Abstract
Osteoarthritis (OA) is the most frequently diagnosed joint disorder worldwide with increasing prevalence and crucial impact on the quality of life of affected patients through chronic pain, decreasing mobility and invalidity. Although some risk factors, such as age, obesity and previous joint injury are well established, the exact pathogenesis of OA on a cellular and molecular level remains less understood. Today, the role of nitrosative and oxidative stress has not been investigated conclusively in the pathogenesis of OA yet. Therefore, the objective of this study was to identify biological substances for oxidative and nitrosative stress, which mirror the degenerative processes in an osteoarthritic joint. 69 patients suffering from a diagnosed knee pain participated in this study. Based on the orthopedic diagnosis, patients were classified into an osteoarthritis group (OAG, n=24) or in one of two control groups (meniscopathy, CG1, n=11; anterior cruciate ligament rupture, CG2, n=34). Independently from the study protocol, all patients underwent an invasive surgical intervention which was used to collect samples from the synovial membrane, synovial fluid and human serum. Synovial biopsies were analyzed histopathologically for synovitis (Krenn-Score) and immunohistochemically for detection of end products of oxidative (8-isoprostane F2α) and nitrosative (3-nitrotyrosine) stress. Additionally, the fluid samples were analyzed for 8-isoprostane F2α and 3-nitrotyrosine by competitive ELISA method. The analyzation of inflammation in synovial biopsies revealed a slight synovitis in all three investigated groups. Detectable concentrations of 3-nitrotyrosine were reported in all three investigated groups without showing any significant differences between the synovial biopsies, fluid or human serum. In contrast, significant increased concentrations of 8-isoprostane F2α were detected in OAG compared to both control groups. Furthermore, our data showed a significant correlation between the histopathological synovitis and oxidative stress in OAG (r=0.728, P<0.01). There were no significant differences between the concentrations of 8-isoprostane F2α in synovial fluid and human serum. The findings of the current study support the hypothesis that oxidative and nitrosative stress are components of the multi-factory pathophysiological formation of OA. It seems reasonable that an inflammatory process in the synovial membrane triggers the generation of oxidative and nitrosative acting substances which can lead to a further degradation of the articular cartilage. Based on correlations between the observed degree of inflammation and investigated biomarkers, especially 8-isoprostane F2α seems to be a novel candidate biomarker for OA. However, due to the finding that also both control groups showed increased concentrations of selected biomarkers, future studies have to validate the diagnostic potential of these biomarkers in OA and in related conditions of the knee joint.
Key words: Oxidative stress, Nitrosative stress, osteoarthritis
Introduction
Osteoarthritis (OA) describes a heterogenic group of joint diseases, which are characterized by a progressive loss of articular cartilage in association with the formation of subchondral sclerosis and inflammation in the synovial compartments. Through the successive impact of these degenerative changes on the physiological function of the joint, patients complain about prolonged pain, decreased mobility and therefore a consecutive decline in quality of life.1 From epidemiological purposes, OA is one of the most frequently diagnosed diseases worldwide, which is especially pronounced in the elderly, reaching prevalence of about 30% in patients aged over 70 years.2,3 Since OA is mostly diagnosed in the knee and hip joint,4 the resulting decline in functionality in association with the current demographic changes presupposes the importance of comprehensive early diagnostic tools and therapeutic interventions.5
Etiologically, OA can be sub grouped in primary or secondary forms. While the causes for the primary OA remain less understood, the pathophysiology of the secondary OA can be roughly classified into metabolic-endocrine (e.g. chondrocalcinosis, hemochromatosis), anatomical (e.g. malformations, instabilities), traumatic or inflammatory origins.3 Basically, the integrity of joints depends on a dynamic balance between assembling and dismantling of the cartilage components (e.g. chondrocytes, extracellular matrix, synovia).6 If this balance is disturbed, chondrocytes produce increased amounts of extracellular components to preserve the physiological function of the joint, which indeed reduces its resistance against physical and biochemical stress.7 Under these conditions, the cartilage gets successively damaged, which can be morphologically interrelated by the presence of tears in the tissue, inducing increased abrasion by asperities, which in association with inflammatory events induce the irreversible loss of the cartilage.8
Since the success of a non-surgical therapy is closely linked to the time point of the initial diagnosis, a lot of research tries to describe the underlying pathomechanisms of OA formation to facilitate novel diagnostic markers for early diagnosis and potential innovative therapeutic approaches. Several studies were able to prove that the degenerative processes of the cartilage tissue, triggered by altered biomechanical patterns, are closely related to pro-inflammatory events which are associated with an increased impact of oxidative and nitrosative stress.9-12
Reactive oxygen species (ROS) are highly reactive chemical species which are generated physiologically during metabolism or even during aerobic ATP generation in the complexes of the mitochondria.13 In OA, the affected chondrocytes as well as the synoviocytes start to produce proinflammatory cytokines (e.g. interleukin- 1β, tumor necrosis factor α)14-16 which were shown to induce an increased expression of prooxidant enzymes in the synovial membrane, like xanthin-oxidase, which in fact is one of the sources for increased amounts of ROS in OA.17 Additionally, reactive nitrogen species (RNS), which are generated by nitric oxide synthases, are able to react directly with ROS by generating highly reactive molecules18,19 which themselves are able to induce DNA fragmentation or even lipid peroxidation (Figure 1).20
Figure 1.
