Significance
Second messengers are employed by all organisms to regulate fundamental behaviors, including biofilm formation, motility, metabolism, and pathogenesis in bacteria. We have identified a phospholipase in the El Tor Vibrio cholerae biotype, responsible for the current cholera pandemic, that is directly activated by the second messenger 3′, 3′-cyclic GMP-AMP (cGAMP). Discovery of this proteinaceous bacterial cGAMP effector sheds light on the functions and basic principles of cGAMP signaling. Both this phospholipase and the cGAMP synthase are encoded within the VSP-1 pathogenicity island, unique to the El Tor biotype, and our findings assign a biochemical function to VSP-1 that may contribute to the epidemiological success of El Tor V. cholerae.
Keywords: second messengers, cyclic dinucleotides, phospholipid metabolism, pathogenicity island, cGAMP
Abstract
Sensing and responding to environmental changes is essential for bacteria to adapt and thrive, and nucleotide-derived second messengers are central signaling systems in this process. The most recently identified bacterial cyclic dinucleotide second messenger, 3′, 3′-cyclic GMP-AMP (cGAMP), was first discovered in the El Tor biotype of Vibrio cholerae. The cGAMP synthase, DncV, is encoded on the VSP-1 pathogenicity island, which is found in all El Tor isolates that are responsible for the current seventh pandemic of cholera but not in the classical biotype. We determined that unregulated production of DncV inhibits growth in El Tor V. cholerae but has no effect on the classical biotype. This cGAMP-dependent phenotype can be suppressed by null mutations in vc0178 immediately 5′ of dncV in VSP-1. VC0178 [renamed as cGAMP-activated phospholipase in Vibrio (CapV)] is predicted to be a patatin-like phospholipase, and coexpression of capV and dncV is sufficient to induce growth inhibition in classical V. cholerae and Escherichia coli. Furthermore, cGAMP binds to CapV and directly activates its hydrolase activity in vitro. CapV activated by cGAMP in vivo degrades phospholipids in the cell membrane, releasing 16:1 and 18:1 free fatty acids. Together, we demonstrate that cGAMP activates CapV phospholipase activity to target the cell membrane and suggest that acquisition of this second messenger signaling pathway may contribute to the emergence of the El Tor biotype as the etiological agent behind the seventh cholera pandemic.
The molecular mechanisms and signal transduction pathways by which bacteria respond and adapt to a changing environment remain some of the most fundamental questions in microbiology. These processes are essential for all bacteria whether they are soil isolates, symbionts, or human and agricultural pathogens. Given the importance of environmental adaptation, it is not surprising that bacteria pursue an abundance of strategies to accomplish this task. Of primary importance to environmental adaptation are second messenger signaling pathways that leverage nucleotide-derived small molecules for the transduction of external signals. The intracellular concentration of these small molecule second messengers imparts global regulatory effects by altering transcription, translation, or even protein activity, leading to changes in bacterial physiology and behavior (1).
We have just begun to fully appreciate the breadth and central importance of nucleotide-derived second messengers to all aspects of bacterial life. The first second messenger system described, cyclic adenosine monophosphate (cAMP), is primarily associated with the regulation of carbon utilization (2, 3). However, cAMP is also a global regulator controlling biofilm formation, virulence, and central metabolism (4–6). Guanosine penta/tetraphosphate (p/ppGpp) has also been studied for decades and is the primary signal modulating the bacterial stringent response (7, 8). In response to nutrient limitation and other stimuli, the level of p/ppGpp dramatically increases, resulting in the inhibition of macromolecular synthesis, a key adaptive behavior for survival under these stress conditions (9, 10). The last 15 y have witnessed the emergence of cyclic dinucleotide second messengers in both bacteria and eukaryotes. Initially, cyclic diguanosine monophosphate (c-di-GMP), the first cyclic dinucleotide identified, was not widely appreciated until the renewed interest in understanding the molecular mechanisms underpinning biofilm formation (11). The second cyclic dinucleotide discovered was cyclic diadenosine monophosphate (c-di-AMP) (12, 13). c-di-AMP signaling plays a major physiological role in the regulation of potassium uptake, as well as other essential cellular processes, such as fatty acid synthesis, cell wall homeostasis, detection of DNA damage, progression of sporulation, and regulation of cell division (12, 14–22).
In 2012, the most recently identified bacterial cyclic dinucleotide signaling molecule, the hybrid 3′, 3′-cyclic GMP-AMP (cGAMP), was discovered in the El Tor biotype of the human pathogen Vibrio cholerae (23). This new class of second messenger is synthesized by the enzyme DncV, encoded at locus vc0179 in the Vibrio seventh pandemic island-1 (VSP-1) pathogenicity island. The expression of dncV is positively influenced by the master virulence regulator, ToxT. cGAMP signaling regulates virulence, chemotaxis, and fatty acid metabolic genes, yet there is no known cGAMP-binding effector in V. cholerae attributed to the control of these processes. Additionally, ectopic expression of dncV has been shown to enhance the transcription and accumulation of three phosphodiesterases capable of degrading cGAMP, named V-cGAP1-3 (24), but the mechanism for this regulation is also unknown. More recently, cGAMP-specific riboswitches were found to regulate the expression of genes mediating a variety of cellular processes, including adhesion and exoelectrogenesis in δ-proteobacteria, such as Geobacter species (25, 26). In addition, a GGDEF-encoding enzyme, which generally makes c-di-GMP, also synthesizes cGAMP and c-di-AMP in Geobacter (27), suggesting that cGAMP signaling has a broad impact on microbial physiology. Metazoans produce an isomer, 2′,3′-cGAMP, that is synthesized by the DncV structural and functional homolog cGAS (28) to stimulate the innate immune system via the receptor STING in response to cytoplasmic double-stranded DNA (29, 30). Its regulatory mechanism has been intensively studied (31). In contrast, almost nothing is known about the proteinaceous receptors of bacterial cGAMP and how this signal regulates the activities of its interacting partners.
