Abstract
Hepatocyte nuclear factor 4-α (HNF4-α) regulates expression of a number of genes in several metabolic organs. The HNF4-α gene has two promoters and encodes at least nine isoforms through differential splicing. In mouse liver, transcription initiates at promoter 2 (P2) during fetal life, but switches to P1 at birth. Developmental and tissue-specific expression of HNF4-α in other organs is largely unknown. Here, we examined expression of P1-and P2-derived transcripts in a number of mouse and rat tissues. Both P1 and P2 were active in mouse fetal liver, but P2-derived isoforms were detected 50% more abundantly than P1 transcripts. Conversely, the adult mouse liver expressed significantly higher levels of P1- than P2-derived mRNA. In contrast, in the rat, P1 was used more predominantly in both fetal and adult liver. Exposure of fetal rats to the synthetic glucocorticoid dexamethasone caused suppression of P2 while enhancing hepatic expression of transcripts from P1. This was associated with increased expression of erythropoietin and phosphoenolpyruvate carboxykinase, which are key HNF4-α targets in the liver. Unlike liver, the kidney and stomach used promoters more selectively, so that only P1-derived isoforms were detected in fetal and adult kidneys in mice or rats, whereas the stomach in both species expressed P2-derived transcripts exclusively. No significant HNF4-α mRNA was detected in the spleen. These data reveal striking developmental and tissue-specific variation in expression of HNF4-α, and indicate that this can be influenced by environmental factors (such as exposure to glucocorticoid excess), with potential pathophysiological consequences.
Key words: Hepatocyte nuclear factor 4-α (HNF4-α), Liver, Kidney, Stomach, Glucocorticoids
INTRODUCTION
Hepatocyte nuclear factor 4-α (HNF4-α), an orphan nuclear receptor of the steroid/thyroid hormone receptor superfamily, has been shown to play a central role in the development and function of a number of metabolic organs, including the liver, pancreas, kidney, stomach, and intestines (12,14–16). In the liver and endocrine pancreas HNF4-α is considered to be at the top of hierarchy of transcription factor cascade that determines tissue-specific gene expression, with up to 50% of transcribable genes in the in these tissues thought to be regulated by HNF4-α (12,14,15). Targeted disruption of the HNF4-α gene causes embryonic lethality associated with impaired gastrulation (2,5). In humans, inactivating mutations of the HNF4-α gene cause maturity-onset diabetes of the young 1 (MODY1), a subgroup of diabetes characterized by an autosomal dominant inheritance and early onset non-insulin-dependent diabetes that results from a single-gene defect causing pancreatic β-cell dysfunction (19). Polymorphisms of the HNF4-α gene are also thought to play a role in the pathogenesis of type 2 diabetes, by altering insulin secretion and predisposing toward hyperglycemia (8,13,20). In the adult kidney (as in fetal liver), HNF4-α has been implicated in regulation of erythropoietin (EPO) synthesis, but the physiological functions of HNF4-α in the gastrointestinal tract are largely unknown.
HNF4-α is regulated through complex and poorly understood mechanisms. The gene has 13 exons and contains two promoters, P1 and P2 (Fig. 1), which encode at least nine distinct isoforms as result of alternate promoter usage and differential splicing. The developmental and physiological relevance of the various HNF4-α isoforms have not been fully explored, but recent data suggest that their expression may vary with development and in a tissue-specific manner. For example, in the mouse, P2-derived transcripts are expressed predominantly in embryonic/fetal liver, while the P1 isoforms increase dramatically in late gestation and predominate in the adult liver (17). Interestingly, this switch in promoter usage can be induced prematurely by exposure to glucocorticoid excess (9,10). This is consistent with known tissue-maturing effects of glucorticoids, which are, for example, classically exploited therapeutically to accelerate lung maturity (and thereby improve neonatal viability) in cases of preterm labor (6).
Figure 1.
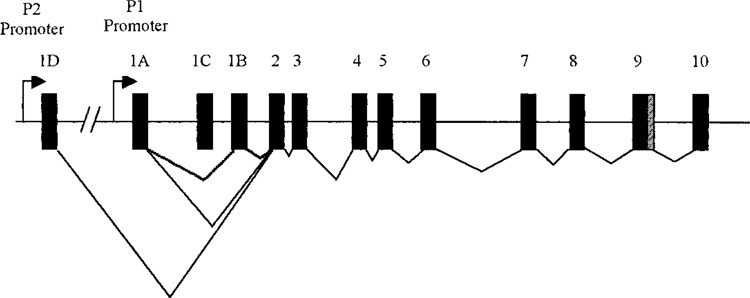
Systematic representation of the HNF4-α gene structure. Exons are shown as boxes. The arrows represent the two alternative promoters and the lines linking exons indicate splicing events.
