Significance
Despite high response rates to treatment with small molecule inhibitors of EGFR tyrosine kinase activity, patients with EGFR-mutant lung adenocarcinomas eventually develop resistance to these drugs. In many cases, the basis of acquired resistance remains unclear. We have used a transposon mutagenesis screen in an EGFR-mutant cell line and clinical genomic sequencing in cases of acquired resistance to identify amplification of YES1 as a targetable mechanism of resistance to EGFR inhibitors in EGFR-mutant lung cancers.
Keywords: YES1, EGFR, ALK, acquired resistance, lung adenocarcinoma
Abstract
In ∼30% of patients with EGFR-mutant lung adenocarcinomas whose disease progresses on EGFR inhibitors, the basis for acquired resistance remains unclear. We have integrated transposon mutagenesis screening in an EGFR-mutant cell line and clinical genomic sequencing in cases of acquired resistance to identify mechanisms of resistance to EGFR inhibitors. The most prominent candidate genes identified by insertions in or near the genes during the screen were MET, a gene whose amplification is known to mediate resistance to EGFR inhibitors, and the gene encoding the Src family kinase YES1. Cell clones with transposon insertions that activated expression of YES1 exhibited resistance to all three generations of EGFR inhibitors and sensitivity to pharmacologic and siRNA-mediated inhibition of YES1. Analysis of clinical genomic sequencing data from cases of acquired resistance to EGFR inhibitors revealed amplification of YES1 in five cases, four of which lacked any other known mechanisms of resistance. Preinhibitor samples, available for two of the five patients, lacked YES1 amplification. None of 136 postinhibitor samples had detectable amplification of other Src family kinases (SRC and FYN). YES1 amplification was also found in 2 of 17 samples from ALK fusion-positive lung cancer patients who had progressed on ALK TKIs. Taken together, our findings identify acquired amplification of YES1 as a recurrent and targetable mechanism of resistance to EGFR inhibition in EGFR-mutant lung cancers and demonstrate the utility of transposon mutagenesis in discovering clinically relevant mechanisms of drug resistance.
Four small molecule tyrosine kinase inhibitors (TKIs) have been approved by the Food and Drug Administration (FDA) for the treatment of EGFR-mutant lung cancers and represent three generations of drug development for this disease: erlotinib and gefitinib (first generation), afatinib (second), and osimertinib (third). Despite high response rates to these agents, the development of acquired resistance almost universally ensues. The mechanisms of acquired resistance can be grouped into target-dependent and target-independent categories. Target-dependent mechanisms are secondary alterations of EGFR that typically affect drug binding by, for example, altering the affinity of the kinase for ATP or by eliminating key sites for covalent bonding between drug and target protein. These include the T790M mutation that confers resistance to first- and second-generation EGFR TKIs (1–4) and the C797S mutation that emerges upon osimertinib treatment (5, 6). Common target-independent mechanisms include amplification of MET and ERBB2 (HER2) as well as small cell transformation (7, 8). However, in ∼30% of cases of acquired resistance to first-generation EGFR TKIs, the underlying mechanisms still remain to be identified. Although target-independent resistance mechanisms are expected to largely overlap between EGFR TKI generations, comprehensive studies of mechanisms of acquired resistance to third-generation TKIs are currently ongoing.
To complement clinical genomic sequencing as a means of identifying mediators of resistance to EGFR inhibition, several different strategies have been employed using cell culture-based systems. Gradual escalation of concentrations of EGFR TKIs applied to EGFR-mutant lung cancer cell lines initially sensitive to the drugs has yielded TKI-resistant cells with clinically relevant mechanisms of resistance, including amplification of MET (9), overexpression of AXL (10), and secondary mutations of EGFR, most notably the T790M mutation (11–13). Forward genetic screens for modifiers of responses to EGFR inhibition, using libraries for RNA interference (14–18), expression of ORFs (16, 19), or CRISPR/Cas9-mediated gene deletion (16, 20), have also identified candidate genes that are implicated in acquired resistance in patients, including NF1, BRAF, AXL, and ERBB2.