The VSP-1 island is only present in the circulating El Tor V. cholerae strains responsible for the seventh pandemic of cholera (1961 to the present) and is absent in the classical isolates that predominated in the previous six pandemics (32). This exclusive acquisition of VSP-1 by the El Tor biotype suggests that the functions encoded in this pathogenicity island are important for the displacement of the classical biotype as the etiological agent driving modern day cholera. A recent study that analyzed whole genome sequences to track the evolution of the El Tor biotype from a nonpathogenic bacterium to a pandemic pathogen found that there were six discrete stages in this process spanning the late 1800s to 1961 (33). During the fifth stage (1925 to 1954) paracholera El Tor strains, which could cause disease but lacked pandemicity, acquired VSP-1, as well as a second genomic island known as VSP-2 and an El Tor-specific variant of cholera toxin, CTXET. During the sixth stage (1954 to 1960) recombination and horizontal gene transfer in the El Tor biotype essentially stopped, and the final 12 SNPs, which have no conspicuous connection to pathogenicity, completed the pandemic maturation of the El Tor biotype. Thus, acquisition of the VSP islands was predicted to be the major event driving the evolution of pandemic El Tor V. cholerae from paracholera and led to the displacement of the classical biotype as the causative agent of cholera. While the genomic modifications in the evolution of El Tor V. cholerae have been identified and elegantly described, the evolutionary advantages that these changes provided are not known.
Here, we show that unregulated ectopic expression of the cGAMP synthase, DncV, in the V. cholerae El Tor biotype inhibits planktonic growth and produces an atypical colony morphology on solid agar. However, these phenotypes associated with overproduction of cGAMP were not observed in classical V. cholerae or Escherichia coli. Mutation of an uncharacterized gene vc0178 [renamed herein as cGAMP-activated phospholipase in Vibrio (capV)], located immediately upstream of dncV in VSP-1, suppresses these cGAMP-induced phenotypes. Moreover, coexpression of dncV and capV in classical V. cholerae and E. coli sensitizes these species to cGAMP overproduction, leading to growth inhibition. Finally, in vitro biochemical assays, microscopy, and whole cell lipid analyses demonstrate that cGAMP directly binds to and activates the serine hydrolase/phospholipase activity of CapV, leading to degradation of cell membrane phospholipids and release of free fatty acids. Together, our results suggest that acquisition of this second messenger and its receptor, both present in the VSP-1 pathogenicity island, leads to changes in cell physiology that could be important in the pandemic evolution of the El Tor biotype. As homologs of capV and dncV are often linked to one another in a variety of bacteria from across the bacterial phylogenetic tree (34), cGAMP signaling and its connection to membrane lipid metabolism may be broadly conserved.
Results
High cGAMP Production Leads to Planktonic Growth Inhibition and Atypical Colony Morphology in El Tor V. cholerae.
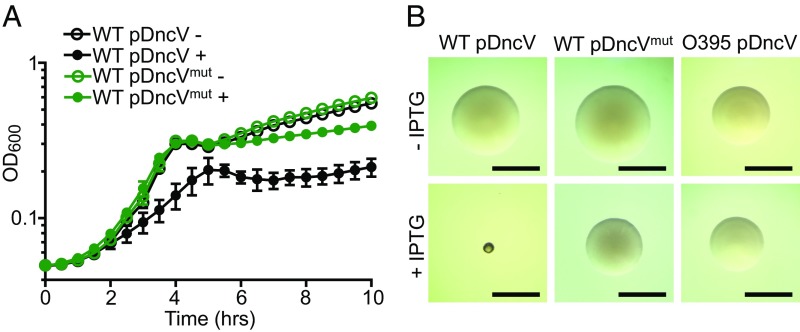
To understand the physiological functions of cGAMP in V. cholerae, we perturbed the intracellular concentration of this signal molecule by either deleting dncV, the gene encoding the cGAMP synthase, or overexpressing dncV from a plasmid. Although a ΔdncV mutant is moderately attenuated in infant mouse colonization (23), we did not observe any in vitro growth phenotype in this mutant. In contrast, when dncV expression was induced ectopically in El Tor V. cholerae WT C6706, under control of the Ptac promoter, planktonic growth was significantly arrested (Fig. 1A). Overexpression of dncVmut, encoding a DncV active site mutant (D131A/D133A), did not lead to substantial growth arrest, suggesting that cGAMP synthesis is responsible for this growth inhibition (Fig. 1A). When grown on a solid agar surface, we also observed the development of a small colony morphology in response to DncV overproduction that was not observed in El Tor V. cholerae producing DncVmut (Fig. 1B). To determine if abnormal accumulation of cGAMP is generally toxic, we overproduced DncV in a classical strain of V. cholerae (O395) and a laboratory strain of E. coli using the same Ptac-inducible plasmid. In both cases, DncV overproduction alone had no impact on planktonic growth or colony morphology, indicating that the observed cGAMP-induced phenotypes were specific to El Tor V. cholerae (Fig. 1B and SI Appendix, Fig. S1).
Fig. 1.
Overproduction of the cGAMP synthase DncV induces planktonic growth arrest and a small colony phenotype in El Tor V. cholerae. (A) Growth curves of El Tor V. cholerae cultures carrying a Ptac-inducible plasmid encoding dncV (pDncV) or a catalytically inactive mutant (pDncVmut), grown in the absence (−) or presence (+) of 100 µM IPTG. Each data point represents the mean ± SD of six biological replicates. (B) Colony morphologies of El Tor V. cholerae with pDncV (Left) or pDncVmut (Middle), or classical V. cholerae strain O395 with pDncV (Right), grown on solid agar plates in the absence (Top) or presence (Bottom) of 100 µM IPTG. (Scale bars: 1 mm.) Two independent plasmid constructs with different copy numbers were tested, and similar results were found (SI Appendix, Fig. S5 and Materials and Methods). Growth curves and colony images are representative of at least three independent experiments.
Both cGAMP-Induced Growth Arrest and Atypical Colony Morphology Require CapV.
We then performed two distinct genetic screens to identify the gene/s necessary for cGAMP-induced growth arrest and atypical colony morphology. Because the V. cholerae El Tor strain overexpressing dncV forms tiny colonies on agar plates containing isopropyl β-d-1-thiogalactopyranoside (IPTG), we reasoned that, under the same inducing conditions, suppressor mutations would restore colony morphology and planktonic growth despite the presence of excessive cGAMP. Thus, an mTn10 transposon library was constructed from a dncV merodiploid strain and screened for clones that developed as regular-sized colonies on plates containing IPTG. We screened over 50,000 transposon mutants and obtained dozens of suppressors; half of these mutants carried mutations in the dncV-overexpression plasmid and were not further studied. The vast majority (>90%) of the remaining suppressors carried a transposon insertion (with different insertion sites and orientations) in vc0178 (capV), a gene immediately 5′ of dncV (gene designation vc0179) in VSP-1. The growth rates and yields of these capV transposon mutants were nearly identical when they were grown with and without IPTG (a representative mutant is shown in Fig. 2A).
