Abstract
Human conjunctival cell lines are useful tools for modeling ocular surface disease and evaluation of ocular drugs and cosmetics. However, gene expression in these cells may not be comparable to primary cultured cells, raising doubts that they could be used as a substitute. We aimed to ascertain the similarities of global gene expression between commonly used cell lines and primary cells using a microarray approach. The Affymetrix U133A chip (>22,000 genes) was used to investigate conjunctival tissue (CT), primary conjunctival epithelial cells (PCEC), two conjunctival epithelial cell lines (IOBA-NHC and ChWK), and HCEC-T, a human corneal epithelial cell line (control). Using principal component analysis, the PCEC profile was clustered more closely to conjunctival tissue than either of the two cell lines. Certain extracellular matrix genes were differentially upregulated in CT compared to PCEC, suggesting presence of fibroblasts in addition to epithelial cells in CT. Overall, 67.3% (95% CI: 66.7–67.9) of transcripts in IOBA-NHC were within 1.5-fold of the corresponding transcripts in PCEC, but only 62.2% (95% CI: 61.5–62.9) in the case of ChWK. In HCEC-T, the proportion was only 58.8% (95% CI 58.1–59.4), suggesting less resemblance to PCEC than the conjunctival epithelial cell lines. The IOBA-NHC profile was more similar to PCEC than ChWK, for all genes and genes concerned with membrane association, communication, development, and regulation of metabolism, especially protein and nucleic acid metabolism. The correlation of normalized gene expression levels was high between either the IOBA-NHC or ChWK and PCEC for genes concerned with cell defense, viral life cycle, antigen presentation, antioxidation, or ubiquitin ligation. In order to evaluate the functional significance of the altered gene expression in IOBA-NHC cells, we evaluated a few proteins important for epithelial differentiation or defense, corresponding to the transcripts for S100A9, TGM2, and TLR4. Protein levels of S100A9 and TGM2 were indeed raised, and TLR4 decreased, in IOBA-NHC compared to PCEC. Gene expression in conjunctival cell lines differs from primary cells, but the profile varies according to functional gene categories. Depending on the methodology of proposed studies, if there is limited availability of PCEC, NHC-IOBA may be more suitable than ChWK, but even then, epithelial differentiation and innate immunity functions in NHC-IOBA may differ from primary cells.
Key words: Human, Cell culture, Gene expression, Microarray, Ocular surface disease
INTRODUCTION
The use of cultured cells for the assessment of ocular surface irritation, instead of using laboratory animals, in the evaluation of cosmetics and the safety of commercial products is increasingly important from the ethical and economic points of view (20,42). Testing of human epidermal health using markers in cultured cells may be the only feasible way to rapidly screen compounds that may be allergenic (8) or pro-inflammatory (28). Apart from these considerations, culturing of cells is now an integral part of the armamentarium of techniques in ocular surface science. Cell culture as a laboratory tool for investigation of diseases has numerous advantages, including homogeneity of cell type, increased reproducibility, as well as definition and control of environmental/experimental parameters. Cell cultures (primary cells from native tissues and cell lines) have been used to study conjunctival pathology including dry eye (10,18,41), infection (44,48), inflammation (13,35), radiation (4), and drug toxicity (7,29). They are also useful for assessment of drug delivery (19,22) and understanding basic processes such as apoptosis (25) and ocular surface immunology (15,23,37).
In particular, ocular surface epithelial cell culture experiments may use primary cultured cells (55) or cell lines, including the Wong-Kilbourne derivative of the Chang cells (ChWK) (5,51) and the more recent University Institute of Applied Ophthalmobiology-Normal Human Conjunctiva (IOBA-NHC) cells (16). Primary cell cultures have the advantages of biological similarity to native tissue either normal or diseased (17), whereas in cell lines there are advantages of greater homogeneity, increased accessibility, and easy propagation, permitting more rapid, high throughput experiments. Although morphological and immunocytochemistry of ChWK (12) and IOBA-NHC (16) cells have been used to compare these cell lines to primary cultured conjunctival epithelial cells, the gene expression patterns of these cells have not been compared using an unbiased global gene expression approach. Here, we show, using a global gene analysis of these cells lines, primary conjunctival epithelial cells and native conjunctival tissue using a gene mi-croarray approach, that ChWK and IOBA-NHC cells are suitable for modeling only selected biological functions of primary conjunctival epithelial cells.
MATERIALS AND METHODS
Cells, Materials, and Subjects
Human conjunctival tissue was obtained from the Pterygium Aetiology and Conjunctival Evaluation (PACE) study (52). This prospective and ongoing study involved patients undergoing surgical excision for pterygium. At the time of surgery, a small portion of the conjunctival patch (approximately 1.3 mm) was biopsied from an area of the superior bulbar conjunctiva uninvolved with the disease. This was rapidly frozen in liquid nitrogen after removal and stored at −150°C. The study was approved by the Institutional Review Board of the Singapore Eye Research Institute. All procedures complied with the Tenets of the Declaration of Helsinki for human research. Written informed consent was obtained from all patients prior to participation in the study.
The culturing of primary conjunctival epithelial cells has previously been described (1). Briefly, human cadaveric conjunctival tissues were obtained from the Singapore Eye Bank and used for the isolation and culture of conjunctival epithelial cells within 16 h of death. All donors were males aged from 51 to 68 years of age with an average of 58 years. Four samples of human primary conjunctival epithelial cells (PCEC), passages P0–P2 were cultured. IOBA-NHC cells were obtained from Margarita Calonge and Yolanda Diebold, IOBA, University of Valla-dolid, Valladolid, Spain. The Wong and Kilbourne derivative of the Chang cells (ChWK) were obtained from the American Type Culture Collection (ATCC CCL-20.2, clone 1-5c-4).
Culture Conditions
Culturing of cell lines was performed as previously described (16). Briefly, cells were cultured in DMEM/F12 supplemented with 1 μg/ml bovine pancreas insulin, 2 ng/ml mouse epidermal growth factor, 0.1 μg/ml cholera toxin, 5 μg/ml streptomycin, 2.5 μg/ml amphotericin B, and 10% fetal bovine serum. Medium was changed very 2–3 days, and cell growth was assessed daily by phase-contrast microscopy.
Microarray Analysis
Microarray chips, related protocols, and equipment for the processing of these chips were from Affymetrix Inc. (Santa Clara, CA). The human genome U133A GeneChip® consisting of more than 22,000 probe sets was used for this study. RNA was extracted as described in the manufacturer’s manual. cRNA generated from 5 μg of total RNA was prepared as described in the sample preparation protocol. In order to obtain sufficient starting RNA from conjunctival tissues, each sample of RNA consists of pooled RNA from four patients’ tissues. Washing and staining of arrays were performed with a GeneChip® Fluidics Station 450 and scanning was performed with the GeneChip® Scanner 3000.
Statistical analysis was performed using the Gene-spring GX 7.3 platform (Agilent Technology, Redwood City, CA). All data underwent probe level Robust Multi-Array Average (RMA) algorithm normalization as well as chip level normalization (30). Correlation analysis was performed as follows. One sample of primary conjunctival epithelial cells was used as reference, and correlation of global gene expression data was performed against each of the four samples of ChWK and each of the five samples of IOBA-NHC cell. As a control, five samples of a less related human corneal epithelial cell line (HCEC-T) were used.
The raw gene expression data (CEL files) for the A549 cell line were downloaded from the NCBIGEO datasets GSM 47472, GSM47468, and GSM 47462 and processed subsequently as for other data as described.
The Pearson correlation coefficient was calculated, as well as the mean, SD, median, minimum, and maximum of the coefficients. The unrelated samples t-test was used to compare the correlation coefficients for the ChWK/PCEC and IOBA-NHC/PCEC associations, with statistical significance set at the level of α = 0.05.
Fold change analysis was used to compare the mean expression levels of various transcripts in the cell lines compared to the primary conjunctival epithelial cells as a reference. The percentage of genes showing a 1.5×-fold change (increase or decrease) relative to PCEC was calculated and tabulated, with the 95% confidence intervals of the proportions.