Transposon-based mutagenesis is another forward genetic approach that can identify mechanisms of drug resistance. This strategy introduces genome-wide insertions of transposons, which have been designed with the potential to induce both gains and losses of endogenous gene function through the action of promoter/enhancer elements and splice acceptor and donor sequences that have been introduced into the transposons (21). Transposon mutagenesis has been used in cell culture-based systems and mouse models to screen for resistance to standard and investigational therapies for a variety of cancers, including paclitaxel (22), fludarabine (23), the PARP inhibitor olaparib (24), the MDM2-TP53 inhibitor HDM201 (25), and the BRAF inhibitors PLX4720 and PLX4032 (26, 27).
Here we report the results of an integrated approach, employing both forward genetic screening with transposon mutagenesis to recover drug-resistant derivatives of an EGFR-mutant lung adenocarcinoma cell line and genomic sequencing data from patients with acquired resistance to define clinically relevant mechanisms of resistance to EGFR inhibition.
Results
A Transposon Mutagenesis Screen for Resistance to EGFR Inhibition in an EGFR-Mutant Lung Adenocarcinoma Cell Line.
To identify mechanisms of resistance to EGFR inhibition, we performed a transposon mutagenesis screen for resistance to the second-generation EGFR TKI afatinib in the EGFR TKI-sensitive PC9 lung adenocarcinoma cell line, which harbors an activating small in-frame deletion in exon 19 of EGFR (Fig. 1A). Because transposon mutagenesis does not generate point mutations, our screen favored the recovery of target-independent mechanisms of resistance over target-dependent mechanisms such as the T790M and C797S second site mutations in EGFR. Although the emergence of some target-independent mechanisms of resistance might be suppressed by off-target TKI inhibition of kinases other than EGFR, we expected several of these mechanisms, including amplification of MET, to emerge repeatedly with successive generations of EGFR TKIs.
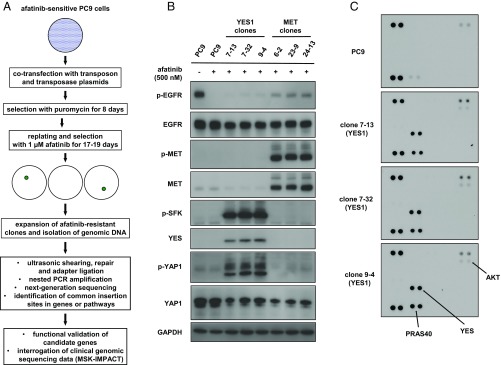
Fig. 1.
A transposon mutagenesis screen in EGFR-mutant PC9 lung adenocarcinoma cells for resistance to afatinib. (A) Flowchart representing the overall design of the screen. (B) Lysates from PC9 cells, YES1 clones, and MET clones treated with or without 500 nM afatinib for 60 min were subjected to immunoblot analysis with antibodies against the indicated proteins. (C) Lysates from PC9 cells and YES1 clones treated with 500 nM afatinib for 60 min were hybridized to human phosphokinase antibody arrays (ARY003B; R&D Systems).
PC9 cells were cotransfected with plasmids encoding a hyperactive piggyBac transposase (28) and a mutagenic transposon, which includes cytomegalovirus (CMV) enhancer and promoter sequences, a splice donor sequence, and a puromycin resistance cassette that provides a selection marker for transposon tagging (22). After selection with puromycin, transposon-tagged cells from 13 independent cotransfections were selected with 1 µM afatinib for 17–19 d. Afatinib-resistant clones were isolated for expansion and preparation of genomic DNA. No resistant clones were observed with non–transposon-tagged parental PC9 cells that were treated in parallel with 1 µM afatinib.
Transposon insertion sites were identified using a modified TraDIS-type method to generate Illumina-compatible libraries from DNA fragments that span the piggyBac sequence and the surrounding genomic DNA (29). Utilizing a custom bioinformatic pipeline with a set of filters based on the number of supporting reads, mean fragment size, and SD of fragment size, we generated a list of 1,927 distinct transposon insertion sites from 188 afatinib-resistant clones. Insertions were predicted to be activating if a transposon was situated near the transcription start site or first intron of a known human gene and was correctly oriented to drive expression of that gene. Genes that were found to be disrupted by insertions in both orientations or throughout the body of the gene were predicted to be inactivated.
MET and YES1 Are the Top Candidate Genes from the Transposon Mutagenesis Screen for Resistance to EGFR Inhibition.
