Significance
Little is known about the cellular origin and the molecular signals that regulate spinal cord ependymal cells. In this report, we characterize Wnt-responsive progenitor cells throughout spinal cord development, showing that they are restricted to the dorsal midline and give rise to dorsal ependymal cells in a spatially restricted pattern. In the postnatal and adult spinal cord, ependymal cells continue to exhibit Wnt/β-catenin signaling activity, which promotes ependymal cell proliferation. This is demonstrated by the genetic elimination of β-catenin and inhibition of Wnt secretion in Wnt-activated ependymal cells in vivo, which result in impaired proliferation. Our results thus reveal the molecular signals underlying the formation and regulation of spinal cord ependymal cells.
Keywords: ependymal cells, spinal cord, Wnt
Abstract
In the adult mouse spinal cord, the ependymal cell population that surrounds the central canal is thought to be a promising source of quiescent stem cells to treat spinal cord injury. Relatively little is known about the cellular origin of ependymal cells during spinal cord development, or the molecular mechanisms that regulate ependymal cells during adult homeostasis. Using genetic lineage tracing based on the Wnt target gene Axin2, we have characterized Wnt-responsive cells during spinal cord development. Our results revealed that Wnt-responsive progenitor cells are restricted to the dorsal midline throughout spinal cord development, which gives rise to dorsal ependymal cells in a spatially restricted pattern. This is contrary to previous reports that suggested an exclusively ventral origin of ependymal cells, suggesting that ependymal cells may retain positional identities in relation to their neural progenitors. Our results further demonstrated that in the postnatal and adult spinal cord, all ependymal cells express the Wnt/β-catenin signaling target gene Axin2, as well as Wnt ligands. Genetic elimination of β-catenin or inhibition of Wnt secretion in Axin2-expressing ependymal cells in vivo both resulted in impaired proliferation, indicating that Wnt/β-catenin signaling promotes ependymal cell proliferation. These results demonstrate the continued importance of Wnt/β-catenin signaling for both ependymal cell formation and regulation. By uncovering the molecular signals underlying the formation and regulation of spinal cord ependymal cells, our findings thus enable further targeting and manipulation of this promising source of quiescent stem cells for therapeutic interventions.
The central canal of the adult mammalian spinal cord is lined by a layer of ependymal cells, which are located at the interface between the parenchyma and the central canal. Ependymal cells serve important sensory and mechanical functions, including signal transduction, movement of cerebral spinal fluid (CSF), and insulation of neural tissues from damaging substances (1, 2). Recent studies have suggested that adult ependymal cells are progenitors that can act as latent neural stem cells (3–8). Among the cell types within the adult spinal cord, ependymal cells are unique in their capacity for proliferation and renewal during homeostasis and in their multipotency both in vitro and in vivo in response to injury (9–16). In response to certain injuries, ependymal cells generate oligodendrocytes and astrocytes that contribute to glial scar formation (14–17). In contrast to ependymal cells in the forebrain, ependymal cells in the spinal cord can self-renew and are not depleted during an injury response. Despite their functional importance, very little is known about the molecular mechanisms underlying ependymal cell formation and regulation.
Neuroepithelial and radial glial cells are the main neural progenitors at different stages of embryonic spinal cord development. Both cell types line the ventricular lumen and have a distinct radial morphology (18). Their cell bodies are located in the ventricular zone and their processes radiate from the ventricle to the pial surface. Neuroepithelial cells are the earliest precursors and are present before the onset of neurogenesis. They give rise to radial glial cells, which then become the predominant progenitors during gliogenesis (19, 20). Eventually, ependymal cells are formed as the ventricle and the ventricular zone retract through the process of obliteration. This process is accompanied by terminal differentiation and exit of radial glial cells from the ventricular zone (18, 19, 21–24).
Compared with our knowledge about ependymal cells in the brain, little is known about the cellular origin of spinal cord ependymal cells. It has been shown that ependymal cells in the brain are derived from radial glial cells; however, a similar lineage relationship has not been definitively established in the spinal cord (25). Furthermore, the regional identities of the neural progenitor cells that give rise to spinal cord ependymal cells remain unclear. Neural progenitors from distinct domains along the dorsal–ventral axis in the developing spinal cord give rise to distinct subclasses of neurons and glial cells, but it remains unknown whether progenitors from all domains or only a subset of them generate ependymal cells (26–29). Based on their selective expression of ventral progenitor markers, it has been suggested that ependymal cells are derived exclusively from ventral neural progenitors; however, without genetic lineage tracing, this hypothesis has not been directly proven (23).
Wnt signaling has been shown to regulate neural progenitor cells in the developing spinal cord. During the early embryonic stages, Wnt ligands, specifically Wnt1 and Wnt3a, are secreted from the dorsal roof plate and act as mitogenic signals by promoting proliferation and inhibiting differentiation of ventricular neural progenitor cells (30–35). The dorsal source of Wnts also patterns the developing spinal cord through the specification of dorsal cell fates (36–39). However, the regional distribution of Wnt-responsive neural progenitor cells at major stages of spinal cord development has not been characterized. The role of Wnt signaling on ventricular neural progenitor cells during late embryonic stages also remains unclear.
In this study, we have systematically characterized Wnt-responsive progenitor cells throughout spinal cord development, showing that they are restricted to the dorsal midline, and give rise to dorsal ependymal cells in a spatially restricted pattern. Using genetic lineage tracing, we revealed the developmental origin of spinal cord ependymal cells. Our findings are contrary to previous reports that pointed to an exclusively ventral origin of ependymal cells, suggesting that ependymal cells may retain positional identities in relation to their neural progenitors, a pattern that is strikingly similar to the established segmental template model of astrocyte generation. Furthermore, we demonstrated that in the postnatal and adult spinal cord, ependymal cells continue to exhibit Wnt/β-catenin signaling activity, which interestingly, no longer follows a spatially restricted pattern. In fact, all ependymal cells in the postnatal and adult spinal cord express the Wnt/β-catenin signaling target gene Axin2, as well as Wnt ligands. Using conditional knockout animals, we further demonstrated that Wnt/β-catenin signaling is required for ependymal cell proliferation and maintenance.
Results
Wnt-Responsive Neural Progenitor Cells Give Rise to Ependymal Cells.
