Abstract
Objectives
During arthroscopic or open joint surgery, articular cartilage may be subjected to mechanical insults by accident or design. These may lead to chondrocyte death, cartilage breakdown and posttraumatic osteoarthritis. We have shown that increasing osmolarity of routinely used normal saline protected chondrocytes against injuries that may occur during orthopedic surgery. Often several liters of irrigation fluid are used during an orthopedic procedure, which is usually kept at room temperature, but is sometimes chilled. Here, we compared the effect of normal and hyperosmolar saline solution at different temperatures on chondrocyte viability following cartilage injury using in vitro and in vivo models of scalpel-induced injury.
Design
Cartilage injury was induced in bovine osteochondral explants and the patellar groove of rats in vivo by a single pass of a scalpel blade in the presence of normal saline (300 mOsm) or hyperosmolar saline solution (600 mOsm, sucrose addition) at 4°C, 21°C, or 37°C. Chondrocytes were fluorescently labeled and visualized by confocal microscopy to assess cell death.
Results
Hyperosmolar saline reduced scalpel-induced chondrocyte death in both bovine and rat cartilage by ~50% at all temperatures studied (4°C, 21°C, 37°C; P < 0.05). Raising temperature of both irrigation solutions to 37°C reduced scalpel-induced cell death (P < 0.05).
Conclusions
Increasing the osmolarity of normal saline and raising the temperature of the irrigation solutions to 37°C reduced chondrocyte death associated with scalpel-induced injury in both in vitro and in vivo cartilage injury models. A hyperosmolar saline irrigation solution at 37°C may protect cartilage by decreasing the risk of chondrocyte death during mechanical injury.
Keywords: cartilage, injury, chondroprotection, chondrocyte, viability, irrigation
Introduction
Articular cartilage has an extraordinary durability and capacity to adapt to the physiological loading patterns associated with normal joint activities.1 However, exposing the tissue to unphysiological mechanical insults can result in chondrocyte death, potentially rendering articular cartilage more vulnerable to degeneration and development of posttraumatic osteoarthritis (PTOA).2 Arthroscopic and open surgical interventions on articular cartilage may subject the tissue to various iatrogenic injuries resulting from cutting, probing, or drilling.3,4 Such injuries may be associated with loss of chondrocyte viability, cartilage damage, and poor lateral integration and therefore potentially affect the surgical outcome.5 Minimizing chondrocyte death resulting from iatrogenic surgical injuries would thus be beneficial in reducing cartilage damage, promote the repair response, and improve integrative healing and patient outcome.5
Various irrigation solutions are used in orthopedic surgical practice to improve visualization of the joint space and cartilage surface. During this process, the synovial fluid is replaced with an artificial solution that has a lower osmotic pressure (~250-300 mOsm) compared with that of normal synovial fluid (~400 mOsm).6 Therefore, chondrocytes are exposed to a marked decrease in extracellular osmolarity during articular surgery, which is likely to affect their response to injury.7 For example, lowering the osmolarity of the irrigation solution increased cell death caused by mechanical cartilage injury however raising osmolarity provided significant chondroprotection against mechanical trauma caused by drilling,4 impact,7 or scalpel cutting.8 Using an in vivo model of scalpel-induced cartilage injury, we have shown that irrigating the joint with hyperosmotic saline solution significantly decreased the extent of chondrocyte death and promoted a cartilage repair response with no harmful effect on other joint tissues.9 Of interest is the observation that in an in vitro cartilage injury model at the site of the scalpel wound, the abnormal morphology of chondrocytes may be normalized by hyperosmolarity.10 In addition, protection of chondrocytes against the exposure of cartilage to static11 or moving air12 has been demonstrated using various hydrating solutions.
Temperature is an important factor that affects cell viability and metabolism13,14; however, the optimum temperature of the irrigation solution used in joint surgery has not yet been reported. Often a high volume (multiple liters) of irrigation fluid is used during an arthroscopic procedure, this is usually kept at room temperature, but is sometimes chilled as cold solutions have been proposed to reduce postoperative pain and inflammation after arthroscopic surgery.15 However, no statistically or clinically significant effect was found in the first 4 postoperative days.16 Moreover, an in vivo study investigating the ultrastructural surface changes of articular cartilage in rat knee joints using scanning electron microscopy, showed an uneven cartilage surface and fibrillation in joints irrigated by saline solution at 4°C and a normal, even, cartilage surface in joints flushed with saline at 37°C.17 It has also been demonstrated that chondrocyte metabolism and RNA synthesis were suppressed on exposure to cold saline solution.14
The aim of this study was therefore to investigate the effect of a normal and hyperosmotic (potentially chondroprotective) saline solution at different temperatures on chondrocyte viability in carefully controlled in vitro and in vivo scalpel-induced models of cartilage injury.
