Abstract
We investigated interindividual variability in gene expression in abdominal subcutaneous (SC) and omental (OM) adipose tissue of 10 massively obese men. Affymetrix human U133A microarrays were used to measure gene expression levels. A total of 6811 probesets generated significant signal in both depots in all samples. Interindividual variability in gene expression was rather low, with more than 90% of transcripts showing a coefficient of variation (CV) lower than 23.6% and 21.7% in OM and SC adipose tissues, respectively. The distributions of CV were similar between the two fat depots. A set of highly variable genes was identified for both tissues on the basis of a high CV and elevated gene expression level. Among the set of highly regulated genes, 18 transcripts were involved in lipid metabolism and 28 transcripts were involved in cell death for SC and OM samples, respectively. In conclusion, gene expression interindividual variability was rather low and globally similar between fat compartments, and the adipose tissue transcriptome appeared as relatively stable, although specific pathways were found to be highly variable in SC and OM depots.
Key words: Adipose tissue, Omental, Subcutaneous, Microarrays, Obese men
INTRODUCTION
A higher risk of obesity-related metabolic diseases has been associated with increased adipose tissue mass in the abdominal region (5,24). Using imaging methods, studies have shown that abdominal, and especially visceral or intra-abdominal obesity, in both men and women, is closely associated with a dyslipidemic state that includes hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol levels, elevated apolipoprotein B, a greater proportion of small, dense low-density lipoprotein (LDL) particles, and increased LDL cholesterol to HDL cholesterol ratio (6). This condition is also associated with hyper-insulinemia and insulin resistance (7,24).
Adipose tissue located within the abdominal cavity has been suggested to be functionally and metabolically distinct from that of the subcutaneous compartment (15,23) and a number of differentially expressed genes encoding important functional properties may underlie abdominal obesity-related disorders (23). Many studies have now used microarray profiling of adipose tissue to investigate gene expression in obesity (2–4,16). Analysis of variability in gene expression has been used to examine specific genes that could be related to adipose tissue function. However, so far only animal data are available (2,3), and no large-scale genomic study has been performed to examine the variability of gene expression in human adipose tissue. In this study, we investigated the interindividual variability in gene expression in abdominal subcutaneous (SC) and omental (OM) adipose tissue samples from 10 nondiabetic, normolipidemic obese men, using previously established microarrays (23).
SUBJECTS AND METHODS
Patient Selection
The study group included 10 massively obese men undergoing biliopancreatic diversion at the Laval Hospital (Quebec City). This surgical procedure involves bypassing the small intestine and diverting the bile and pancreatic juice to the distal ileum, which produces maldigestion and selective malabsorption essentially for fat and starch (17). Following clinical examination, none of the patients had identified chronic diseases such as cardiomyopathy and endocrine disorders. Body weight was stable at the time of study and no subject had been on a diet or involved in a weight reduction program in the last 6 months. All patients provided informed written consent prior to their inclusion in the study. Adipose tissue samples were obtained at the beginning of the surgery from the abdominal subcutaneous wall (close to the umbilicus) and from the greater omentum. Body weight, height, and waist and hip circumferences were measured according to standardized procedures.
RNA Extraction, Reverse Transcription, and Probe Preparation
Adipose tissue samples were homogenized in Trizol reagent and centrifuged to separate the lipid fraction. Total RNA was prepared from the cleared homogenate according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). RNA was repurified using RNEasy mini columns (Qiagen, Hilden, Germany). RNA integrity was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Probes for microarray experiments were prepared using 10 μg of total RNA and hybridized overnight to Affymetrix HG-U133A Gene Chips (Affymetrix, Santa Clara, CA). Nonspecifically bound probe was removed by washing using the Agilent GeneChip Fluidics Station 400. Detection of specifically bound probes was performed by incubating the arrays with a biotinylated anti-streptavidin antibody (Vector Laboratories, Burlingame, CA) prior to staining with SAPE (streptavidin phycoerthryin; Molecular Probes, Eugene, OR). Detailed protocols for probe synthesis and hybridization reactions have been previously described (20). Real-time RT-PCR was used for confirmation with a subset of genes (23).
Array Data Extraction and Analysis
The arrays were scanned using an Agilent Gene Array Scanner and raw data were extracted from scanned images and scaled to 1000 units mean intensity using Microarray Analysis D-Chip software (PM-MM model). A significant signal was considered when the DChip software indicated a “present” call based on the modified algorithm of Microarray Suite analysis software 4 (Affymetrix). Interindividual variance in mean expression level and coefficient of variation (CV) calculations were performed for each transcript from the normalized signal obtained in both fat depots of all 10 subjects of the study (one array per fat sample, for a total of 20 arrays). Nonparametric Spearman rank correlation coefficients were computed to quantify associations between variance and mean expression levels or CV and mean expression levels in SC and OM fat samples either combined or separately. Log-10 transformation for all variables was used to normalize values. SC and OM variances or CVs were compared among fat depots by paired t-test. The selection of the most variable genes in SC and OM fat samples was based on the following criteria: 1) probesets that generated significant signal (“present” call) in both depots in all 10 subjects (n = 6811); 2) probesets that were in the top 2 percentile of CV for each depot (n = 136 for SC and OM); and 3) probesets that generated a mean expression level that was in the upper tertile (n = 68 in each depot). In addition, we examined variability in the probesets that generated significant signal (“present” call) in at least one and up to nine individuals.
Biological Pathway Analyses
Cellular pathways related to these transcripts were identified using the Kegg database (http://www.genome.ad.jp/kegg/) and genecards (http://www.genecards.org/). The Ingenuity Pathway Analysis System (Ingenuity® Systems, www.ingenuity.com) was also used to visualize gene expression data in the context of biological pathways. Two input files were uploaded in the Ingenuity Pathway Analysis system considering: 1) probesets highly variable in SC tissues (n = 68) and 2) probesets highly variable in OM tissues (n = 68). Analyses were performed on both files individually, and a comparison analysis was also performed.
RESULTS
Men of the study were 17.0 to 45.0 years old and were in the morbid obesity range with BMI values ranging from 44.7 to 80.7 kg/m2. They were characterized by a normal lipid profile, and were slightly hypertensive (9). Of the 22,283 probesets present on the array, significant signal (“present” call) was obtained for 6,811 probesets in both fat compartments of all 10 subjects. A total of 9,076 and 8,590 probesets generated significant signal (“present” call) in at least one and up to nine individuals in OM and SC samples, respectively.