The data discussed in this publication have been deposited in NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE8633.
Western Blot
Western blots were performed as previously published (49). Briefly, total cell lysates for PCEC or IOBA-NHC (40 μg) were loaded on SDS-PAGE, transferred to nitrocellulose membrane, and blotted with primary antibodies against S100A9 (mouse monoclonal 1C10, Abnova, Walnut, CA), TGM2 (rabbit polyclonal Ab421, Abcam, Cambridge, MA), TLR4 (rabbit polyclonal, H-80, Santa Cruz, CA) at a dilution of 1:500. All primary antibody incubation was performed overnight at 4°C and blotted with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz, CA) at 1:5000 dilution.
Survey of Literature
The NCBI Pubmed database was searched for articles containing “IOBA” or Chang conjunctiva cells (combination of these words or ChWK) in their abstracts. The results were manually curated to exclude review articles and to determine the predominant experimental strategy and outcome. Forty-four articles were found using the “Chang” cell line, so our analysis was restricted to articles after 2000 and for articles available on the Pubmed up to April 30, 2007. The correlation analyses and the fold change analyses were repeated with Gene Ontology categories representing subsets of genes implicated in these published articles.
RESULTS
Analysis of Conjunctival Tissue and Primary Cultured Conjunctival Epithelial Cells
Global gene expression profiling, using principal component analysis, shows that PCEC have a pattern more similar to normal conjunctival tissue (CT) compared to either one of the conjunctival epithelial cell lines (Fig. 1). In Figure 1, the PCEC samples are spatially closer in the 3D scatter diagram to the tissue samples than the cell lines. In addition, a clear plane could be seen separating the cell lines from PCEC and the conjunctival tissue samples. Nevertheless, one would expect differences between the gene expression in CT and PCEC because the conjunctival biopsy will inevitably include some subepithelial stromal tissue together with the conjunctival epithelium, which may include fibroblasts, vascular endothelial cells, and blood cells. In order to evaluate the differences between PCEC and conjunctival tissue, we examined the possible upregulation of genes related to production of extracellular matrix in the conjunctival tissue compared to PCEC. We found that collagen types (1, 3, 6, and 18) were significantly upregulated (p < 0.05) by more than two times in conjunctival tissue compared to PCEC (Table 1). The same was also observed for other genes containing “extracellular matrix” within the Gene Ontology (GO) descriptions (Table 1).
Figure 1.
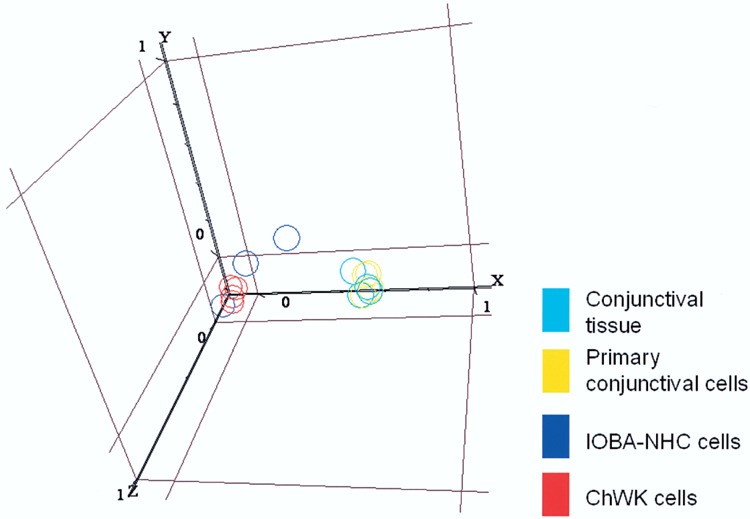
Global gene expression profiling in conjunctival tissue and conjunctival epithelial cells. Three-dimensional scatter plot showing the first three principal components in the x, y, and x axes, respectively. Principal component analysis (PCA) on the global gene expression data was performed for the four types of samples analyzed. Samples for different conditions are illustrated in different colors. The PCA procedure and generation of the scatter plot were performed using the Genespring GX 7.3 platform (Agilent Technology, Redwood City, CA). The option “PCA on conditions” was selected under “Tools.” The gene list was set to “All genes” and 16 experimental samples were selected, with the “mean centering and scaling” method used. On each of the axes of the scatter diagram, the data were set to “expression profile” with “linear” as the graph mode. Out of five principal components computed by the software, the first three principal components account for 31.4%, 13.5%, and 10% of the variance in the expression data, respectively, with a cumulative 54.9% for the three components.
TABLE 1.
EXTRACELLULAR MATRIX GENES UPREGULATED IN CONJUNCTIVAL TISSUE COMPARED TO PRIMARY CONJUNCTIVAL EPITHELIAL CELLS
| Probe ID | Gene Symbol | Genbank Accession | Name | Fold Change |
|---|---|---|---|---|
| 219087_at | ASPN | NM_017680 | Asporin (leucine rich repeat class 1) | 2.581 |
| 215016_x_at | BPAG1 | BC004912 | Bullous Pemphigoid antigen 1 | 2.715 |
| 211896_s_at | DCN | AF138302 | Decorin | 13.53 |
| 201893_x_at | DCN | AF138300 | Decorin | 8.868 |
| 211813_x_at | DCN | AF138303 | Decorin | 5.078 |
| 207977_s_at | DPT | NM_001937 | Dermatopontin | 2.217 |
| 201843_s_at | EFEMP1 | NM_004105 | EGF-containing tubulin-like extracellular matrix protein 1 | 3.185 |
| 202994_s_at | FBLN1 | Z95331 | Fibulin 1 | 2.111 |
| 202766_s_at | FBN1 | NM_000138 | Fibrillin 1 | 2.254 |
| 201744_s_at | LUM | NM_002345 | Lumican | 7.242 |
| 202310_s_at | COL1A1 | K01228 | Collagen Type I | 2.473 |
| 202403_s_at | COL1A2 | AA788711 | Collagen Type I | 6.012 |
| 202404_s_at | COL1A2 | NM_000089 | Collagen Type I | 4.725 |
| 215076_s_at | COL3A1 | AU144167 | Collagen Type III | 4.815 |
| 201852_x_at | COL3A1 | AI813758 | Collagen Type III | 4.649 |
| 213428_s_at | COL6A1 | AA292373 | Collagen Type VI | 2.542 |
| 201438_at | COL6A3 | NM_004369 | Collagen Type VI | 2.132 |
| 209082_s_at | COL18A1 | AFO18081 | Collagen Type VIII | 2.603 |
We also found that three of the genes containing “blood” as a search term in the GO description were significantly upregulated (p < 0.05) by at least twofold in conjunctival tissue compared to PCEC. These were the genes coding for interleukin 20 receptor alpha subunit (NM_014432), phospholipid scramblase 4 (NM_020353), and ectonucleoside triphosphate diphosphohydrolase 1 (NM_001776). These transcripts were upregulated in conjunctival tissue compared to PCEC by 5.4-, 4.6-, and 2.0-fold, respectively.
Comparison of Conjunctival Epithelial Cell Lines With Primary Cultured Conjunctival Epithelial Cells
In order to compare epithelial cells from cell lines with a similarly homogenous sample of epithelial cells, the PCEC was used as a reference in the evaluation of the cell lines.
Using a fold change analysis method, the genes in IOBA-NHC and ChWK cells were stratified into two categories: within 1.5-fold of the level of expression in the primary cells or outside this level of expression. The percentages of the genes that were within 1.5-fold are shown in two columns in bold in Table 2, corresponding to the two types of cell lines.
TABLE 2.