Materials and methods
In Vitro Cartilage Injury Model
Bovine osteochondral strips were harvested from the metacarpophalangeal joints of 3-year old cows ( Fig. 1A ) under aseptic conditions. These were then trimmed to produce rectangular explants of approx. 5 mm × 4 mm,8 immediately placed in serum-free Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Paisley, UK) and used within 30 minutes. The serum-free medium was used to provide more controlled environment for the cells and to eliminate the variance that may occur due to variability of serum composition. The osteochondral explants were immersed for 5 minutes in either normal saline (NaCl 300 mOsm, Baxter Healthcare Ltd, Berkshire, UK) or hyperosmolar saline solution (600 mOsm, 105 g sucrose addition to 1 L normal saline)8,9 and maintained at 4°C, 21°C, or 37°C. In this study, sucrose was the preferred osmolyte as it is impermeable, not metabolised by chondrocytes, relatively benign and thus one experimental variable. The addition of NaCl to raise osmolarity on the other hand can potentially alter a range of processes including the activity of sodium-dependent transport processes and membrane potential, and thus it would be difficult to separate out the effects of osmolarity from sodium-dependent effects.9
Figure 1.
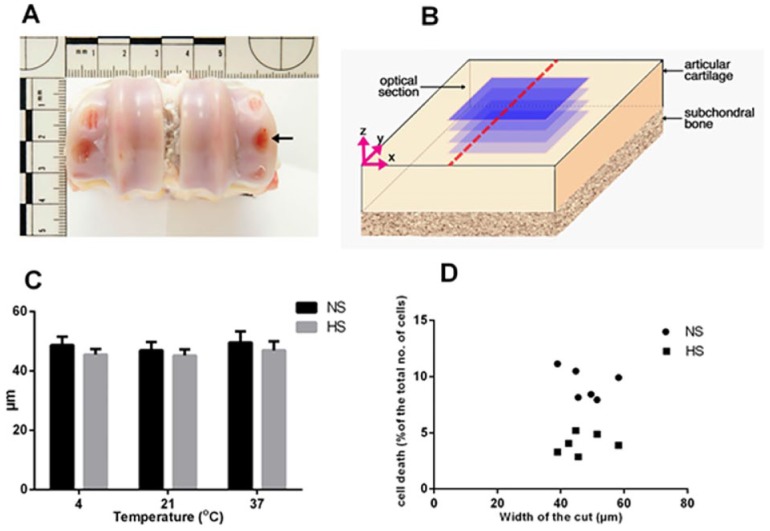
Generation and validation of an in vitro model of scalpel injury. (A) Bovine metacarpophalangeal joint with areas of exposed bone where osteochondral explants were removed (arrow). (B) Schematic drawing of the scalpel cut (broken red line) and the optical sections in blue, each acquired at 10-µm intervals. (C) Measurement of the cut width in osteochondral bovine explants exposed to normal saline solution (NS) or hyperosmolar saline solution (HS) at the indicated temperatures. Data are expressed as mean ± SEM, [6(36)]. (D) Scatterplots of percentage cell death in different explants against the width of the scalpel-induced cut in bovine osteochondral explants irrigated with either normal saline or hyperosmotic saline at 21°C.
The osmolarity of the solutions was measured by freezing point osmometer (Vitech Scientific Ltd, Horsham, UK). Explants were wounded with fresh No. 11 scalpel blades to produce a single partial thickness longitudinal cut in the middle of explant ( Fig. 1B ) and then returned to the designated solution for a further 5 minutes after injury before being assessed for cell viability.8 Control uninjured explants were exposed to normal or hyperosmolar saline solution at 4°C, 21°C, or 37°C for 10 minutes before staining and fixation for microscopic analysis to determine the effect of raised osmolarity and temperature of the irrigation solution on chondrocyte viability.
In Vivo Scalpel Injury Model
Injury to the rat knee joint cartilage was induced as previously described.9 Briefly, male Sprague-Dawley rats (8 weeks old) were anesthetized using 3% isoflurane. The patella was dislocated laterally after medial parapatellar arthrotomy to expose the patellar groove. The articular cartilage was then wounded along the groove by a single pass of a fresh No. 11 scalpel blade held in a standard holder, and applied under its own weight. Joints were irrigated for 5 minutes before, and 5 minutes after the induction of the cartilage injury by normal saline (300 mOsm) or hyperosmolar saline solution (600 mOsm) at 4°C, 21°C, or 37°C to allow the in situ chondrocytes to respond to altered osmolarity18 (6 joints from separate animals were used in each group). Note that the open joint area was irrigated with the test solutions, and that a true lavage involving the closed joint with fluid applied under pressure was not performed. The patella was then relocated and the wound sutured in layers with coated vicryl 6-0 (polyglactin 910, Ethicon, Livingston, UK). Animals were sacrificed immediately after surgery, and knee joints were dissected and assessed for chondrocyte viability. Contralateral joints were subjected to arthrotomy and patellar dislocation without inducing cartilage injury (sham-operated) and irrigated with either normal or hyperosmolar saline solution at 4°C, 21°C, or 37°C for 10 minutes before dissection and staining. All procedures were approved by the Local Ethics Committee and Animals (Scientific Procedures) Act 1986 UK Home Office.