Figure 1 shows the correlations between gene expression variance or CV (% variance) and mean gene expression levels for all 6,811 positive signals, regardless of the fat depot (Fig. 1A), in the OM (Fig. 1B) or SC (Fig. 1C) fat compartments. As expected, highly expressed genes had higher absolute variance in their expression levels as reflected by a positive correlation between mean transcript expression levels and absolute gene expression variance. However, mean gene expression levels were negatively correlated with CV, indicating slightly higher variability at low expression levels. The distribution of CV in gene expression in OM and SC is shown in Figure 2. The left panels show the 6,811 probesets that generated significant signal in both compartments in all 10 fat samples. More than 90% of clones showed a CV lower than 23.6% and 21.7% in OM and SC adipose tissues, respectively. The right panels show CV distributions of genes that generated significant signal (“present” call) in at least one and up to nine individuals in the OM and SC fat samples. More than 90% of clones showed a CV lower than 22.0% and 20.3% in OM and SC samples, respectively. No difference in CV was observed between fat depots in both subsets of transcripts.
Figure 1.
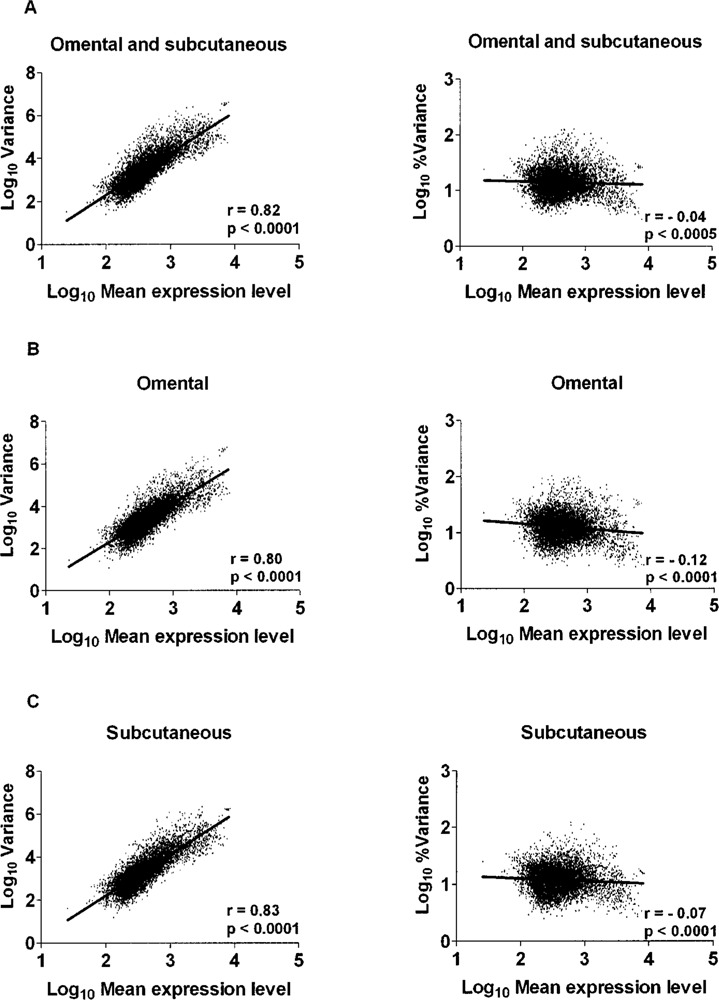
Correlations between gene expression variance or coefficient of variation (% variance) and mean gene expression levels for all 6,811 positive signals regardless of fat depot (A), or in the OM (B) or SC (C) fat compartments.
Figure 2.
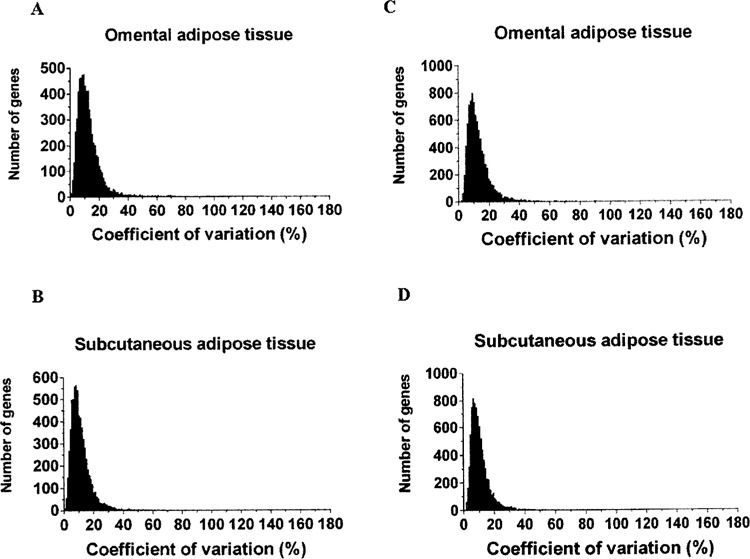
Distribution of coefficients of variation in OM or SC adipose tissue of (A, B) 6,811 probesets that generated significant signal in both fat depots in all 10 subjects, and (C, D) 9,076 and 8,590 probesets that only presented significant signal in at least one and up to nine individuals. Interindividual variability was similar in both sets of transcripts. No difference in CV distribution was observed between fat depots.
Among the 6,811 probesets that generated significant signal (“present” call) in both depots in all 10 subjects, we selected probesets that were in the top 2 percentile of CV in each depot, and then identified the ones (68 probesets in each depot) that were in the upper tertile of mean gene expression level (Tables 1 and 2). Sixty-three genes were obtained in both fat compartments. Selected pathways with highly variable transcripts in SC and OM adipose tissue are shown in Table 3. Some pathways were highly variable in both fat depots, including pathways of hematopoietic cell lineage, the Fc epsilon RI signaling pathway, genes involved in glycerophospholipid metabolism, leukocyte transendothelial migration, and the GnRH signaling pathway (PLA2G2A and TFRC). Conversely, several pathways were highly variable only in OM or SC adipose tissue samples. Transcripts related to the Jak-STAT, Wnt, adipocytokine, apoptosis, and MAPK signaling pathways were more variable among OM samples. We also found that pyruvate kinase (PKM2), a transcript related to insulin signaling, glycolysis/gluconeogenesis and type 2 diabetes, was more variable among SC samples. The Ingenuity Pathway Analysis system revealed that 18 transcripts in the SC dataset were involved in lipid metabolism, which was clearly the top function associated with this dataset, whereas 28 transcripts were associated with cell death in OM fat (Table 4).
TABLE 1.