COMPARISON OF GENE EXPRESSION PROFILES
| GO No. | Total No. | IOBA-NHC % 1.5× (95% CI) | ChWK % <1.5× (95% CI) | |
|---|---|---|---|---|
| Overall | 22,283 | 67.3 (66.7–67.9) | 62.2 (61.5–62.9) | |
| Membrane bound organelles | 43,227 | 5,899 | 61.6 (60.3–62.9) | 57.0 (55.7–58.3) |
| Nucleus | 5,634 | 4,140 | 62.9 (61.4–64.4) | 58.1 (56.6–59.7) |
| Extracellular region | 5,576 | 1,119 | 70.8 (67.9–73.5) | 66.5 (63.6–69.3) |
| Chromosome | 5,694 | 249 | 53.0 (46.5–54.4) | 49.0 (42.5–55.5) |
| Response to wounding | 9,611 | 477 | 66.9 (62.3–71.1) | 61.6 (57.0–66.1) |
| Response to stress | 6,950 | 1,091 | 62.2 (59.2–65.2) | 56.7 (53.7–59.8) |
| Response to inflammation | 6,954 | 235 | 67.2 (60.6–70.2) | 61.3 (54.6–67.6) |
| Cell communication | 7,154 | 4,121 | 72.2 (70.8–73.6) | 67.0 (65.5–68.4) |
| Transcription regulator | 30,528 | 1,636 | 71.9 (69.6–74.1) | 67.8 (65.4–70.1) |
| Transporter | 5,215 | 1,953 | 66.5 (64.3–68.6) | 62.4 (60.1–64.6) |
| Development | 7,275 | 2,152 | 72.0 (70.0–73.9) | 66.5 (64.5–68.6) |
| Differentiation | 30,154 | 315 | 72.4 (66.9–77.3) | 64.8 (59.1–70.1) |
| Motor activity | 3,774 | 167 | 67.7 (59.8–74.7) | 61.7 (53.7–69.1) |
| Cellular defense | 6,968 | 128 | 69.5 (60.5–77.3) | 58.6 (49.4–67.3) |
| Viral life cycle | 16,032 | 43 | 55.8 (39.7–70.8) | 55.8 (39.7–70.8) |
| Antigen presentation | 19,882 | 47 | 63.8 (48.2–77.1) | 59.6 (44.0–73.5) |
| Antioxidant | 16,209 | 43 | 48.8 (33.3–64.5) | 48.8 (33.3–64.6) |
| Ubiquitin ligase complex | 151 | 33 | 57.6 (39.1–74.3) | 45.5 (28.3–63.7) |
| Regulation of metabolism | 19,222 | 1,752 | 69.1 (66.8–71.3) | 64.6 (62.2–66.8) |
| Lipid metabolism | 6,629 | 649 | 64.9 (61.0–68.6) | 60.7 (56.7–64.5) |
| Protein metabolism | 19,538 | 2,853 | 63.3 (61.5–65.1) | 56.6 (54.8–58.5) |
| Carbohydrate metabolism | 5,975 | 467 | 63.4 (58.7–67.8) | 58.9 (54.2–63.5) |
| Nucleic acid metabolism | 6,139 | 2,961 | 62.3 (60.5–64.1) | 57.8 (56.0–59.6) |
| Organic acid metabolism | 6,082 | 469 | 56.7 (52.0–61.3) | 50.1 (45.4–54.8) |
The transcript level of various classes of genes in each of the two types of conjunctival cell lines (IOBA-NHC and ChWK) was compared with the corresponding transcripts in the primary conjunctival epithelial cells using fold change analysis. Total No.: number of genes in that Gene Ontology (GO) category in the GeneChip. 1.5×: 50% increased or decreased from the level in primary cells. CI: confidence interval of the proportion.
The main finding (first row Table 2) was that in IOBA-NHC cells 67.3% (95% CI 66.7–67.9) of the genes were within 1.5-fold of the expression level in PCEC, whereas in ChWK, the corresponding proportion was less, at 62.2% (95% CI 61.5–62.9). The difference in proportion was statistically significant (p < 0.05).
Due to biological variation, one would not expect 100% of genes to be within 1.5-fold even when comparing different samples/batches of the same cell type such as PCEC. When comparing one sample of PCEC with the other samples of PCEC, 79% (95% CI 78.5–79.6) of genes were expressed within a 1.5-fold (Table 3, first row, right-hand column). In this regard, neither IOBA-NHC nor ChWK was completely ideal as a substitute for PCEC, because 67.3% and 62.2% were each significantly lower than 79%, respectively (Table 4, indicated by asterisks in first row, columns 4 and 5 from left).
TABLE 3.
COMPARISON OF EXPRESSION PROFILE BETWEEN UNRELATED CELL LINE (HCEC-T) AND PRIMARY CONJUNCTIVAL EPITHELIAL CELLS (PCEC)
| Total No. | HCEC-T vs. PCEC | PCEC vs. PCEC | |||
|---|---|---|---|---|---|
| % <1.5× | 95% CI | % <1.5× | 95% CI | ||
| Overall | 22,283 | 58.8 | 58.1, 59.4 | 79.0 | 78.5, 79.6 |
| Membrane bound organelles | 5,899 | 56.9 | 55.6, 58.2 | 76.7 | 75.6, 77.8 |
| Nucleus | 4,140 | 56.2 | 54.7, 57.7 | 77.2 | 76.0, 78.5 |
| Extracellular region | 1,119 | 59.8 | 56.9, 62.7 | 79.3 | 76.8, 81.7 |
| Chromosome | 249 | 49.0 | 42.8, 55.2 | 73.9 | 67.8, 79.3 |
| Response to wounding | 477 | 57.0 | 52.6, 61.5 | 77.5 | 73.4, 81.2 |
| Response to stress | 1,091 | 54.1 | 51.1, 57.0 | 75.0 | 72.3, 77.6 |
| Response to inflammation | 235 | 58.7 | 52.4, 65.0 | 77.8 | 71.8, 82.9 |
| Cell communication | 4,121 | 60.7 | 59.2, 62.2 | 81.5 | 80.2, 82.6 |
| Transcription regulator | 1,636 | 59.5 | 57.1, 61.9 | 82.0 | 80.1, 83.9 |
| Transporter | 1,953 | 61.0 | 58.8, 63.1 | 80.7 | 78.9, 82.5 |
| Development | 2,152 | 60.8 | 58.7, 62.8 | 80.0 | 78.3, 81.7 |
| Differentiation | 315 | 57.5 | 52.0, 62.9 | 80.9 | 76.0, 85.1 |
| Motor activity | 167 | 53.3 | 45.7, 60.9 | 76.0 | 68.6, 82.2 |
| Cell defense | 128 | 56.3 | 47.7, 64.8 | 83.5 | 75.6, 89.4 |
| Viral life cycle | 43 | 55.8 | 41.0, 70.7 | 74.4 | 58.2, 86.1 |
| Antigen presentation | 47 | 51.1 | 36.8, 65.4 | 65.9 | 50.3, 78.9 |
| Antioxidant | 43 | 60.5 | 45.9, 75.1 | 65.1 | 48.7, 78.7 |
| Ubiquitin ligase | 33 | 51.5 | 34.5, 68.6 | 69.6 | 50.8, 84.9 |
| Regulation metabolism | 1,752 | 59.1 | 56.8, 61.4 | 80.8 | 78.9, 82.7 |
| Lipid metabolism | 649 | 57.2 | 53.4, 61.0 | 77.3 | 73.8, 80.5 |
| Protein metabolism | 2,853 | 57.6 | 55.7, 59.4 | 78.0 | 76.5, 79.6 |
| Carbohydrate metabolism | 467 | 56.5 | 52.0, 61.0 | 78.1 | 74.0, 81.8 |
| Nucleic acid metabolism | 2,961 | 56.5 | 54.7, 58.3 | 76.9 | 75.4, 78.4 |
| Organic acid metabolism | 469 | 60.3 | 55.9, 64.8 | 73.5 | 69.2, 77.5 |
TABLE 4.