Detailed developmental and tissue-specific expression of HNF4-α have not been investigated in other organs or species. We therefore examined tissue-specific expression of P1 and P2 transcripts in a number of fetal and adult organs in mice and rats. Additionally, in order to determine whether glucocorticoid-induced changes in HNF4-α expression have functional significance, we exposed fetal rats to the synthetic glucocorticoid dexamethasone and examined hepatic mRNA expression of HNF4-α target genes, notably erythropoietin (EPO) and phosphoenolpyruvate carboxykinase (PEPCK).
MATERIALS AND METHODS
Animals
All experiments were carried out under UK Home Office animal license. Wistar rats (200–250 g; Harlan UK Ltd, Bicester, UK) and C57BL/6J mice (12 weeks old; Charles River, UK) were maintained under conditions of controlled lighting (lights on 0700–1900 h) and temperature (22°C) and allowed free access to food (standard laboratory chow; B.S.&S Scotland Ltd. Edinburgh) and tap water. Animals (five per group) were time mated, and the day on which the vaginal plug was visualized was designated embryonic day 0 (E0). Dams were killed by decapitation in a fed state, and fetal (E15 and E19 in mice; E15 and E21 in rats) tissues (liver, kidney, stomach, and spleen) were removed and snap frozen in liquid nitrogen and stored at −80°C for subsequent analyses. In a different cohort of animals, tissues were collected from adult (6 months old) male mice and rats in a fed state.
Dexamethasone Administration
In the study involving prenatal glucocorticoid exposure, animals were treated with dexamethasone as previously described (11). In brief, time-mated pregnant rats (four per group) were given daily subcutaneous injections of dexamethasone (100 μg/kg−1 day−1, dissolved in 4% ethanol/0.9% saline, 200 μg/ml) or vehicle during the last week (day 15 to 21) of pregnancy. Pregnant dams were killed at day 21 of gestation and livers were collected from two fetuses, selected at random, per dam.
Real-Time RT-PCR
Tissue fragments were homogenized in TRIZOL solution (Invitrogen, Paisley, UK) and total RNA extracted as per the manufacturer’s instruction. RNA was quantified spectrophotometrically at OD260 using a nanodrop machine and RNA integrity was checked by agarose electrophoresis. cDNA was then synthesized from 1 μg of RNA using the Quantitect Reverse Transcription kit (Qiagen), which incorporates a gDNA removal step prior to cDNA synthesis. Real-time PCR quantification was carried out with the Lightcycler 480 real-time PCR system (Roche Applied Science). Total rat HNF4-α and EPO mRNA were detected using predesigned TaqMan Gene Expression Assay primers and probes (Applied Biosystems); assay ID for HNF4-α: Rn00573309_m1; assay ID for EPO: Rn01481376_m1. Primers for detection of P1 and P2 HNF4-α transcripts were custom ordered from Applied Biosystems, and the same set of primers was used to detect HNF4-α mRNA in mice and rats. P1 transcripts: forward primer GACATG GACATGGCTGACTACG; reverse primer CAGAAGGGAGGCTTGACGA; reporter sequence ACGT GTCATAAGGACTCGC-FAM. P2 transcripts: forward primer CTTGGTCATGGTCAGTGTGAAC; backward primer AGGCTGTTGGATGAATTGAG GTT; reporter sequence ACGTGTCATAAGGACT CGC-FAM. GAPDH was used to normalize the mRNA levels of the genes of interest, and predesigned primers were obtained from TaqMan Gene Expression Assay (Assay ID Mm03302249_g1 for mouse and Rn01775763_g1 for rat). Serial 1:2 dilutions of stock cDNA (pooled from all samples) were used to create a standard curve. No-template negative controls (nuclease-free water) and negative RT controls were added to the real-time plate. Each sample was run in duplicate and the mean value of the duplicates was used to calculate the transcript level.
Statistics
All data are expressed as mean ± SEM. Data were compared using unpaired Student’s t-tests. Values were considered significant when p < 0.05.
