Abstract
Objective:
To evaluate the efficacy and safety of an intraarticular injection of Cingal (Anika Therapeutics, Inc., Bedford, MA) compared with Monovisc (Anika Therapeutics, Inc., Bedford, MA) or saline for the treatment of knee osteoarthritis.
Design:
This multicenter, double-blind, saline-controlled clinical trial randomized subjects with knee osteoarthritis (Kellgren-Lawrence grades I-III) to a single injection of Cingal (4 mL, 88 mg hyaluronic acid [HA] plus 18 mg triamcinolone hexacetonide [TH]), Monovisc (4 mL, 88 mg HA), or saline (4 mL, 0.9%). The primary efficacy outcome was change in WOMAC (Western Ontario and McMaster Universities Arthritis Index) Pain Score through 12 weeks with Cingal versus saline. Secondary outcomes included Patient and Evaluator Global Assessments, OMERACT-OARSI Responder index, and WOMAC Total, Stiffness, and Physical Function scores through 26 weeks.
Results:
A total of 368 patients were treated (Cingal, n = 149; Monovisc, n = 150; saline, n = 69). Cingal improvement from baseline was significantly greater than saline through 12 weeks (P = 0.0099) and 26 weeks (P = 0.0072). WOMAC Pain was reduced by 70% at 12 weeks and by 72% at 26 weeks with Cingal. Significant improvements were found in most secondary endpoints for pain and function at most time points through 26 weeks. At 1 and 3 weeks, Cingal was significantly better than Monovisc for most endpoints; Cingal and Monovisc were similar from 6 weeks through 26 weeks. A low incidence of related adverse events was reported.
Conclusions:
Cingal provides immediate and long-term relief of osteoarthritis-related pain, stiffness, and function, significant through 26 weeks compared to saline. Cingal had similar immediate advantages compared with HA alone, while showing benefit comparable to HA at 6 weeks and beyond.
Keywords: intraarticular delivery, therapeutic delivery, clinical trial, general, osteoarthritis, diagnosis, knee, joint involved
Introduction
Osteoarthritis (OA) is the most common joint disorder globally,1 affecting over 27 million people in the United States2 and 40 million people in Europe.3 It is characterized by a decreased concentration of hyaluronic acid (HA) in synovial fluid4 and a slow degradation of cartilage,5 as well as joint pain and significant functional limitations.6 The economic burden of OA is high, with health care expenditures of over $185 billion annually in the United States due to OA.2 The incidence of OA is projected to rise; and nearly 1 in 2 people are projected to develop symptomatic knee OA by age 85,7 leading to over an estimated 3.5 million knee replacement surgeries by 2030.8
Intraarticular corticosteroid injections to treat knee OA are recommended in clinical practice guidelines of medical associations.3,9 In addition, a review of 28 trials found statistically significant short-term pain reduction for up to 3 weeks post injection.10 Intraarticular HA injections are also used to reduce osteoarthritic pain and improve joint function by supplementing the synovial fluid with exogenous HA (“viscosupplementation”) to provide lubrication and mechanical support. A meta-analysis of 54 randomized clinical trials of intraarticular HA versus placebo to treat OA knee pain demonstrated that HA was efficacious between 4 weeks and 6 months.11
Thus, corticosteroids provide shorter term pain relief compared with HA, which provides longer term pain relief with an onset of pain reduction occurring over several weeks.11 Adding a corticosteroid to HA could then potentially alleviate OA pain more immediately in those receiving HA prior to the onset of pain relief with HA. Several small randomized trials have shown that the combination of a corticosteroid and HA versus HA alone results in significant short-term pain relief from the corticosteroid with the longer term pain relief from the HA.12-14
A new product, Cingal (Anika Therapeutics, Inc., Bedford, MA), was developed to deliver the short-term pain relief of an approved corticosteroid, triamcinolone hexacetonide (TH), with the sustained pain relief of a commercial cross-linked HA viscosupplement, Monovisc (Anika Therapeutics, Inc., Bedford, MA). Cingal is a single, 4-mL, intraarticular injection containing 88 mg HA and 18 mg of TH. The objective of this clinical trial was to demonstrate the safety and effectiveness of Cingal for relief of joint pain and symptoms in knee OA patients. It was hypothesized that Cingal’s pain reduction would be superior to saline through 12 and 26 weeks, and also be superior to Monovisc for short-term pain relief.