Fig. 2.
The El Tor VSP-1 gene capV is necessary for cGAMP-induced growth arrest and small colony morphology. (A) Growth curves of El Tor V. cholerae WT and capV::Tn, each carrying pDncV, grown in the absence (−) or presence (+) of 100 µM IPTG. (B) Colony morphologies of the classical V. cholerae strain O395 carrying both pDncV and either an empty vector (Left) or one of two unique El Tor genomic cosmids containing VSP-1 (pCCD7, Middle; pCCD13, Right). Strains were grown on solid agar plates in the absence (Top) or presence (Bottom) of 100 µM IPTG. (Scale bars: 1 mm.) (C) Growth curves of El Tor V. cholerae WT and ΔcapV, each carrying pDncV, grown in the absence (−) or presence (+) of 100 µM IPTG. (D) Colony morphologies of El Tor V. cholerae WT and ΔcapV, each carrying pDncV, grown on solid agar plates in the absence (Top) or presence (Bottom) of 100 µM IPTG. (Scale bars: 1 mm.) (E) Growth curves of El Tor V. cholerae ΔcapV mutants carrying a Ptac-inducible plasmid encoding both dncV and either capV (pCapV-DncV) or a catalytically inactive mutant (pCapVmut-DncV), grown in the absence (−) or presence (+) of 100 µM IPTG to induce expression of the polycistronic transcript. Each data point in the growth curves represents the mean ± SD of six biological replicates. Growth curves and colony images are representative of at least three independent experiments.
As a complementary approach to identify the cGAMP receptor/s that mediate growth arrest and small colony morphology, we screened for genes from the El Tor strain that sensitized classical V. cholerae to DncV overproduction. This approach would allow us to identify genes in this pathway that may not be amenable to transposon mutagenesis, such as essential genes. To perform this screen, a random cosmid library that encoded ∼20 kb El Tor genomic fragments was introduced into the classical strain O395 expressing dncV from a plasmid. We identified two unique cosmids that sensitized classical V. cholerae to growth inhibition by cGAMP overproduction and determined that each contained the VSP-1 pathogenicity island harboring capV. Small colony morphologies were also observed in these classical V. cholerae strains maintaining either of the VSP-1–containing cosmids upon overexpression of dncV on agar plates (Fig. 2B). These results further suggest that capV is the gene necessary for cGAMP-induced growth arrest and small colony morphology in El Tor V. cholerae.
capV is the first ORF in a putative four-gene operon that also includes dncV and downstream genes vc0180 and vc0181, both of which encode putative proteins of unknown function. VC0180 contains E1 and E2 domains similar to eukaryotic ubiquitin-ligase enzymes while VC0181 contains a JAB domain similar to eukaryotic isopeptidases. To clarify the role of capV in cGAMP-dependent growth inhibition and test for polarity, we introduced single in-frame deletions of capV, dncV, vc0180, and vc0181 on the chromosome of El Tor V. cholerae. Only deletion of capV suppressed cGAMP-dependent growth phenotypes resulting from ectopic expression of dncV (Fig. 2 C and D and SI Appendix, Fig. S2), indicating that the ability of mutations in capV to suppress cGAMP-induced growth arrest is not due to polar effects on downstream genes. We also observed that coexpression of capV and dncV from a common plasmid restored cGAMP-dependent growth arrest in the ∆capV mutant (Fig. 2E). Likewise, coexpression of capV and dncV was sufficient to induce planktonic growth arrest in classical V. cholerae and E. coli, which are otherwise unperturbed by the independent expression of either gene alone (SI Appendix, Fig. S1).
Sequence analysis suggested that CapV carries a canonical patatin domain, appearing to belong to a class of PLA2 phospholipases containing a conserved serine residue in the active site (35). We tested if the putative serine hydrolase activity of CapV is required for growth arrest caused by cGAMP overproduction by generating a catalytically inactive S62A variant of the protein (CapVmut). Coexpression of capVmut and dncV in a ΔcapV El Tor strain did not elicit cGAMP-induced growth arrest, demonstrating that CapV catalytic activity is required for this cGAMP-dependent phenotype (Fig. 2E). Together, these results indicate that DncV and CapV are necessary and sufficient to cause growth inhibition. Our data are consistent with a model that the current El Tor V. cholerae pandemic strains employ cGAMP as a second messenger to regulate the serine hydrolase/phospholipase activity of CapV.
cGAMP Binds CapV and Stimulates Its Activity.
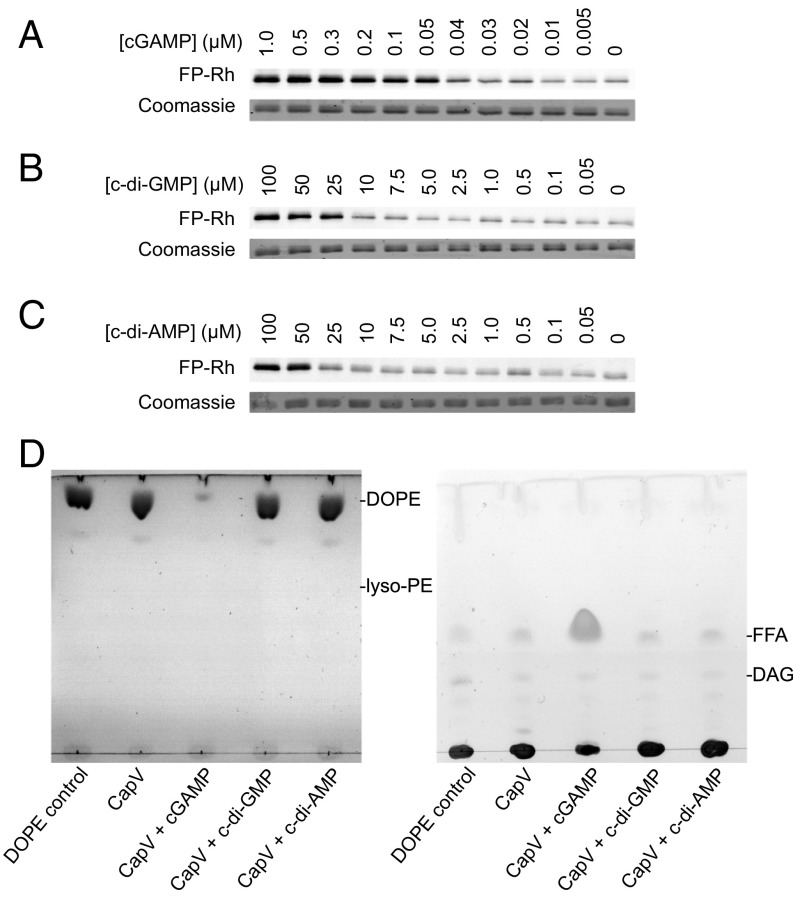