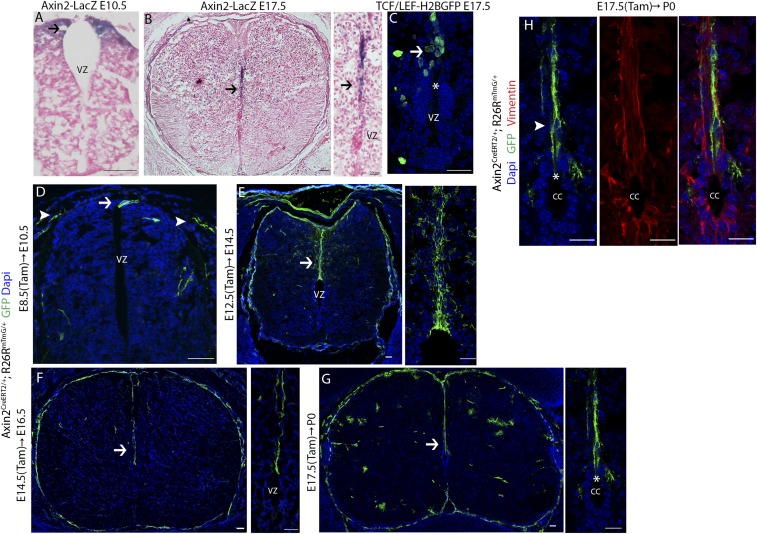
We began by identifying Wnt-responsive cells during the various stages of spinal cord development. Axin2 is regarded as a universal target gene of Wnt/β-catenin signaling, and its expression is used as a marker for pathway activity. We therefore examined Axin2-LacZ reporter embryos at the beginning of neurogenesis [embryonic day 10.5 (E10.5)] and at the time when the ventricular zone becomes the ependymal layer (E17.5) (22). In E10.5 embryos, we observed LacZ signal near the roof plate at the dorsal midline of the developing neural tube (Fig. 1A, arrow). In E17.5 embryos, the ventricle has undergone obliteration and reduced to a ring. LacZ signal was detected along the dorsal midline above the ventricle (Fig. 1B, arrows). Consistent with this observation, the Wnt/β-catenin signaling reporter line, TCF/Lef-H2B:GFP, also showed active Wnt/β-catenin signaling along the dorsal midline and above the ventricle at E17.5 (Fig. 1C, arrow). These results demonstrate that Wnt-responsive cells reside along the dorsal midline at both early and late stages of spinal cord development.
Fig. 1.
Dorsal midline neural progenitor cells are Wnt responsive throughout spinal cord development. (A) Histologic X-gal stained sections of spinal cord from E10.5 Axin2-LacZ mice counterstained with Nuclear Fast Red. Arrow indicates Axin2-LacZ+ cells. (B) Histologic X-gal stained sections of spinal cord from E17.5 Axin2-LacZ mice counterstained with Nuclear Fast Red. Axin2-LacZ+ cells concentrate along the dorsal midline. Arrow indicates Axin2-LacZ+ cells. (C) The central canal of the spinal cord from E17.5 TCF/Lef:H2B-GFP. GFP+ cells concentrate along the dorsal midline. Dotted line outlines the central canal. Asterisk indicates the gap at the dorsalmost portion. (D) Cross-sectional image of Axin2CreERT2/+; Rosa26mTmG/+ spinal cord labeled at E8.5 and analyzed at E10.5. Axin2CreERT2 marks dorsal midline neuroepithelial cells. Arrow indicates GFP+ neuroepithelial cells. Arrowheads indicate GFP+ neural crest cells. (E and F) Cross-sectional images of Axin2CreERT2/+; Rosa26mTmG/+ spinal cord labeled at E12.5 and analyzed at E14.5 (E), or labeled at E14.5 and analyzed at E16.5 (F). GFP+ radial glial cells span the entire dorsal midline. (G) Dorsal midline radial glial cells retain radial processes that span the entire length from the central canal to the dorsal pial surface and are labeled with GFP upon tamoxifen administration at E17.5. (H) GFP+ radial glial cells labeled at E17.5 and analyzed at P0 express Vimentin. (Scale bar, 50 µm.)
To examine the precise cellular identities of Wnt-responsive cells in the developing spinal cord, we crossed Axin2CreERT2 mice with the Rosa26mTmG reporter strain to pulse label Wnt-responsive cells at various time points—E8.5–E10.5, E12.5–E14.5, and E17.5–postnatal day 0 (P0)—corresponding to the presence of the types of predominant neural progenitors at each time point (40, 41). In Axin2CreERT2/+; Rosa26mTmG/+ mice, a small subset of Axin2+ cells are stochastically labeled with membrane GFP upon low-dose tamoxifen administration and induction of Cre activity (42, 43). We first administered low-dose tamoxifen to pregnant females bearing Axin2CreERT2/+; Rosa26mTmG/+ embryos at E8.5. Two days after tamoxifen administration (E10.5), embryos were examined for the presence of GFP+ cells. During this time window, neuroepithelial cells are the predominant neural progenitor cells (18). We detected GFP+ neuroepithelial cells by their characteristic radial morphology near the roof plate along the midline of the developing spinal cord (Fig. 1D, arrow). In addition, GFP+ cells within the neural crest population were also detected based on their morphology and location (Fig. 1D, arrowhead). Thus, Axin2CreERT2 marks dorsal midline neuroepithelial cells in the early spinal cord. This expression pattern of the pathway target gene Axin2 corresponds to the spatially restricted expression of the Wnt ligands, Wnt1 and Wnt3a, at the dorsal midline, as reported previously (30, 31).
Subsequently, tamoxifen was administered at E12.5, and embryos were analyzed at E14.5. At this time, the ventricle is reduced in size along with an increase in the distance between the ventricle and the dorsal pial surface. At this stage, radial glial cells have become the predominant neural progenitor cell population (19). Interestingly, we detected GFP+ radial glial cells that spanned the entire dorsal midline (Fig. 1E and SI Appendix, Fig. S1) as indicated by their morphology. Additional Wnt-responsive cells in the gray matter were also labeled, which are likely differentiated glial cells. When we administered tamoxifen at E14.5, and analyzed the embryos at E16.5, we found that dorsal midline radial glial cells remained Wnt responsive (Fig. 1F and SI Appendix, Fig. S1). Their processes have further elongated to accompany the continued reduction of the ventricle. Interestingly, it has been reported that by E16, the majority of the radial glial cells have undergone terminal differentiation, while those that remain exhibit a “cruciform” pattern, concentrating only along the dorsal midline and horizontally along the lateral midline (18). Our findings thus suggest that only the remaining radial glial cells along the dorsal midline, and not those along the lateral midline, in E16.5 spinal cords correlate with active Wnt signaling.
It has been shown that the dorsal midline radial glial cells still retain their radial glial morphology at P0, while the majority of the radial glial cells have lost their processes and undergone terminal differentiation (18). When we administered tamoxifen at E17.5, and analyzed newborn mice at P0, we found that these dorsal midline radial glial cells (as shown by cytoplasmic Vimentin staining) remained Wnt-responsive (Fig. 1 G and H and SI Appendix, Fig. S1). A number of Wnt-responsive fibrous and protoplasmic astrocytes in the white matter and gray matter were also observed. In P0 spinal cords, the ventricle is reduced to a ring and will be referred to hereafter as the central canal. The central canal becomes lined by ependymal cells, except dorsally, where a gap remains (22, 44). Interestingly, the cell bodies of Wnt-responsive radial glial cells were found above the gap (Fig. 1H, arrowhead), while their processes span the entire length from the central canal to the dorsal pial surface (Fig. 1 G and H). Even though the phenomenon of a gap at the dorsalmost portion of the central canal when it is first formed has been observed previously, its functional significance has not been characterized. This phenomenon will be further discussed in the following paragraphs. The above results from pulse labeling Wnt-responsive cells at various time points collectively suggest that dorsal midline neural progenitor cells are Wnt responsive throughout spinal cord development.