Methods
Study Design
This was a prospective, multicenter, randomized, double-blind, placebo-controlled, active-comparator clinical trial conducted at 30 sites in Europe and Canada. Eligible subjects with radiologically confirmed knee OA were randomized 2:2:1 to a single injection of Cingal (4 mL, 88 mg HA plus 18 mg TH), Monovisc (4 mL, 88 mg HA), or saline (4 mL, 0.9%). The randomization code for each site was generated by an independent biostatistician using a block size of 5. All study syringes and cartons were identical, and the treating physicians, evaluators, radiologists who reviewed the X-ray images, sponsor, study monitors, and subjects remained blinded throughout the trial, until the database was locked.
A physical exam, medical history, blood/urine/pregnancy tests, bilateral knee X-rays, concomitant medications, and the Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaire were completed at screening. Subjects were instructed to discontinue all analgesics for the duration of the study, except rescue medication (acetaminophen/paracetamol), which could be used for up to 48 hours prior to visits.
At baseline, the treating physician prepped the subject’s index knee, penetrated the joint space using a sterile 18-21 gauge needle and syringe, aspirated to dryness, and retained the aspirate for visual inspection and volume measurement. Study treatment was then injected into the index knee using standard intraarticular injection techniques.
Each site received approval from the Ethics Committee and Competent Authority to begin the study and all subjects were consented prior to enrollment. The study was conducted in accordance with Good Clinical Practices as required by the International Conference on Harmonisation and the Declaration of Helsinki, and was registered with ClinicalTrials.gov (NCT01891396).
Study Population
Study participants were 40 to 75 years of age with a body mass index (BMI) ≤ 40 kg/m2, and Kellgren-Lawrence (K-L) OA grade I, II, or III in the index knee as determined by X-ray.15 At baseline, subjects had to have a WOMAC pain score ≥40 mm and ≤90 mm in the affected knee and ≤30 mm in the contralateral knee on a 100-mm visual analog scale (VAS).
Key exclusion criteria that could interfere with the study assessments included certain joint disorders, some medical condition(s), or prior knee treatments (including HA or steroid injections in the index knee in the past 6 months); and taking medications that could interfere with the procedure, healing, and/or assessments. A subject was excluded if the synovial fluid aspirate volume was >20 mL or there was visual evidence of cloudiness, crystals, or blood. Pregnant women were also excluded.
Study Outcomes
At baseline and follow-up visits, a physical exam of both knees including range of motion (ROM), and the following were completed: the WOMAC, including Total, Pain, Stiffness, and Physical Function scores16; Evaluator and Patient Global Assessments; the OMERACT-OARSI Responder Index17; and the EuroQol.18 Subject diaries were also reviewed for concomitant medications and adverse events (AEs) at all follow-ups. Subject follow-up occurred at 1, 3, 6, 12, 18, and 26 weeks following the injection.