Our genetic evidence suggested that the phospholipase activity of CapV might be directly activated by binding to cGAMP as we identified no additional proteins necessary for cGAMP-induced growth arrest by either genetic screen. We tested this hypothesis in vitro by determining if the CapV active-site serine can be covalently labeled by a reactive rhodamine-labeled fluorophosphonate probe (FP-Rh), a commonly used suicide substrate to monitor serine hydrolase activity, in the presence of cGAMP or other related nucleotides (36, 37). Purified His6-tagged CapV was covalently labeled with this serine hydrolase probe in the presence of as low as 50 nM cGAMP, and the labeling increased in a dose-dependent manner (Fig. 3A). While DncV was previously shown to synthesize all three known cyclic dinucleotides in vitro (23), it preferentially catalyzed the production of cGAMP over c-di-GMP and c-di-AMP. Under our standard growth conditions (LB, 37 °C, OD ≈ 1), we determined that both cGAMP and c-di-AMP were below the level of detection before induction of dncV expression. After 30 min of induction in a ∆capV mutant, the average intracellular concentrations of cGAMP and c-di-AMP increased to 1.2 and 0.2 µM, respectively (SI Appendix, Fig. S3A). Induction of dncV expression, however, did not change the concentration of c-di-GMP in the cell, which decreased from ∼0.3 to ∼0.1 µM over the course of the experiment in both conditions (SI Appendix, Fig. S3A). We also assayed CapV labeling with FP-Rh in the presence of both c-di-GMP and c-di-AMP and found that they were about 1,000-fold less active than cGAMP (Fig. 3 B and C). Labeling of CapV by FP-Rh was only observed with at least 25 µM c-di-GMP or 50 µM c-di-AMP. This level of c-di-GMP has been observed when V. cholerae is grown in M9 medium to late-exponential phase, but not in rich medium such as LB (38), and c-di-AMP is not regularly detected in V. cholerae. Therefore, c-di-GMP and c-di-AMP are both unlikely to be a physiologically relevant signal for CapV activation under our tested growth conditions. Moreover, CapV labeling is specific for cGAMP as other related nucleotides (cAMP, GTP, ATP, dGTP, and dATP) did not increase CapV labeling when present at 1.75 µM or 1 mM (SI Appendix, Fig. S3B).
Fig. 3.
cGAMP binding directly induces CapV phospholipase activity. (A–C) Covalent labeling (Top) of the CapV active-site serine by a reactive rhodamine-labeled fluorophosphonate probe (FP-Rh). 1.6 µM His6-tagged CapV was mixed with both FP-Rh and (A) different concentrations of cGAMP or (B) c-di-GMP or (C) c-di-AMP. (Bottom) Coomassie blue staining of CapV. (D) Thin-layer chromatographic analysis of polar (Left) and neutral (Right) lipids released from the in vitro degradation of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) by 500 nM purified CapV in the presence of 1 µM cGAMP or other cyclic dinucleotides. DAG, diacylglyceride; FFA, free fatty acid; lyso-PE, lysophosphatidylethanolamine.
We then tested if purified CapV displayed bona fide phospholipase activity by providing a phospholipid substrate, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), in the presence and absence of different cyclic dinucleotides. Only the addition of 1 µM cGAMP, but not equimolar concentrations of c-di-GMP or c-di-AMP, was capable of stimulating CapV to catalyze the degradation of DOPE (Fig. 3D, Left) resulting in the release of free fatty acids (FFAs) (Fig. 3D, Right), but not of lyso-PE, suggesting that both acyl groups of the lipid are susceptible to the lipase. This result not only further demonstrates CapV’s capacity to distinguish between cyclic dinucleotide ligands but also shows that the enzyme is a phospholipase capable of degrading a biologically relevant phospholipid in a cGAMP-dependent manner. Finally, we used microscale thermophoresis (MST) to confirm that purified CapV directly bound to cGAMP with a Kd of 8.6 ± 1.9 μM (SI Appendix, Fig. S3C). Together, our genetic and biochemical data support a model whereby cGAMP functions as a specific activator of CapV catalytic activity. Thus, DncV, cGAMP, and CapV constitute a functional cGAMP signaling pathway in V. cholerae.
High cGAMP Production Results in the Degradation of Phosphatidylethanolamine and Phosphatidylglycerol in El Tor V. cholerae.
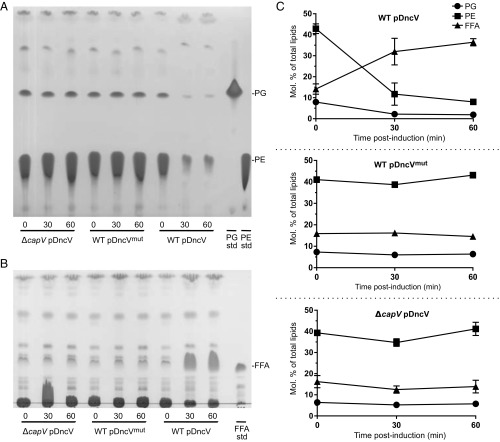
To test if cGAMP regulates the activity of CapV in vivo, total lipids were extracted from WT El Tor V. cholerae overexpressing dncV and dncVmut and the ΔcapV mutant overexpressing dncV (SI Appendix, Fig. S4). Extracted lipids were then resolved by TLC and stained with iodine. We observed that phosphatidylethanolamine (PE) and phosphatidylglycerol (PG), the major phospholipids in V. cholerae (39), were rapidly degraded when DncV production was induced in the presence of CapV (Fig. 4 A and C and SI Appendix, Fig. S4B). Overproduction of DncVmut in WT or of DncV in ΔcapV had no effect on gross phospholipid pools, indicating that the observed phospholipid degradation in the WT El Tor V. cholerae strain was dependent on both the production of cGAMP and CapV activity.
Fig. 4.