LIST OF THE 68 OM ADIPOSE TISSUE TRANSCRIPTS IN UPPER TERTILE OF MEAN EXPRESSION LEVEL AND TOP 2 PERCENTILE OF THE COEFFICIENT OF VARIATION
| Probeset | Symbol | Description | Cytogen. Band | Accession | % CV |
|---|---|---|---|---|---|
| 217739_s_at | PBEF1 | Pre-B-cell colony-enhancing factor | 7q22.2 | NM_005746 | 100.5 |
| 202241_at | TRIB1 | Phosphoprotein regulated by mitogenic pathways | 8q24.13 | NM_025195 | 95.0 |
| 204472_at | GEM | GTP binding protein overexpressed in skeletal muscle | 8q13-q21 | NM_005261 | 91.3 |
| 202643_s_at | TNFAIP3 | Tumor necrosis factor, alpha-induced protein 3 | 6q23 | AI738896 | 86.6 |
| 202644_s_at | TNFAIP3 | Tumor necrosis factor, alpha-induced protein 3 | 6q23 | NM_006290 | 83.3 |
| 202637_s_at | ICAM1 | Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor | 19p13.3-p13.2 | AI608725 | 78.8 |
| 202638_s_at | ICAM1 | Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor | 19p13.3-p13.2 | NM_000201 | 46.6 |
| 212724_at | RND3 | Ras homolog gene family, member E | 2q23.3 | BG054844 | 77.6 |
| 36711_at | MAFF | V-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | 22q13.1 | AL021977 | 76.3 |
| 204007_at | FCGR3B | Fc fragment of IgG, low affinity IIIb, receptor for (CD16) | 1q23 | J04162 | 71.4 |
| 202917_s_at | S100A8 | S100 calcium binding protein A8 (calgranulin A) | 1q21 | NM_002964 | 70.9 |
| 203574_at | NFIL3 | Nuclear factor, interleukin 3 regulated | 9q22 | NM_005384 | 66.0 |
| 221541_at | CRISPLD2 | Hypothetical protein DKFZp434B044 | 16q24.1 | AL136861 | 64.2 |
| 221477_s_at | MGC5618 | Hypothetical protein MGC5618 | BF575213 | 63.3 | |
| 202388_at | RGS2 | Regulator of G-protein signaling 2, 24kD | 1q31 | NM_002923 | 63.1 |
| 217546_at | MT1M | Metallothionein 1M | 16q13 | R06655 | 62.2 |
| 200798_x_at | MCL1 | Myeloid cell leukemia sequence 1 (BCL2-related) | 1q21 | NM_021960 | 61.3 |
| 200797_s_at | MCL1 | Myeloid cell leukemia sequence 1 (BCL2-related) | 1q21 | AI275690 | 39 |
| 207574_s_at | GADD45B | Growth arrest and DNA-damage-inducible, beta | 19p13.3 | NM_015675 | 61.1 |
| 208152_s_at | DDX21 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 21 | 10q21 | NM_004728 | 60.7 |
| 204881_s_at | UGCG | UDP-glucose ceramide glucosyltransferase | 9q31 | NM_003358 | 60.1 |
| 201325_s_at | EMP1 | Epithelial membrane protein 1 | 12p12.3 | NM_001423 | 60.1 |
| 201502_s_at | NFKBIA | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha | 14q13 | AI078167 | 59.5 |
| 202672_s_at | ATF3 | Activating transcription factor 3 | 1q32.3 | NM_001674 | 59.1 |
| 209193_at | PIM1 | Pim-1 oncogene | 6p21.2 | M24779 | 59.0 |
| 201858_s_at | PRG1 | Proteoglycan 1, secretory granule | 10q22.1 | J03223 | 56.0 |
| 203411_s_at | LMNA | Lamin A/C | 1q21.2-q21.3 | NM_005572 | 55.9 |
| 212086_x_at | LMNA | Lamin A/C | 1q21.2-q21.3 | AK026584 | 51.2 |
| 201631_s_at | IER3 | Immediate early response 3 | 6p21.3 | NM_003897 | 54.4 |
| 202391_at | BASP1 | Brain abundant, membrane attached signal protein 1 | 5p15.1-p14 | NM_006317 | 53.7 |
| 208836_at | ATP1B3 | ATPase, Na+/K+ transporting, beta 3 polypeptide | 3q23 | U51478 | 53.3 |
| 207332_s_at | TFRC | Transferrin receptor (p90, CD71) | 3q29 | NM_003234 | 53.1 |
| 208691_at | TFRC | Transferrin receptor (p90, CD71) | 3q29 | BC001188 | 52.5 |
| 200800_s_at | HSPA1A | Heat shock 70kD protein 1A | 6p21.3 | NM_005345 | 51.9 |
| 208470_s_at | HPR | Haptoglobin-related protein | 16q22.1 | NM_020995 | 51.7 |
| 208151_x_at | DDX17 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 17 | 22q13.1 | NM_030881 | 51.2 |
| 209340_at | UAP1 | UDP-N-acteylglucosamine pyrophosphorylase 1 | 1q23.3 | S73498 | 51.1 |
| 202581_at | HSPA1B | Heat shock 70kD protein 1B | 6p21.3 | NM_005346 | 50.8 |
| 200768_s_at | MAT2A | Methionine adenosyltransferase II, alpha | 2p11.2 | BC001686 | 49.6 |
| 214038_at | CCL8 | Chemokine (C-C motif) ligand 8 | 17q11.2 | AI984980 | 48.6 |
| 201466_s_at | JUN | V-jun sarcoma virus 17 oncogene homolog (avian) | 1p32-p31 | NM_002228 | 48.0 |
| 201739_at | SGK | Serum/glucocorticoid regulated kinase | 6q23 | NM_005627 | 47.5 |
| 200666_s_at | DNAJB1 | DnaJ (Hsp40) homolog, subfamily B, member 1 | 19p13.2 | NM_006145 | 47.5 |