RESULTS
HNF4-α Transcripts in Mouse Liver
Fetal liver in mice expressed both P1 and P2 HNF4-α mRNA (Fig. 2). In accord with previous reports (17), P2 transcripts were expressed more abundantly (50% higher) than P1-derived isoforms. In sharp contrast, in adult mice, hepatic expression of P1-driven transcript was significantly higher than that of mRNA derived from the P2 promoter (Fig. 2).
Figure 2.
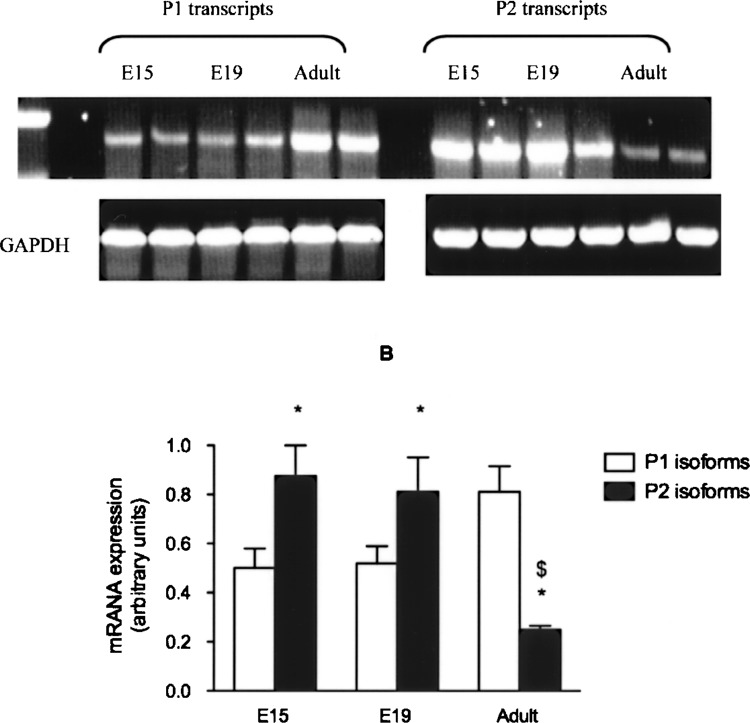
Expression of P1 and P2 HNF4-α isoforms in mouse liver. HNF4-α mRNA from mouse fetal (E15 and E19) and adult liver was measured by real-time RT-PCR using P1- or P2-specific primers. GAPDH was used as control housekeeping gene. (A) RT-PCR products separated on agarose gel and (B) relative mRNA content (mean ± SEM) in the liver at different ages. *p < 0.05 versus P1; $p < 0.05 versus fetal (E15, E19).
HNF4-α Expression in Rat Liver
In the rat, transcripts resulting from usage of the P1 promoter were expressed in significantly higher amounts than the P2-derived isoforms, in both fetal and adult livers. Expression of P2 transcripts fell significantly towards the end of gestation (between E15 and E21), and were hardly detectable in the adult liver (Fig. 3).
Figure 3.
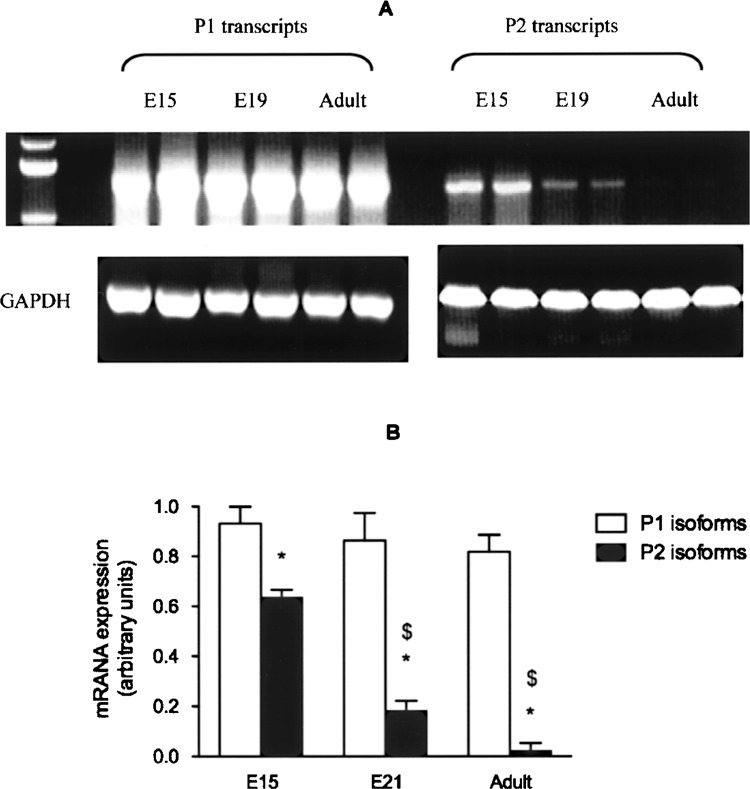
Expression of P1 and P2 HNF4-α isoforms in rat liver. HNF4-α mRNA from rat fetal (E15 and E21) and adult liver was measured by real-time RT-PCR using P1- or P2-specific primers. GAPDH was used as control housekeeping gene. (A) PCR products separated on agarose gel and (B) relative mRNA content (mean ± SEM) in the liver at different ages. *p < 0.05 versus P2; $p < 0.05 versus E15.
