redfern P. H. Potentiation of the time-dependent, antidepressant-induced changes in the agonistic behaviour of resident rats by the 5-HT1A receptor antagonist, WAY. Behav. Pharmacol. 8(6–7):585–606; 1997. [DOI] [PubMed] [Google Scholar]
- 82. Nibuya M.; Nestler E. J.; Duman R. S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 16(7):2365–2372; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nilsson M.; Perfilieva E.; Johansson U.; Orwar O.; Eriksson P. S. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 39(4):569–578; 1999. [DOI] [PubMed] [Google Scholar]
- 84. Peterson D. A. Stem cells in brain plasticity and repair. Curr. Opin. Pharm. 2:34–42; 2002. [DOI] [PubMed] [Google Scholar]
- 85. Pham K.; Nacher J.; Hof P. R.; McEwen B. S. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 17(4):879–886; 2003. [DOI] [PubMed] [Google Scholar]
- 86. Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol. Psychol. 48(8):766–777; 2000. [DOI] [PubMed] [Google Scholar]
- 87. Rakic P. Adult neurogenesis in mammals: An identity crisis. J. Neurosci. 22(3):614–618; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rakic P. Neurogenesis in adult primate neocortex: An evaluation of the evidence. Nat. Rev. Neurosci. 3(1):65–71; 2002. [DOI] [PubMed] [Google Scholar]
- 89. Sairanen M.; Lucas G.; Ernfors P.; Castren M.; Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 25(5):1089–1094; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Santarelli L.; Saxe M.; Gross C.; Surget A.; Battaglia F.; Dulawa S.; Weisstaub N.; Lee J.; Duman R.; Arancio O.; Belzung C.; Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301(5634):805–809; 2003. [DOI] [PubMed] [Google Scholar]
- 91. Sapolsky R. M. Depression, antidepressants, and the shrinking hippocampus. Proc. Natl. Acad. Sci. USA 98(22):12320–12322; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sapolsky R. M. Glucocorticoids, stress, and their adverse neurological effects: Relevance to aging. Exp. Gerontol. 34(6):721–732; 1999. [DOI] [PubMed] [Google Scholar]
- 93. Sapolsky R. M. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol. Psychol. 56(3):137–139; 2004. [DOI] [PubMed] [Google Scholar]
- 94. Scharfman H.; Goodman J.; Macleod A.; Phani S.; Antonelli C.; Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 192:348–356; 2005. [DOI] [PubMed] [Google Scholar]
- 95. Schecter L. E.; Ring R. H.; Beyer C. E. E.; Hughes Z. A.; Khawaja X.; Malberg J. E.; Rosenzweig-Lipson S. Innovative approaches for the development of anti-depressant drugs: Current and future strategies. NeuroRx 2:590–611; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sheline Y. I. 3D MRI studies of neuroanatomic changes in unipolar major depression: The role of stress and medical comorbidity. Biol. Psychol. 48(8):791–800; 2000. [DOI] [PubMed] [Google Scholar]
- 97. Shirayama Y.; Chen A. C.; Nakagawa S.; Russell D. S.; Duman R. S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 22(8):3251–3261; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shors T. J.; Townsend D. A.; Zhao M.; Kozorovitskiy Y.; Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12(5):578–584; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Song H. J.; Stevens C. F.; Gage F. H. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat. Neurosci. 5(5):438–445; 2002. [DOI] [PubMed] [Google Scholar]
- 100. Thomas R. M.; Peterson D. A. A neurogenic theory of depression gains momentum. Mol. Interv. 3(8):441–444; 2003. [DOI] [PubMed] [Google Scholar]
- 101. Thomas R. M.; Urban J. H.; Peterson D. A. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult hippocampus. Exp. Neurol. 201(2):308–315; 2006. [DOI] [PubMed] [Google Scholar]
- 102. Thomas R. M.; Hotsenpiller G.; Peterson D. A. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J. Neurosci. 27(11):2734–2743; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. van Praag H.; Christie B. R.; Sejnowski T. J.; Gage F. H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 96(23):13427–13431; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. van Praag H.; Schinder A. F.; Christie B. R.; Toni N.; Palmer T. D.; Gage F. H. Functional neurogenesis in the adult hippocampus. Nature 415(6875):1030–1034; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vega C.; Peterson D. A. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat. Methods 2(3):167–169; 2005. [DOI] [PubMed] [Google Scholar]
- 106. Zhang H. T.; Huang Y.; Jin S. L.; Frith S. A.; Suvarna N.; Conti M.; O’Donnell J. M. Antidepressant-like profile and reduced sensitivitiy to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology 27(4):587–595; 2002. [DOI] [PubMed] [Google Scholar]