| 213629_x_at | MT1F | Metallothionein 1F (functional) | 16q13 | BF246115 | 47.0 |
| 217165_x_at | MT1F | Metallothionein 1F (functional) | 16q13 | M10943 | 41.2 |
| 200831_s_at | SCD | Stearoyl-CoA desaturase (delta-9-desaturase) | 10q23-q24 | AA678241 | 46.7 |
| 212185_x_at | MT2A | Metallothionein 2A | 16q13 | NM_005953 | 46.5 |
| 200704_at | LITAF | Lipopolysaccharide-induced TNF factor | 16p13.13 | AB034747 | 45.7 |
| 211456_x_at | LOC645745 | Metallothionein 1H-like protein | 1q43 | AF333388 | 45.5 |
| 208581_x_at | MT1X | Metallothionein IX | 16q13 | NM_005952 | 44.9 |
| 202238_s_at | NNMT | Nicotinamide N-methyltransferase | 11q23.1 | NM_006169 | 44.6 |
| 204419_x_at | HBG2 | Hemoglobin, gamma G | 11p15.5 | NM_000184 | 44.6 |
| 221841_s_at | KLF4 | Kruppel-like factor 4 (gut) | 9q31 | BF514079 | 44.0 |
| 202081_at | IER2 | Immediate early response 2 | 19p13.13 | NM_004907 | 43.9 |
| 203649_s_at | PLA2G2A | Phospholipase A2, group IIA (platelets, synovial fluid) | 1p35 | NM_000300 | 43.6 |
| 220046_s_at | CCNL1 | Cyclin L1 | 3q25.32 | NM_020307 | 43.2 |
| 205100_at | GFPT2 | Glutamine-fructose-6-phosphate transaminase 2 | 5q34-q35 | NM_005110 | 43.1 |
| 201289_at | CYR61 | Cysteine-rich, angiogenic inducer, 61 | 1p31-p22 | NM_001554 | 42.8 |
| 215499_at | LOC651423 | Similar to mitogen-activated protein kinase kinase 3 isoform A | 17q11.2 | AA780381 | 42.3 |
| 201473_at | JUNB | Jun B proto-oncogene | 19p13.2 | NM_002229 | 42.0 |
| 205516_x_at | CIZ1 | CDNK1A interacting zinc finger protein | 9q34.1 | NM_012127 | 42.0 |
| 221651_x_at | IGKC | Immunoglobulin kappa constant | 2p12 | BC005332 | 41.3 |
| 204745_x_at | MT1G | Metallothionein 1G | 16q13 | NM_005950 | 41.3 |
| 200881_s_at | DNAJA1 | DnaJ (Hsp40) homolog, subfamily A, member 1 | 9p13-p12 | NM_001539 | 41.2 |
| 217753_s_at | RPS26 | Ribosomal protein S26 | 12q13 | NM_001029 | 40.7 |
| 218520_at | TBK1 | TANK-binding kinase 1 | 12q14.1 | NM_013254 | 40.4 |
| 200989_at | HIF1A | Hypoxia-inducible factor 1, alpha subunit (basic helix-loop-helix transcription factor) | 14q21-q24 | NM_001530 | 39.7 |
| 212859_x_at | MT1E | Metallothionein 1E (functional) | 16q13 | BF217861 | 39.2 |
TABLE 2.
LIST OF THE 68 SC ADIPOSE TISSUE TRANSCRIPTS IN THE UPPER TERTILE OF MEAN EXPRESSION LEVEL AND TOP 2 PERCENTILE OF THE COEFFICIENT OF VARIATION
| Probeset | Symbol | Description | Cytogen. Band | Accession | % CV |
|---|---|---|---|---|---|
| 212657_s_at | IL1RN | Interleukin 1 receptor antagonis | 2q14.2 | U65590 | 121.4 |
| 209875_s_at | SPP1 | Secreted phosphoprotein 1 (osteopontin, bone sialoprotein I, early T-lymphocyte activation 1) | 4q21-q25 | M83248 | 111.9 |
| 209395_at | CHI3L1 | Chitinase 3-like 1 (cartilage glycoprotein-39) | 1q32.1 | M80927 | 107.9 |
| 203936_s_at | MMP9 | Matrix metalloproteinase 9 (gelatinase B, 92kD gelatinase, 92kD type IV collagenase) | 20q11.2-q13.1 | NM_004994 | 92.0 |
| 208691_at | TFRC | Transferrin receptor (p90, CD71) | 3q29 | BC001188 | 79.9 |
| 207332_s_at | TFRC | Transferrin receptor (p90, CD71) | 3q29 | NM_003234 | 66.3 |
| 201952_at | ALCAM | Activated leucocyte cell adhesion molecule | 3q13.1 | AA156721 | 78.8 |
| 201422_at | IFI30 | Interferon, gamma-inducible protein 30 | 19p13.1 | NM_006332 | 75.5 |
| 201850_at | CAPG | Capping protein (actin filament), gelsolin-like | 2p11.2 | NM_001747 | 71.1 |
| 201847_at | LIPA | Lipase A, lysosomal acid, cholesterol esterase (Wolman disease) | 10q23.2-q23.3 | NM_000235 | 64.2 |
| 201720_s_at | LAPTM5 | Lysosomal-associated multispanning membrane protein 5 | 1p34 | AI589086 | 62.6 |
| 201721_s_at | LAPTM5 | Lysosomal-associated multispanning membrane protein 5 | 1p34 | NM_006762 | 60.7 |
| 203523_at | LSP1 | Lymphocyte-specific protein 1 | 11p15.5 | NM_002339 | 62.6 |
| 213274_s_at | CTSB | Cathepsin B | 8p22 | AA020826 | 61.9 |
| 200838_at | CTSB | Cathepsin B | 8p22 | NM_001908 | 57.5 |
| 200839_s_at | CTSB | Cathepsin B | 8p22 | NM_001908 | 44.9 |
| 213275_x_at | CTSB | Cathepsin B | 8p22 | W47179 | 38.9 |
| 219454_at | EGFL6 | EGF-like-domain, multiple 6 | Xp22 | NM_015507 | 60.8 |
| 202803_s_at | ITGB2 | Integrin, beta 2 (complement component 3 receptor 3 and 4 subunit) | 21q22.3 | NM_000211 | 59.2 |