COMPARISONS BETWEEN CELL LINES AND ASPECTS OF REPRODUCIBILITY
| IOBA vs. ChWK | IOBA vs. HCEC-T | ChWK vs. HCEC-T | IOBA vs. PCEC | ChWK vs. PCEC | HCEC-T vs. PCEC | |
|---|---|---|---|---|---|---|
| Overall | * | * | * | * | * | * |
| Membrane bound organelles | * | * | * | * | * | |
| Nucleus | * | * | * | * | ||
| Extracellular region | * | * | * | * | * | |
| Chromosome | * | * | * | |||
| Response to wounding | * | * | * | * | ||
| Response to stress | * | * | * | * | ||
| Response to inflammation | * | * | * | * | ||
| Cell communication | * | * | * | * | * | * |
| Transcription regulator | * | * | * | * | * | |
| Transporter | * | * | * | * | ||
| Development | * | * | * | * | * | * |
| Differentiation | * | * | * | |||
| Motor activity | * | * | ||||
| Cell defense | * | * | ||||
| Viral life cycle | ||||||
| Antigen presentation | ||||||
| Antioxidant | ||||||
| Ubiquitin ligase | ||||||
| Regulation metabolism | * | * | * | * | * | * |
| Lipid metabolism | * | * | * | * | ||
| Protein metabolism | * | * | * | * | * | |
| Carbohydrate metabolism | * | * | * | |||
| Nucleic acid metabolism | * | * | * | * | * | |
| Organic acid metabolism | * | * | * |
Note: Cell type A versus cell type B does not refer to direct comparison between cell type A and cell type B (refer to text for explanation). IOBA: IOBA-NHC cell line, ChWK: Chang cell line WK derivative, PCEC: primary conjunctival epithelial cell, HCEC-T: unrelated corneal epithelial cell line.
p < 0.05.
However, the proportions (67.3% and 62.2%) are both significantly greater than 58.8% (Table 3, bold percentage in first row), confirming that the two conjunctival cell lines (IOBA-NHC and ChWK, respectively) resemble PCEC more than the less-related human corneal epithelial cell line (HCEC-T). The statistical significance of these relationships is indicated by asterisks in the first row, columns 2 and 3 in Table 4.
The proportions 67.3% appear rather close to 62.2% (Table 2, bold percentages, first row). However, these proportions are significantly different (Table 2, first row, evidenced by nonoverlapping 95% confidence intervals). To illustrate the statistical significance or otherwise of the proportions in the bold columns in Table 2, Table 4 (first column from left) is constructed with asterisks indicated whenever there is a significant difference (p < 0.05). IOBA-NHC was more similar to PCEC than ChWK (Table 4, asterisks in the first column) in genes concerned with membrane binding, cell communication, development, regulation of metabolism, in particular regulation of protein and nucleic acid metabolism.
Not surprisingly, the expression levels of genes in the less-related human corneal epithelial cell line (HCEC-T) were significantly less similar to PCEC than comparing PCEC to PCEC itself (Table 3, lower percentages in the left compared to right bold columns). The last column in Table 4 illustrates that this relationship was significant for all GO categories apart from genes concerned with viral life cycle, antigen presentation, antioxidant, and ubiquitin ligase. The reason for lack of statistical significance in these four categories is the relatively small number (less than 50) of genes present in each of these categories on the U133A GeneChip used in this study.
To explore the gene expression profile of another unrelated cell line (bronchial epithelial cell A549) in place of HCEC-T, we repeated the above analyses and showed this in Tables S2 (available at http://www.seri.com.sg/publications/others/Table S2.doc) and S3 (available at http://www.seri.com.sg/publications/others/Table S3.doc). In general, the same conclusions can be drawn as in Tables 3 and 4.
We also use a different method to compare the gene expression profiles in IOBA-NHC or ChWK
cells with PCEC (Table 5). This method used the correlation of paired expression data between either IOBA-NHC or ChWK against PCEC. The information provided by this analysis is different from that in Tables 2 and 3. A large percentage of genes expressed within 1.5-fold could still be observed with low correlation and vice versa.
TABLE 5.
COMPARISON OF GENE EXPRESSION BETWEEN CONJUNCTIVAL CELL LINES AND PRIMARY CONJUNCTIVAL EPITHELIAL CELLS USING CORRELATION
| GO No. | IOBA (n = 5) vs. PCEC | ChWK (n = 4) vs. PCEC | |||||
|---|---|---|---|---|---|---|---|
| Median r | Min | Max | Median r | Min | Max | ||
| Overall | 0.322 | 0.319 | 0.326 | 0.312 | 0.310 | 0.315 | |
| Membrane bound organelles | 43,227 | 0.551 | 0.542 | 0.557 | 0.548 | 0.545 | 0.553 |
| Nucleus | 5,634 | 0.51 | 0.495 | 0.518 | 0.510 | 0.507 | 0.516 |
| Extracellular region | 5,576 | 0.11 | 0.096 | 0.114 | 0.080 | 0.076 | 0.094 |
| Chromosome | 5,694 | 0.618 | 0.613 | 0.638 | 0.640 | 0.613 | 0.666 |
| Response to wounding | 9,611 | 0.157 | 0.14 | 0.175 | 0.128 | 0.121 | 0.139 |
| Response to stress | 6,950 | 0.225 | 0.221 | 0.227 | 0.207 | 0.198 | 0.211 |
| Response to inflammation | 6,954 | −0.0115 | −0.031 | 0.003 | −0.040 | −0.048 | −0.035 |
| Cell communication | 7,154 | 0.348 | 0.335 | 0.354 | 0.345 | 0.323 | 0.355 |
| Transcription regulator | 30,528 | 0.441 | 0.411 | 0.47 | 0.442 | 0.436 | 0.448 |
| Transporter | 5,215 | 0.373 | 0.363 | 0.38 | 0.369 | 0.35 | 0.381 |
| Development | 7,275 | 0.208 | 0.2 | 0.221 | 0.188 | 0.18 | 0.195 |
| Differentiation | 30,154 | 0.208 | 0.2 | 0.221 | 0.188 | 0.18 | 0.195 |
| Motor activity | 3,774 | 0.66 | 0.589 | 0.794 | 0.654 | 0.603 | 0.717 |
| Cell defense | 6,968 | 0.785 | 0.774 | 0.809 | 0.782 | 0.761 | 0.805 |
| Viral life cycle | 16,032 | 0.776 | 0.723 | 0.811 | 0.810 | 0.788 | 0.822 |
| Antigen presentation | 19,882 | 0.776 | 0.724 | 0.784 | 0.775 | 0.764 | 0.804 |
| Antioxidant | 16,209 | 0.738 | 0.682 | 0.753 | 0.736 | 0.727 | 0.739 |
| Ubiquitin ligase | 151 | 0.769 | 0.719 | 0.798 | 0.771 | 0.701 | 0.816 |
| Regulation metabolism | 19,222 | 0.462 | 0.430 | 0.477 | 0.442 | 0.431 | 0.448 |
| Lipid metabolism | 6,629 | 0.334 | 0.312 | 0.35 | 0.400 | 0.393 | 0.420 |
| Protein metabolism | 19,538 | 0.586 | 0.572 | 0.595 | 0.562 | 0.556 | 0.567 |
| Carbohydrate metabolism | 5,975 | 0.482 | 0.446 | 0.505 | 0.502 | 0.474 | 0.571 |
| Mucleic acid metabolism | 6,139 | 0.547 | 0.543 | 0.563 | 0.548 | 0.541 | 0.551 |
| Organic acid metabolism | 6,082 | 0.443 | 0.423 | 0.471 | 0.414 | 0.396 | 0.432 |
Table values show Pearson correlation coefficients (r) where 1.0 = perfect correlation, 0.0 = no correlation, −1.0 = negative correlation.
The correlation analysis was useful for GO categories with relatively few genes (30–50 each). The correlation of gene expression levels, using PCEC as a reference was generally high (r > 0.70) for either IOBA-NHC or ChWK cells in the categories described as “viral life cycle,” “antigen presentation,” “antioxidant,” and “ubiquitin ligase” (Table 5). However, correlation varied in different gene categories; for example, the genes involved in the inflammatory response were poorly correlated to PCEC in either of the cell lines.