Overproduction of the cGAMP synthase DncV results in degradation of phosphatidylethanolamine (PE) and phosphatidylglycerol (PG) and release of free fatty acids (FFAs) in a CapV-dependent fashion. TLC plates of (A) polar lipids and (B) neutral lipids extracted at 0, 30, and 60 min postinduction with 1 mM IPTG from El Tor V. cholerae WT carrying pDncV or pDncVmut and from the El Tor V. cholerae ΔcapV mutant carrying pDncV. Plates are representative of three independent experiments. The PE, PG, and FFA standards are dioleoyl-PE, dioleoyl-PG, and linoleic acid, respectively. (C) Quantification (molar percentage of total lipids) by GC of PG, PE, and free fatty acids (FFAs) from A and B. Graphs show the mean of three independent experiments.
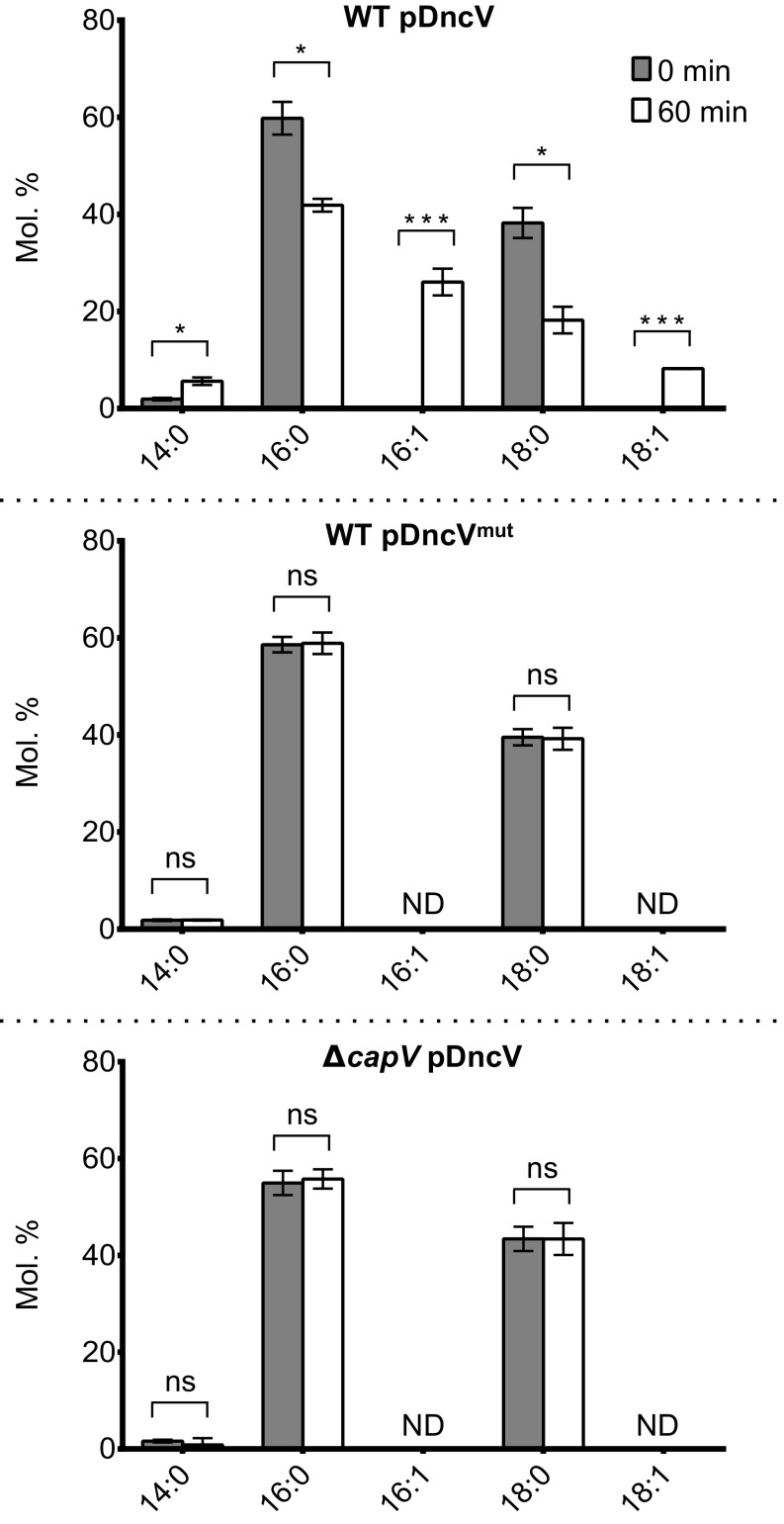
PE and PG represent two broad classes of phospholipids characterized by the presence of a common head group and a phosphoglycerol moiety with two acyl groups of variable lengths and degrees of saturation giving rise to different molecular species. The degradation of PE and PG by cGAMP-activated CapV significantly increased the FFAs in the afflicted cells (Fig. 4 B and C). To understand whether activated CapV preferentially degraded specific molecular species of PE and PG, we used gas chromatography to quantify the total lipid extracts and the acyl composition of TLC-resolved PE, PG, and FFAs from all three strains. Ectopic expression of dncV in the WT El Tor V. cholerae strain led to a profound change in the composition of the FFA pool. Before dncV induction, the FFA pool was mainly composed of species with 16:0 (carbons:double bonds) and 18:0 tails. However, after 60 min of dncV overexpression, 16:1 and 18:1 FFAs, which were originally undetectable, made up ∼30% of the FFA pool (Fig. 5). Moreover, overproduction of DncV in the WT El Tor V. cholerae resulted in a decrease in the molar ratio of PE and PG phospholipids containing 16:1 unsaturated FFAs that was not seen in the ΔcapV or dncVmut overexpression strains (SI Appendix, Fig. S4B). These results suggest that CapV preferentially degrades PE and PG species containing unsaturated acyl groups in a cGAMP-dependent manner leading to the accumulation of cytosolic 16:1 and 18:1 FFAs.
Fig. 5.
Overproduction of the cGAMP synthase DncV results in accumulation of 16:1 and 18:1 free fatty acids (FFAs) in the cell. Shown is the proportion (molar percentage) of fatty acid species present in the FFA fractions collected from El Tor V. cholerae WT carrying pDncV (Top) or pDncVmut (Middle) and from El Tor V. cholerae ΔcapV carrying pDncV (Bottom). Each data point represents the mean ± SD of three independent experiments (*P < 0.01, ***P < 0.0001). ND, not detected; ns, not significant.