Cell Viability Assay
In situ chondrocyte viability was determined by standard methods.8,9 Briefly, bovine osteochondral explants or rat knee joints were incubated with 5-chloromethyl-fluorescein diacetate (CMFDA) and propidium iodide (PI) (1 hour; both 10 µmol/L, Invitrogen, Paisley, UK) to label live/dead cells respectively. Following labeling, samples were fixed in 4% formalin (Fisher Scientific, Loughborough, UK) overnight and then stored in phosphate buffered saline (PBS) at 4°C prior to confocal imaging.
Cartilage Imaging by Confocal Laser Scanning Microscopy
Fluorescently labeled and fixed osteochondral explants and rat knee joints were anchored to the base of a Petri dish with Blu-Tack (Bostik Ltd, Leicester, UK), immersed in PBS with the articular surface facing upward. Consecutive series of axial optical sections of the labeled in situ chondrocytes were acquired using a Zeiss Axioskop LSM510 confocal laser scanning microscope (CLSM; Carl Zeiss Ltd, Cambridge, UK) at 10-µm intervals ( Fig. 1B ). A standard multitrack protocol involving excitation by argon (excitation wavelength = 488 nm) and helium-neon (excitation wavelength = 543 nm) lasers, with band-pass filters (500-550 nm) and long-pass filters (>560 nm) was used to detect fluorescence emitted from CMFDA and PI and therefore allow the visualization of live and dead cells, respectively, within each optical section. Laser power, detector gain, and sensitivity were manually adjusted before acquiring each image to capture the optimal signal from each section without pixel saturation. A 3-dimensional reconstruction of all optical sections was then created using imaging software (Velocity 4·0, UK). The region of interest (ROI) applied for the in vivo experiments was 200 µm from the center of the scalpel injury (x-axis) × 921 µm (y-axis) × 40 µm (z-axis), whereas for the in vitro experiments the ROI was 150 × 921 × 60 µm. The quantification of in situ chondrocyte death after injury was then determined within these ROIs ( Fig. 1B ).
Live and dead cells were identified in the green and red channels, respectively, by thresholding voxel (volumetric pixel) intensity. A histogram of measured values for all objects in each channel was used to set the percentage threshold for the intensity (upper limit =100%; lower limit >5%) and the percentage cell death (PCD = 100 × number of dead cells/number of dead and live cells) calculated in the ROI. The width of the injury was measured by LSM imaging software (Carl Zeiss Ltd) at 100-µm intervals along the y-axis using the CLSM images. In all samples, 10 widths per projection were taken to calculate the mean width.
Statistical Analysis
Data analyses were performed using GraphPad Prism (version 6.0, GraphPad Software, Inc., San Diego, CA, USA). All data were presented as mean ± standard error of mean (SEM) where N refers to the number of individual knee joints analyzed from separate animals and n to the total number of bovine explants with data shown as [N(n)]. Pearson product-moment correlation was used to test the relation between the cut width and cell death and 1-way analysis of variance (ANOVA) followed by a post hoc Holm Sidak correction were used to compare normal or hyperosmolar saline solution groups across all temperatures. Differences were considered statistically significant when P < 0.05.
Results
Assessment of Chondrocyte Viability Following Exposure to Varying Osmolarities and Temperatures
Control uninjured bovine osteochondral explants and sham-operated rat knee joints were irrigated with either normal or hyperosmolar saline solution at 4°C, 21°C, or 37°C to evaluate the effect of increasing osmolarity and temperature on cell viability. The PCD in articular cartilage was negligible (<1%) under all conditions in both in vitro and in vivo experiments (see Fig. 2B ).
Figure 2.
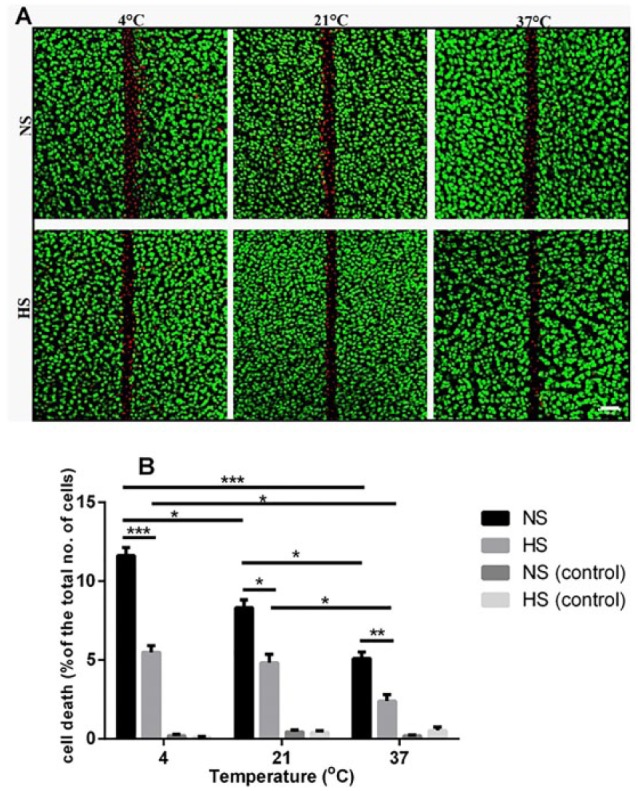
Effect of the hyperosmolar saline solution on chondrocyte death in injured osteochondral explants at different temperatures in vitro. (A) Axial confocal laser scanning microscopy (CLSM) reconstructions of 5-chloromethyl-fluorescein diacetate (CMFDA) and propidium iodode (PI) labeled chondrocytes in injured bovine explants exposed to normal saline solution (NS) or hyperosmolar saline solution (HS) at the indicated temperatures. Scale bar = 50 µm. (B) Pooled data for percentage cell death of explants exposed to normal saline (NS) or hyperosmolar saline solution (HS) at 4°C, 21°C, or 37°C. Data are expressed as mean ± SEM, [5(30)] for each data set. *P < 0.05, **P < 0.01, ***P < 0.001.