In order to shed some light on the genes that were expressed differently between a conjunctival cell line and PCEC, we tabulated the list of 55 genes that demonstrated a change of at least 1.5-fold in IOBA-NHC cells compared to primary conjunctival cells (Table S1: available at http://www.seri.com.sg/publications/others/Table S1.xls). For these genes, the exact number of fold of up- or downregulation is illustrated in the table. Some examples include the S100A8 (NM_002964) and S100A9 (NM_002965), which are important inflammatory markers that can be noninvasively monitored in human tears, and interleukin-1α (M15329), an important proinflammatory cytokine.
In Table 5, genes concerned with cellular defense and protein metabolism, relatively high correlation coefficients (>0.55) were obtained for IOBA-NHC or ChWK against PCEC, which agreed with the relatively high percentages in the corresponding rows in Table 2.
In order to evaluate the functional relevance of the transcript changes, we performed Western blots to relatively quantify the levels of three proteins that are expressed in the PCEC and IOBA-NHC cells corresponding to the transcripts evaluated in the micro-array (Fig. 2). S100A9 was selected for this purpose because of its importance in differentiation and regulation of calcium-associated stress signal transduction (21), and its transcript levels were greatly increased in IOBA-NHC (Table S1: available at http://www.seri.com.sg/publications/others/Table S1.xls) compared to PCEC cells. The same was also true for transglutaminase (TGM)-2, which was expressed at 3.1-, 2.6-, 2.7-, and 1.1-fold higher in the IOBA-NHC cells, compared to the PCEC for the probesets 201042_at, 211003_x_at, 211573_x_at, and 216183_at, respectively. TGM2 is an important molecule involved in wound healing, apoptosis, and regulation of wound healing, oncogenesis and tumor metastasis, and expressed in the eye (39,45). On the other hand, the Toll-like receptor (TLR)-4 expression was lower in the IOBA-NHC cells compared to PCEC (Table S1: available at http://www.seri.com.sg/publications/others/Table S1.xls). TLR-4 is an important pathogen recognition receptor molecule in the innate defense of the eye (36). Figure 2 shows that the protein level changes in the three proteins were consistent with differences in transcript levels, confirming the functional significance of our study.
Figure 2.
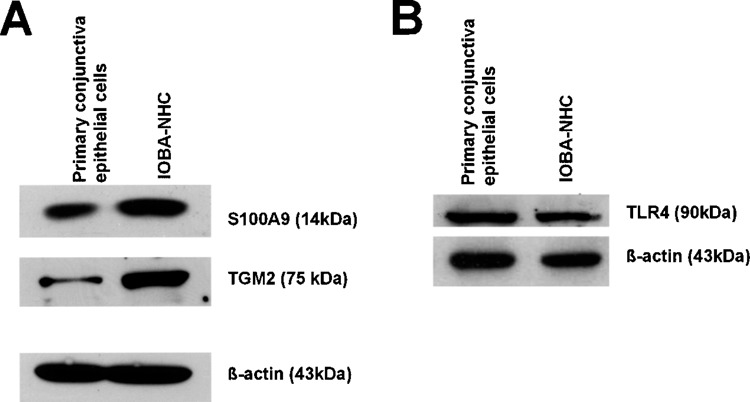
Protein levels in primary cultured conjunctival epithelial cells compared to the IOBA-NHC. Western blots performed on total cell lysate showing that evaluated proteins are higher in the IOBA-NHC (A) or lower in the IOBA-NHC (B) compared to primary conjunctival epithelial cells.
DISCUSSION
PCEC resemble the native conjunctival tissue more than any of the cell lines. This is not surprising, considering that the ChWK is artificially (51), whereas the IOBA-NHC cells spontaneously (16), immortalized. The IOBA-NHC cells appear to resemble PCEC more than the ChWK cells, especially for the genes involved in cell communication and development. Although the PCEC expectedly show a greater resemblance to the conjunctival tissue than any cell line, the gene expression profile was still distinct from the tissue as other cell types/components may be present in the tissue but not the primary cells.
A high level of genes in cellular defense showed similarities in expression profile between IOBA-NHC and primary cells, validating the use of IOBA-NHC in the previous studies in this field (Table 6). As possible differences in the gene expression data between cell lines and primary epithelial cells may be dependent on gene function category, we highlighted the methodology of some studies employing cell lines in Table 6. It is interesting that a large number of such studies deal with microbial infection and, indeed, the correlation of the gene expression levels between IOBA and PCEC was relatively high (r > 0.70) in the categories of cellular defense and viral life cycle-related genes. When there were disparities in the gene expression levels between NHC-IOBA and PCEC in important entities such as S100A9, TGM2, and TLR4, which play important roles in the biology of conjunctiva epithelium, the transcript level differences were mirrored by protein level differences, suggesting that our approach is valid and that even in the areas of differentiation and innate immunity, definite differences in molecular processes do exist between NHC-IOBA and PCEC.
TABLE 6.
PREVIOUS STUDIES EMPLOYING IOBA-NHC AND ChWK CELL LINES
| Study | Purpose | Biological Process |
|---|---|---|
| IOBA-NHC | ||
| Buron 2006 (4) | UV and drug toxicity | Apoptosis |
| Corrales 2001 (7) | Drug toxicity | Cell viability |
| Diebold 1998 (15) | Cytokine stimulation | Immunity |
| Diebold 2003 (16) | Cytokine stimulation | Morphology, proliferation |
| Diebold 2007 (19) | Drug delivery | Nanoparticle penetration |
| Enriquez de Salamanca 2005 (23) | Cytokine stimulation | Membrane receptor expression |
| Enriquez de Salamanca 2006 (22) | Drug delivery, toxicity | Drug uptake, cell viability |
| Narayanan 2006 (41) | Cytokine stimulation | Cytokine expression |
| Talreja 2005 (48) | LPS stimulation | TLR signaling |
| ChWK (only 21/44 shown) | ||
| Garweg 2006 (26) | Drug toxicity | Cell proliferation |
| Gallyas 2006 (25) | Viral cytopathy | Apoptosis |
| Chen 2006 (6) | Cytokine stimulation | Signaling, Immunity |
| Buron 2006 (4) | As above | |
| Talreja 2005 (48) | As above | |
| Guenoun 2005 (29) | Drug toxicity | Antioxidant, free radicals |
| De Saint Jean 2004 (9) | Cytokine stimulation | Differentiation |
| Zhan 2003 (55) | Cytokine stimulation | Immunity |
| Papa 2003 (44) | Drug toxicity | Cell proliferation |
| Jendrossek 2003 (31) | Bacterial infection | Apoptosis, immunity |
| Sinniah 2002 (47) | Toxicology | Cell morphology |
| Fillon 2002 (24) | Bacterial infection | Apoptosis |
| Debbasch 2002 (13) | Drug toxicity | Cell viability, free radicals |
| Wu 2001 (53) | Viral infection | Membrane proteins |
| Ochiai 2001 (43) | Drug toxicity | Growth factor expression |
| Jendrossek 2001 (32) | Bacterial infection | Signaling, apoptosis |
| Debbasch 2001 (12) | Drug toxicity | Apoptosis, free radicals |
| Debbasch 2001 (14) | Drug toxicity | Apoptosis, free radicals |
| Blom 2001 (3) | Bacterial infection | Immunity |
| Minor 2000 (40) | Bacterial infection | Carbohydrate metabolism |
| De Saint Jean 2000 (11) | Cytokine stimulation | Apoptosis |
A SV40 immortalized human corneal epithelial cell line, as described previously, and that expressed cornea-specific, 64-kDa cytokeratin in addition to five major insoluble proteins, was used as a control because this cell line has no conjunctival epithelial cell origin, although it is still an ocular surface epithelial cell type (2).
Not surprisingly, the gene expression profile for HCEC-T was significantly different from PCEC, and from IOBA-NHC cells. Some gene categories in HCEC-T (nuclear genes and differentiation), however, showed no significant difference in expression from ChWK cells, suggesting that some features of ChWK gene expression profile resembles the HCEC-T, perhaps related to the method of immortalization.