The WOMAC Pain score is composed of 5 questions, 4 of which assess pain after physical activities. The WOMAC Pain, Stiffness, and Physical Function subscales were developed in 1982 and have been fully validated.16 The Global Assessment is a single question answered on a 100-mm VAS: “Considering all the ways the osteoarthritis in your (study) knee affects you, what is your assessment of how much your knee is bothering you today?” which was answered by the subjects and the evaluators. The EuroQol (EQ-5D) questionnaire assesses overall health on a 100-mm VAS in which 0 = worst health imaginable and 100 = best health imaginable.18
The primary efficacy endpoint was the change from baseline in WOMAC Pain score through 12 weeks posttreatment comparing the Cingal group with the saline group. Secondary efficacy endpoints comparing Cingal with saline included changes in the OMERACT-OARSI Responder Index through 12 weeks, WOMAC Pain score through 26 weeks, and Evaluator and Patient Global Assessments through 12 and 26 weeks. Other secondary efficacy endpoints compared Cingal with Monovisc for changes at 1 and 3 weeks for the WOMAC Pain score, and Evaluator and Patient Global Assessments. Exploratory efficacy endpoints for Cingal versus saline through 26 weeks included Total WOMAC, and WOMAC Stiffness and Physical Function, OMERACT-OARSI Responders, EuroQol (EQ-5D) VAS Health Scale, rescue medication use, and ROM changes.
Safety was measured by the incidence, timing, severity, and relationship to the study medication for all AEs in the safety population (defined as all treated subjects). AEs were coded according to Medical Dictionary for Regulatory Activities (MedDRA).
Statistical Analysis
Sample size was calculated based on a difference in the mean responses between Cingal and saline of 10 mm (100-mm scale) based on previous trials of viscosupplements.19 Assuming 90% power to detect the difference between Cingal and saline at a 5% significance level and a 15% dropout rate, 147 subjects each should be enrolled in the Cingal and Monovisc groups and 74 in the saline group, for a total of 368 subjects.
Summary statistics and statistical analyses were performed using SAS software (version 9.4, Cary, NC). The analyses were based on data pooled across study centers, after poolability testing determined appropriateness. A significance level of 5% was used for all analyses.
Data were analyzed using analysis of variance, without covariates or other factors assumed in the model. Discrete variables, such as the OMERACT-OARSI score, were analyzed using a Fisher’s exact test. No adjustments for multiplicity were done.
All efficacy endpoints were analyzed using the intent to treat (ITT) population (all randomized subjects who received study treatment) and the multiple imputation methodology, which uses a mixed effects, repeated measures model to predict missing values. Secondary analyses were conducted on the per protocol (PP) population (all subjects who completed the 12-week visit and did not have major protocol violations).
Results
Subject Disposition and Demographics
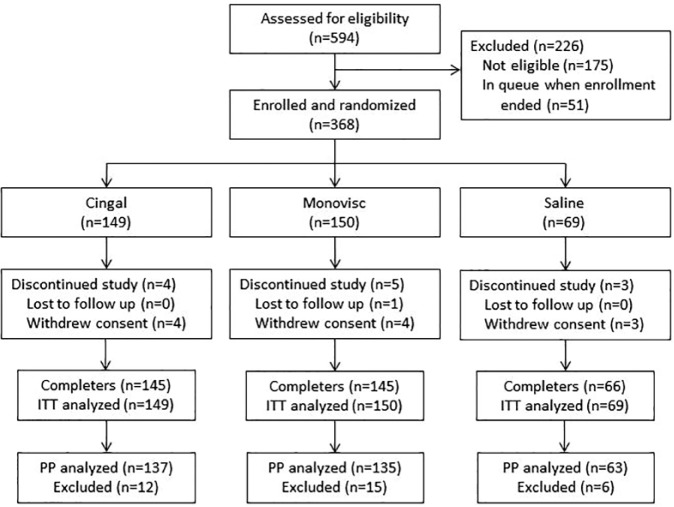
A total of 368 subjects were enrolled and randomized to Cingal (n = 149), Monovisc (n = 150), or saline (n = 69; Fig. 1 ). The safety and ITT populations were identical since all subjects received the study injection in accordance with randomization. Most subjects (n = 356; 96.7%) completed the study; 12 subjects did not complete the study ( Fig. 1 ). One subject was lost to follow-up and 11 subjects withdrew consent; no withdrawals were due to an AE ( Fig. 1 ). The PP population consisted of 335 (91.0%) subjects (Cingal [n = 137], Monovisc [n = 135], or saline [n = 63]); 33 subjects were excluded from the PP analysis ( Fig. 1 ). Most were excluded due to major protocol violation (n = 29) with others due to study withdrawal (n = 5); one subject who withdrew also had a major protocol violation.