Development of an In Vitro Cartilage Injury Model
Cartilage injury in osteochondral bovine explants was induced by fresh scalpel blades to produce a single longitudinal partial thickness cut in the middle of explant ( Fig. 1A and B ). To assess the reproducibility of the cut across all the experimental conditions, the mean width was calculated from 10 measurements taken at 100-μm intervals. The width of the defect was 47.1 ± 6.5 µm (mean ± SD; n = 36) with no significant difference between the groups (P > 0.05; Fig. 1C ). Within this range, there was no significant correlation between the cut width and cell death (P > 0.05; Fig. 1D ) for samples irrigated in either normal or hyperosmolar saline. This suggested that this standardized injury produced a reproducible zone of cell death and therefore would allow further assessment of the effect of solution osmolarity and temperature on chondrocyte viability.
Hyperosmolarity and Physiological Temperature Reduce Chondrocyte Death due to Scalpel-Induced Injury in Bovine Cartilage
Axial CLSM projection images showed a band of cell death at/around the edges of scalpel-induced injury in bovine osteochondral explants ( Fig. 2A ). Exposing cartilage explants to hyperosmolar saline solution significantly (P < 0.001, P < 0.05, P < 0.01 for 4°C, 21°C, and 37°C, respectively) reduced cell death by approximately 50% within the specified ROI in injured explants compared with normal saline solution at all temperatures 4°C: 11.6% ± 0.2% (saline), 5.4% ± 0.4% (hyperosmolar); 21°C: 8.3% ± 0.4% (saline), 4.8% ± 0.2% (hyperosmolar); 37°C: 5.1% ± 0.3% (saline), 2.3% ± 0.1% (hyperosmolar) ( Fig. 2B ). Increasing the temperature of the normal saline solution from 4°C to 21°C resulted in 1.4-fold reduction in the PCD (P < 0.05) and warming to 37°C further reduced the PCD associated with scalpel-induced injury compared with 21°C (P < 0.05). The overall protection by raising temperature from 4°C to 37°C for saline irrigation was by approximately 2.3-fold (P < 0.001; Fig. 2B ). Similarly, raising the temperature of the hyperosmolar saline solution to 37°C significantly decreased PCD by 2.4-fold compared with 4°C (P < 0.05 by ANOVA) ( Fig. 2B ). It should be noted that in control (uninjured) cartilage explants, there was no significant effect of either the normal saline solution or hyperosmotic saline solution on chondrocyte viability and no significant difference between these solutions ( Fig. 2B ). Thus overall, the use of the hyperosmotic irrigating solution at 37°C in the in vitro injury model decreased the PCD by about 5-fold compared with the PCD present with saline irrigation solution at 4°C.
The Chondroprotective Effect of Hyperosmolar Saline Solution and Increased Temperature in an In Vivo Model of Cartilage Injury
The PCD in the specified ROI used for the in vivo sham-operated joints irrigated at different osmotic pressures and temperatures was negligible (PCD = 0.45% ± 0.12% [6(36)]) under all conditions. Analysis of the CLSM images of injured cartilage showed approximately 50% reduction of chondrocyte death in joints irrigated with hyperosmolar saline solution compared with normal saline solution at all temperatures [4°C: 20% ± 0.4% (saline), 10.7% ± 1.9% (hyperosmolar saline solution); 21°C: 19% ± 0.9% (saline), 11% ± 0.3% (hyperosmolar saline solution); 37°C: 14% ± 1.1% (saline), 7% ± 1.0% (hyperosmolar saline solution) (P < 0.001 for each pair) ( Fig. 3 )]. Irrigation of the joints prior to and after injury with warm (37°C) saline solution significantly reduced cell death by ~30% compared with cold saline solution (4°C) or normal saline kept at 21°C (P < 0.001) ( Fig. 3B ).
Figure 3.