Comparison With Previously Published Data
There were no previous data on the global gene expression comparison between PCEC and cell lines. However, the differential expression of a selected set of proteins or markers has been previously evaluated. For example, after stimulation by IFN-γ and TNF-α, ChWK cells had less upregulation of inflammatory genes when compared to PCEC (12). ChWK cells were also cytokeratin-4 negative, whereas PCEC demonstrated obvious expression of this well-known conjunctival epithelial differentiation marker (12). Immortalized rabbit lacrimal acinar cells were shown to express higher levels of proteins such as vimentin, compared to primary lacrimal acinar cells (46), but this study only used immunolabeling of proteins and did not address transcript levels. A glial cell line has been compared to oligodendrocytes, but only with respect to myelin-associated glycoproteins and glycolipids (54). As these studies are focused on an arbitrary set of markers they cannot address the relative differences in different functional or biological subsets. In the nonocular literature, global gene profiling has been performed for cancer-derived cell lines compared to primary chondrocytes (27), normal leukocytes (50), or Schwann cells (34), while one study compared cancer cell lines to clinical cancer specimens (38). These studies primarily address the differences in diseased cells compared to normal cells, not the differences between a nontransfected primary cell equivalent with its natural counterpart.
The reasons for the ChWK and IOBA-NHC cells to retain potential for continuous proliferation after repeated passaging are not completely known. The ChWK cells may have been contaminated by HeLa cells (33). The IOBA-NHC, though not contaminated by Langerhans cells, endothelial cells, or fibroblasts, may have been exposed to an unknown oncogenic stimulus, because it is heteroploid, with a near triploid chromosome number (65+/−4) and no Y chromosome despite the original source of a male human donor (16). The reasons for the immortalization are likely to explain the deviation of the gene expression of these cells from PCEC.
Strengths and Limitations
Because this is a microarray experiment with a global human gene array, we were unbiased in the selection of genes to compare. We accept there is some arbitrary selection of the panel of specific genes in some analyses. However, we have selected these categories based on the type of studies that had been conducted using these specific conjunctival cell lines in the literature.
The main limitation to this article is the restriction of the analysis to gene expression data and limiting the functional evaluation to only three proteins expressed in the IOBA-NHC and PCEC. Our data would have been more valuable if it can be supplemented by proteomics profiling and other functional assays. We only use IOBA-NHC and ChWK cells from a single batch. It is theoretically possible that, over the years, some mutations and changes of characteristics may have occurred after the propagation of these cells. Therefore, there may be some limitations to the extrapolation of our data to other IOBA-NHC and ChWK cells.
Potential Applications
Researchers should exercise caution in the selection of conjunctival cell lines in their research. Figure 3 provides some guidelines for the selection of study samples in research related to the ocular surface. For example, data on the general response of cells in IOBA-NHC and ChWK cells to inflammation may not be applicable to native conjunctival tissue. However, given the limited availability of human tissue and PCEC, specific questions and cellular mechanisms related to inflammation, such as characteristics of cell communication and defense may still be properly addressed in these cells. In such cases, particular caution must be exercised when studying transcript levels of certain markers of inflammation such as S100A8 in cell lines, because these could vary tremendously from the corresponding primary cultured cells.
Figure 3.
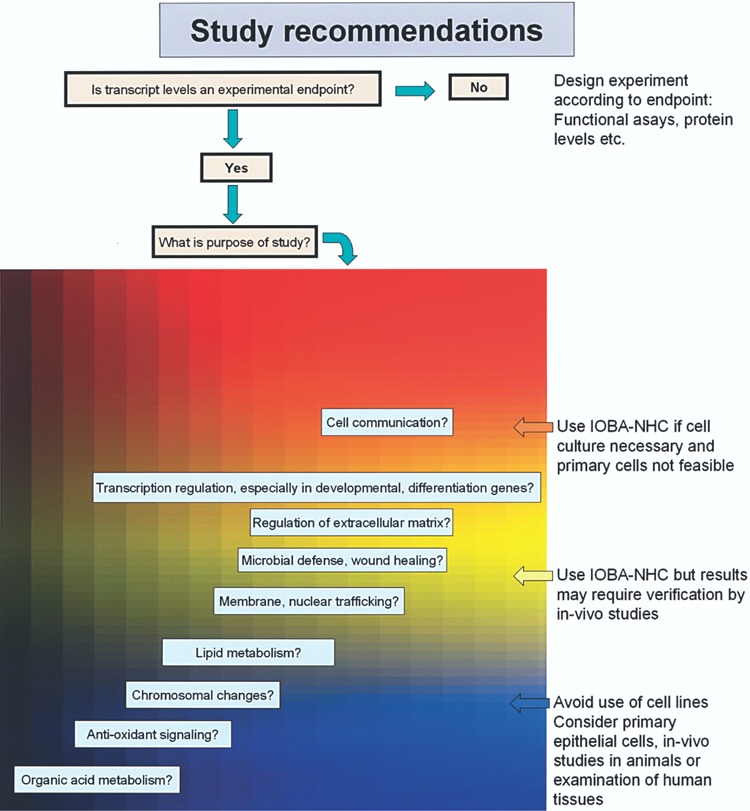
Selection of cells for ocular surface research. This chart summarizes how investigators can select appropriate cells in the ocular surface research in the future, based on the gene expression data in the current study.
In nonocular research, the vast majority of the scientific literature that utilized cell lines did not include a parallel assessment of the induced changes in equivalent native cells, which may not be available. The findings in these studies may or may not apply to human disease processes, depending on the area of biology involved, and should be addressed in their specific context.
CONCLUSIONS
Conjunctival cell lines do not resemble native conjunctival tissue as much as primary cultured conjunctival epithelial cells, which also differed from native tissue in some ways. However, when laboratory and industrial settings dictate the use of conjunctival cell lines, scientists should be aware that there are some differences in the gene expression profile of IOBA-NHC and ChWK cells, and studies using one of these cell types may be more valid than the other depending on the aim and context of the experiment.
ADDITIONAL FILES
The data discussed in this publication have been deposited in NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE8633.
Table S1: Genes in the inflammatory response category and the mean number of fold of alteration of expression in IOBA-NHC cells relative to primary conjunctival epithelial cells. Microsoft excel file (available at http://www.seri.com.sg/publications/others/Table S1.xls)
Table S2: Comparison of gene expression profile between bronchial epithelial cell line (A549) and PCEC. Internal epithelial conjunctival control: this refers to the comparison of one sample of PCEC against other samples of PCEC. Microsoft word file (available at http://www.seri.com.sg/publications/others/Table S2.doc)
Table S3: Comparisons between cell lines and aspects of reproducibility. IOBA: IOBA-NHC cell line, ChWK: Chang cell line WK derivative, PCEC: primary conjunctival epithelial cell, and A549: unrelated bronchial epithelial cell line. Microsoft word file (available at http://www.seri.com.sg/publications/others/Table S3.doc)
ACKNOWLEDGMENTS
L.T. performed the analysis for the data and drafted the manuscript. R.W.B. conceived of the study, participated in the coordination of study, and helped to draft the manuscript. J.P.G. performed the cell cultures and RNA extraction for the study. M.E.S., M.C., and Y.D. carried out the initial experiments for the characterization of the IOBA-NHC cell line, and participated in the design of the study. All authors read and approved the final manuscript. This study was funded by Biomedical Research Council grant: BMRC 03/1/35/19/231, Singapore Eye Research Institute Grant R502/51/2006, and an unrestricted grant from Allergan, Inc. The funding bodies did not play a role in the study design, in the collection, analysis, and interpretation of data, nor in the writing of the manuscript; but BMRC has approved the manuscript for publication. We would like to thank Dr. Li Jing, Singapore Eye Research Institute, for her help in the study on TLR4.