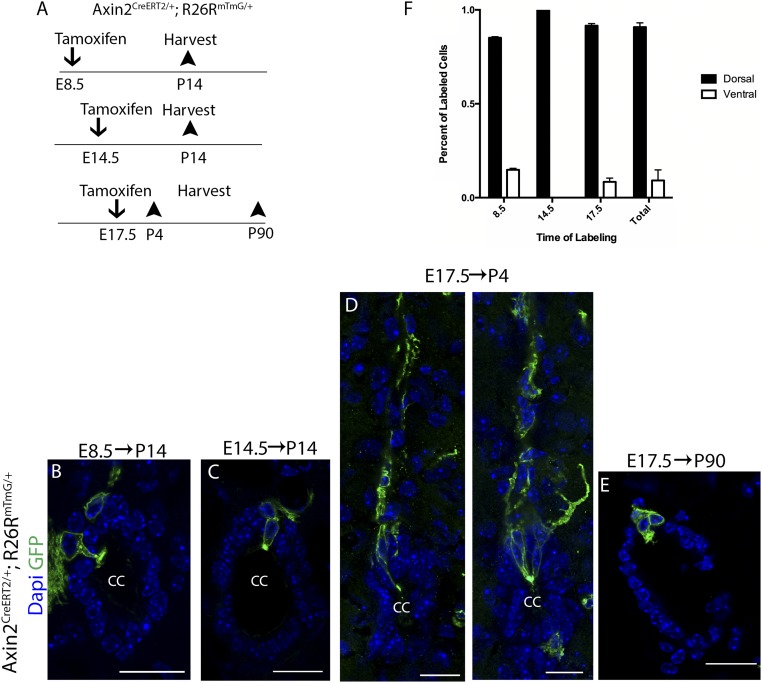
To directly test whether Wnt-responsive neural progenitors are related to mature ependymal cells, we followed the fate of Wnt-responsive neuroepithelial cells and radial glial cells by injecting tamoxifen into pregnant females bearing Axin2CreERT2/+; Rosa26mTmG/+ embryos at the same time points as shown above (Fig. 1) and traced them into postnatal stages. This allows us to investigate whether the labeled Axin2+ progenitor cells give rise to mature ependymal cells. We first injected tamoxifen into pregnant females bearing Axin2CreERT2/+; Rosa26mTmG/+ embryos at E8.5 and allowed their progenies to develop until P14 (Fig. 2A). As shown in Fig. 1D, Wnt-responsive neuroepithelial cells found at E8.5 are located near the roof plate along the midline of the developing spinal cord and are sparsely labeled upon low dose of tamoxifen administration. Upon tracing to P14, we detected GFP+ ependymal cells around the central canal (Fig. 2B and SI Appendix, Fig. S2), indicating that Wnt-responsive neuroepithelial cells give rise to ependymal cells. Next, we labeled a subset of Wnt-responsive radial glial cells found at E14.5 with tamoxifen injection (as shown in Fig. 1F) and analyzed the spinal cords at P14. GFP+ ependymal cells were again detected around the central canal (Fig. 2C and SI Appendix, Fig. S2).
Fig. 2.
Wnt-responsive neural progenitor cells give rise to ependymal cells. (A) Timeline for the lineage tracing experiments. (B) Wnt-responsive dorsal neuroepithelial cells labeled at E8.5 generate dorsal ependymal cells when analyzed at P14. (C) Wnt-responsive radial glial cells labeled at E14.5 generate ependymal cells when analyzed at P14. (D) Wnt-responsive radial glial cells labeled at E17.5 transform into ependymal cells by P4. (E) Wnt-responsive radial glial cells labeled at E17.5 transform into adult ependymal cells that persist for an extended period of time. (F) Quantification of labeled dorsal versus ventral cells upon tracing from embryonic stages (total number of traced animals = 6, P < 0.0001). (Scale bar, 50 µm.)
To further examine the transition from radial glial cells to ependymal cells, we proceeded to label a subset of Wnt-responsive radial glial cells at E17.5 (as shown in Fig. 1 G and H), and analyzed the spinal cords at P4. Labeled cells gave rise to dorsal ependymal cells that retained prominent radial processes which extended toward the dorsal pial surface (Fig. 2D and SI Appendix, Fig. S2) and filled in the remaining gap of the central canal that was observed at P0. This result suggests a direct transformation from radial glial cells to ependymal cells. Furthermore, the morphology of these newly generated ependymal cells is consistent with that of radial ependymal cells, which are most often found at the dorsal and ventral poles (13). Finally, when dorsal midline radial glial cells marked at E17.5 were traced out well into adulthood (P90), we again observed labeled ependymal cells, suggesting that radial glial cells at the dorsal midline give rise to ependymal cells that persist for an extended period of time in the adult spinal cord (Fig. 2E and SI Appendix, Fig. S2). Quantification of the percentage of labeled cells that were found at dorsal versus ventral portion of the central canal when traced from the above embryonic stages shows that labeled dorsal midline neural progenitors give rise primarily to dorsal ependymal cells (Fig. 2F). Our findings thus demonstrate that, contrary to previous reports that suggested an exclusively ventral origin of ependymal cells (23, 45), dorsal midline neural progenitors also give rise to ependymal cells and notably do so in a spatially restricted manner. This suggests that ependymal cells may retain positional identities in relation to their neural progenitors. Furthermore, our results reveal that the cell bodies of the dorsal midline radial glial cells are the last to be integrated into the ependymal layer, which occurs in the first postnatal week.
Our combined results indicate that Wnt signaling is selectively activated in neural progenitor cells at the dorsal midline during all major developmental stages. In particular, Wnt-responsive radial glial cells persist into the first postnatal week before giving rise to dorsal pole ependymal cells that retain prominent radial processes, highlighting a unique cellular origin of radial ependymal cells. Radial ependymal cells at the dorsal pole are generated last among all ependymal cells, consistent with previous findings that the generation of ependymal cells in the lateral ventricles of the brain followed a ventral-to-dorsal direction (25). Previous reports hypothesize an exclusively ventral origin of ependymal cells; our findings demonstrate that dorsal neural progenitors give rise to dorsal ependymal cells, suggesting that ependymal cells may retain positional identities in relation to their neural progenitors. This pattern of ependymal cell generation is strikingly similar to the established segmental template model of astrocyte generation, where astrocytes are allocated to particular spatial domains following a segmental template in accordance with their embryonic sites of origin in the ventricular zone (29, 46, 47).