| 202902_s_at | CTSS | Cathepsin S | 1q21 | NM_004079 | 58.2 |
| 203337_x_at | ITGB1BP1 | Integrin beta 1 binding protein 1 | 2p25.2 | NM_004763 | 55.4 |
| 212737_at | GM2A | GM2 ganglioside activator | 5q31.3-q33.1 | AL513583 | 52.5 |
| 209122_at | ADFP | Adipose differentiation-related protein | 9p22.1 | BC005127 | 51.3 |
| 208607_s_at | SAA2 | Serum amyloid A2 | 11p15.1-pl4 | NM_030754 | 51.2 |
| 205516_x_at | CIZ1 | CDKN1A interacting zinc finger protein 1 | 9q34.1 | NM_012127 | 49.7 |
| 202546_at | VAMP8 | Vesicle-associated membrane protein 8 (endobrevin) | 2p12-p11.2 | NM_003761 | 49.6 |
| 200766_at | CTSD | Cathepsin D (lysosomal aspartyl protease) | 11p15.5 | NM_001909 | 48.4 |
| 202409_at | IGF2 | Insulin-like growth factor 2 (somatomedin A) | 11p15.5 | X07868 | 48.1 |
| 204122_at | TYROBP | TYRO protein tyrosine kinase binding protein | 19q13.1 | NM_003332 | 46.8 |
| 218520_at | TBK1 | TANK-binding kinase 1 | 12q14.1 | NM_013254 | 46.6 |
| 214456_x_at | SAA1 | Serum amyloid A1 | 11p15.1 | M23699 | 46.6 |
| 204232_at | FCER1G | Fc fragment of IgE, high affinity I, receptor for; gamma polypeptide | 1q23 | NM_004106 | 46.3 |
| 221651_x_at | IGKC | Immunoglobulin kappa constant | 2p12 | BC005332 | 46.2 |
| 201141_at | GPNMB | Glycoprotein (transmembrane) nmb | 7p15 | NM_002510 | 46.0 |
| 201201_at | CSTB | Cystatin B (stefin B) | 21q22.3 | NM_000100 | 45.9 |
| 221269_s_at | SH3BGRL3 | SH3 domain binding glutamic acid-rich protein like 3 | 1p35-p34.3 | NM_031286 | 45.3 |
| 203649_s_at | PLA2G2A | Phospholipase A2, group IIA (platelets, synovial fluid) | 1p35 | NM_000300 | 42.9 |
| 218540_at | THTPA | Thiamine triphosphatase | 14q11.2 | NM_024328 | 42.1 |
| 209659_s_at | CDC16 | CDC16 cell division cycle 16 homolog (S. cerevisiae) | 13q34 | AF164598 | 41.9 |
| 20083l_s_at | SCD | Stearoyl-CoA desaturase (delta-9-desaturase) | 10q23-q24 | AA678241 | 41.6 |
| 213553_x_at | APOC1 | Apolipoprotein C-I | 19q13.2 | W79394 | 41.4 |
| 213101_s_at | ACTR3 | ARP3 actin-related protein 3 homolog (yeast) | 2q14.1 | Z78330 | 40.0 |
| 202605_at | GUSB | Glucuronidase, beta | 7q21.11 | NM_000181 | 39.7 |
| 201050_at | PLD3 | Phospholipase D family, member 3 | 19q13.2 | NM_012268 | 39.6 |
| 202399_s_at | AP3S2 | Adaptor-related protein complex 3, sigma 2 subunit | 15q26.1 | NM_005829 | 39.5 |
| 202404_s_at | COL1A2 | Collagen, type I, alpha 2 | 7q22.1 | NM_000089 | 39.5 |
| 201108_s_at | THBS1 | Thrombospondin 1 | 15q15 | BF055462 | 38.9 |
| 201470_at | GSTO1 | Glutathione-S-transferase omega 1 | 10q25.1 | NM_004832 | 38.8 |
| 201251_at | PKM2 | Pyruvate kinase, muscle | 15q22 | NM_002654 | 38.5 |
| 200078_s_at | ATP6V0B | ATPase, H+ transporting, lysosomal 21kDa, V0 subunit b | 1p32.3 | BC005876 | 37.9 |
| 213515_x_at | HBG1 | Hemoglobin, gamma A | 11p15.5 | AI133353 | 37.7 |
| 217118_s_at | C22orf9 | Chromosome 22 open reading frame 9 | 22q13.31 | AK025608 | 37.6 |
| 203382_s_at | APOE | Apolipoprotein E | 19q13.2 | NM_000041 | 37.6 |
| 201944_at | HEXB | Hexosaminidase B (beta polypeptide) | 5q13 | NM_000521 | 37.2 |
| 209390_at | TSC1 | Tuberous sclerosis 1 | 9q34 | AF013168 | 37.0 |
| 203381_s_at | APOE | Apolipoprotein E | 19q13.2 | N33009 | 36.8 |
| 201005_at | CD9 | CD9 molecule | 12p13.3 | NM_001769 | 36.7 |
| 218109_s_at | MFSD1 | Major facilitator superfamily domain containing 1 | 3q25.33 | NM_022736 | 36.5 |
| 203920_at | NR1H3 | Nuclear receptor subfamily 1, group H, member 3 | 11p11.2 | NM_005693 | 36.4 |
| 207168_s_at | H2AFY | H2A histone family, member Y | 5q31.3-q32 | NM_004893 | 36.4 |
| 208998_at | UCP2 | Uncoupling protein 2 (mitochondrial, proton carrier) | 11q13.4 | U94592 | 35.7 |
| 207977_s_at | DPT | Dermatopontin | 1q12-q23 | NM_001937 | 35.7 |
| 203416_at | CD53 | CD53 molecule | 1p13 | NM_000560 | 35.7 |
| 201954_at | ARPC1B | Actin related protein 2/3 complex, subunit 1B, 41 kDa | 7q22.1 | NM_005720 | 35.6 |
| 201525_at | APOD | Apolipoprotein D | 3q26.2-qter | NM_001647 | 35.4 |
| 209183_s_at | C10orf10 | Chromosome 10 open reading frame 10 | 10q11.21 | AL136653 | 35.0 |
| 202087_s_at | CTSL | Cathepsin L | 9q21-q22 | NM_001912 | 34.9 |
| 217753_s_at | RPS26 | Ribosomal protein S26 | 12q13 | NM_001029 | 34.7 |
TABLE 3.