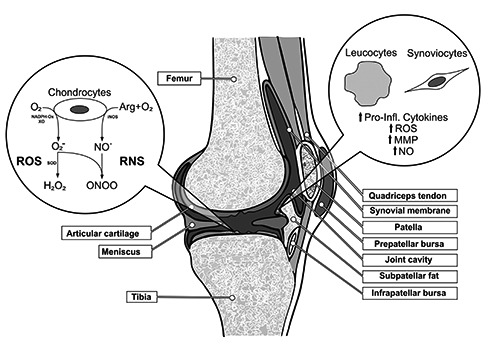
Pathological formation of reactive oxygen species (ROS) and reactive nitrosative species (RNS) in osteoarthritis (OA) of the knee. Additionally, this figure shows the influence of migrated leukocytes and affected synoviocytes in OA pathophysiology. Abbreviations: O2 = oxygen, O2 – = superoxide anion, H2O2 = hydrogen peroxide, NO = nitric oxide, ONOO- = peroxynitrite anion, NADPH-Ox = NADPH-oxidase, XO = xanthine oxidase, SOD = superoxide dismutase, iNOS = inducible nitric oxide synthase, Arg = arginine, MMP = matrix metalloproteinases.
Research in OA was able to show that the amount of RNS as well as the corresponding synthases are upregulated in chondrocytes during the inflammation phases of OA.21 In consideration of further confounders in the pathology of OA, ROS as well as RNS are able to decrease the synthesis capacity of collagen and proteoglycans which thereby supports the tissue breakdown by associated upregulation of matrix metalloproteinases and promotes the maintenance of the pro-inflammatory environment. 14-16
Since this biochemical cascades are also evident in an early stage of OA, the quantification of end-products generated by oxidative and nitrosative stress could be an unexplored diagnostic marker for the determination of the extent of joint damage. Therefore, the aim of the present study was to identify novel biomarkers for degenerative changes of the knee joint, based on the pathophysiological impact of oxidative and nitrosative stress.
Materials and Methods
Study design
The present study focused on the quantification of oxidative and nitrosative stress in OA of the knee. For comparison, patients suffering from OA with consecutive total knee arthroplasty (TKA) surgery were compared to patients with acute knee pain diagnosis and symptom-free patients. The study was composed in a cross-sectional design, where samples from the synovial membrane, synovial fluid as well as human serum were collected during invasive surgery and subsequently analyzed for end products of oxidative and nitrosative stress.
Subjects
Sixty-nine patients who were treated for different knee conditions in our orthopedics department participated in this study. All recruited patients ran through a comprehensive anamnesis (inter alia: size, weight, pain intensity by a visual analog scale, VAS) and physical examination, followed by an X-ray of the affected knee joint. Based on the summarized diagnostic findings (evaluation of the symptomatology, calculation of the cartilage damage, X-ray classification after Kellgren and Lawrence),22 the patients were classified into three groups:
The first group (osteoarthritis group, OAG) consist of patients with a diagnosed OA of the knee joint, who reported chronic knee pain with symptoms lasting longer than six months (n=24). The second group (control group 1, CG1) contains patients suffering from acute knee pain, which consisted less than six months and were free of symptoms before. This particular group acts as a control group and mostly involved patients with meniscal lesions (medial and lateral meniscus) (n=11). The third group acts as a second control group (CG2) which contained patients who were mostly in clinical therapy for an anterior cruciate ligament (ACL) reconstruction during the inflammation-free interval and therefore by definition symptom free at the time of surgery (n=34). Detailed information about the groups are summarized in Table 1.
Table 1.