Ependymal Cells Are Wnt Responsive and Wnt Producing in the Postnatal and Adult Spinal Cord.
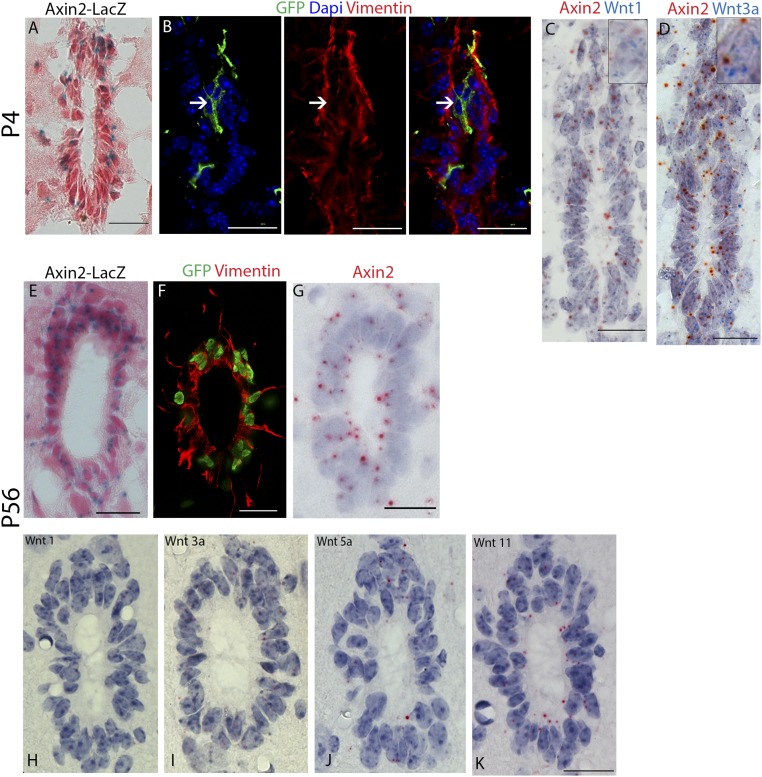
We have shown that Wnt-responsive neural progenitors give rise to ependymal cells; however, whether ependymal cells continue to exhibit Wnt/β-catenin signaling activity in the postnatal and adult spinal cord is not known. We first analyzed Axin2-LacZ reporter mice at P4 and detected LacZ signal in cells lining the central canal (Fig. 3A). To pinpoint these Axin2+ cells, we marked a subset of these cells by administering tamoxifen to Axin2CreERT2/+; Rosa26mTmG/+ mice at P4 and analyzed the spinal cord 2 d later. Labeled cells lining the central canal also expressed the ependymal cell marker Vimentin, suggesting that ependymal cells in the early postnatal spinal cord are Wnt responsive (Fig. 3B).
Fig. 3.
Ependymal cells are Wnt responsive and Wnt producing in the postnatal and adult spinal cord. (A) Histologic X-gal stained sections of spinal cord from P4 Axin2-LacZ mice counterstained with Nuclear Fast Red. (B) Axin2CreERT2/+; Rosa26mTmG/+ spinal cord. Labeled cells at P4 express the ependymal cell marker Vimentin. Arrows indicated GFP+ Vimentin+ ependymal cells. (C and D) In situ hybridization against Axin2, Wnt1, and Wnt3a on spinal cord sections from P4 wild-type mice. (E) Histologic X-gal stained sections of spinal cord from adult (P56) Axin2-LacZ mice counterstained with Nuclear Fast Red. (F) Vimentin (red) staining on spinal cord sections from adult (P56) TCF/Lef:H2B-GFP mice. (G) In situ hybridization against Axin2 on spinal cord sections from adult (P56) wild-type mice. (H–K) In situ hybridization against Wnt1, Wnt3a, Wnt5a, and Wnt11 on spinal cord sections from adult (P56) wild-type mice. (Scale bar, 50 µm.)
When examining the source of Wnt ligands by double labeling in situ hybridization, we found that Axin2-expressing ependymal cells are also the source of the Wnt ligands, Wnt1 and Wnt3a, previously described as mitogenic Wnt ligands that promote neural progenitor proliferation (Fig. 3 C and D) (32, 33, 36). Thus, our results suggested that ependymal cells in the early postnatal spinal cord are both Wnt responsive and Wnt producing.
When we examined Wnt/β-catenin signaling activity in adult reporter mice, we detected LacZ signal in all ependymal cells of Axin2-LacZ animals (Fig. 3E) and GFP signal in ependymal cells of TCF/Lef:H2B-GFP animals (Fig. 3F), suggesting that Wnt/β-catenin signaling continues to be activated. Moreover, Axin2 mRNA expression was detected in all ependymal cells by in situ hybridization (Fig. 3G), as well as expression of Wnt1 and Wnt3a (Fig. 3 H and I), in addition to Wnt5a and Wnt11 (Fig. 3 J and K). Our results thus suggest that shortly after birth, ependymal cells become both Wnt responsive and Wnt producing and remain so throughout the animal’s lifetime.
Dorsal Ependymal Cells Expand at a Higher Rate Under Homeostasis.
Previous studies have established that unlike ependymal cells at the lateral ventricles of the brain, spinal cord ependymal cells proliferate under homeostatic conditions (2, 4, 13, 14, 48) to maintain the ependyma. To further interrogate the dynamics of ependymal cell proliferation, we used a similar lineage tracing approach to examine the contribution of pulse-labeled cells to the ependyma over time.