Expression of HNF4-α in Mouse Kidney, Stomach, and Spleen
Expression of HNF4-α P1- and P2-derived mRNA was also measured in fetal and adult kidney, stomach, and spleen. Mouse kidney, in fetuses and adult animals, expressed abundant amounts of P1-derived mRNA (Fig. 4A; data not shown for fetal expression). However, the kidney did not express P2 transcripts. In sharp contrast to the kidney, the stomach expressed exclusively the P2 isoforms, both in fetal life and adulthood (Fig. 4A; data not shown for fetal expression). We did not detect any HNF4-α mRNA (neither P1 nor P2 derived) in the spleen.
Figure 4.
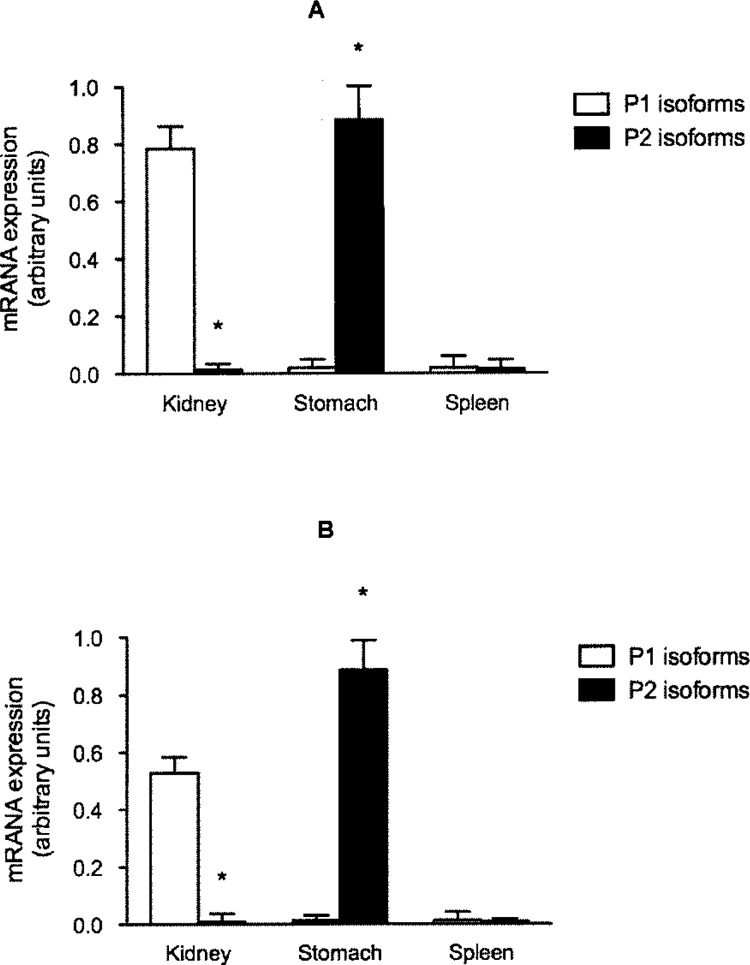
Expression of P1 and P2 HNF4-α isoforms in kidney, stomach, and spleen. HNF4-α mRNA isolated from adult mouse (A) and rat (B) kidney, stomach, or spleen was analyzed by real-time RT-PCR using P1- or P2-specific primers. GAPDH was used as control housekeeping gene. *p < 0.05 P1 versus P2.