Demographic characteristics of the three investigated groups.
| OA-Group | Control Group 1 | Control Group 2 | P-values | |
|---|---|---|---|---|
| Subjects (n) | 24 | 11 | 34 | |
| Age (years) | 48.58±2.94 | 52.91±5.01 | 29.74±0.79 | <0.001* |
| Sex (w/m in %) | 41.7 / 58.3 | 36.4 / 63.6 | 58.8 / 41.2 | 0.282 |
| Height (cm) | 171.80±2.06 | 172.89±2.81 | 172.46±1.65 | 0.945 |
| Weight (kg) | 84.25±3.87 | 78.89±5.80 | 85.11±3.73 | 0.679 |
| BMI (kg/m²) | 28.42±1.07 | 26.22±1.39 | 28.50±1.03 | 0.477 |
| Pain (VAS 0-100 mm) | 2.1±0.28 | 1.44±0.29 | 2.07±0.19 | 0.352 |
*significant group difference (P<0.05).
Blood samples
During the pre-operative preparation a venous blood sample of each patient was collected. The samples were subsequently centrifuged, the serum aliquoted and stored at −80°C until analysis.
Synovial biopsies and synovial fluid samples
During arthroscopy (CG1, CG2), respectively TKA surgery (OAG), samples of the synovial membrane as well as the synovial fluid were taken. The fluid samples were collected by an arthroscopic lavage technique subsequently aliquoted and stored at −80°C for later investigation. The taken biopsies were transferred to a 0.9% NaCl solution which was followed by a transfer to a specific tissue freezing medium (Leica Biosystems, Nussloch, Germany) for storing at −80°C and later production of frozen section and immunohistochemical analysis.
Frozen sections and staining
Frozen sections were generated by a cryotome (Mikrotom-Kryostat CM3050S, Leica Biosystems, Nussloch, Germany) with a section thickness of 10 μm. Subsequently, the sections were transferred to uncoated microscope slides (SuperFrost Plus, Thermo Scienific, Waltham, Massachusetts, USA) and dyed with hematoxylin- eosin (HE). For immunohistochemistry, sections were also transferred to specific adhesive microscope slides (Polylysine slides, Thermo Scientific, Waltham, Massachusetts, USA) until analysis.
Quantification of inflammation in synovial biopsies
The histological quantification of inflammation was done by analyzation of the HE-stained frozen sections. Specifically the synovial stroma, the epithelial cells (fibrocytes, fibroblasts, endothelial cells) as well as the migrated leukocytes were evaluated. The histopathological examination was done according to Krenn et al.,23 in which the morphological changes in the stroma and the covering cell layer are used for the calculation of a specific score which is conferrable to the stage of synovitis (0 points = no synovitis; 9 points = high-grade synovitis).
Immunohistochemical detection of 3-nitrotyrosine und 8-isoprostane F2α in synovial biopsies
Primary, frozen sections were incubated with 3% H2O2 in PBS for 20 minutes at room temperature. After three times washing with PBS, the primary antibody was transferred and sections were incubated overnight at 4°C. For quantification of nitrosative stress, a monoclonal mice 3-nitrotyrosine antibody (Millipore, Billerica, Massachusetts, USA) was used, whereas a polyclonal goat 8-isoprostane F2α antibody (Oxford Biomedical Research, Oxford, England) was applied for determination of oxidative stress. After overnight incubation and three times washing with PBST (PBS + 1% Triton X 100), a corresponding secondary biotinylated antibody was transferred and samples were incubated for 45 minutes at room temperature. Subsequently after washing, samples were incubated with AB-complex (BioScience GmbH, Ransbach-Baumbach, Germany) for 20 minutes. For densitometry, peroxidase substrate 3,3‘diaminobenzidine (DAB, Sigma-Aldrich, Saint Louis, Missouri, USA) was applied, quantified by light microscopy (Axioskop 2, Carl Zeiss Microscopy GmbH, Cologne, Germany) and recorded as digital images by an associated digital camera (AxioCam MRc, Carl Zeiss Microscopy GmbH, Cologne, Germany). For analyzation, the software ImageJ 1.47v (Image Processing and Analysis in Java, Wayne Rasband) was used, which measured the staining intensity of three synoviocytes of each dyed section in comparison to background signal. Based on the staining difference between background and selected synoviocyte, it was possible to measure the absolute staining intensity of the cells. This intensity stands for the amount of antigenantibody- complexes and therefore as a semi quantitative degree for our investigated antigens (3-nitrotyrosine and 8-isoprostane F2α). Statistical analyses were done by using the arithmetic average staining intensity.