SELECTED PATHWAYS WITH HIGHLY VARIABLE TRANSCRIPTS IN OM AND SC ADIPOSE TISSUE, BASED ON THE COEFFICIENT OF VARIATION IN GENE EXPRESSION LEVEL
| Omental Adipose Tissue | Subcutaneous Adipose Tissue | ||||
|---|---|---|---|---|---|
| Pathway | No. of Genes | Symbols | Pathway | No. of Genes | Symbols |
| Aminosugars metabolism | 2 | UAP1, GFPT2 | Aminosugars metabolism | 1 | HEXB |
| Antigen processing and presentation | 2 | HSPA1A, HSPA1B | Antigen processing and presentation | 4 | IFI30, CTSB**, CTSS, CTSL |
| Arachidonic acid metabolism | 1 | PLA2G2A | Arachidonic acid metabolism | 1 | PLA2G2A |
| Cell adhesion molecules (CAMs) | 1 | ICAM1* | Cell adhesion molecules (CAMs) | 2 | ITGB2, ALCAM |
| Cell communication | 1 | LMNA* | Cell communication | 3 | SPP1, COL1A2, THBS1 |
| Cell cycle | 1 | GADD45B | Cell cycle | 1 | CDC16 |
| Fc epsilon RI signaling pathway | 2 | PLA2G2A, MAP2K3 | Fc epsilon RI signaling pathway | 2 | PLA2G2A, FCER1G |
| Focal adhesion | 1 | JUN | Focal adhesion | 3 | SPP1, COL1A2, THBS1 |
| Glycan structures—biosynthesis 2 | 1 | UGCG | Glycan structures—degradation | 2 | GUSB,HEXB |
| Glycerophospholipid metabolism | 1 | PLA2G2A | Glycerophospholipid metabolism | 1 | PLA2G2A |
| GnRH signaling pathway | 2 | PLA2G2A, MAP2K3 | GnRH signaling pathway | 1 | PLA2G2A |
| Hematopoietic cell lineage | 1 | TFRC* | Hematopoietic cell lineage | 2 | TFRC, CD9 |
| Leukocyte transendothelial migration | 1 | ICAM1* | Leukocyte transendothelial migration | 3 | ITGB2, MMP9, TFRC |
| Linoleic acid metabolism | 1 | PLA2G2A | Linoleic acid metabolism | 1 | PLA2G2A |
| Long-term depression | 1 | PLA2G2A | Long-term depression | 1 | PLA2G2A |
| MAPK signaling pathway | 6 | GADD45B, HSPA1A, HS-PA1B, JUN, PLA2G2A, MAP2K3 | MAPK signaling pathway | 1 | PLA2G2A |
| mTOR signaling pathway | 1 | HIF1A | mTOR signaling pathway | 1 | TSC1 |
| Natural killer cell mediated cytotoxicity | 2 | ICAM1*, FCGR3B | Natural killer cell mediated cytotoxicity | 3 | ITGB2, FCER1G, TYROBP |
| Ribosome | 1 | RPS26 | Ribosome | 1 | RPS26 |
| Toll-like receptor signaling pathway | 4 | NFKBIA, MAP2K3, | Toll-like receptor signaling pathway | 1 | TBK1 |
| VEGF signaling pathway | 1 | PLA2G2A | VEGF signaling pathway | 1 | PLA2G2A |
| Wnt signaling pathway | 1 | JUN | Alkaloid biosynthesis II | 1 | LIPA |
| Adipocytokine signaling pathway | 1 | NFKBIA | Alzheimer’s disease | 1 | APOE* |
| Apoptosis | 1 | NFKBIA | ATP synthesis | 1 | ATP6V0B |
| B cell receptor signaling pathway | 2 | NFKBIA, JUN | Bile acid biosynthesis | 1 | LIPA |
| Cytokine–cytokine receptor interaction | 1 | CCL8 | Carbon fixation | 1 | PKM2 |
| Epithelial cell signaling in Helicobacter pylori infection | 1 | NFKBIA | Cholera—infection | 1 | ATP6V0B |
| Glutamate metabolism | 1 | GFPT2 | ECM-receptor interaction | 3 | SPP1, COL1A2, THBS1 |
| Glycosphingolipid metabolism | 1 | UGCG | Globoside metabolism | 1 | HEXB |
| Jak-STAT signaling pathway | 1 | PIM1 | Glutathione metabolism | 1 | GSTOl |
| Methionine metabolism | 1 | MAT2A | Glycerolipid metabolism | 1 | LIPA |
| Nicotinate and nicotinamide metabolism | 2 | NNMT, PBEF1 | Glycolysis/gluconeogenesis | 1 | PKM2 |
| Selenoamino acid metabolism | 1 | MAT2A | Glycosaminoglycan degradation | 2 | GUSB, HEXB |
| T cell receptor signaling pathway | 2 | NFKBIA, JUN | Insulin signaling pathway | 2 | PKM2, TSC1 |
| Metabolism of xenobiotics by cytochrome P450 | 1 | GSTOl | |||
| Neurodegenerative Disorders | 1 | APOE* | |||
| N-Glycan degradation | 1 | HEXB | |||
| Oxidative phosphorylation | 1 | ATP6V0B | |||
| Pentose and glucuronate interconversions | 1 | GUSB | |||
| Porphyrin and chlorophyll metabolism | 1 | GUSB | |||
| Pyruvate metabolism | 1 | PKM2* | |||
| Regulation of actin cytoskeleton | 2 | ITGB2, ARPC1B | |||
| SNARE interactions in vesicular transport | 1 | VAMP8 | |||
| Starch and sucrose metabolism | 1 | GUSB | |||
| TGF-beta signaling pathway | 1 | THBS1 | |||
| Thiamine metabolism | 1 | THTPA | |||
| Type II diabetes mellitus | 1 | PKM2 | |||
| Ubiquitin mediated proteolysis | 1 | CDC16 | |||
Two probesets generated similar results for these genes.
TABLE 4.
GENES ASSOCIATED WITH THE TOP FUNCTION IN THE OM AND SC DATASETS CONTAINING HIGHLY VARIABLE TRANSCRIPTS
| Category/Process | Genes |
|---|---|
| Omental adipose tissue: cell death | |
| Cell death | ATF3, CYR61, DNAJB1, EMP1, GADD45B, HIF1A, HSPA1A, HSPA1B, ICAM1, IER3, JUN, JUNB, KLF4, MAP2K3, MCL1, MT1X, MT2A, NFIL3, NFKBIA, PBEF1, PIM1, PRG1, S100A8, SGK, TBK1, TFRC, TNFAIP3, UGCG |
| Apoptosis | ATF3, CYR61, GADD45B, HIF1A, HSPA1A, HSPA1B, ICAM1, IER3, JUN, KLF4, MAP2K3, MCL1, MT2A, NFIL3, NFKBIA, PBEF1, PIM1, PRG1, S100A8, SGK, TBK1, TFRC, TNFAIP3, UGCG |
| Killing | HSPA1A, HSPA1B, S100A8 |
| Cell viability | LMNA, MCL1, MT2A, NFKBIA, TNFAIP3, UGCG |
| Cytotoxicity | FCGR3B, MT2A, TNFAIP3 |
| Survival | CYR61, HIF1A, HSPA1B, JUN, MCL1, NFIL3, NFKBIA, PIM1, UGCG |
| Colony survival | JUN |
| Inhibition | HSPA1B, IER3, MCL1 |
| Activation-induced cell death | ICAM1 |
| Subcutaneous adipose tissue: lipid metabolism | |
| Storage | ADFP, GM2A, LIPA, SCD |
| Quantity | APOC1, APOE, CTSS, FCER1G, LIPA, NR1H3, PLA2G2A, SCD, UCP2, IL1RN |
| Synthesis | APOE, CD9, FCER1G, NR1H3, PLA2G2A, SCD |
| Release | CTSB, FCER1G, IL1RN, PLA2G2A |
| Modification | APOE, PLA2G2A, SCD, UCP2, ITGB2 |
| Efflux | APOE, NR1H3, SAA1 |
| Hydrolysis | GM2A, HEXB, PLA2G2A |
| Accumulation | APOE, NR1H3, IL1RN, UCP2, APOC1 |
| Production | APOE, IL1RN, ITGB2, PLA2G2A, NR1H3 |
| Activation | NR1H3 |
| Esterification | APOE, SCD |
| Oxidation | APOE, SCD, UCP2 |
| Peroxidation | APOE, PLA2G2A |
| Co-capping | ITGB2 |
| Uptake | APOC1, APOE |
| Metabolism | APOC1, APOD, IL1RN, PLA2G2A, SCD |
| Exchange | APOC1 |
| Liberation | PLA2G2A |
| Degradation | GM2A, PLA2G2A |
| Desaturation | SCD |
| Secretion | APOE, SCD |
| Steroidogenesis | APOE |
| Transport | ADFP |
DISCUSSION
Regional fat distribution accounts for an important part of the association between obesity and related metabolic complications. In the present study, we used SC and OM adipose tissue samples from 10 obese men for microarray hybridizations, and measured expression levels for ∼22,200 probesets. We studied the interindividual variability of gene expression in both depots, and attempted to identify highly variable transcripts or pathways in these fat compartments. Interindividual variability in gene expression in both depots in all subjects was rather low. In addition, no difference in the distribution of CVs was observed among fat depots. This provides evidence that gene expression within abdominal OM and SC adipose tissue samples is relatively homogenous, and indirectly suggests that primary characteristics of adipose tissue from both the SC and OM compartments are relatively similar. Several studies have now used microarrays to investigate gene expression profiling of adipose tissue in rodents (2–4,16) and humans (12,15,23). However, no study had examined human adipose tissue gene expression variability. Individual analyses of adipose tissue gene expression within a homogeneous study group or population might help to identify possible new functional links between different genes. This is the largest microarray study of human SC and OM fat performed to date and the first to provide information on the interindividual variability of gene expression in human adipose tissue.