Expression of HNF4-α in Rat Kidney, Stomach, and Spleen
Expression of HNF4-α in rat kidney, stomach, or spleen was similar to that observed in the mouse, with the kidney exclusively expressing P1 isoforms whereas only P2 transcripts were detected in the stomach (Fig. 4B; data not shown for fetal expression). As in the mouse, rat spleen did not express appreciable HNF4-α mRNA.
Effect of Dexamethasone Treatment on HNF4-α and its Target Genes
Prenatal dexamethasone treatment caused a marked increase in expression of HNF4-α in fetal liver (Fig. 5). In order to test whether this was of functional significance, we measured expression of PEPCK and EPO, which are its important downstream targets of HNF4-α. Dexamethasone treatment was associated with marked elevations for PEPCK and EPO expression mRNAs (Fig. 5). In contrast, expression of mRNA for glucokinase, a gene in the glycolytic pathway and not a normal target of HNF4-α, was not affected by exposure to dexamethasone.
Figure 5.
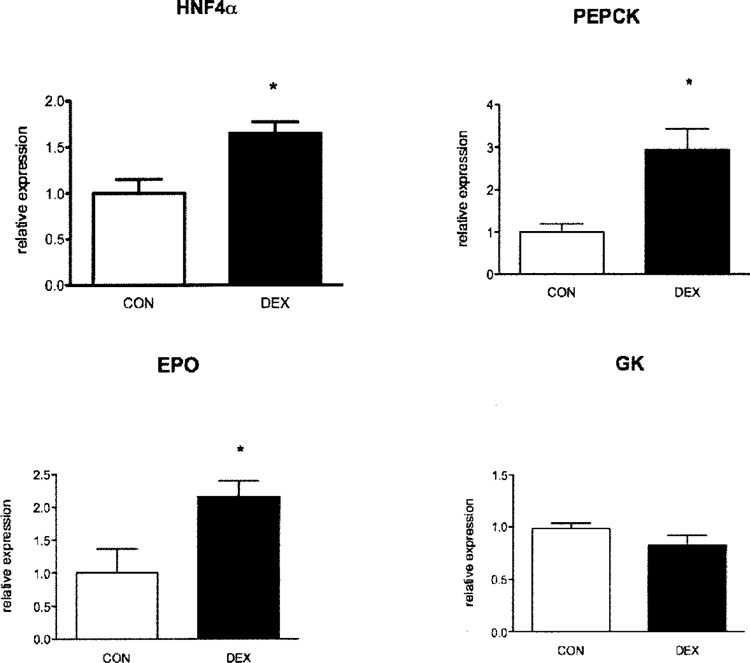
Effect of dexamethasone on HNF4-α and its target genes. Hepatic expression of HNF4-α, PEPCK, EPO, and glucokinase (GK) mRNA was measured in E21 fetuses of dams that received dexamethasone (DEX) or vehicle (CON) during the last week of pregnancy. Results (mean ± SEM) represent mRNA levels relative to control animals (n = 8 per group). *p < 0.05 versus control.
DISCUSSION
The HNF4-α gene can yield up to nine isoforms as a result of alternate promoter usage and differential splicing (14). Previous studies in mice have suggested that these isoforms are expressed in a tissue-specific manner, and that, at least in the liver, expression is dependent on the stage of development (9,14,17). Here, we have extended these observations to the rat. As in the mouse, rat fetal liver expressed both P1- and P2-derived HNF4-α isoforms. However, unlike in mice, It was:the P1 (rather than P2) transcripts that were expressed in abundance. Indeed, hepatic expression of P2-derived isoforms in rat liver fell dramatically towards the end of gestation and was hardly detectably postnatally. The reasons for this discrepancy are unclear, but may reflect differences in ontogenic maturation between the two species. In the other organs, usage of the two HNF4-α promoters followed a more distinct pattern, so that only one of the promoters was used exclusively in a given tissue, in both fetal and adult life. Thus, only P1 transcripts were detected in fetal and adult kidney, whereas the stomach expressed exclusively P2-derived mRNA. These data are in accord with recent studies that showed that the adult kidney expresses high levels of P1-derived HNF4-α mRNA (1,9). Additionally, our data indicate that this distinct pattern, of exclusive use of P1 and P2 promoters in kidney and stomach, respectively, is set in utero.