Quantification of protein content in synovial fluid
The protein content of frozen stored synovial fluid was measured by bicinchoninic acid protein assay (BCA, Micro BCATM Protein Assay Kit, Pierce/Thermo Scientific, Waltham, Massachusetts, USA) and was performed in accordance to Smith et al.24
Quantification of 3-nitrotyrosine and 8-isoprostane F2α in synovial fluid and human serum
For quantification of oxidative and nitrosative end products in synovial fluid and human serum an enzyme-linked immunosorbent assay (ELISA) was applied. Examination of 3-nitrotyrosine was done by OxiSelectTM Nitrotyrosine ELISA kit (Cell Biolabs Inc., San Diego, California, USA). In the same manner, OxiSelectTM 8-iso-Prostaglandin F2α ELISA kit (Cell Biolabs Inc., San Diego, California, USA) was used for evaluation of 8-isoprostane F2α. Photospectrometric data were statistically analyzed by GraphPad Prism version 5 (GraphPad Software Inc., San Diego, California, USA).
Statistical analysis
Frequency distributions were assessed using χ2-test. Pearson’s correlation coefficients were calculated to estimate correlations between the assessed parameters. To compare biomarkers between two groups, ttests were used. Assessment for normality of data was carried out by Kolmogorov- Smirnov test. As the normality condition was fulfilled, a one-way ANOVA was used to determine differences between all three groups. In case of rejected normality or a too small analyzable sample size (n<10) the Kruskal-Wallis test was performed as a nonparametric alternative to the one-way ANOVA. When a significant group effect was found by ANOVA, Tukey’s post hoc tests were performed. Statistical significance was set at P<0.05 for all analyses and means with respective standard deviations are used to present data (M±SD), both in tables and running text. Vertical bars in figures represent standard error of means (SEM). All statistical analyses were performed using SPSS version 17 (SPSS Inc., Chicago, Illinois, USA).
Results
Demographic characteristics and stage of OA
Subjective pain intensity (VAS scale, 0-100mm) did not show a statistical significant correlation with the stage of OA according to Kellgren-Lawrence by Pearson’s correlation coefficient, in either medial (r=-0.039, P=0.77), lateral (r=0.063, P=0.65) or femoropatellar compartment (r=0.202, P=0.14). Within the CG1, arthroscopically seen meniscal lesions of the medial meniscus showed a very large correlation to the perceived pain intensity of the patients (r=-0.756, P=0.02), which was not reportable for lesions of the lateral meniscus (r=0.478, P=0.19).
Concerning the BMI of the patients and the stage of OA after Kellgren-Lawrence a moderate correlation for the medial (r=0.315, P<0.01), lateral (r=0.267, P=0.03) as well as for the femoropatellar compartment (r=0.48, P<0.001) existed. Application of this comparison to the CG1 showed no statistical correlation between the BMI of the patients and the degree of meniscal lesion (medial meniscus: r=0.087, P=0.83; lateral meniscus: r=-0.183, P=0.64).
Degree of synovial inflammation
The degree of inflammation in the investigated synovial biopsies was quantified by synovitis-score after Krenn and colleagues. 23 Based on the histopathologic changes in the HE-stained samples this score was applied for the synovial cover cell layer thickness and conformity, the cell density of the stroma and the amount of leukocyte infiltration. The total count has a range from 0 points (no synovitis) to a maximum of 9 points (high-grade synovitis). An inter-group comparison by ANOVA showed no significant differences between the three investigated groups OAG (0.96±0.22), CG1 (0.27±0.16) and CG2 (0.79±0.21) (P=0.28), representing a low grade synovitis in all three investigated groups.
No significant correlations between the calculated synovitis-score and baseline characteristics of the OAG were found for pain intensity (r=-0.057, P=0.39), BMI (r=0.357, P=0.06) or extend of OA after Kellgren-Lawrence in all compartments (medial: r=0.147, P=0.24; lateral: r=0.147, P=0.24, femoropatellar: r=0.35, P=0.43).
Immunohistochemical detection of 3-nitrotyrosine und 8-isoprostane F2α in synovial biopsies
The quantitative detection of 3-nitrotyrosine by immunohistochemical staining showed comparable values in the three groups [OAG: n=4 (17%), 38.07±4.45; CG1: n=3 (27%), 45.48±3.08; CG2: n=5 (15%), 34.09 ±7.03, P=0.48].
The detection of 8-isoprostane F2α representing oxidative stress showed a trend of higher values in the OAG [n=4 (17%), 77.54 ±5.13[ compared to both control groups [n=3 (27%), CG1: 63.99±2.43; CG2: n=5 (15%), 63.57±1.54], due to the low sample size this difference was not significant (P=0.12).