Figure 1.
Patient disposition.
Demographic and baseline characteristics were not statistically significant different among the treatment groups ( Table 1 ). Subjects had a mean age of 58.3 years and a mean BMI of 28.7 kg/m2, most were Caucasian, and more than half (60%) had a K-L OA grade of II ( Table 1 ).
Table 1.
Demographic and Baseline Characteristics.
| Characteristic | Cingal (n = 149) | Monovisc (n = 150) | Saline (n = 69) | Combined (n = 368) | P value |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 57.5 ± 8.4 | 59.2 ± 8.6 | 58.0 ± 9.0 | 58.3 ± 8.6 | 0.2337 |
| Gender, n (%) | |||||
| Male | 52 (34.9) | 51 (34.0) | 18 (26.1) | 121 (32.9) | 0.4122 |
| Female | 97 (65.1) | 99 (66.0) | 51 (73.9) | 247 (67.1) | |
| Race, n (%) | |||||
| Caucasian | 149 (100) | 149 (99.3) | 69 (100) | 367 (99.7) | 1.0000 |
| Other | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.3) | |
| BMI, kg/m2, mean ± SD | 28.9 ± 4.7 | 28.4 ± 4.5 | 29.1 ± 4.5 | 28.7 ± 4.6 | 0.5047 |
| K-L OA grade, n (%) | |||||
| Grade I | 36 (24.2) | 24 (16.0) | 17 (24.6) | 77 (20.9) | |
| Grade II | 84 (56.4) | 98 (65.3) | 38 (55.1) | 220 (59.8) | |
| Grade III | 29 (19.4) | 27 (18.0) | 14 (20.3) | 70 (19.0) | |
| Grade IV | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.3) | |
| Baseline WOMAC Pain (index knee; mm) | 58.9 ± 12.3 | 61.0 ± 11.7 | 58.8 ± 10.6 | 59.7 ± 11.8 | 0.2252 |
| Baseline WOMAC Pain (contralateral knee; mm) | 11.5 ± 11.5 | 11.9 ± 12.7 | 10.3 ± 8.3 | 11.4 ± 11.5 | 0.6365 |
BMI = body mass index; K-L = Kellgren-Lawrence grade; OA = osteoarthritis; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Effects on Study Outcomes
The Cingal group had significantly lower WOMAC Pain scores than the saline group through 12 weeks (primary endpoint) and at each individual time point in the ITT population (except 6 weeks; Table 2 ; Fig. 2 ); similar results were found in the PP population. Changes from baseline to 12 weeks for Cingal versus saline were −40.2 mm versus −31.0 mm (P = 0.0099) in the ITT population and −40.3 mm versus −32.2 mm (P = 0.0029) in the PP population ( Table 3 ). The Cingal group also had significantly lower WOMAC Pain scores than the saline group through 26 weeks (P = 0.0072) in the ITT population, with a similar result in the PP population. Percent improvements from baseline in WOMAC Pain with Cingal, saline, and Monovisc were 70%, 52%, and 64%, respectively, at 12 weeks, and 72%, 56%, and 65%, respectively, at 26 weeks. The effect sizes of Cingal compared with saline for reduction in WOMAC Pain from baseline (calculated by the standardized mean difference) were 0.45 at 12 weeks and 0.41 at 26 weeks. Similar results were found for the ITT and PP populations.
Table 2.