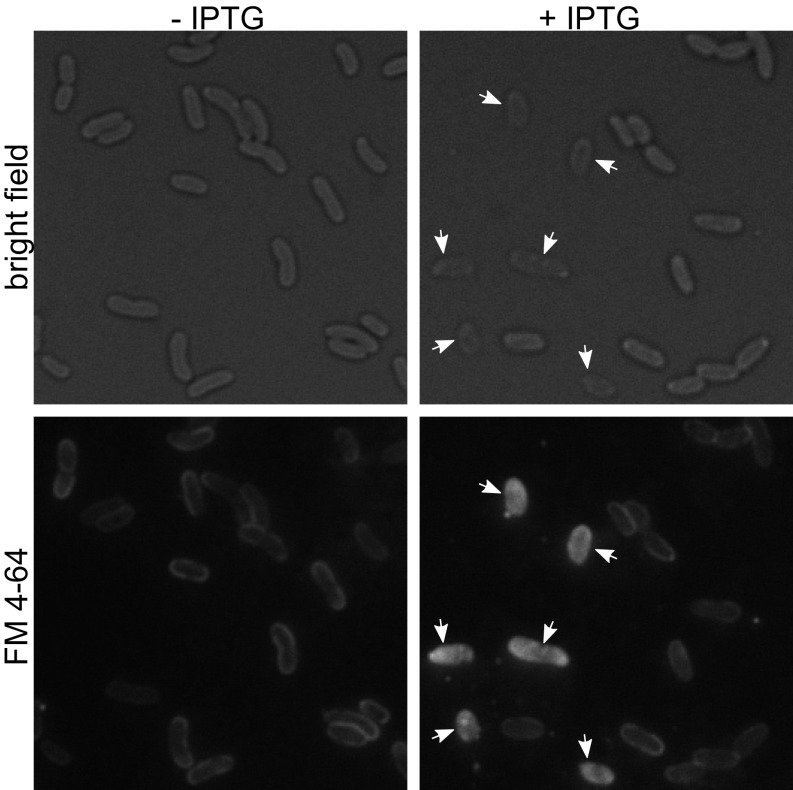
Finally, we used fluorescence microscopy to visualize the effect of dncV overexpression and CapV activation on El Tor V. cholerae cell membranes. Rapid degradation of phospholipids from the membrane could compromise membrane integrity and increase permeability. Using the lipophilic dye FM 4-64, which selectively stains cell membranes, we assessed the impact on the overall cell membrane structure of WT El Tor V. cholerae cells following dncV overexpression (40, 41). Uninduced cells appeared as normal comma-shaped V. cholerae under bright-field microscopy, and the FM 4-64 staining was localized to the cell periphery (Fig. 6, Left). Following ectopic expression of dncV, “ghost cells,” which are more translucent than normal cells with bright-field microscopy, appeared within 30 min of dncV overexpression and became a substantial proportion of the visualized population 2 h after induction (Fig. 6, Right). While the shape of most of these ghost cells remained normal, the interior of the cells was flooded with FM 4-64 dye, indicating that cell membrane integrity had been compromised following dncV overexpression. This microscopy study further implicates the cell membrane is the target of the DncV, cGAMP, CapV signaling pathway in El Tor V. cholerae.
Fig. 6.
Overproduction of the cGAMP synthase DncV is detrimental to membrane integrity. Images depict the cell morphology (Top, bright field) and membrane integrity (Bottom, FM 4-64) of El Tor V. cholerae WT carrying pDncV 2 h postinduction with (Right) or without (Left) 100 µM IPTG. Expression of dncV leads to a loss of cellular integrity (Top Right) and altered fluorescent labeling of the cell membrane (Bottom Right). Arrows indicate affected ghost cells. Images are representative of six independent experiments.
Discussion
The hybrid dinucleotide second messenger, cGAMP, was first discovered in El Tor V. cholerae and is involved in the regulation of a number of processes, including virulence, chemotaxis, and fatty acid metabolism (23). There is very little understanding of the direct targets of bacterial cGAMP or how this signal regulates its interacting partners. Here, we identify CapV, a previously uncharacterized phospholipase, as a proteinaceous cGAMP receptor in V. cholerae, demonstrate that this small molecule interaction activates the enzyme, and show that this activity leads to the degradation of specific phospholipid components from the cell membrane.
Results from our biochemical and whole-cell assays (Figs. 3–6) show that cGAMP directly and specifically activates CapV phospholipase activity. We note that it is possible that the 8.6 ± 1.9 μM Kd for cGAMP binding to CapV, as determined in vitro using MST, underestimates the in vivo binding affinity for numerous reasons, including, among other possibilities, the absence of phospholipid substrate. However, we found that cGAMP concentration reached 1.2 µM in dncV-expressing cells (SI Appendix, Fig. S3A), and the Kd determined by MST is in line with previous in vitro measurements of cyclic dinucleotide (e.g., c-diGMP) binding to some target receptors (42–45). While additional studies are required to measure the effects of phospholipid substrate on CapV-cGAMP binding affinity, it is clear that CapV phospholipase activity is directly and specifically triggered by cGAMP.
Both DncV and CapV are encoded in the VSP-1 genomic island that was acquired by the current El Tor V. cholerae strains (32). Our results and others’ (23) suggest that the acquisition of DncV and cGAMP signaling provides El Tor with additional biochemical capacities that are likely important in the evolution of pandemic El Tor V. cholerae. While we now understand that cGAMP interacts with CapV to form a functional cGAMP signaling pathway, it is still unclear how this contributes to the in vitro and in vivo fitness of the current V. cholerae pandemic strains. Our current study demonstrates that full activation of CapV by cGAMP results in growth inhibition due to phospholipid degradation; however, it is very likely that CapV is activated to various extents during the V. cholerae life cycle. We hypothesize that, in part, cGAMP regulation of CapV remodels the cell membrane of El Tor V. cholerae to adapt to different membrane stresses.
Moreover, unsaturated fatty acids (USFAs) are key regulators of membrane fluidity (46), and their abundance plays a role in homeoviscous adaptation (47). We speculate that the specific removal of USFAs bound to PE and PG would make the cell membrane more rigid and this cGAMP-CapV degradative process may provide El Tor V. cholerae with a fitness advantage when confronted with fluctuations in ambient temperature (48). USFAs have also been shown to negatively impact the transcriptional activity of the master virulence transcriptional activator, ToxT (49–51). The cGAMP-CapV signaling network is part of the ToxT regulon (23), and this signal transduction system may have contributed to the emergence and persistence of pandemic El Tor by releasing USFAs to alter the dynamics of virulence gene expression. Clinical manifestations of El Tor during human infections are reported to be less severe than those of classical strains, consistent with cGAMP-CapV negatively regulating virulence factor expression (52, 53).