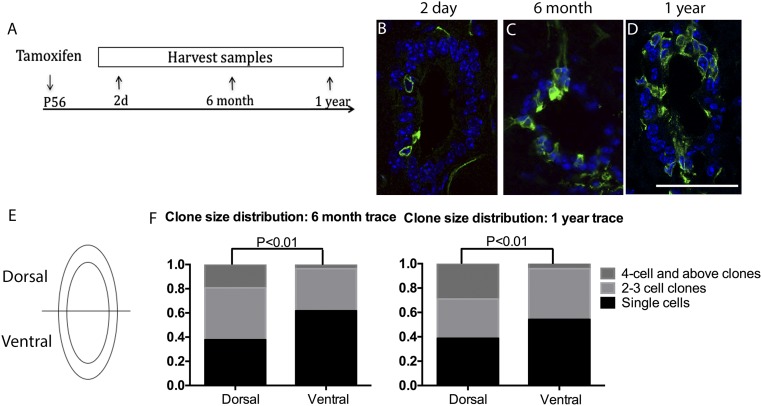
We initiated labeling of Axin2CreERT2/+; Rosa26mTmG/+ mice at P56 and analyzed the spinal cord 2 d, 6 mo, and 1 y after (Fig. 4A). In the initial pulse-labeled (2 d posttamoxifen treatment) mice, singly labeled ependymal cells were observed at a low frequency in all sections examined (Fig. 4B). Over time, the population of labeled ependymal cells expanded into clones (defined as a cluster of similarly labeled cells) that remained in the ependymal layer (Fig. 4 C and D). This is consistent with previous observations that ependymal cells are lineage restricted during homeostasis (13, 14). Interestingly, we observed larger clones originating from dorsal ependymal cells compared with ventral cells, which frequently remain as single cells. Upon quantification of the number and size of clones in the dorsal versus ventral ependyma, we found that 6 mo after initial labeling, the dorsal ependyma is enriched for clones containing four or more cells, whereas the ventral ependyma contained significantly more single cells (Fig. 4 E and F). This difference was maintained 1 y after initial labeling (Fig. 4F), consistent with a previous report showing a higher number of Ki67+ cells at the dorsal pole compared with the ventral pole of the ependyma (4). Thus, our lineage tracing study shows that ependymal cells expand clonally and remain in the ependyma. Our results further highlight the heterogeneity in the proliferative potential of ependymal cells and suggest that dorsal ependymal cells expand at a higher rate than ventral ependymal cells.
Fig. 4.
Dorsal ependymal cells expand at a higher rate under homeostasis. (A) Experimental schedule for the lineage tracing experiments. (B–D) Cross-sectional images of GFP (green) immunostaining and DAPI (blue) of Axin2CreERT2/+; Rosa26mTmG/+ spinal cord traced for 2 d, 6 mo, or 1 y from P56. (Scale bar, 100 um.) (E) Schematic of the dorsal and ventral domains used for quantification. (F) Dorsal versus ventral GFP+ clone size and number quantification of 6-mo (Left) and 1-y traced (Right) spinal cords. In each column, fractions of single cell clones, two- to three-cell clones, and four cells and above clones are represented by different colors described in the legends. n = 3 animals per time point.
Wnt/β-Catenin Signaling Is Required for Ependymal Proliferation in the Postnatal and Adult Spinal Cord.
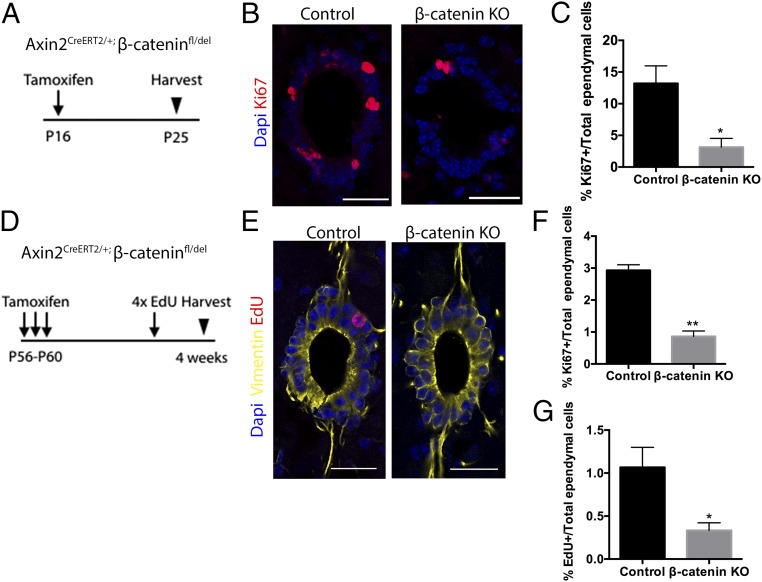
To test the functional requirement for Wnt signaling in Axin2+ ependymal cells, both during postnatal growth and adult homeostasis, we conditionally deleted the β-catenin gene in Axin2-expressing cells upon tamoxifen injection either at P16 or at P56–P60 using the Axin2-CreERT2/+; B-cat fl/del mouse (49). The tissues were then analyzed at P25 or P88, respectively. The P56–P60 mice also received four doses of EdU before tissue harvest (Fig. 5A).
Fig. 5.
Wnt/β-catenin signaling is required for ependymal proliferation in the postnatal and adult spinal cord. (A and D) Experimental schedule used for β-catenin conditional knockout experiments. (B) Representative Ki67 (red) DAPI (blue) immunostaining images of spinal cords from Axin2CreERT2/+; β-cateninfl/+ (control) and mutant Axin2CreERT2/+; β-cateninfl/del (β-cat KO) littermates that received tamoxifen at P16. (Scale bar, 50 um.) (C) The percentages of Ki67+ ependymal cells out of total ependymal cells in control and KO animals. Control, n = 3; β-cat KO, n = 3. (E) Representative EdU (red) Vimentin (Yellow), and DAPI (blue) immunostaining images of spinal cords from Axin2CreERT2/+; β-cateninfl/+ (control) and mutant Axin2CreERT2/+; β-cateninfl/del (β-cat KO) littermates that received tamoxifen at P56–P60. (Scale bar, 50 um.) (F) The percentages of Ki67+ ependymal cells out of total ependymal cells in control and KO animals. Control, n = 4; β-cat KO, n = 4. (G) The percentages of EdU+ ependymal cells out of total ependymal cells in control and KO animals. *P < 0.05; **P < 0.01.
Compared with age-matched controls, proliferation rates of ependymal calls in β-catenin knockout mice that received a tamoxifen injection at P16 were found to be significantly reduced as indicated by Ki67 immunostaining (Fig. 5 B and C). Similarly, ependymal cells showed slower rates of proliferation in β-catenin knockout mice when deletion was induced at P56, as both the percentage of Ki67+ ependymal cells and EdU+ ependymal cells were reduced in the knockouts compared with the controls (Fig. 5 E–G).
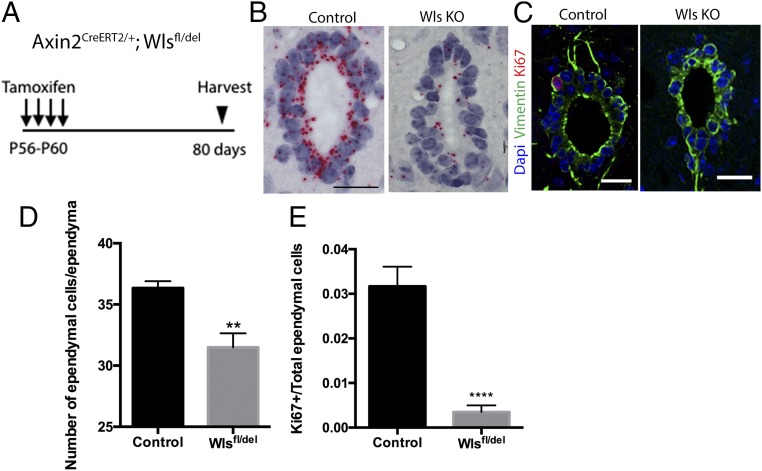
To test whether Wnt ligands are required for ependymal cell proliferation, we first took into consideration that the overlapping expression of multiple Wnt ligands by ependymal cells likely suggested functional redundancy. However, Wntless (Wls) is a Wnt-specific transporter molecule required for proper secretion of all Wnt ligands (50). We therefore conditionally deleted the Wntless gene in Axin2+ ependymal cells by injecting control mice (Axin2-CreERT2/+; Wlsfl/+) and conditional knockout mice (Axin2-CreERT2/+; Wls fl/del) with tamoxifen and analyzed the spinal cords after 80 d (Fig. 6A).