WOMAC Pain Score: Change in from Baseline Over Time (ITT Population).
| Baseline, Mean ± SD (mm) | Difference from Baseline (mean ± SD, mm) |
||||||
|---|---|---|---|---|---|---|---|
| Week 1 | Week 3 | Week 6 | Week 12 | Week 18 | Week 26 | ||
| Cingal | 59.0 ± 12.3 | −34.6 ± 20.8 | −40.1 ± 20.1 | −40.5 ± 20.7 | −41.1 ± 20.5 | −40.5 ± 20.4 | −42.4 ± 18.7 |
| Monovisc | 61.0 ± 11.7 | −29.6 ± 21.4 | −34.9 ± 21.7 | −39.2 ± 20.1 | −39.0 ± 21.9 | −38.5 ± 23.8 | −39.5 ± 22.8 |
| Saline | 58.8 ± 10.6 | −26.6 ± 18.2 | −31.4 ± 18.8 | −35.5 ± 20.2 | −30.8 ± 23.7 | −31.4 ± 24.2 | −32.9 ± 23.6 |
| P value Cingal vs. saline | - | 0.0080 | 0.0039 | 0.0908 | 0.0013 | 0.0059 | 0.0027 |
| P value Cingal vs. Monovisc | - | 0.0367 | 0.0289 | 0.5572 | 0.4103 | 0.4452 | 0.2525 |
WOMAC = Western Ontario and McMaster Universities Arthritis Index; ITT = intent to treat.
Figure 2.
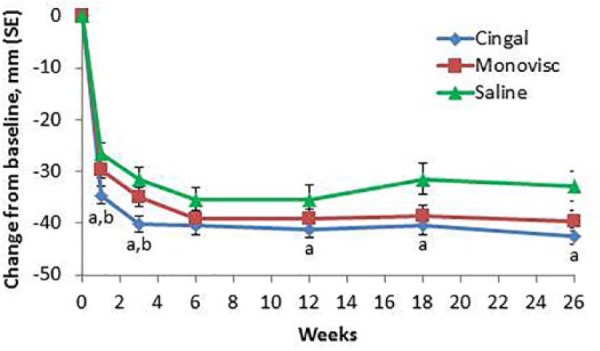
Mean changes from baseline for the WOMAC Pain score with Cingal, Monovisc, or saline over time in the ITT population. aP < 0.01 versus placebo; bP < 0.05 versus Monovisc.
Table 3.
Primary Endpoint Analysis: WOMAC Pain Score through 12 Weeks for Cingal versus Saline.
| Parameter | Treatment | Mean Improvement from BL (mm) | Mean Difference Cingal vs. Saline (mm) | 95% Confidence Interval | P Value* |
|---|---|---|---|---|---|
| ITT population | Cingal | −40.2 | −9.1 | −15.2, −3.1 | 0.0099 |
| Saline | −31.0 | ||||
| PP population | Cingal | −40.3 | −8.1 | −13.2, −3.0 | 0.0029 |
| Saline | -32.2 |
WOMAC = Western Ontario and McMaster Universities Arthritis Index; ITT = intent to treat; PP = per protocol.
P value for mean difference between Cingal and saline.
Cingal was also superior to Monovisc for pain relief at 1 and 3 weeks. The absolute differences in WOMAC Pain from baseline over time are shown in Figure 2 . Percent improvements from baseline in WOMAC Pain with Cingal, saline, or Monovisc were 59%, 45%, and 49%, respectively, at 1 week, and 68%, 53%, and 57%, respectively, at 3 weeks.