Giles et al. (39) showed that polyunsaturated fatty acids from bile and other natural sources can be incorporated into the V. cholerae membrane, resulting in alterations of the membrane phospholipid profile in this bacterium (39). These findings suggest that V. cholerae undergoes membrane remodeling during infection of the small intestine. The expression of both capV and dncV are indirectly induced by ToxT in V. cholerae (23), suggesting that CapV and DncV might participate in membrane remodeling in the presence of bile during infection. However, it should be noted that incorporation of exogenous FFAs occurs in both El Tor and classical strains; thus, cGAMP signaling must provide additional advantages to the El Tor biotype.
Burroughs et al. (34) recently performed a comprehensive bioinformatic analysis of the genes associated with members of the DncV nucleotide synthase family. They determined that these synthases are frequently associated with specific classes of effector proteins, including patatin-like phospholipases homologous to CapV. The phylogenetic distribution of these gene elements suggested that they moved by horizontal gene transfer, and their analysis found a DncV ortholog linked to a phospholipase in Verrucomicrobia, α‐, β‐, δ‐, and γ‐proteobacteria, Paenibacillus, cyanobacteria, bacteroidetes, and actinobacteria (34). These authors predicted that the synthases might directly control the activity of the associated phospholipases, and our results in El Tor V. cholerae support this prediction. Thus, cGAMP regulation of a phospholipase to modify bacterial membranes may be widespread.
Materials and Methods
Strains and Plasmids.
All bacterial strains and plasmids used in this study are listed in SI Appendix, Table S1. PCR primers used are listed in SI Appendix, Table S2. V. cholerae mutants were constructed with allelic exchange using plasmid pKAS32 as described previously (54). Details of strain construction, growth analysis, and suppressor screens can be found in SI Appendix.
Serine Hydrolase Labeling Assay.
The serine hydrolase activity of His6-tagged CapV purified from E. coli was probed with a fluorescently labeled suicide substrate FP-Rh based on a procedure previously described (55). Details of assays can be found in SI Appendix.
Phospholipase Assay and Lipid Analysis.
In vitro lipase assays using 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (dioleoyl-PE) (Avanti Polar Lipids) as the lipase substrate with His6-tagged CapV were assembled similar to a procedure previously described (56). Extraction and analysis of V. cholerae lipids was based on a previously published protocol (57). Details of these assays can be found in SI Appendix.
Detailed information of all other experimental procedures is provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Andrew Camilli, Victor DiRita, and members in the W.-L.N. and C.M.W. laboratories for helpful comments and suggestions; Jing-ping Cong for construction of the El Tor cosmid library; Benjamin Pursley for construction of pMMB67EH-DncV; and Christopher Rhoades, Lucas Demey, and Morgan Miller for research assistance. We appreciate the generous gift of FP-Rh from Aimee Shen. W.-L.N., M.S.R., L.A.H., and B.J.O. were supported in part by NIH Grants GM007310, AI007329, and AI121337. C.M.W., G.B.S., M.E.P., A.-K.K., and L.V.B. were supported in part by NIH Grants GM109259, GM110444, and AI130554, and NSF Grant MCB-1253684. M.B.N. and A.K. were supported in part by NIH Grants GM109259 and AI125452. C.B. and K.W. were supported by Department of Energy Grants DE-FG02-98ER20305 and DE-FG02-91ER20021 and by AgBioResearch, Michigan State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801233115/-/DCSupplemental.
References
- 1.Hengge R, Gründling A, Jenal U, Ryan R, Yildiz F. Bacterial signal transduction by cyclic di-GMP and other nucleotide second messengers. J Bacteriol. 2016;198:15–26. doi: 10.1128/JB.00331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makman RS, Sutherland EW. Adenosine 3′,5′-Phosphate in Escherichia coli. J Biol Chem. 1965;240:1309–1314. [PubMed] [Google Scholar]
- 4.D’Ari R, Jaffé A, Bouloc P, Robin A. Cyclic AMP and cell division in Escherichia coli. J Bacteriol. 1988;170:65–70. doi: 10.1128/jb.170.1.65-70.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong JC, Yildiz FH. Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J Bacteriol. 2008;190:6646–6659. doi: 10.1128/JB.00466-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen S, Young GM. Essential role for cyclic AMP and its receptor protein in Yersinia enterocolitica virulence. Infect Immun. 2002;70:3665–3672. doi: 10.1128/IAI.70.7.3665-3672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashel M, Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969;221:838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- 8.Haseltine WA, Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci USA. 1973;70:1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol. 2015;13:298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Bittner AN, Wang JD. Diversity in (p)ppGpp metabolism and effectors. Curr Opin Microbiol. 2015;24:72–79. doi: 10.1016/j.mib.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Gründling A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011;7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Y, Helmann JD. Analysis of the role of Bacillus subtilis σ(M) in β-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Microbiol. 2012;83:623–639. doi: 10.1111/j.1365-2958.2011.07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep. 2011;12:594–601. doi: 10.1038/embor.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witte G, Hartung S, Büttner K, Hopfner KP. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell. 2008;30:167–178. doi: 10.1016/j.molcel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Li W, He ZG. DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in Mycobacterium smegmatis. J Biol Chem. 2013;288:3085–3096. doi: 10.1074/jbc.M112.428110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai Y, et al. Cyclic di-AMP impairs potassium uptake mediated by a cyclic di-AMP binding protein in Streptococcus pneumoniae. J Bacteriol. 2014;196:614–623. doi: 10.1128/JB.01041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrigan RM, et al. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc Natl Acad Sci USA. 2013;110:9084–9089. doi: 10.1073/pnas.1300595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gundlach J, et al. Control of potassium homeostasis is an essential function of the second messenger cyclic di-AMP in Bacillus subtilis. Sci Signal. 2017;10:eaal3011. doi: 10.1126/scisignal.aal3011. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, et al. Structural studies of potassium transport protein KtrA Regulator of Conductance of K+ (RCK) C domain in complex with cyclic diadenosine monophosphate (c-di-AMP) J Biol Chem. 2015;290:16393–16402. doi: 10.1074/jbc.M115.641340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moscoso JA, et al. Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter. J Bacteriol. 2015;198:98–110. doi: 10.1128/JB.00480-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies BW, Bogard RW, Young TS, Mekalanos JJ. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell. 2012;149:358–370. doi: 10.1016/j.cell.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J, et al. Identification and characterization of phosphodiesterases that specifically degrade 3′3′-cyclic GMP-AMP. Cell Res. 2015;25:539–550. doi: 10.1038/cr.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson JW, et al. Control of bacterial exoelectrogenesis by c-AMP-GMP. Proc Natl Acad Sci USA. 2015;112:5389–5394. doi: 10.1073/pnas.1419264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren A, et al. Structural basis for molecular discrimination by a 3′,3′-cGAMP sensing riboswitch. Cell Rep. 2015;11:1–12. doi: 10.1016/j.celrep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallberg ZF, et al. Hybrid promiscuous (Hypr) GGDEF enzymes produce cyclic AMP-GMP (3′, 3′-cGAMP) Proc Natl Acad Sci USA. 2016;113:1790–1795. doi: 10.1073/pnas.1515287113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kranzusch PJ, et al. Structure-guided reprogramming of human cGAS dinucleotide linkage specificity. Cell. 2014;158:1011–1021. doi: 10.1016/j.cell.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato K, Omura H, Ishitani R, Nureki O. Cyclic GMP-AMP as an endogenous second messenger in innate immune signaling by cytosolic DNA. Annu Rev Biochem. 2017;86:541–566. doi: 10.1146/annurev-biochem-061516-044813. [DOI] [PubMed] [Google Scholar]
- 32.Dziejman M, et al. Comparative genomic analysis of Vibrio cholerae: Genes that correlate with cholera endemic and pandemic disease. Proc Natl Acad Sci USA. 2002;99:1556–1561. doi: 10.1073/pnas.042667999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu D, et al. Origins of the current seventh cholera pandemic. Proc Natl Acad Sci USA. 2016;113:E7730–E7739. doi: 10.1073/pnas.1608732113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burroughs AM, Zhang D, Schäffer DE, Iyer LM, Aravind L. Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological conflicts, immunity and signaling. Nucleic Acids Res. 2015;43:10633–10654. doi: 10.1093/nar/gkv1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: Classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 36.Kidd D, Liu Y, Cravatt BF. Profiling serine hydrolase activities in complex proteomes. Biochemistry. 2001;40:4005–4015. doi: 10.1021/bi002579j. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Patricelli MP, Cravatt BF. Activity-based protein profiling: The serine hydrolases. Proc Natl Acad Sci USA. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koestler BJ, Waters CM. Exploring environmental control of cyclic di-GMP signaling in Vibrio cholerae by using the ex vivo lysate cyclic di-GMP assay (TELCA) Appl Environ Microbiol. 2013;79:5233–5241. doi: 10.1128/AEM.01596-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giles DK, Hankins JV, Guan Z, Trent MS. Remodelling of the Vibrio cholerae membrane by incorporation of exogenous fatty acids from host and aquatic environments. Mol Microbiol. 2011;79:716–728. doi: 10.1111/j.1365-2958.2010.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fishov I, Woldringh CL. Visualization of membrane domains in Escherichia coli. Mol Microbiol. 1999;32:1166–1172. doi: 10.1046/j.1365-2958.1999.01425.x. [DOI] [PubMed] [Google Scholar]
- 41.Pogliano J, et al. A vital stain for studying membrane dynamics in bacteria: A novel mechanism controlling septation during Bacillus subtilis sporulation. Mol Microbiol. 1999;31:1149–1159. doi: 10.1046/j.1365-2958.1999.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol. 2008;69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merighi M, Lee VT, Hyodo M, Hayakawa Y, Lory S. The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol Microbiol. 2007;65:876–895. doi: 10.1111/j.1365-2958.2007.05817.x. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava D, Harris RC, Waters CM. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J Bacteriol. 2011;193:6331–6341. doi: 10.1128/JB.05167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitney JC, et al. Dimeric c-di-GMP is required for post-translational regulation of alginate production in Pseudomonas aeruginosa. J Biol Chem. 2015;290:12451–12462. doi: 10.1074/jbc.M115.645051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguilar PS, de Mendoza D. Control of fatty acid desaturation: A mechanism conserved from bacteria to humans. Mol Microbiol. 2006;62:1507–1514. doi: 10.1111/j.1365-2958.2006.05484.x. [DOI] [PubMed] [Google Scholar]
- 47.Ernst R, Ejsing CS, Antonny B. Homeoviscous adaptation and the regulation of membrane lipids. J Mol Biol. 2016;428:4776–4791. doi: 10.1016/j.jmb.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 48.de Mendoza D. Temperature sensing by membranes. Annu Rev Microbiol. 2014;68:101–116. doi: 10.1146/annurev-micro-091313-103612. [DOI] [PubMed] [Google Scholar]
- 49.Childers BM, et al. N-terminal residues of the Vibrio cholerae virulence regulatory protein ToxT involved in dimerization and modulation by fatty acids. J Biol Chem. 2011;286:28644–28655. doi: 10.1074/jbc.M111.258780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plecha SC, Withey JH. Mechanism for inhibition of Vibrio cholerae ToxT activity by the unsaturated fatty acid components of bile. J Bacteriol. 2015;197:1716–1725. doi: 10.1128/JB.02409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowden MJ, et al. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc Natl Acad Sci USA. 2010;107:2860–2865. doi: 10.1073/pnas.0915021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bart KJ, Huq Z, Khan M, Mosley WH. Seroepidemiologic studies during a simultaneous epidemic of infection with El Tor Ogawa and classical Inaba Vibrio cholerae. J Infect Dis. 1970;121(Suppl):S17–S24. doi: 10.1093/infdis/121.supplement.s17. [DOI] [PubMed] [Google Scholar]
- 53.Khan M, Shahidullah M. Cholera due to the E1 Tor biotype equals the classical biotype in severity and attack rates. J Trop Med Hyg. 1980;83:35–39. [PubMed] [Google Scholar]
- 54.Skorupski K, Taylor RK. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 55.Adams CM, Eckenroth BE, Putnam EE, Doublié S, Shen A. Structural and functional analysis of the CspB protease required for Clostridium spore germination. PLoS Pathog. 2013;9:e1003165. doi: 10.1371/journal.ppat.1003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang K, Froehlich JE, Zienkiewicz A, Hersh HL, Benning C. A plastid phosphatidylglycerol lipase contributes to the export of acyl groups from plastids for seed oil biosynthesis. Plant Cell. 2017;29:1678–1696. doi: 10.1105/tpc.17.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Benning C. Arabidopsis thaliana polar glycerolipid profiling by thin layer chromatography (TLC) coupled with gas-liquid chromatography (GLC) J Vis Exp. 2011;49:2518. doi: 10.3791/2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.