REFERENCES
- 1. Ang L. P.; Tan D. T.; Phan T. T.; Li J.; Beuerman R.; Lavker R. M. The in vitro and in vivo proliferative capacity of serum-free cultivated human conjunctival epithelial cells. Curr Eye Res. 28:307–317; 2004. [DOI] [PubMed] [Google Scholar]
- 2. Araki-Sasaki K.; Ohashi Y.; Sasabe T.; Hayashi K.; Watanabe H.; Tano Y.; Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 36:614–621; 1995. [PubMed] [Google Scholar]
- 3. Blom A. M.; Rytkonen A.; Vasquez P.; Lindahl G.; Dahlback B.; Jonsson A. B. A novel interaction between type IV pili of Neisseria gonorrhoeae and the human complement regulator C4B-binding protein. J. Immunol. 166:6764–6770; 2001. [DOI] [PubMed] [Google Scholar]
- 4. Buron N.; Micheau O.; Cathelin S.; Lafontaine P. O.; Creuzot-Garcher C.; Solary E. Differential mechanisms of conjunctival cell death induction by ultraviolet irradiation and benzalkonium chloride. Invest. Ophthalmol. Vis. Sci. 47:4221–4230; 2006. [DOI] [PubMed] [Google Scholar]
- 5. Chang R. S. M. Continuous subcultivation of epithelial-like cells from normal human tissues. Proc. Soc. Exp. Biol. Med. 87:440–443; 1954. [DOI] [PubMed] [Google Scholar]
- 6. Chen J. T.; Chen C. H.; Horng C. T.; Chien M. W.; Lu D. W.; Liang J. B.; Tai M. C.; Chang Y. H.; Chen P. L.; Chen Y. H. Glucosamine sulfate inhibits proinflammatory cytokine-induced icam-1 production in human conjunctival cells in vitro. J. Ocul. Pharmacol. Ther. 22:402–416; 2006. [DOI] [PubMed] [Google Scholar]
- 7. Corrales R. M.; Diebold Y.; Callejo S.; Calonge M.; Herreras J. M.; Saez V.; Mayo A. In vitro toxicity of non-preserved artificial-tear formulations. Arch. Soc. Esp. Oftalmol. 76:613–619; 2001. [PubMed] [Google Scholar]
- 8. Corsini E.; Sheasgreen J.; Marinovich M.; Galli C. L. Use of differential display-polymerase chain reaction to identify genes selectively modulated by chemical allergens in reconstituted human epidermis. Toxicol. In Vitro 16:427–431; 2002. [DOI] [PubMed] [Google Scholar]
- 9. Debbasch C.; Brignole F.; Pisella P. J.; Warnet J. M.; Rat P.; Baudouin C. Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 42:642–652; 2001. [PubMed] [Google Scholar]
- 10. Debbasch C.; De La Salle S. B.; Brignole F.; Rat P.; Warnet J. M.; Baudouin C. Cytoprotective effects of hyaluronic acid and Carbomer 934P in ocular surface epithelial cells. Invest. Ophthalmol. Vis. Sci. 43:3409–3415; 2002. [PubMed] [Google Scholar]
- 11. Debbasch C.; Pisella P. J.; De Saint Jean M.; Rat P.; Warnet J. M.; Baudouin C. Mitochondrial activity and glutathione injury in apoptosis induced by unpreserved and preserved beta-blockers on Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 42:2525–2533; 2001. [PubMed] [Google Scholar]
- 12. De Saint Jean M.; Baudouin C.; Di Nolfo M.; Roman S.; Lozato P.; Warnet J. M.; Brignole F. Comparison of morphological and functional characteristics of primary-cultured human conjunctival epithelium and of Wong-Kilbourne derivative of Chang conjunctival cell line. Exp. Eye Res. 78:257–274; 2004. [DOI] [PubMed] [Google Scholar]
- 13. De Saint Jean M.; Brignole F.; Bringuier A. F.; Bauchet A.; Feldmann G.; Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 40:619–630; 1999. [PubMed] [Google Scholar]
- 14. De Saint Jean M.; Debbasch C.; Rahmani M.; Brignole F.; Feldmann G.; Warnet J. M.; Baudouin C. Fas- and interferon gamma-induced apoptosis in Chang conjunctival cells: Further investigations. Invest. Ophthalmol. Vis. Sci. 41:2531–2543; 2000. [PubMed] [Google Scholar]
- 15. Diebold Y.; Calonge M.; Carretero V.; Fernandez N.; Herreras J. M. Expression of ICAM-1 and HLA-DR by human conjunctival epithelial cultured cells and modulation by nedocromil sodium. J. Ocul. Pharmacol. Ther. 14:517–531; 1998. [DOI] [PubMed] [Google Scholar]
- 16. Diebold Y.; Calonge M.; Enriquez de Salamanca A.; Callejo S.; Corrales R. M.; Saez V.; Siemasko K. F.; Stern M. E. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest. Ophthalmol. Vis. Sci. 44:4263–4274; 2003. [DOI] [PubMed] [Google Scholar]
- 17. Diebold Y.; Calonge M.; Fernandez N.; Lazaro M. C.; Callejo S.; Herreras J. M.; Pastor J. C. Characterization of epithelial primary cultures from human conjunctiva. Graefes Arch. Clin. Exp. Ophthalmol. 235:268–276; 1997. [DOI] [PubMed] [Google Scholar]
- 18. Diebold Y.; Corrales R. M.; Calonge M.; Saez M. V.; Callejo S.; Lazaro M. C.; Herreras J. M. Human conjunctival epithelium in culture: A tool to assay new therapeutic strategies for dry eye. Adv. Exp. Med. Biol. 506:307–311; 2002. [DOI] [PubMed] [Google Scholar]
- 19. Diebold Y.; Jarrin M.; Saez V.; Carvalho E. L.; Orea M.; Calonge M.; Seijo B.; Alonso M. J. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP). Biomaterials 28:1553–1564; 2007. [DOI] [PubMed] [Google Scholar]
- 20. Doucet O.; Lanvin M.; Thillou C.; Linossier C.; Pupat C.; Merlin B.; Zastrow L. Reconstituted human corneal epithelium: A new alternative to the Draize eye test for the assessment of the eye irritation potential of chemicals and cosmetic products. Toxicol. In Vitro 20:499–512; 2006. [DOI] [PubMed] [Google Scholar]
- 21. Eckert R. L.; Broome A. M.; Ruse M.; Robinson N.; Ryan D.; Lee K. S100 proteins in the epidermis. J. Invest. Dermatol. 123:23–33; 2004. [DOI] [PubMed] [Google Scholar]
- 22. Enriquez de Salamanca A.; Diebold Y.; Calonge M.; Garcia-Vazquez C.; Callejo S.; Vila A.; Alonso M. J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: Toxicity, uptake mechanism and in vivo tolerance. Invest. Ophthalmol. Vis. Sci. 47:1416–1425; 2006. [DOI] [PubMed] [Google Scholar]
- 23. Enriquez de Salamanca A.; Siemasko K. F.; Diebold Y.; Calonge M.; Gao J.; Juarez-Campo M.; Stern M. E. Expression of muscarinic and adrenergic receptors in normal human conjunctival epithelium. Invest. Ophthalmol. Vis. Sci. 46:504–513; 2005. [DOI] [PubMed] [Google Scholar]
- 24. Fillon S.; Lang F.; Jendrossek V. Pseudomonas aeruginosa triggered apoptosis of human epithelial cells depends on the temperature during infection. Cell Physiol. Biochem. 12:207–214; 2002. [DOI] [PubMed] [Google Scholar]
- 25. Gallyas E.; Seprenyi G.; Sonkoly E.; Mandi Y.; Kemeny L.; Megyeri K. Vesicular stomatitis virus induces apoptosis in the Wong-Kilbourne derivative of the Chang conjunctival cell line. Graefes Arch. Clin. Exp. Ophthalmol. 244:717–724; 2006. [DOI] [PubMed] [Google Scholar]
- 26. Garweg J. G.; Wegmann-Burns M.; Goldblum D. Effects of daunorubicin, mitomycin C, azathioprine and cyclosporin A on human retinal pigmented epithelial, corneal endothelial and conjunctival cell lines. Graefes Arch. Clin. Exp. Ophthalmol. 244:382–389; 2006. [DOI] [PubMed] [Google Scholar]
- 27. Gebauer M.; Saas J.; Sohler F.; Haag J.; Soder S.; Pieper M.; Bartnik E.; Beninga J.; Zimmer R.; Aigner T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1beta. Osteoarthritis Cartilage 13:697–708; 2005. [DOI] [PubMed] [Google Scholar]