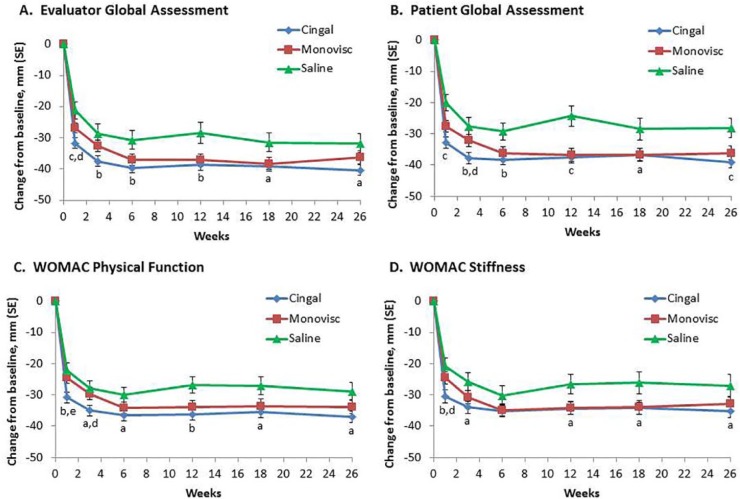
Cingal was superior to saline at most time points and to Monovisc at 1 and 3 weeks for changes from baseline for Global Assessments, and WOMAC Stiffness and Physical Function ( Fig. 3 ), as well as in the EuroQol. From 6 weeks until the end of the study, outcomes with Cingal and Monovisc were similar. The OMERACT-OARSI Responder Index also showed significantly more responders with Cingal versus saline at 1 week (89% vs. 75%; P = 0.0161), which was maintained until 26 weeks (93% vs. 84%; P = 0.0463), except at 3 weeks. While significantly more responders were observed with Cingal versus Monovisc at 1 week (89% vs. 79%; P = 0.0280) and 3 weeks (92% vs. 83%; P = 0.0340), similar percentages of responders were found starting at 6 weeks up until 26 weeks. In addition, Cingal demonstrated superiority over saline in most other secondary and exploratory endpoints (P < 0.05), and over Monovisc at 1 and 3 weeks for most secondary endpoints (P < 0.05), in the ITT and PP populations.
Figure 3.
Mean changes from baseline for Evaluator (A) and Patient (B) Global Assessments and WOMAC Physical Function (C) and Stiffness (D) with Cingal, Monovisc, or saline over time in the ITT population. aP < 0.05, bP < 0.01, cP < 0.001 versus placebo; dP < 0.05, eP < 0.01 versus Monovisc.
Safety
Twenty-three percent of subjects overall experienced an AE: 24.2% with Cingal, 24.7% with Monovisc, and 17.4% with saline. There were no statistically significant differences among the groups for type or rate of events. The most common AEs were headache (15.7%), arthralgia (12.9%), spinal pain (8.3%), back pain (6.0%), and nasopharyngitis (5.1%). Over 99% of AEs were considered mild or moderate in severity.
Six AEs were related to study medication: arthralgia with Cingal (n = 2) and Monovisc (n = 2), peripheral edema with Cingal (n = 1), and rash with Monovisc (n = 1). All related AEs resolved without sequelae. Five serious AEs were reported in 4 subjects (Cingal [n = 2], saline [n = 2]). None of the serious AEs were considered related to treatment and resolved without sequelae. No deaths occurred during the study.
Discussion
We report on the safety and efficacy of Cingal, a novel treatment for the pain and symptoms of knee OA, which combines cross-linked HA from the commercial viscosupplement, Monovisc, with an established corticosteroid, TH. This combination of HA and TH resulted in both rapid and long-term effects, as shown by significantly better pain reduction and functional improvement compared with saline as early as 1 and 3 weeks after injection, which were maintained until 26 weeks. In addition, the effectiveness of Cingal is apparent in its broad success for improving primary and secondary endpoints, including pain and function, as well as both physician- and patient-assessed outcomes.
Results from this study also demonstrate a degree of benefit with Cingal that is greater than that reported in prior viscosupplement studies,6,19-21 including the 72% improvement in WOMAC Pain at 26 weeks and 92% proportion of OMERACT-OARSI responders through 26 weeks. In addition, the rapid pain relief provided by Cingal, demonstrated by a 58% improvement in WOMAC Pain at 1 week and 67% improvement at 3 weeks, is unmatched compared with prior trials of TH alone (20 mg) in knee OA.22,23
Cingal also verified its design rationale, demonstrating that the addition of a corticosteroid to HA provided significant early pain relief over the first few weeks relative to Monovisc. This finding is similar to those of other studies in which a corticosteroid was added to an HA product, showing that the corticosteroid provided an advantage of more immediate pain relief while patients waited for the onset of pain relief from the HA.12-14 A separate meta-analysis examining randomized, clinical trials of intraarticular HA versus placebo found that HA starts to become efficacious over several weeks.11
The clinical significance of Cingal’s effect on OA knee pain is apparent by its rapid response, its consistency of effect across all outcome measures, and other factors. The earlier onset of pain relief (statistically significant changes of WOMAC pain at 1 and 3 weeks) with the addition of TH to HA in Cingal demonstrates the product’s clinical meaningfulness. The advantage of pain reduction with Cingal over Monovisc was a 17% relative improvement at 1 week and 15% relative improvement at 3 weeks.