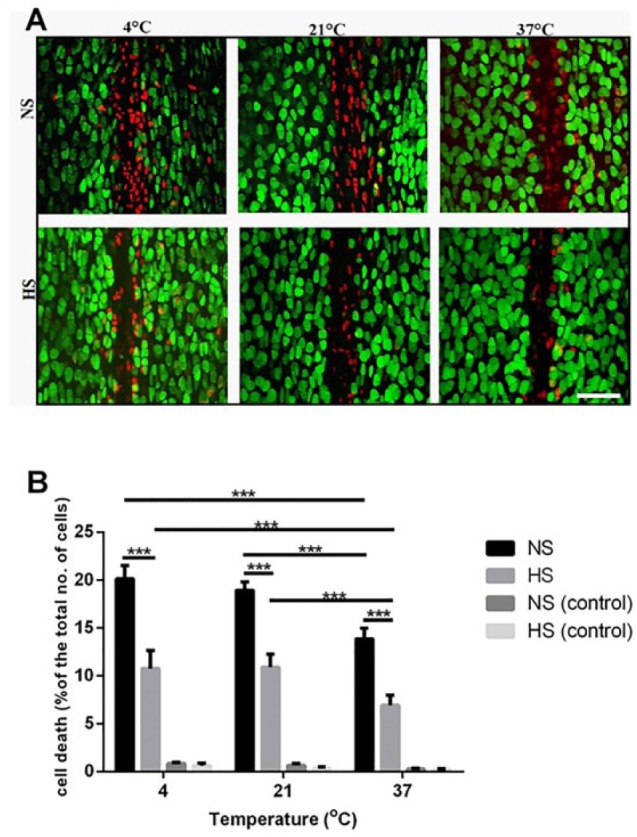
The chondroprotective effect of hyperosmotic saline solution and increased irrigation temperature in an in vivo model of scalpel-induced cartilage injury (A) Axial confocal laser scanning microscopy (CLSM) projection images of fluorescently labeled chondrocytes in injured cartilage exposed to normal saline solution (NS) or hyperosmolar saline solution (HS) at the indicated temperatures. Scale bar = 50µm. (B) The percentage of cell death in injured and uninjured control joints irrigated with hyperosmolar saline solution or normal saline solution at 4°C, 21°C, or 37°C. Data are expressed as mean ± SEM, [6(12)] for each data set. *P < 0.05, **P < 0.01, ***P < 0.001.
Raising the temperature of the hyperosmolar saline solution to 37°C also significantly decreased the PCD in injured joints compared with this solution maintained at either 4°C (P < 0.001) or 21°C (P < 0.001) ( Fig. 3B ). It is notable that in control (uninjured) in vivo cartilage, there was no significant effect of either the normal saline solution or hyperosmotic saline solution on chondrocyte viability and no significant difference between these solutions ( Fig. 2B ). In summary, in this in vivo model of cartilage injury, the use of the hyperosmotic irrigating solution at 37°C decreased the PCD by approximately 3-fold compared with that present with saline irrigation solution at 4°C.
Discussion
An isotonic NaCl saline solution at room temperature is commonly used for joint irrigation during orthopedic and arthroscopic procedures to provide a clear bloodless surgical field. However, this is likely to alter the temperature and extracellular osmolarity of in situ chondrocytes and may increase their sensitivity to mechanical trauma8 and adversely affect cartilage metabolism.14,19 Here, we investigated the effect of increasing osmolarity and temperature of a normal standard saline irrigation solution on chondrocyte viability using in vitro and in vivo models of cartilage injury. Irrigation with hyperosmolar saline solution significantly reduced cell death following scalpel-induced injury in both in vitro bovine and in vivo rat articular cartilage by approximately 50% at all temperatures examined. In addition, increasing the temperature of both irrigation solutions to 37°C significantly reduced cell death after mechanical injury. This suggests that in response to mechanical perturbation of articular cartilage, warm (37°C) hyperosmolar saline is chondroprotective compared with standard irrigation with normal saline at room (21°C) or cold (4°C) temperature.
In the in vitro experiments, a reproducible single linear cut was induced in osteochondral bovine cartilage explants using fresh scalpel blades. This minimized the variation in applied force and led to a consistent amount of cell death that was not correlated with minor variations in the width of the cut ( Fig. 1C and D ). This meant that the effect of altering solution temperature and osmolarity on cell death could be studied with greater sensitivity and reproducibility. Similarly, a validated in vivo model of scalpel-induced cartilage injury was chosen to represent partial thickness sharp mechanical trauma to articular cartilage.9
Irrigating healthy uninjured articular cartilage with either normal or hyperosmolar saline solution at 4°C, 21°C, or 37°C did not affect cell viability in either in vitro or in vivo models ( Figs. 2B and 3B ). However, following sharp mechanical trauma to bovine and rat articular cartilage, chondrocyte death was significantly influenced by both changes in the osmolarity and temperature of the irrigation solution. The PCD present in the hyperosmolar solution at 37°C could be reduced by over 5-fold in vitro ( Fig. 2B ) and by approximately 3-fold in vivo ( Fig. 3B ) compared with a normal saline irrigation at 4°C. For the ROI used for the in vivo experiments, it is estimated that approximately 20% of the joint’s cartilage surface was studied. The single scalpel injury occurring in the presence of normal saline at 4°C caused 20% cell death (i.e., 4% of the total joint surface) and this was reduced to 7% by hyperosmotic saline at 37°C (i.e., about 1.3% of the total joint surface).