Taken together, these studies reveal that regulation of HNF4-α expression is highly complex. The physiological relevance of the various HNF4-α isoforms is unknown. Differential promoter usage might enable tighter modulation of HNF4-α activity during development or in specific tissues. This notion is supported by data suggesting that the isoforms vary in their ability to recruit cofactors, which may result in differences in their activation functions (18). For example, P2-derived transcripts have been shown to be more potent at transactivating genes involved in fetal hepatocyte function (such as α-fetoprotein), whereas P1 transcripts are more efficient at facilitating transactivation of genes characteristic of mature hepatocyte function (17).
Exposing fetal rats or hepatoma cell lines to dexamethasone induces precocious hepatic expression of the “adult” (P1) promoter-derived transcripts, while suppressing activity of the “fetal” or P2 promoter (9,10). In the present study, we demonstrated that these changes were associated with marked increases in hepatic expression of PEPCK and EPO, which are important targets of HNF4-α. PEPCK is the rate-controlling enzyme of gluconeogenesis and its increased expression would lead to increased glucose production from the liver (3). Similarly, EPO plays a key role in regulation of erythropoiesis, and the liver is the major source EPO during fetal life (4,7). These data indicate that HNF-α expression can be influenced by environmental factors, and that this could have significant pathophysiological consequences.
The mechanisms that regulate tissue-specific and developmental expression of the HNF4-α isoforms are unknown. The reciprocal relationship between P2 and P1 transcripts in the liver (with P2 present in embryonic liver but mainly extinguished in adulthood where P1 transcripts are high) and the exclusive use P1 and P2 promoters in the kidney and stomach, respectively, suggests that products of one promoter might influence activity of the other. This hypothesis is supported by recent data showing that P1 transcripts can repress the P2 promoter (1). However, this hypothesis will require critical examination, perhaps with conditional expression of the products of these alternate promoters. Our data provide intriguing insight to stimulate such studies.
ACKNOWLEDGMENTS
This study was supported by Medical Research Scotland. M.N. is a recipient of an MRC fellowship. We thank members of the Endocrine Unit, particularly Prof. K. E. Chapman, for helpful advice and many useful discussions.
REFERENCES
- 1. Briancon N.; Bailly A.; Clotman F.; Jacquemin P.; Lemaigre F. P.; Weiss M. C. Expression of the alpha7 isoform of hepatocyte nuclear factor (HNF) 4 is activated by HNF6/OC-2 and HNF1 and repressed by HNF4alpha1 in the liver. J. Biol. Chem. 279:33398–33408; 2004. [DOI] [PubMed] [Google Scholar]
- 2. Chen W. S.; Manova K.; Weinstein D. C.; Duncan S. A.; Plump A. S.; Prezioso V. R.; Bachvarova R. F.; Darnell J. E. Jr. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 8:2466–2477; 1994. [DOI] [PubMed] [Google Scholar]
- 3. Consoli A.; Nurjhan N. Contribution of gluconeogenesis to overall glucose output in diabetic and nondiabetic men. Ann. Med. 22:191–195; 1990. [DOI] [PubMed] [Google Scholar]
- 4. Dame C.; Fahnenstich H.; Freitag P.; Hofmann D.; Abdul-Nour T.; Bartmann P.; Fandrey J. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood 92:3218–3225; 1998. [PubMed] [Google Scholar]
- 5. Duncan S. A.; Nagy A.; Chan W. Murine gastrulation requires HNF-4 regulated gene expression in the visceral endoderm: Tetraploid rescue of Hnf-4(-/-) embryos. Development 124:279–287; 1997. [DOI] [PubMed] [Google Scholar]
- 6. Kattner E.; Metze B.; Waiss E.; Obladen M. Accelerated lung maturation following maternal steroid treatment in infants born before 30 weeks gestation. J. Perinat. Med. 20:449–457; 1992. [DOI] [PubMed] [Google Scholar]