The significance of the shorter term pain relief with Cingal is also supported by the consistency of significant improvements in WOMAC Pain, Function and Stiffness and Total WOMAC and at 1 and 3 weeks (except stiffness at 1 week), as well as the Evaluator and Patient Global Assessments. In addition, the short-term pain relief gained with TH in Cingal can be considered clinically significant when factors to elucidate clinical significance in pain clinical trials, as recommended by IMMPACT,24 are taken into account, including statistical significance of the primary efficacy analysis, magnitude of improvement in the primary efficacy outcome, responder analysis results, treatment effect size, rapidity of onset of treatment benefit, results from secondary efficacy endpoint, and convenience. Last, clinical meaningfulness can also be directly shown by the OMERACT-OARSI Responder Index results, which showed a significantly higher number of responders with Cingal versus Monovisc at both 1 and 3 weeks.
Safety of Cingal in this study was demonstrated by a low incidence of AEs (n = 6), including those related to treatment, which all resolved without sequelae. In addition, no serious AEs were considered related to Cingal.
One of the limitations of our study was that follow-up ended at 26 weeks. Since Cingal achieved its largest improvement in WOMAC Pain at 26 weeks, further investigation is warranted into the benefits over longer timeframes. Another limitation was that saline demonstrated a marked impact, which was consistent with that seen in other published knee OA viscosupplementation studies,6 possibly due to the effects of joint lavage and cleansing of joint debris.25 Despite this, however, Cingal demonstrated statistical and clinical superiority over saline in most efficacy measures for both ITT and PP populations, and across measurements of pain and symptoms, including stiffness and physical function.
In conclusion, the results of our study confirm the safety and effectiveness of a single intraarticular injection of Cingal for rapid and long-term relief of joint pain and symptoms in patients with OA of the knee.
Footnotes
Acknowledgments and Funding: The authors acknowledge the contribution to the conduct of the study from the following participating investigators, who contributed to the patient recruitment and data collection: Valentin Stoyanov (Department of Orthopaedics and Traumatology, Multiprofile Hospital for Active Treatment, Lyulin Hospital, Sofia, Bulgaria); Timothy Deakon (Oakville Sports Injury Clinic, Oakville, Ontario, Canada); Ivan Wong (QEII Health Sciences Centre, Halifax, Nova Scotia, Canada); Vasil Yablanski (Tokuda Hospital, Sofia, Bulgaria); Vladimir Stavrev (Multiprofile Hospital for Active Treatment “Sveti Georgi,” Plovdiv, Bulgaria); Plamen Kinov (Multiprofile Hospital for Active Treatment “Tsaritsa Yoanna,” Sofia, Bulgaria); Olga Sleglova (Institute of Rheumatology, Prague, Czech Republic); Rene Moster (Revmacentrum MUDr. Mostera, s.r.o., Brno, Czech Republic); Gyorgy Gruber (G&V Pharma-Med Bt., Mako, Hungary); Andras Kortvelyessy (Medidea Bt., Kiskunfélegyháza, Hungary); Artur Gadek (CenterMed Kraków Sp. z.o.o., Krakow, Poland); Artur Racewicz (Osteo-Medic s.c., Białystok, Poland). The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study sponsor, Anika Therapeutics Inc., funded the clinical study, participated in the study design, and assisted in the drafting of the article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Laszlo Hangody and Gary Stevens have been paid consultants for Anika Therapeutics, Inc. but received no direct funding for this work. The other authors report no conflicts of interest.