SC and OM fat have been demonstrated as being very different in terms of lipolysis, cytokine secretion, and linking to disease risks such as insulin resistance and dyslipidemia (6,19,22). This wide heterogeneity among individuals could potentially be reflected by different patterns of gene expression in each fat depot. We measured some variability in gene expression in the present analysis. However, the adipose tissue transcriptome appeared as relatively stable, because interindividual variability was rather low, with more than 90% of clones showing a CV lower than 23.6% and 21.7% in OM and SC adipose tissues, respectively, in the 6,811 probsets that generated significant signal in both fat depots in all 10 subjects. Variability in the probesets that were silenced in at least one and up to nine individuals out of 10 in both depots showed similar variability, and no difference in distributions of CVs was observed between fat depots. Interestingly, Boeuf et al. (2) obtained strikingly similar data when analyzing the individual variability of gene expression in subcutaneous white and brown adipose tissue of hamsters. They found that individual variability of gene expression in both types of fats was also low, with more than 80% of clones showing a CV lower than 30%. These results led the authors to conclude that gene expression in adipose tissue was rather robust and stable for animals, under identical environmental conditions. In the present study, gene expression variability was very consistent with that observed by Boeuf et al (2). We suggest that even in human subjects not under controlled physiological, metabolic, and environmental situations, adipose tissue gene expression is relatively homogeneous. Our results also indicate that the larger portion of genes in SC and OM adipose tissue have stable expression and suggest that only a few pivotal genes might be responsible for the demonstrated regional differences in adipose tissue physiology and related complications.
Among the set of highly variable transcripts, we found that genes in SC samples were mostly involved in lipid metabolism. We also found that a transcript related to insulin signaling, PKM2, was more variable among SC than OM samples. Insulin increases glucose uptake in muscle and fat, and promotes the storage of substrates in fat, liver, and muscle by stimulating lipogenesis, glycogen and protein synthesis, and by inhibiting lipolysis, glycogenolysis, and protein breakdown. PKM2 is a glycolytic enzyme that catalyzes the transfer of a phosphoryl group from phosphoenolpyruvate (PEP) to ADP, generating ATP (13). Kim et al. (14) examined mice with tissue-specific overexpression of LPL and their findings indicated a direct and causative relationship between the accumulation of intracellular fatty acid-derived metabolites and insulin resistance mediated via alterations in the insulin signaling pathway. This phenomenon has been suggested to occur as a means to prevent further fat accumulation in a given tissue, the reduction in insulin action seen in insulin-resistant states affecting metabolic fuel partitioning (8). High variability of SC adipose tissue genes of fat storage and insulin signaling may reflect high variability in the capacity to store fat in this depot in the presence of energy excess.
Cytokines regulate several aspects of adipose tissue metabolism (11,18). Some possibly mediate their responses through activation of the JAK-STAT pathway. We found a transcript related to JAK-STAT pathway that was more variable among OM samples. Another interesting finding of this study is that transcripts related to the MAPK, Wnt, and adipocytokine signaling pathways, and to apoptosis were also more variable among OM samples. These transcripts were PIM1, NFKBIA, JUN, PLA2G2A, HSPA1A, HSPA1B, GADD45B, and MAP2K3. Transcripts related to these cellular processes are involved in cell cycle, apoptosis, growth, proliferation, fate determination, development, immunity, and ubiquitin-mediated proteolysis. The proto-oncogene PIM1 has been shown to prevent the normal process of apoptosis, acting as a cell survival factor. GADD45B is involved in cell cycle arrest, apoptosis, signal transduction, and cell survival. The human HSPA multigene family encodes several highly conserved proteins that are expressed in response to heat shock and a variety of other stress stimuli, including oxidative free radicals and toxic metal ions (21). At the same time, we found that among highly variable genes in OM adipose tissue samples, 28 transcripts showing high variability were involved in cell death. These results suggest high interindividual variability in programmed OM fat cell death.
Obesity has been recently suggested as a pro-inflammatory state (10), and white adipose tissue is no longer considered an inert tissue mainly devoted to energy storage but is emerging as an active participant in regulating physiologic and pathologic processes, including immunity and inflammation. Many of these cellular pathways were highly variable in both fat depots. For example, pathways of hematopoietic cell lineage, the Fc epsilon RI signaling pathway, glycerophospholipid metabolism, and leukocyte transendothelial migration included highly variable genes in both fat compartments. Two main genes were responsible for this finding (PLA2G2A, TFRC). PLA2G2A plays an important role in a variety of cellular processes, including the production of precursors for inflammatory reactions. Furthermore, it is a key enzyme in eicosanoid synthesis and is therefore an interesting candidate gene in the context of inflammation (1). TFRC encodes the transferrin receptor, which plays an important role in controlling cell growth through iron uptake. Both genes are involved in inflammation, proliferation, growth, and oncogenesis. Our results may reflect high variability in inflammatory responses in both fat compartments in obesity.
In summary, our data demonstrated that interindividual variability of gene expression in abdominal SC and OM adipose tissue samples from obese men was rather low. Future studies are required to investigate relations between different phenotypes (such as obesity, insulin, blood lipids) and expression of these transcripts.