It is important to note that although this study provided proof of principle that increasing the osmolarity and temperature of normal saline reduced chondrocyte death associated with scalpel-induced injury, it has certain limitations. We emphasise that the results obtained from our experimental model/procedures cannot be regarded as translational to those occurring during orthopedic surgery and additionally only a single outcome measure, that is, that of chondrocyte viability, was assessed. The cartilage was only briefly exposed to the different irrigation solutions to elucidate the immediate influence of varying osmolarity and temperatures on the extent of cell death following cartilage injury. Previous studies have established that chondrocytes sense and respond within 5 minutes.18 Thus, it is in the early phase following the addition of the chondroprotective solution possibly as a result of chondrocyte shrinkage7,18 that influences the extent of chondrocyte death following mechanical injury. Research using an in vivo animal model has shown that short-term exposure of articular cartilage to hyperosmolar solution (600 mOsm; 5 minutes before and 5 minutes after mechanical injury) influenced long-term chondrocyte viability and cartilage regeneration by matrix constituents.9 This significantly improved the cartilage repair score compared to normal saline (300 mOsm) irrigation and this is why this short-term exposure was used in our present work.
There have been relatively few studies on the effect of longer term exposure of intact articular cartilage to irrigation solutions of differing osmolarity and temperature, and the influence on other cartilage structural and biological properties, for example, gene expression, synthetic and catabolic activities remain to be evaluated. However, in a porcine osteochondral explant model, exposure to saline irrigation solution at room temperature had a detrimental effect on chondrocyte metabolism and RNA synthesis.14 In addition, a recent report using a canine shoulder arthroscopy model20 demonstrated no deleterious effect on chondrocyte viability or cartilage hydration after 2hrs of irrigation with a hyperosmolar saline (600 mOsm by raised NaCl). Clearly, further work on the influence of long-term exposure of chondroprotective solutions on cartilage properties, extracellular matrix metabolism, and chondrocyte viability is warranted.
It is likely that unless care is taken, normal joint temperature will be reduced during articular surgery as a result of irrigation solution temperature, the length of the surgical procedure and exposure to the ambient conditions of the operating theatre. For example, it has been demonstrated that intra-articular temperature decreases from ~35°C at the beginning of anterior cruciate ligament (ACL) reconstruction or menisectomy surgery to ~25°C toward the end of the procedure after about 1.5hrs.21 These changes might have detrimental effects on the structural, physiological and biomechanical properties of articular cartilage, and potentially increase the sensitivity of chondrocytes to mechanical injury. In addition, exposure of articular cartilage to cold saline significantly altered the ultrastructure of the articular surface.22 During thermal chondroplasty, the temperature of the lavage solution influenced chondrocyte viability and surface contouring.22 The use of cool lavage solution (22°C) led to a significant increase in the depth of chondrocyte death and roughening of articular surface compared to a 37°C solution.22 Thus, the use of warmed, rather than cool, irrigation solutions has the potential to limit these deleterious effects if used for joint lavage.
The mechanism underlying the chondroprotective effect of warmed hyperosmolar saline against mechanical injury is unclear. This could be due to changes in matrix mechanics and/or direct effects on chondrocytes or chondrocyte-matrix interactions rendering them less sensitive to scalpel-induced injury. Previous work has demonstrated that raised temperature increased the dynamic and equilibrium stiffness of cartilage23 and therefore at 37°C the extracellular matrix at the injury edge could be stiffer and more resistant to disruption when injured by the scalpel. Although raising osmolarity might have a similar effect, the injury width was identical for scalpel-induced trauma with both saline and hyperosmotic saline irrigation over the temperature range studied ( Fig. 1C ). It is probable that a more direct action of temperature and osmolarity on chondrocytes or chondrocyte-matrix interactions are more important than their effects on bulk properties of cartilage. Changes to osmolarity affect the sensitivity and viability of chondrocytes following exposure to injurious stimuli.7-9 Thus, raising the osmolarity surrounding cartilage will decrease chondrocyte volume,18 membrane transport activity24 and lead to cytoskeletal reorganization.25 Changes to chondrocyte volume can initiate intracellular signaling cascades, including regulation of gene expression,25 metabolic activity,19 and calcium concentration,26 with the latter