Quantification of 3-nitrotyrosine und 8-isoprostane F2α in synovial fluid and human serum
Due to the very small concentrations of 3-nitrotyrosine und 8-isoprostane F2α in both fluids of some patients, not all 69 synovial and serum samples were able to detect measurable values of the biomarkers. Especially in the diagnostics of the synovial membrane, only a small amount of samples were able to reach detectable concentrations of questioned biomarkers. Supposedly due to the sampling technique by arthroscopic lavage, the ELISA method was only able to detect valid 3-nitrotyrosine concentrations in 13 of 69 synovial samples. Within the analyzed samples, no significant differences between the OAG (n=3 (13%), 7332.23 ±5895.39 nM), CG1 (n=4 (36%), 2915.15 ±2451.85 nM) and CG2 (n=6 (18%), 539.07±340.39 nM) were found. Accordingly, the quantification of oxidative stress marked by 8-isoprostane F2α in synovial fluid was only successful in 29 of 69 synovial fluid samples. Similarly to the nitrosative stress indicator 3-nitrotyrosine, also the oxidative biomarker showed no significant differences between the groups (OAG: n=9 (38%), 30094.22±2334.35 pg/mL; CG1: n=5 (45%), 16450.29±5806.01 pg/mL; CG2: n=15 (44%), 16522.13±5342.21 pg/mL).
Detectable concentrations of 3-nitrotyrosine and 8-isoprostane F2α in human serum were recorded in 37 of 69 and 48 of 69 samples, respectively. For nitrosative stress, there was no significant difference between the OAG [n=14 (58%), 43.45±9.01 nM] and both control groups [CG1: n=7 (64%), 32.50±11.34 nM, P=0.24; CG2: n=16 (47%), 74.56 ±20.233 nM, P=0.1] in the 3-nitrotyrosine level. Additionally, the comparison between both control groups revealed no significant difference (P=0.1). In context of oxidative stress, the concentrations of 8-isoprostane F2α in the serum were descriptively higher in OAG [n=16 (67%), 3152.3±500.4 pg/mL] than in comparison to CG1 [n=5 (45%), 1723.04±367.46 pg/mL] and CG2 [n=27 (79%), 2875.44 ±281.99 pg/mL], but these differences were not significant (OAG vs. CG1, P=0.07; OAG vs. CG2, P=0.3; Table 2).
Table 2.
Overview of calculated scores and sample analysis for 3-nitrotyrosine and 8-isoprostane of the three investigated groups. Number in parentheses indicates the analyzable sample size for the specific marker within the group. No significant differences between groups were found.
| Subjects (n) | 24 | 11 | 34 |
|---|---|---|---|
| Synovitis score | 0.96±0.22 | 0.27±0.16 | 0.79±0.21 |
| Immunohistochemical staining | |||
| 3-nitrotyrosine | 38.07±4.45(4) | 45.48±3.08(3) | 34.09±7.03(5) |
| 8-isoprostane | 77.54±5.13(4) | 63.99±2.43(3) | 63.57±1.54(5) |
| Synovial concentrations | |||
| 3-nitrotyrosine (nM) | 7332.23±5895.39(3) | 2915.15±2451.85(4) | 539.07±340.39(6) |
| 8-isoprostane (pg/mL) | 30094.22±2334.35(9) | 16450.29±5806.01(5) | 16522.13±5342.21(15) |
| Serum concentrations | |||
| 3-nitrotyrosine (nM) | 43.45±9.01(14) | 32.50±11.34(7) | 74.56±20.233(16) |
| 8-isoprostane (pg/mL) | 3152.3±500.4(16) | 1723.04±367.46(5) | 2875.44±281.99(27) |
Discussion
The main goal of the present study was the identification and quantification of biomarkers for oxidative and nitrosative stress in the pathology of OA of the knee. In fact, we postulated that local damage of the synovial membrane presupposes the appearance of specific biomarkers in the synovial fluid, which could simultaneously be detectable in the blood system by mechanical compression/ decompression of the cartilage or after transport through the lymphatic system.25 Furthermore, in order to classify the appearance of biomarkers, the findings were compared to two control groups, representing on the one hand an acute pain symptomatology (CG1, meniscus lesion) and on the other hand a roughly pain free knee pathology (CG2, ACL-reconstruction). Therefore, samples from synovial membrane, synovial fluid as well as blood serum were investigated to evaluate the level of inflammation and to provide evidences for end products of oxidative (8- isoprostane F2α) and nitrosative (3-nitrotyrosine) stress.