ACKNOWLEDGMENTS
We thank Vicky Drapeau, Suzy Laroche, and members of the Department of Surgery of Laval Hospital and Drs. Frédéric-Simon Hould, Odette Lescelleur, and Simon Marceau for collaboration in tissue and specimen sampling. Marie-Claude Vohl, André Tchernof, and Yohan Bossé are funded by the Fonds de la Recherche en Santé du Québec (MVC) and the Canadian Institutes of Health Research (AT and YB). This work was partially supported by the Donald B. Brown Research Chair on Obesity, the FRSQ-Réseau en santé cardiovasculaire, Genome Québec, and Genome Canada.
REFERENCES
- 1. Bertsch T.; Aufenanger J. Phospholipase A2 in inflammatory bowel disease. Gut 41:859–860; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boeuf S.; Keijer J.; Franssen-Van Hal N. L.; Klaus S. Individual variation of adipose gene expression and identification of covariated genes by cDNA microarrays. Physiol. Genomics 11:31–36; 2002. [DOI] [PubMed] [Google Scholar]
- 3. Boeuf S.; Klingenspor M.; Van Hal N. L.; Schneider T.; Keijer J.; Klaus S. Differential gene expression in white and brown preadipocytes. Physiol. Genomics 7:15–25; 2001. [DOI] [PubMed] [Google Scholar]
- 4. Bullen J. W. Jr.; Ziotopoulou M.; Ungsunan L.; Misra J.; Alevizos I.; Kokkotou E.; Maratos-Flier E.; Stephanopoulos G.; Mantzoros C. S. Short-term resistance to diet-induced obesity in A/J mice is not associated with regulation of hypothalamic neuropeptides. Am. J. Physiol. Endocrinol. Metab. 287:E662–E670; 2004. [DOI] [PubMed] [Google Scholar]
- 5. Chan D. C.; Watts G. F.; Sussekov A. V.; Barrett P. H.; Yang Z.; Hua J.; Song S. Adipose tissue compartments and insulin resistance in overweight-obese Caucasian men. Diabetes Res. Clin. Pract. 63:77–85; 2004. [DOI] [PubMed] [Google Scholar]
- 6. Despres J. P.; Moorjani S.; Ferland M.; Tremblay A.; Lupien P. J.; Nadeau A.; Pinault S.; Theriault G.; Bouchard C. Adipose tissue distribution and plasma lipoprotein levels in obese women. Importance of intra-abdominal fat. Arteriosclerosis 9:203–210; 1989. [DOI] [PubMed] [Google Scholar]
- 7. Despres J. P.; Nadeau A.; Tremblay A.; Ferland M.; Moorjani S.; Lupien P. J.; Theriault G.; Pinault S.; Bouchard C. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes 38:304–309; 1989. [DOI] [PubMed] [Google Scholar]
- 8. Eckel R. H. Insulin resistance: An adaptation for weight maintenance. Lancet 340:1452–1453; 1992. [DOI] [PubMed] [Google Scholar]
- 9. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497; 2001. [DOI] [PubMed] [Google Scholar]
- 10. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115:911–999; 2005. [DOI] [PubMed] [Google Scholar]
- 11. Fried S. K.; Bunkin D. A.; Greenberg A. S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 83:847–850; 1998. [DOI] [PubMed] [Google Scholar]
- 12. Gomez-Ambrosi J.; Catalan V.; Diez-Caballero A.; Martnez-Cruz L. A.; Gil M. J.; Garcia-Foncillas J.; Cienfuegos J. A.; Salvador J.; Mato J. M.; Fruhbeck G. Gene expression profile of omental adipose tissue in human obesity. FASEB J. 18:215–217; 2004. [DOI] [PubMed] [Google Scholar]
- 13. Ikeda Y.; Noguchi T. Allosteric regulation of pyruvate kinase M-2 isozyme involves a cysteine residue in the intersubunit contact. J. Biol. Chem. 273:12227–12233; 1998. [DOI] [PubMed] [Google Scholar]
- 14. Kim J. K.; Fillmore J. J.; Chen Y.; Yu C.; Moore I. K.; Pypaert M.; Lutz E. P.; Kako Y.; Velez-Carrasco W.; Goldberg I. J.; Breslow J. L.; Shulman G. I. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl. Acad. Sci. USA 98:7522–7527; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Linder K.; Arner P.; Flores-Morales A.; Tollet-Egnell P.; Norstedt G. Differentially expressed genes in visceral or subcutaneous adipose tissue of obese men and women. J. Lipid Res. 45:148–154; 2004. [DOI] [PubMed] [Google Scholar]
- 16. Lopez I. P.; Marti A.; Milagro F. I.; Zulet Md Mde L.; Moreno-Aliaga M. J.; Martinez J. A.; De Miguel C. DNA microarray analysis of genes differentially expressed in diet-induced (cafeteria) obese rats. Obes. Res. 11:188–194; 2003. [DOI] [PubMed] [Google Scholar]
- 17. Marceau P.; Biron S.; Hould F. S.; Marceau S.; Simard S.; Thung S. N.; Kral J. G. Liver pathology and the metabolic syndrome X in severe obesity. J. Clin. Endocrinol. Metab. 84:1513–1517; 1999. [DOI] [PubMed] [Google Scholar]
- 18. Mohamed-Ali V.; Pinkney J. H.; Coppack S. W. Adipose tissue as an endocrine and paracrine organ. Int. J. Obes. Relat. Metab. Disord. 83:1145–1158; 1998. [DOI] [PubMed] [Google Scholar]
- 19. Motoshima H.; Wu X.; Sinha M. K.; Hardy V. E.; Rosato E. L.; Barbot D. J.; Rosato F. E.; Goldstein B. J. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J. Clin. Endocrinol. Metab. 87:5662–5667; 2002. [DOI] [PubMed] [Google Scholar]
- 20. Novak J. P.; Sladek R.; Hudson T. J. Characterization of variability in large-scale gene expression data: Implications for study design. Genomics 79:104–113; 2002. [DOI] [PubMed] [Google Scholar]
- 21. Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 46:959–961; 1986. [DOI] [PubMed] [Google Scholar]
- 22. Tchernof A.; Belanger C.; Morisset A. S.; Richard C.; Mailloux J.; Laberge P.; Dupont P. Regional differences in adipose tissue metabolism in women: Minor effect of obesity and body fat distribution. Diabetes 55:1353–1360; 2006. [DOI] [PubMed] [Google Scholar]
- 23. Vohl M. C.; Sladek R.; Robitaille J.; Gurd S.; Marceau P.; Richard D.; Hudson T. J.; Tchernof A. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes. Res. 12:1217–1222; 2004. [DOI] [PubMed] [Google Scholar]
- 24. Wajchenberg B. L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 21:697–738; 2000. [DOI] [PubMed] [Google Scholar]