Fig. 6.
Ependymal cell-derived Wnt proteins are required for ependymal cell proliferation and ependyma maintenance. (A) Experimental schedule used for Wntless conditional knockout experiments. (B) In situ hybridization against Wntless of the control and the Wls KO spinal cords. (Scale bar, 50 um.) (C) Representative Ki67 (red), Vimentin (green), and DAPI (blue) immunostaining images of spinal cords from control and Wls KO spinal cords. (Scale bar, 50 um.) (D) The numbers of total ependymal cells in control and Wls KO spinal cords. (E) The percentages of Ki67+ ependymal cells out of total ependymal cells in control and Wls KO animals. Control, n = 4; Wls KO, n = 4. **P < 0.01; ****P < 0.0001.
As shown by in situ hybridization, Wls expression in the ependymal cells of mutant mice was reduced compared with the controls, confirming Wls deletion in the mutant ependymal cells (Fig. 6B). Upon quantification of over 100 sections across multiple animals, we found that the number of Vimentin+ ependymal cells was significantly lower in Wlsfl/del mice (Fig. 6 C and D). This result suggested a proliferation defect in ependymal cells of the mutant mice and that ependymal cell-derived Wnt ligands are required to maintain the tissue integrity of the ependyma. Indeed, the percentage of Ki67+ ependymal cells in Wlsfl/del mice was significantly reduced compared with that of the controls (Fig. 6E). Our observations indicate that both Wnt ligands and β-catenin which are expressed in ependymal cells are required for promoting ependymal cell proliferation and maintaining ependymal tissue integrity.
Discussion
Our lineage tracing results demonstrate that, similar to the lateral ventricles of the brain, radial glial cells give rise to ependymal cells in the spinal cord (25). We demonstrate that dorsal midline neural progenitor cells are Wnt responsive throughout spinal cord development and give rise to ependymal cells in a spatially restricted pattern. Although the majority of embryonic radial glial cells have undergone terminal differentiation and retracted their radial processes by birth, we find that Wnt-responsive radial glial cells do not transform into ependymal cells until the first postnatal week and retain radial processes after the transformation. These dorsal ependymal cells are consistent with the previously described radial ependymal cells found at the dorsal and ventral poles, which are thought to represent the more progenitor-like population in the ependymal layer (4, 51). Contrary to previous reports, which suggested that all ependymal cells are derived from ventral neural progenitors (23, 45), our results highlight the previously unappreciated heterogeneity in the embryonic origin of these long-term progenitor cells of the adult spinal cord. Our model of ependymal cell generation is strikingly similar to the established segmental template model of astrocyte generation, where astrocytes are allocated to particular spatial domains following a segmental template in accordance with their embryonic sites of origin in the ventricular zone (29, 46, 47). Future experiments tracking the contribution of progenitors from different dorsal–ventral domains to the ependyma are needed to further test whether a segmental template model holds true for ependymal cell generation.
Compared with the developing spinal cord, relatively little is known about Wnt ligand expression in the adult spinal cord. Previous reports suggested that Wnt proteins are expressed in the adult spinal cord; however, cell-type-specific information was relatively lacking (52, 53). In this study, we show that Wnt/β-catenin signaling is activated in ependymal cells and is a key regulator of ependymal proliferation in the postnatal and adult spinal cord. Our work thus uncovers the functional significance of Wnt/β-catenin signaling in ependymal cell regulation. Although it was reported that Notch1 is expressed in the ependymal cells, its involvement in ependymal cell regulation during homeostasis has not been explored (54).
Building on the knowledge that ependymal cells proliferate at a basal level during adult homeostasis (2, 4, 13, 14, 48), we employed lineage tracing to monitor the turnover and clonal expansion of ependymal cells and revealed heterogeneity in proliferation among dorsal and ventral cells. We showed that overall, dorsal ependymal cells yielded larger clones, potentially suggesting that the dorsal ependymal population represents a progenitor subset of ependymal cells that proliferate at higher rates than other ependymal cells.
We hypothesize that the differences in proliferative potential of ependymal cells are cell intrinsic. We show that dorsal ependymal cells are derived from Wnt-responsive radial glial cells and are generated last among all ependymal cells. This unique, delayed, embryonic origin may endow them with a more progenitor-like state and a higher proliferative potential. Alternatively, asymmetric distribution of Wnt signaling activity, with higher dorsal versus ventral activity, may account for the difference in proliferative potential. Finally, opposing signals expressed in the ventral ependymal cells may counteract the mitogenic activity of Wnts. Interestingly, it has been reported that Sonic Hedgehog (Shh), expressed by ventral ependymal cells (5, 55), may exert an inhibitory effect on ventral cells. The counteracting signals from the dorsal and ventral ependymal zone therefore recapitulate the mitogen distribution in the embryonic ventricular zone, suggesting a continuity between the regulation of the adult progenitor zone and that of the early embryonic progenitor zone.
In a recent gene expression profiling study of ependymomas, CNS tumors derived from ependymal cells (56, 57), up-regulation of Wnt5a and Wnt11 was found to be associated with the highest grade of ependymomas. Moreover, additional genes of the Wnt pathway were also highly expressed or increased their expression, including Frizzled, Disheveled, β-catenin, TCF3, as well as Wnt target genes BIRC5, CCND1, FOSL1, c-MYC, and TP53 (58). These findings further support our conclusion that Wnts are key regulators of ependymal proliferation and suggest that aberrant regulation of Wnt/β-catenin signaling may lead to uncontrolled proliferation of ependymal cells and formation of ependymomas.
Finally, many reports have highlighted the potential of spinal cord ependymal cells as a promising pool of quiescent stem cells to treat spinal cord injury (11, 12, 15, 17, 59–61). As a source of glial scar astrocytes with beneficial functions, it is important to augment or modulate their injury response to further improve the outcome. Our findings provide insights for utilizing the endogenous potential of these cells and for designing regenerative strategies that are based on appropriate modulation of endogenous signaling responses.
Materials and Methods
Animals.