potentially interacting with other pathways such as those that generate reactive oxygen species (ROS), which control chondrocyte viability.27 Reducing temperature also has a variety of effects directly on cells, including increased membrane viscosity and stability28 decreased permeability28 and partial disassembly of spindle microtubules and cortical microfilaments.29,30 It is notable that the chondrocyte death at the site of matrix disruption reported here ( Figs. 2 and 3 ) is similar to that observed following impact injury where cell death was localized around the cartilage cracks and not present in impacted areas without cracks.7,31 Indirect effects of reduced temperature and osmolarity on cell viability on the interactions between matrix proteins, chondrocyte integrins, and cytoplasmic elements may also be crucial as they are known to be essential for chondrocyte survival.32
In the short-term experiments reported here, warmed hyperosmolar saline was chondroprotective following mechanical injury. However, the long-term protection of cartilage integrity, stimulation of wound healing and the quality of the repair tissue remains to be investigated. Inhibiting chondrocyte death at the wound edge reduces matrix loss and enhances cartilage integration.33 We have previously shown that hyperosmotic lavage at room temperature resulted in a more developed repair tissue in vivo after 8 weeks compared with normal saline9 when applied before and after injury. It is therefore conceivable that the extra protection provided by warm irrigation solution may lead to further improvement in cartilage repair and lateral integration. Although this study used clearly defined and reproducible models of scalpel-induced injury, it is important to note that the nature, magnitude, and direction of any mechanical injury occurring during animal or human orthopedic surgery may be more complex and variable, with both blunt and sharp trauma arising from the use of various surgical tools such as osteotomes, pins, and screws. Consequently, the level of chondroprotection provided by raised osmolarity and temperature of the irrigation solution might differ from that reported here. The safety of a hyperosmolar saline solution in joint irrigation has been previously assessed in an in vivo rat model9 with no deleterious effects on any of the synovial joint soft tissues, for example, muscles, synovium, ligaments and menisci9 beyond those observed for normal saline irrigation. Thus, while irrigation with warmed hyperosmolar saline is a relatively simple chondroprotective procedure without adverse effect, further studies are needed to determine whether this solution is appropriate to preserve chondrocyte viability and promote cartilage repair following the injurious stimuli induced by other surgical instruments when applied to in vitro models or in vivo surgery.
This study provided evidence that in our animal models, increasing the osmolarity and temperature of the saline irrigation solution significantly reduced the extent of chondrocyte death associated with scalpel-induced injury. Furthermore, both the saline irrigation solution and the hyperosmolar solution had no detectable effects on the viability of uninjured cartilage. The use of this relatively simple and cheap hyperosmotic irrigation solution at 37°C may provide an effective strategy for conferring chondroprotection against mechanical injury both in vivo and in vitro.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Arthritis Research (UK) grant number 19665.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from the Local Ethics Committee and Animals (Scientific Procedures) Act 1986 UK Home Office (PPL 60/4052).
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
References
- 1. Urban JP. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994;33(10):901-8. [DOI] [PubMed] [Google Scholar]
- 2. Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43(3-4):517-21. [PubMed] [Google Scholar]
- 3. Vega J, Golanó P, Peña F. Iatrogenic articular cartilage injuries during ankle arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1304-10. doi: 10.1007/s00167-014-3237-5. [DOI] [PubMed] [Google Scholar]
- 4. Farhan-Alanie MM, Hall AC. Temperature changes and chondrocyte death during drilling in a bovine cartilage model and chondroprotection by modified irrigation solutions. Int Orthop. 2014;38(11):2407-12. [DOI] [PubMed] [Google Scholar]
- 5. Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12(3):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgarten M, Bloebaum RD, Ross SD, Campbell P, Sarmiento A. Normal human synovial fluid: osmolality and exercise-induced changes. J Bone Joint Surg Am. 1985;67(9):1336-9. [PubMed] [Google Scholar]
- 7. Bush PG, Hodkinson PD, Hamilton GL, Hall AC. Viability and volume of in situ bovine articular chondrocytes—changes following a single impact and effects of medium osmolarity. Osteoarthritis Cartilage 2005;13(1):54-65. [DOI] [PubMed] [Google Scholar]
- 8. Amin AK, Huntley JS, Bush PG, Simpson AH, Hall AC. Osmolarity influences chondrocyte death in wounded articular cartilage. J Bone Joint Surg Am. 2008;90(7):1531-42. [DOI] [PubMed] [Google Scholar]