- 28. Giacomoni P. U. Ageing, science and the cosmetics industry. The micro-inflammatory model serves as a basis for developing effective anti-ageing products for the skin. EMBO Rep. 6(Spec. No.):S45–48; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guenoun J. M.; Baudouin C.; Rat P.; Pauly A.; Warnet J. M.; Brignole-Baudouin F. In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest. Ophthalmol. Vis. Sci. 46:4594–4599; 2005. [DOI] [PubMed] [Google Scholar]
- 30. Irizarry R. A.; Hobbs B.; Collin F.; Beazer-Barclay Y. D.; Antonellis K. J.; Scherf U.; Speed T. P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264; 2003. [DOI] [PubMed] [Google Scholar]
- 31. Jendrossek V.; Fillon S.; Belka C.; Muller I.; Puttkammer B.; Lang F. Apoptotic response of Chang cells to infection with Pseudomonas aeruginosa strains PAK and PAO-I: Molecular ordering of the apoptosis signaling cascade and role of type IV pili. Infect. Immun. 71:2665–2673; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jendrossek V.; Grassme H.; Mueller I.; Lang F.; Gulbins E. Pseudomonas aeruginosa-induced apoptosis involves mitochondria and stress-activated protein kinases. Infect. Immun. 69:2675–2683; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lavappa K. S.; Macy M. L.; Shannon J. E. Examination of ATCC stocks for HeLa marker chromosomes in human cell lines. Nature 259:211–213; 1976. [DOI] [PubMed] [Google Scholar]
- 34. Lee P. R.; Cohen J. E.; Tendi E. A.; Farrer R.; De Vries G. H.; Becker K. G.; Fields R. D. Transcriptional profiling in an MPNST-derived cell line and normal human Schwann cells. Neuron Glia Biol. 1:135–147; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J.; Raghunath M.; Tan D.; Lareu R. R.; Chen Z.; Beuerman R. W. Defensins HNP1 and HBD2 stimulation of wound-associated responses in human conjunctival fibroblasts. Invest. Ophthalmol. Vis. Sci. 47:3811–3819; 2006. [DOI] [PubMed] [Google Scholar]
- 36. Li J.; Shen J.; Beuerman R. W. Expression of tolllike receptors in human limbal and conjunctival epithelial cells. Mol. Vis. 13:813–822; 2007. [PMC free article] [PubMed] [Google Scholar]
- 37. Liu S.; Li J.; Tan D. T.; Beuerman R. W. Expression and function of muscarinic receptor subtypes on human cornea and conjunctiva. Invest. Ophthalmol. Vis. Sci. 48:2987–2996; 2007. [DOI] [PubMed] [Google Scholar]
- 38. Mehta J. P.; O’Driscoll L.; Barron N.; Clynes M.; Doolan P. A microarray approach to translational medicine in breast cancer: How representative are cell line models of clinical conditions? Anticancer Res. 27:1295–1300; 2007. [PubMed] [Google Scholar]
- 39. Mehta K.; Fok J. Y.; Mangala L. S. Tissue transglutaminase: From biological glue to cell survival cues. Front. Biosci. 11:173–185; 2006. [DOI] [PubMed] [Google Scholar]
- 40. Minor S. Y.; Banerjee A.; Gotschlich E. C. Effect of alpha-oligosaccharide phenotype of Neisseria gonorrhoeae strain MS11 on invasion of Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cells. Infect. Immun. 68:6526–6534; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Narayanan S.; Miller W. L.; McDermott A. M. Conjunctival cytokine expression in symptomatic moderate dry eye subjects. Invest. Ophthalmol. Vis. Sci. 47:2445–2450; 2006. [DOI] [PubMed] [Google Scholar]
- 42. Nguyen D.; Beuerman R. W. Three-dimensional construct of the human corneal epithelium for in vitro toxicology. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 43. Ochiai H.; Ochiai Y.; Chihara E. Tranilast inhibits TGF-A1 secretion without affecting its mRNA levels in conjunctival cells. Kobe J. Med. Sci. 47:203–209; 2001. [PubMed] [Google Scholar]
- 44. Papa V.; Leonardi A.; Getuli C.; Pacelli V.; Russo P.; Milazzo G. Effect of ofloxacin and netilmicin on human corneal and conjunctival cells in vitro. J. Ocul. Pharmacol. Ther. 19:535–545; 2003. [DOI] [PubMed] [Google Scholar]
- 45. Raghunath M.; Cankay R.; Kubitscheck U.; Fauteck J. D.; Mayne R.; Aeschlimann D.; Schlotzer-Schrehardt U. Transglutaminase activity in the eye: Cross-linking in epithelia and connective tissue structures. Invest. Ophthalmol. Vis. Sci. 40:2780–2787; 1999. [PubMed] [Google Scholar]
- 46. Saarloos M. N.; Husa M. R.; Jackson R. S. 2nd; Ubels J. L. Intermediate filament, laminin and integrin expression in lacrimal gland acinar cells: Comparison of an immortalized cell line to primary cells, and their response to retinoic acid. Curr. Eye Res. 19:439–449; 1999. [DOI] [PubMed] [Google Scholar]
- 47. Sinniah K.; Paauw J.; Ubels J. Investigating live and fixed epithelial and fibroblast cells by atomic force microscopy. Curr. Eye Res. 25:61–68; 2002. [DOI] [PubMed] [Google Scholar]
- 48. Talreja J.; Dileepan K.; Puri S.; Kabir M. H.; Segal D. M.; Stechschulte D. J.; Dileepan K. N. Human conjunctival epithelial cells lack lipopolysaccharide responsiveness due to deficient expression of MD2 but respond after interferon-gamma priming or soluble MD2 supplementation. Inflammation 29:170–181; 2005. [DOI] [PubMed] [Google Scholar]
- 49. Tong L.; Li J.; Chew J.; Tan D.; Beuerman R. Phospholipase D in the human ocular surface and in pterygium. Cornea 27:693–698; 2008. [DOI] [PubMed] [Google Scholar]
- 50. Ulger C.; Toruner G. A.; Alkan M.; Mohammed M.; Damani S.; Kang J.; Galante A.; Aviv H.; Soteropoulos P.; Tolias P. P.; Schwalb M. N.; Dermody J. J. Comprehensive genome-wide comparison of DNA and RNA level scan using microarray technology for identification of candidate cancer-related genes in the HL-60 cell line. Cancer Genet. Cytogenet. 147:28–35; 2003. [DOI] [PubMed] [Google Scholar]
- 51. Wei C. I.; Chen J. Y.; Kao I. Y.; Pan I. H. Chromosome study and sex determination of Chang human conjunctival cell line. Taiwan Yi Xue Hui Za Zhi 73:267–276; 1974. [PubMed] [Google Scholar]
- 52. Wong Y. W.; Chew J.; Yang H.; Tan D. T.; Beuerman R. Expression of insulin-like growth factor binding protein-3 in pterygium tissue. Br. J. Ophthalmol. 90:769–772; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu E.; Fernandez J.; Fleck S. K.; Von Seggern D. J.; Huang S.; Nemerow G. R. A 50-kDa membrane protein mediates sialic acid-independent binding and infection of conjunctival cells by adenovirus type 37. Virology 279:78–89; 2001. [DOI] [PubMed] [Google Scholar]
- 54. Yim S. H.; Farrer R. G.; Quarles R. H. Expression of glycolipids and myelin-associated glycoprotein during the differentiation of oligodendrocytes: Comparison of the CG-4 glial cell line to primary cultures. Dev. Neurosci. 17:171–180; 1995. [DOI] [PubMed] [Google Scholar]
- 55. Zhan H.; Towler H. M.; Calder V. L. The immuno-modulatory role of human conjunctival epithelial cells. Invest. Ophthalmol. Vis. Sci. 44:3906–3910; 2003. [DOI] [PubMed] [Google Scholar]