Axin2CreERT2 mice were previously described (40). Axin2-LacZ mice were a gift from W. Birchmeier, Max Delbruck Center for Molecular Medicine, Berlin (62). Rosa26mTmG mice (41), β-cateninex2-6-fl mice (49), and TCF/Lef:H2B-GFP mice, where six binding sites for TCF/Lef are used to drive expression of the H2B-GFP cassette in the cell nucleus (63), were obtained from The Jackson Laboratory. β-Cateninex2-6-del mice, in which the β-catenin gene has been deleted ubiquitously, were generated by crossing β-cateninex2-6-fl mice with Vasa-Cre mice. All experiments were approved by the Stanford University Animal Care and Use Committee and performed according to NIH guidelines.
Immunostaining.
For embryos and early postnatal mice, the spinal column containing the spinal cord was dissected and fixed in 4% (wt/vol) paraformaldehyde in PBS at 4 °C overnight. The tissue was then washed in PBS, incubated in 30% sucrose solution in PBS for 48 h at 4 °C, and embedded in optimal cutting temperature compound (OCT). The spinal cord of adult mice was fixed through intracardial perfusion for 10 min at room temperature with 4% (wt/vol) paraformaldehyde in PBS. The spinal column containing the spinal cord was then postfixed in 4% (wt/vol) paraformaldehyde in PBS at 4 °C overnight. The spinal cord was removed from the spinal column and incubated in 30% sucrose solution in PBS for 48 h at 4 °C and embedded in OCT. Frozen samples were sectioned at 12 μm using CryoJane (Leica Microsystems). Cryosections were incubated in blocking buffer [10% (vol/vol) normal donkey serum, 0.2% Triton X-100 in PBS] at room temperature and stained with primary and secondary antibodies, then mounted in Prolong Gold with DAPI mounting medium (Life Technologies). The primary antibodies used were chicken anti-GFP (1:1,000; Abcam), rat anti-Ki67 (1:200; eBioscience), and chicken anti-Vimentin (1:500; Millipore). For EdU staining, cryosections were incubated with the Click-iT EdU Alexa Fluor 647 Imaging Kit (Life Technologies) prepared according to manufacturer’s instructions and mounted in Prolong Gold with DAPI mounting medium (Life Technologies).
X-Gal Staining.
For embryos and early postnatal mice, the spinal column containing the spinal cord was dissected and fixed in 1% (wt/vol) paraformaldehyde in PBS at 4 °C overnight. The tissue was then washed in PBS, incubated in 30% sucrose solution in PBS for 48 h at 4 °C, and embedded in OCT. The spinal cord of adult mice was fixed through intracardial perfusion for 10 min at room temperature with 4% (wt/vol) paraformaldehyde in PBS. The spinal column containing spinal cord was then postfixed in 1% (wt/vol) paraformaldehyde in PBS at 4C overnight. Spinal cord was dissected from the spinal column, washed in PBS, incubated in 30% sucrose solution in PBS for 48 h at 4 °C, and embedded in OCT.
Frozen samples were sectioned at 12 μm using CryoJane (Leica Microsystems) and postfixed with 0.1% (wt/vol) paraformaldehyde on ice, washed in detergent rinse (PBS with 2 mM MgCl2 0.01% sodium deoxycholate, and 0.02% Nonidet P-40) and stained in staining solution (PBS with 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet P-40, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/mL X-gal) in a dark room at room temperature overnight. Sections were then washed and counterstained with Nuclear Fast Red (Vector Laboratories), followed by dehydration sequentially with an ethanol gradient (50%, 70%, 90%, and 100%).
RNA in Situ Hybridization.
Tissues were fixed in 10% (vol/vol) neutral buffered formalin at room temperature for 24 h, dehydrated, and embedded in paraffin. Tissue sections cut at 5-µm thickness were processed for RNA in situ detection using the RNAscope 2.5 HD Assay-RED Kit according to the manufacturer’s instructions (ACDBio) (64). Sequences of the probes used in the study are as follows: Axin2 (NM_015732, 330–1287), Wnt1 (NM_021279.4, 1204–2325), Wnt3a (NM_009522.2, 667–1634), Wnt5a (NM_009524.3, 200–1431), Wnt11 (NM_009519.2, 818–1643), and Wls (NM_026582.4, 500–1477).
Microscopy and Imaging.
Fluorescent and bright-field images were obtained using a Leica SP8 confocal microscope and a Zeiss Axio Imager Z2.
Lineage Tracing Studies.
All mice received i.p. injection of 5–20 mg/mL stock solutions of tamoxifen (Sigma) dissolved in corn oil/10% (vol/vol) ethanol, corresponding to specific doses per gram of body weight as specified below. Female mice pregnant with Axin2-CreERT2/+ R26RmTmG/+ embryos were injected with a single dose of tamoxifen at a dosage of 0.5 mg/25 g of the mother’s body weight. Four-day (P4) and 2-mo-old (P56) mice received a single dose of tamoxifen, totaling 4 mg/25 g body weight. Control mice were injected with the filtered corn oil/ethanol vehicle only.
Conditional Knockout of β-Catenin.
Control Axin2CreERT2/+; β-catenin fl/+ mice and mutant Axin2CreERT2/+; β-catenin fl/del mice either received one dose of tamoxifen corresponding to 4 mg/25 g body weight on P16 and were analyzed at P25, or received three doses of tamoxifen corresponding to 4 mg/25 g body weight at P56–P60 every other day, and were analyzed 4 wk after, as this regimen was previously found by our laboratory to sufficiently induce β-catenin knockout driven by Axin2CreERT2/+ (65). With mice that received tamoxifen or vehicle control at P56–P60, four daily doses of EdU were administered before animals were killed.
Conditional Knockout of Wntless.
Control Axin2-CreERT2/+; Wlsfl/+ mice and mutant Axin2-CreERT2/+; Wls fl/del mice received four doses of tamoxifen corresponding to 4 mg/25 g body weight every other day from P56 to P62. Spinal cords were harvested and processed 80 d after the last injection.
Statistical Analysis.
All statistical analyses were performed using the Student’s t test using GraphPad Prism 6 software. Results are presented as mean ± SEM. Statistical significance was set at *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Supplementary Material
Acknowledgments
We thank members of the R.N. laboratory for valuable input and discussions. L.X. was supported by the Stanford Graduate Fellowship. R.N. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803297115/-/DCSupplemental.