- 9. Eltawil NM, Howie SE, Simpson AH, Amin AK, Hall AC. The use of hyperosmotic saline for chondroprotection: implications for orthopaedic surgery and cartilage repair. Osteoarthritis Cartilage. 2015; 23(3):469-77. [DOI] [PubMed] [Google Scholar]
- 10. Karim A, Hall AC. Hyperosmolarity normalizes serum-induced changes to chondrocyte properties in a model of cartilage injury. Eur Cell Mater. 2016;31:205-20. [DOI] [PubMed] [Google Scholar]
- 11. Farr J, Mathew LM, Stoker AM, Sherman SL, Cook JL. Effects on exposed articular cartilage during open surgical procedures: a comparison of various fluids in an animal model. Arthroscopy. 2015;31(1):113-7. [DOI] [PubMed] [Google Scholar]
- 12. Paterson SI, Amin AK, Hall AC. Airflow accelerates bovine and human articular cartilage drying and chondrocyte death. Osteoarthritis Cartilage. 2015;23:257-65. [DOI] [PubMed] [Google Scholar]
- 13. Alibegović A, Balažic J, Petrovič D, Hribar G, Blagus R, Drobnič M. Viability of human articular chondrocytes harvested postmortem: changes with time and temperature of in vitro culture conditions. J Forensic Sci. 2014;59(2):522-8. [DOI] [PubMed] [Google Scholar]
- 14. Kocaoglu B, Martin J, Wolf B, Karahan M, Amendola A. The effect of irrigation solution at different temperatures on articular cartilage metabolism. Arthroscopy 2011;27(4):526-31. [DOI] [PubMed] [Google Scholar]
- 15. Glenn RE, Jr, Spindler KP, Warren TA, McCarty EC, Secic M. Cryotherapy decreases intraarticular temperature after ACL reconstruction. Clin Orthop Relat Res. 2004;421:268-72. [DOI] [PubMed] [Google Scholar]
- 16. Fincher AL, Wood WG, O’Connor DP. Intraoperative arthroscopic cold irrigation solution does not affect postoperative pain and swelling. J Athl Train. 2004;39(1):12-16. [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng SC, Jou IM, Chern TC, Wang PH, Chen WC. The effect of normal saline irrigation at different temperatures on the surface of articular cartilage: an experimental study in the rat. Arthroscopy. 2004;20(1):55-61. [DOI] [PubMed] [Google Scholar]
- 18. Bush PG, Hall AC. Passive osmotic properties of in situ human articular chondrocytes within non-degenerate and degenerate cartilage. J Cell Physiol. 2005;204(1):309-19. [DOI] [PubMed] [Google Scholar]
- 19. Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154(2):262-70. [DOI] [PubMed] [Google Scholar]
- 20. Capito NM, Smith MJ, Stoker AM, Werner N, Cook JL. Hyperosmolar irrigation compared with a standard solution in a canine shoulder arthroscopy model. J Shoulder Elbow Surg. 2015;24:1243-8. [DOI] [PubMed] [Google Scholar]
- 21. Zaffagnini S, Allen AA, Suh JK, Fu FH. Temperature changes in the knee joint during arthroscopic surgery. Knee Surg Sports Traumatol Arthrosc. 1996;3(4):199-201. [DOI] [PubMed] [Google Scholar]
- 22. Lu Y, Edwards RB, 3rd, Nho S, Cole BJ, Markel MD. Lavage solution temperature influences depth of chondrocyte death and surface contouring during thermal chondroplasty with temperature-controlled monopolar radiofrequency energy. Am J Sports Med. 2002;30(5):667-73. [DOI] [PubMed] [Google Scholar]
- 23. June RK, Fyhrie DP. Temperature effects in articular cartilage biomechanics. J Exp Biol. 2010;213(Pt 22):3934-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerrigan MJP, Hook CSV, Qusous A, Hall AC. Regulatory volume increase (RVI) by in situ and isolated bovine articular chondrocytes. J Cell Physiol. 2006;209:481-92. [DOI] [PubMed] [Google Scholar]
- 25. Hung CT, LeRoux MA, Palmer GD, Chao PH, Lo S, Valhmu WB. Disparate aggrecan gene expression in chondrocytes subjected to hypotonic and hypertonic loading in 2D and 3D culture. Biorheology. 2003;40(1-3):61-72. [PubMed] [Google Scholar]
- 26. Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech. 2001;34(12):1527-35. [DOI] [PubMed] [Google Scholar]
- 27. Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quinn PJ. Effects of temperature on cell membranes. Symp Soc Exp Biol. 1988;42:237-58. [PubMed] [Google Scholar]
- 29. Almeida PA, Bolton VN. The effect of temperature fluctuations on the cytoskeletal organisation and chromosomal constitution of the human oocyte. Zygote. 1995;3:357-65. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki H, Kumai T, Matsuzaki M. Effect of temperature decline on the cytoskeletal organisation of the porcine oocyte. J. Mammal Ova Res. 2007;24:107-13. [Google Scholar]
- 31. Lewis JL, Deloria LB, Oyen-Tiesma M, Thompson RC, Jr, Ericson M, Oegema TR., Jr. Cell death after cartilage impact occurs around matrix cracks. J Orthop Res. 2003;21(5):881-7. [DOI] [PubMed] [Google Scholar]
- 32. Cao L, Lee V, Adams ME, Kiani C, Zhang Y, Hu W, et al. Beta-integrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol. 1999;18(4):343-55. [DOI] [PubMed] [Google Scholar]
- 33. Gilbert SJ, Singhrao SK, Khan IM, Gonzalez LG, Thomson BM, Burdon D, et al. Enhanced tissue integration during cartilage repair in vitro can be achieved by inhibiting chondrocyte death at the wound edge. Tissue Eng Part A. 2009;15(7):1739-49. [DOI] [PubMed] [Google Scholar]