- 7. Koury S. T.; Bondurant M. C.; Koury M. J.; Semenza G. L. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood 77:2497–2503; 1991. [PubMed] [Google Scholar]
- 8. Love-Gregory L. D.; Wasson J.; Ma J.; Jin C. H.; Glaser B.; Suarez B. K.; Permutt M. A. A common polymorphism in:the upstream promoter region of the hepatocyte nuclear factor-4 alpha gene on chromosome 20q is associated with type 2 diabetes and appears to contribute to the evidence for linkage in an ashkenazi jewish population. Diabetes 53:1134–1140; 2004. [DOI] [PubMed] [Google Scholar]
- 9. Nakhei H.; Lingott A.; Lemm I.; Ryffel G. U. An alternative splice variant of the tissue specific transcription factor HNF4alpha predominates in undifferentiated murine cell types. Nucleic Acids Res. 26:497–504; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nyirenda M. J.; Dean S.; Lyons V.; Chapman K. E.; Seckl J. R. Prenatal programming of hepatocyte nuclear factor 4alpha in the rat: A key mechanism in the ‘foetal origins of hyperglycaemia’? Diabetologia 49:1412–1420; 2006. [DOI] [PubMed] [Google Scholar]
- 11. Nyirenda M. J.; Lindsay R. S.; Kenyon C. J.; Burchell A.; Seckl J. R. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J. Clin. Invest. 101:2174–2181; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Odom D. T.; Zizlsperger N.; Gordon D. B.; Bell G. W.; Rinaldi N. J.; Murray H. L.; Volkert T. L.; Schreiber J.; Rolfe P. A.; Gifford D. K.; Fraenkel E.; Bell G. I.; Young R. A. Control of pancreas and liver gene expression by HNF transcription factors. Science 303:1378–1381; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silander K.; Mohlke K. L.; Scott L. J.; Peck E. C.; Hollstein P.; Skol A. D.; Jackson A. U.; Deloukas P.; Hunt S.; Stavrides G.; Chines P. S.; Erdos M. R.; Narisu N.; Conneely K. N.; Li C.; Fingerlin T. E.; Dhanjal S. K.; Valle T. T.; Bergman R. N.; Tuomilehto J.; Watanabe R. M.; Boehnke M.; Collins F. S. Genetic variation near the hepatocyte nuclear factor-4 alpha gene predicts susceptibility to type 2 diabetes. Diabetes 53:1141–1149; 2004. [DOI] [PubMed] [Google Scholar]
- 14. Sladek F. M.; Seidel S. D. Hepatocyte nuclear factor 4a. In: Burris T. P.; McCabe E. R. B., eds. Nuclear receptors and genetic disease. London: Academic Press; 2001:309–361. [Google Scholar]
- 15. Stoffel M.; Duncan S. A. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc. Natl. Acad. Sci. USA 94:13209–13214; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taraviras S.; Monaghan A. P.; Schutz G.; Kelsey G. Characterization of the mouse HNF-4 gene and its expression during mouse embryogenesis. Mech. Dev. 48:67–79; 1994. [DOI] [PubMed] [Google Scholar]
- 17. Torres-Padilla M. E.; Fougere-Deschatrette C.; Weiss M. C. Expression of HNF4alpha isoforms in mouse liver development is regulated by sequential promoter usage and constitutive 3′ end splicing. Mech. Dev. 109:183–193; 2001. [DOI] [PubMed] [Google Scholar]
- 18. Torres-Padilla M. E.; Sladek F. M.; Weiss M. C. Developmentally regulated N-terminal variants of the nuclear receptor hepatocyte nuclear factor 4alpha mediate multiple interactions through coactivator and corepressor-histone deacetylase complexes. J. Biol. Chem. 277:44677–44687; 2002. [DOI] [PubMed] [Google Scholar]
- 19. Yamagata F.; Furuta H.; Oda N.; Kaisaki P. J.; Menzel S.; Cox N. J.; Fajans S. S.; Signorini S.; Stoffel M.; Bell G. I. Mutation in hepatic nuclear factor-4a gene in maturity-onset diabetes of the young (MODY 1). Nature 384:458–460; 1996. [DOI] [PubMed] [Google Scholar]
- 20. Zhu Q.; Yamagata K.; Miura A.; Shihara N.; Horikawa Y.; Takeda J.; Miyagawa J.; Matsuzawa Y. T130I mutation in HNF-4alpha gene is a loss-of-function mutation in hepatocytes and is associated with late-onset type 2 diabetes mellitus in Japanese subjects. Diabetologia 46:567–573; 2003. [DOI] [PubMed] [Google Scholar]