References
- 1.Bruni JE. Ependymal development, proliferation, and functions: A review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Alfaro-Cervello C, Soriano-Navarro M, Mirzadeh Z, Alvarez-Buylla A, Garcia-Verdugo JM. Biciliated ependymal cell proliferation contributes to spinal cord growth. J Comp Neurol. 2012;520:3528–3552. doi: 10.1002/cne.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton LK, Truong MKV, Bednarczyk MR, Aumont A, Fernandes KJL. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience. 2009;164:1044–1056. doi: 10.1016/j.neuroscience.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Hugnot J-P, Franzen R. The spinal cord ependymal region: A stem cell niche in the caudal central nervous system. Front Biosci. 2011;16:1044–1059. doi: 10.2741/3734. [DOI] [PubMed] [Google Scholar]
- 6.Göritz C, Frisén J. Neural stem cells and neurogenesis in the adult. Cell Stem Cell. 2012;10:657–659. doi: 10.1016/j.stem.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Sabelström H, Stenudd M, Frisén J. Neural stem cells in the adult spinal cord. Exp Neurol. 2014;260:44–49. doi: 10.1016/j.expneurol.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Grégoire C-A, Goldenstein BL, Floriddia EM, Barnabé-Heider F, Fernandes KJL. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia. 2015;63:1469–1482. doi: 10.1002/glia.22851. [DOI] [PubMed] [Google Scholar]
- 9.Weiss S, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dervan AG, Roberts BL. Reaction of spinal cord central canal cells to cord transection and their contribution to cord regeneration. J Comp Neurol. 2003;458:293–306. doi: 10.1002/cne.10594. [DOI] [PubMed] [Google Scholar]
- 11.Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006;498:525–538. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meletis K, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnabé-Heider F, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Sabelström H, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 16.North HA, Pan L, McGuire TL, Brooker S, Kessler JA. β1-Integrin alters ependymal stem cell BMP receptor localization and attenuates astrogliosis after spinal cord injury. J Neurosci. 2015;35:3725–3733. doi: 10.1523/JNEUROSCI.4546-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren Y, et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci Rep. 2017;7:41122. doi: 10.1038/srep41122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon SS, McDermott KW. Morphology and differentiation of radial glia in the developing rat spinal cord. J Comp Neurol. 2002;454:263–271. doi: 10.1002/cne.10427. [DOI] [PubMed] [Google Scholar]
- 19.Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–197. doi: 10.1002/glia.20166. [DOI] [PubMed] [Google Scholar]
- 20.McDermott KW, Barry DS, McMahon SS. Role of radial glia in cytogenesis, patterning and boundary formation in the developing spinal cord. J Anat. 2005;207:241–250. doi: 10.1111/j.1469-7580.2005.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Böhme G. Formation of the central canal and dorsal glial septum in the spinal cord of the domestic cat. J Anat. 1988;159:37–47. [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata T, et al. Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci. 1997;17:9212–9219. doi: 10.1523/JNEUROSCI.17-23-09212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu H, et al. Molecular mapping of the origin of postnatal spinal cord ependymal cells: Evidence that adult ependymal cells are derived from Nkx6.1+ ventral neural progenitor cells. J Comp Neurol. 2003;456:237–244. doi: 10.1002/cne.10481. [DOI] [PubMed] [Google Scholar]
- 24.Sevc J, Daxnerová Z, Miklosová M. Role of radial glia in transformation of the primitive lumen to the central canal in the developing rat spinal cord. Cell Mol Neurobiol. 2009;29:927–936. doi: 10.1007/s10571-009-9377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spassky N, et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- 27.Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 28.Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 29.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson DG, Bailes JA, McMahon AP. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987;50:79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- 31.Roelink H, Nusse R. Expression of two members of the Wnt family during mouse development–Restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991;5:381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson ME, Krumlauf R, McMahon AP. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- 33.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 34.Zechner D, et al. Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 35.Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–2649. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- 38.Zechner D, et al. Bmp and Wnt/beta-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev Biol. 2007;303:181–190. doi: 10.1016/j.ydbio.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 39.Ille F, et al. Wnt/BMP signal integration regulates the balance between proliferation and differentiation of neuroepithelial cells in the dorsal spinal cord. Dev Biol. 2007;304:394–408. doi: 10.1016/j.ydbio.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 40.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 42.Bowman AN, van Amerongen R, Palmer TD, Nusse R. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin-responsive neural stem cells. Proc Natl Acad Sci USA. 2013;110:7324–7329. doi: 10.1073/pnas.1305411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sevc J, Matiašová A, Kútna V, Daxnerová Z. Evidence that the central canal lining of the spinal cord contributes to oligodendrogenesis during postnatal development and adulthood in intact rats. J Comp Neurol. 2014;522:3194–3207. doi: 10.1002/cne.23590. [DOI] [PubMed] [Google Scholar]
- 45.Masahira N, et al. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev Biol. 2006;293:358–369. doi: 10.1016/j.ydbio.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 46.Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133:510–522. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai H-H, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horner PJ, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 50.Bänziger C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 51.Fiorelli R, Cebrian-Silla A, Garcia-Verdugo JM, Raineteau O. The adult spinal cord harbors a population of GFAP-positive progenitors with limited self-renewal potential. Glia. 2013;61:2100–2113. doi: 10.1002/glia.22579. [DOI] [PubMed] [Google Scholar]
- 52.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 53.González-Fernández C, Fernández-Martos CM, Shields SD, Arenas E, Javier Rodríguez F. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 2014;31:565–581. doi: 10.1089/neu.2013.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto S, et al. Transcription factor expression and Notch-dependent regulation of neural progenitors in the adult rat spinal cord. J Neurosci. 2001;21:9814–9823. doi: 10.1523/JNEUROSCI.21-24-09814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu K, McGlynn S, Matise MP. Floor plate-derived sonic hedgehog regulates glial and ependymal cell fates in the developing spinal cord. Development. 2013;140:1594–1604. doi: 10.1242/dev.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moynihan TJ. Ependymal tumors. Curr Treat Options Oncol. 2003;4:517–523. doi: 10.1007/s11864-003-0052-5. [DOI] [PubMed] [Google Scholar]
- 57.Poppleton H, Gilbertson RJ. Stem cells of ependymoma. Br J Cancer. 2007;96:6–10. doi: 10.1038/sj.bjc.6603519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palm T, et al. Expression profiling of ependymomas unravels localization and tumor grade-specific tumorigenesis. Cancer. 2009;115:3955–3968. doi: 10.1002/cncr.24476. [DOI] [PubMed] [Google Scholar]
- 59.Moreno-Manzano V, et al. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells. 2009;27:733–743. doi: 10.1002/stem.24. [DOI] [PubMed] [Google Scholar]
- 60.Stenudd M, Sabelström H, Frisén J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2014;72:235–237. doi: 10.1001/jamaneurol.2014.2927. [DOI] [PubMed] [Google Scholar]
- 61.Li X, et al. Regenerative potential of ependymal cells for spinal cord injuries over time. EBioMedicine. 2016;13:55–65. doi: 10.1016/j.ebiom.2016.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrer-Vaquer A, Piliszek A. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang F, et al. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takase HM, Nusse R. Paracrine Wnt/β-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc Natl Acad Sci USA. 2016;113:E1489–E1497. doi: 10.1073/pnas.1601461113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.