Anamnestic data and their diagnostic potential
The success of clinical interventions in OA is highly depending on the early diagnosis. Therefore, researchers try to find easy collectable data (e.g. anamnestic parameters) or even minimal invasive parameters, which are able to reflect the severity and extent of the OA. Thus, we compared values of perceived pain and BMI with the stage of disease. In context of perceived pain, our findings agreed with former findings of the literature which show no significant correlation between the subjective pain intensity and the severity of the disease in all three investigated groups.26,27 Since the impact of metabolic disorders and overweight are also well established factors of influence in the pathology of OA formation, the correlation between size and weight (illustrated by the BMI) of our patients with their degree of arthrosis are of particular interest. Based on these findings we are able to support the hypothesis that an increased BMI is positively correlated with the degree of arthrosis after Kellgren-Lawrence in all three compartments (medial, lateral, femoropatellar).22 In addition, also the histological evaluation of synovitis after Krenn et al. showed a positive correlation between BMI and synovitis score.23 In fact, there is no valid pathophysiological statement about this interrelation to date.
Nitrosative stress in OA
Previous studies were able to show that increased concentrations of nitrotyrosine are detectable in patients suffering from OA which is supported by our present findings. 28 However, our data showed that synovial biopsies of all three investigated groups indeed contained detectable concentrations of biomarkers for nitrosative stress, but without indicating significant differences. By comparison to previous literature, Sandhu and colleagues reported about crucial differences between nitrotyrosine concentrations of patients with OA and patients with rheumatoid arthritis (RA).28 The increased amount of nitrotyrosine in RA patients was also associated with an increased degree of inflammation in comparison to the OA group. Since our study revealed no significant differences in inflammation between the OA group and both control groups, it can be suggested that our control groups were not applicable as such. Support for this assumption comes from Misko and colleagues,29 which were able to show increased concentrations of 3- nitrotyrosine in synovial fluid of patients with OA, meniscal lesions and ACL-ruptures in comparison to a healthy control group. Although, our data reports similar results for the investigated groups, the presented concentrations have to be seen as approximate values, because the sampling method by arthroscopic lavage presuppose false low concentrations which were mathematically extrapolated after BCA assay. In addition, we suppose that the sampling method was causative for the decreased analyzable samples size by ELISA for both, nitrosative and oxidative, biomarkers. Therefore, nitrotyrosine measurements of synovial biopsies have to be compared to a healthy control group in the future to illustrate their diagnostic potential. However, the detection of nitrotyrosine in synovial fluid seems to be an interesting biomarker for an inflammation associated joint disorder, without showing particular sensitivity for OA.
Similar results were obtained by measurements of nitrotyrosine in human serum. Although, several studies reported about significant increased concentrations of 3- nitrotyrosine (μM-range) in OA patients compared to healthy subjects,30-32 the present data were on the one hand much lower (nM-range) and on the other hand not different from our investigated groups. Within the background of different established methods and detection thresholds, our results are well in line with findings by Kaur & Halliwell, who were additionally able to report that the measurable concentrations of patients with RA were significantly higher than in patients with OA.33 Although, we were able to support our hypothesis that the biomarker for nitrosative stress is supposedly originated by joint degradation due to greater concentrations in the synovial fluid of a symptomatic knee compared to human serum, the investigation of 3-nitrotyrosine in human serum seems to be more reasonable in context of RA than OA.34
Oxidative stress in OA
For identification of oxidative stress in OA the impact of ROS on lipid peroxidation was measured by 8-isoprostane F2α. The current findings show that the concentrations of 8-isoprostane F2α in synovial biopsies seem to be higher in OA patients compared to both control groups, but the analyzable sample size was too small to detect a significant difference. Since both control groups revealed detectable concentrations of 8-isoprostane F2α, we concluded that both groups are not equitable with a healthy patient population in the first place and in addition, that all three clinical indications (OA, meniscus lesion, ACL-lesion) are affected by an increased oxidative stress.35 These findings were supported by a detectable histopathological synovitis in all three investigated groups and by findings of Grigolo et al. who reported increased lipid oxidation in OA patients compared to a healthy control group and RA patients, independently from a rise in NO production as a source for nitrosative stress.35 Based on the significant correlation between the histopathological synovitis and oxidative stress (r=0.728, P<0.01), we suggest a considerable pathophysiological impact of the processes in the synovial membrane on the pro-inflammatory environment. Therefore, it seems reasonable that next to the cartilage and bone tissue also the synovial membrane plays a key role in the pathophysiological formation of OA (Figure 1).16,36,37
Based on the increased concentrations of 8-isoprostane F2α in the synovial membrane we postulated that these local oxidative damages provide detectable concentrations of the biomarker in the synovial fluid and in human serum as well. The current results showed a detectable concentration of 8-isoprostane F2α in both fluids in all investigated groups, which is well in line with findings by Basu et al. who documented similar results in patients suffering with OA and RA.38 In reference to several former studies, our results also failed to indicate a significant correlation between the concentrations of 8-isoprostane F2α in synovial fluid and human serum.38,39 Additionally to a high inter-individuality in 8-isoprostane F2α levels in patients and healthy populations, 38,40,41 several influencing factors, such as anti-inflammatory drug intake of the patient, the small sample size or different applied laboratory methods, could be responsible for the low correspondence between our current and former findings in literature. Summarized, this study was able to show an indirect increase of oxidative stress in all three investigated groups which was evident in synovial biopsies, synovial fluid and human serum. Consequently, enhanced non-enzymatically oxidative processes induced by ROS seem to take an important part in formation of chronic diseases (OA, RA) as well as in acute lesions of the knee joint (meniscus lesion, ACLlesion).