Ethical Approval: Ethical approval for this study was obtained from the Office of Health Authorization and Administrative Procedures, Department of Medical Devices, Budapest, Hungary (reference number: 30006/2013/OTIG).
Informed Consent: Written informed consent was obtained from all subjects before the study.
References
- 1. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60:3546-53. [DOI] [PubMed] [Google Scholar]
- 3. Conaghan PG, Kloppenburg M, Schett G, Bijlsma JW; EULAR Osteoarthritis Ad Hoc Committee. Osteoarthritis research priorities: a report from a EULAR ad hoc expert committee. Ann Rheum Dis. 2014;73:1442-5. [DOI] [PubMed] [Google Scholar]
- 4. Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanism of action. Arthritis Res Ther. 2003;5:54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stitik TP, Levy JA. Viscosupplementation (biosupplementation) for osteoarthritis. Am J Phys Med Rehabil. 2006;85(Suppl):S32-S50. [DOI] [PubMed] [Google Scholar]
- 6. Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-5. [DOI] [PubMed] [Google Scholar]
- 9. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363-88. [DOI] [PubMed] [Google Scholar]
- 10. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005328. [DOI] [PubMed] [Google Scholar]
- 11. Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis—meta-analysis. Osteoarthritis Cartilage. 2011;19:611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Campos GC, Rezende MU, Pailo AF, Frucchi R, Camargo OP. Adding triamcinolone improves viscosupplementation: a randomized clinical trial. Clin Orthop Relat Res. 2013;471:613-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozturk C, Atamaz F, Hepguler S, Argin M, Arkun R. The safety and efficacy of intraarticular hyaluronan with/without corticosteroid in knee osteoarthritis: 1-year, single-blind, randomized study. Rheumatol Int. 2006;26:314-9. [DOI] [PubMed] [Google Scholar]
- 14. Caborn D, Rush J, Lanzer W, Parenti D, Murray C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31:333-43. [PubMed] [Google Scholar]
- 15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-40. [PubMed] [Google Scholar]
- 17. Pham T, Van Der Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30:1648-54. [PubMed] [Google Scholar]
- 18. Fransen M, Edmonds J. Reliability and validity of the EuroQol in patients with osteoarthritis of the knee. Rheumatology (Oxford). 1999;38:807-13. [DOI] [PubMed] [Google Scholar]
- 19. Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20:350-6. [DOI] [PubMed] [Google Scholar]
- 20. Karlsson J, Sjogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology. 2002;41:1240-8. [DOI] [PubMed] [Google Scholar]
- 21. Neustadt D, Caldwell J, Bell M, Wade J, Gimbel J. Clinical effects of intraarticular injection of high molecular weight hyaluronan (Orthovisc) in osteoarthritis of the knee: a randomized, controlled, multicenter trial. J Rheumatol. 2005;32:1928-36. [PubMed] [Google Scholar]
- 22. Pyne D, Ioannou Y, Mootoo R, Bhanji A. Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone hexacetonide and methylprednisolone acetate. Clin Rheumatol. 2004;23:116-20. [DOI] [PubMed] [Google Scholar]
- 23. Frias G, Caracuel MA, Escudero A, Rumbao J, Perez-Gujo V, del Carmen CM, et al. Assessment of the efficacy of joint lavage versus joint lavage plus corticoids in patients with osteoarthritis of the knee. Curr Med Res Opin. 2004;20:861-7. [DOI] [PubMed] [Google Scholar]
- 24. Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238-44. [DOI] [PubMed] [Google Scholar]
- 25. Rosseland LA, Helgesen KG, Breivik H, Stubhaug A. Moderate-to-severe pain after knee arthroscopy is relieved by intraarticular saline: a randomized controlled trial. Anesth Analg. 2004;98:1546-51. [DOI] [PubMed] [Google Scholar]