Conclusions
The findings of the current study support the hypothesis that oxidative and nitrosative stress are components of the multi-factorial pathophysiological formation of OA, which has already been shown for other chronic-degenerative diseases such as diabetes mellitus,42 Alzheimer’s disease 43 or several cardiovascular conditions. 44 In context of OA, it seems reasonable that an inflammatory process in the synovial membrane triggers the generation of oxidative and nitrosative acting substances which may lead to a further degradation of the articular cartilage. Based on correlations between the degree of inflammation and the radiological scores for OA according to Kellgren-Lawrence, 3-nitrotyrosine und 8-isoprostan F2α seem to be novel candidate biomarkers for OA. However, due to the finding that also both control groups showed increased concentrations of selected biomarkers, future studies have to validate the diagnostic potential of the biomarkers in OA and in other medical conditions of the knee joint (meniscus lesion, ACL-lesion).
Funding Statement
Funding: none.
References
- 1.Hunter DJ. Osteoarthritis. Best Pract Res Clin Rheumatol 2011;25:801-14. [DOI] [PubMed] [Google Scholar]
- 2.Delmas PD, Anderson M. Launch of the bone and joint decade 2000-2010. Osteoporos Int 2000;11:95-7. [DOI] [PubMed] [Google Scholar]
- 3.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006;20:3-25. [DOI] [PubMed] [Google Scholar]
- 4.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the health, aging and body composition study. J Am Geriatr Soc 2003;51:323-30. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Prevalence of self-reported arthritis or chronic joint symptoms among adults—United States, 2001. MMWR Morb Mortal Wkly Rep 2002;51:948-50. [PubMed] [Google Scholar]
- 6.Eyre DR. Collagens and cartilage matrix homeostasis. Clin Orthop Relat Res 2004:S118-22. [DOI] [PubMed] [Google Scholar]
- 7.Hackenbroch MH. Arthrosen: Basiswissen zu Klinik, Diagnostik und Therapie; 44 Tabellen. Stuttgart: Thieme; 2002. [Google Scholar]
- 8.Kohn D, Adam F, Wirth CJ, eds. Knie: 67 Tabellen. Orthopadie und orthopadische Chirurgie,/hrsg. von Carl Joachim Wirth. Stuttgart: Thieme; 2005. [Google Scholar]
- 9.Henrotin YE, Bruckner P, Pujol JPL. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr Cartil 2003;11:747-55. [DOI] [PubMed] [Google Scholar]
- 10.Martin JA, Brown TD, Heiner AD, Buckwalter JA. Chondrocyte senescence, joint loading and osteoarthritis. Clin Orthop Relat Res 2004:S96-103. [DOI] [PubMed] [Google Scholar]
- 11.Martin JA, Brown T, Heiner A, Buckwalter JA. Post-traumatic osteoarthritis: The role of accelerated chondrocyte senescence. Biorheology 2004;41:479-91. [PubMed] [Google Scholar]
- 12.Greenwald RA. Oxygen radicals, inflammation, and arthritis: Pathophysiological considerations and implications for treatment. Semin Arthritis Rheum 1991;20:219-40. [DOI] [PubMed] [Google Scholar]
- 13.Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans 2003;31:1300-1. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002;39:237-46. [PubMed] [Google Scholar]
- 15.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum 2001;44:1237-47. [DOI] [PubMed] [Google Scholar]
- 16.Krasnokutsky S, Samuels J, Abramson SB. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis 2007;65:222-8. [PubMed] [Google Scholar]
- 17.Clavijo-Cornejo D, Martinez-Flores K, Silva-Luna K, et al. The overexpression of nalp3 inflammasome in knee osteoarthritis is associated with synovial membrane prolidase and NADPH Oxidase 2. Oxid Med Cell Longev 2016;2016:1472567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol 1997;15:323-50. [DOI] [PubMed] [Google Scholar]
- 19.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol 1996;271:C1424-37. [DOI] [PubMed] [Google Scholar]
- 20.Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: Reaction pathways and antioxidant protection. Arterioscler Thromb Vasc Biol 2000;20:1716-23. [DOI] [PubMed] [Google Scholar]
- 21.Fermor B, Weinberg JB, Pisetsky DS, et al. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res 2001;19:729-37. [DOI] [PubMed] [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krenn V, Morawietz L, Burmester GR, Haupl T. Synovialitis-Score: Histopathologisches Graduierungsschema rheumatischer und nicht-rheumatischer Synovialitiden. Z Rheumatol 2005; 64:334-42. [DOI] [PubMed] [Google Scholar]
- 24.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985;150:76-85. [DOI] [PubMed] [Google Scholar]
- 25.Junqueira LCU, Carneiro J, Kelley RO, eds. Histologie: Mit 14 Tabellen. 5th ed. Springer-Lehrbuch. Berlin: Springer; 2002. [Google Scholar]
- 26.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365:965-73. [DOI] [PubMed] [Google Scholar]
- 27.Tornbjerg SM, Nissen N, Englund M, et al. Structural pathology is not related to patient-reported pain and function in patients undergoing meniscal surgery. Br J Sports Med 2017;51:525-30. [DOI] [PubMed] [Google Scholar]
- 28.Sandhu JK, Robertson S, Birnboim HC, Goldstein R. Distribution of protein nitrotyrosine in synovial tissues of patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 2003; 30:1173-81. [PubMed] [Google Scholar]
- 29.Misko TP, Radabaugh MR, Highkin M, et al. Characterization of nitrotyrosine as a biomarker for arthritis and joint injury. Osteoarthr Cartil 2013;21:151-6. [DOI] [PubMed] [Google Scholar]
- 30.Ersoy Y, Ozerol E, Baysal O, et al. Serum nitrate and nitrite levels in patients with rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis. Ann Rheum Dis 2002;61:76-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karan A, Karan MA, Vural P, et al. Synovial fluid nitric oxide levels in patients with knee osteoarthritis. Clin Rheumatol 2003;22:397-9. [DOI] [PubMed] [Google Scholar]
- 32.Suantawee T, Tantavisut S, Adisakwattana S, et al. Upregulation of inducible nitric oxide synthase and nitrotyrosine expression in primary knee osteoarthritis. J Med Assoc Thai 2015;98:S91-7. [PubMed] [Google Scholar]
- 33.Kaur H, Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett 1994;350:9-12. [DOI] [PubMed] [Google Scholar]
- 34.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis 1992;51:1219-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grigolo B, Roseti L, Fiorini M, Facchini A. Enhanced lipid peroxidation in synoviocytes from patients with osteoarthritis. J Rheumatol 2003;30: 345-7. [PubMed] [Google Scholar]
- 36.Shah R, Raska K, Tiku ML. The presence of molecular markers of in vivo lipid peroxidation in osteoarthritic cartilage: A pathogenic role in osteoarthritis. Arthritis Rheum 2005;52:2799-807. [DOI] [PubMed] [Google Scholar]
- 37.Samuels J, Krasnokutsky S, Abramson SB. Osteoarthritis: A tale of three tissues. Bull NYU Hosp Jt Dis 2008;66:244-50. [PubMed] [Google Scholar]
- 38.Basu S, Whiteman M, Mattey DL, Halliwell B. Raised levels of F(2)-isoprostanes and prostaglandin F(2alpha) in different rheumatic diseases. Ann Rheum Dis 2001;60:627-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaturvedi V, Handa R, Rao DN, Wali JP. Estimation and significance of serum and synovial fluid malondialdehyde levels in rheumatoid arthritis. Indian J Med Res 1999;109:170-4. [PubMed] [Google Scholar]
- 40.Abou-Raya A, el-Hallous D, Fayed H. 8-Isoprostaglandin F2 alpha: A potential index of lipid peroxidation in systemic lupus erythematosus. Clin Invest Med. 2004;27:306-11. [PubMed] [Google Scholar]
- 41.Ames PR, Alves J, Murat I, et al. Oxidative stress in systemic lupus erythematosus and allied conditions with vascular involvement. Rheumatology 1999;38:529-34. [DOI] [PubMed] [Google Scholar]
- 42.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol 2003;17:24-38. [DOI] [PubMed] [Google Scholar]
- 43.Schrag M, Mueller C, Zabel M, et al. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol Dis 2013; 59:100-10. [DOI] [PubMed] [Google Scholar]
- 44.El Assar M, Angulo J, Rodriguez-Manas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 2013;65:380-401. [DOI] [PubMed] [Google Scholar]