Because the period between transfection and selection with afatinib was sufficient to allow one or more rounds of cell division of transposon-tagged cells, several clones from each transfection exhibited identical insertion sites, consistent with derivation from a common transfected progenitor. In selecting candidate genes for functional analysis, we therefore prioritized them based on the number of different insertions per gene and the number of independent transfections in which these insertions were discovered. The most promising candidate genes are listed in Table 1. The top two candidates were MET, encoding a receptor tyrosine kinase that is a known mediator of resistance, and YES1, encoding a Src family kinase (SFK). (Because there is no YES2 gene and no other SFK gene name contains numerals, the authors suggested to the Human Genome Organisation (HUGO) Gene Nomenclature Committee that the gene name be changed from YES1 to YES. The committee did not agree to this change, noting that the use of “yes” in literature searches recovers numerous unrelated items. Regardless of the arguments for and against either YES1 or YES as a gene name, the continued use of both YES1 and YES within the scientific community necessitates the inclusion of both terms in literature searches to ensure retrieval of all publications that are relevant to the gene.) All but one of the 188 clones harbored insertions in MET (78 clones), YES1 (58 clones), or both genes (51 clones). In 29 clones, insertions were only found in MET out of the candidate genes listed in Table 1, and 45 clones had insertions in only YES1 among these same candidate genes. The one clone that lacked insertions in either MET or YES1 instead had insertions predicted to be activating in SOS1 and RABGAP1L. Mutations in SOS1 were recently found to be significantly enriched in lung adenocarcinoma samples without known driver alterations (30). As expected, ERBB2, another gene whose amplification is known to mediate resistance to erlotinib (31), was absent from the candidate list, reflecting the fact that afatinib also inhibits ERBB2 (32).
Table 1.
Candidate genes from a transposon mutagenesis screen for resistance to afatinib in the EGFR-mutant PC9 lung adenocarcinoma cell line
| Gene name | Predicted functional effect of insertions | No. of distinct insertion sites | No. of independent transfections with insertions | Total no. of clones with insertions |
| MET | Activating | 19 | 12 | 129 |
| YES1 | Activating | 15 | 8 | 109 |
| FYN | Activating | 14 | 9 | 30 |
| GSK3B | Inactivating | 7 | 4 | 8 |
| SOS1 | Activating | 6 | 12 | 37 |
| DNM3 | Inactivating | 4 | 8 | 24 |
| NAV2 | Inactivating | 4 | 5 | 15 |
| EPS8 | Activating | 4 | 2 | 6 |
| KRAS | Activating | 4 | 2 | 5 |
| MYOF | Inactivating | 3 | 4 | 19 |
| RABGAP1L | Activating | 3 | 3 | 15 |
| RAF1 | Activating | 3 | 3 | 6 |
| RABGAP1 | Activating | 3 | 3 | 4 |
Candidate genes from a transposon mutagenesis screen for resistance to afatinib in the EGFR-mutant PC9 lung adenocarcinoma cell line. A total of 1,927 distinct transposon insertion sites were identified in 188 afatinib-resistant PC9 clones from 13 independent transfections. Insertions were predicted to be activating if a transposon was situated near the transcription start site or first intron of a known human gene and was correctly oriented to drive expression of that gene. Genes that were found to be disrupted by insertions in both orientations or throughout the body of the gene were predicted to be inactivated.
Transposon Insertions in YES1 Result in High Expression and Phosphorylation of YES1.
We selected three clones with activating insertions in MET and another three with insertions in YES1—hereafter referred to as MET clones and YES1 clones—for further characterization alongside parental PC9 cells. All six clones were maintained in growth medium containing 500 nM afatinib and lacked insertions in the other candidate genes listed in Table 1. To determine the levels of MET and YES1 proteins and phosphorylation of those proteins, we performed a series of immunoblots on cell lysates (Fig. 1B). High levels of phosphorylated MET were detected in MET clones. YES1 clones exhibited high levels of YES1, phosphorylated SFKs, and phosphorylated Yes-associated protein 1 (YAP1). Because the phospho-SFK antibody does not distinguish between different SFKs, we analyzed cell lysates from YES1 clones using a phosphokinase array that specifically measures phosphorylation of YES, SRC, FYN, and four other SFKs (Fig. 1C). In all three YES1 clones, only phosphorylation of YES1 was detected among these seven SFKs. A similar survey using receptor tyrosine kinase (RTK) arrays showed phosphorylation of MET and ERBB3 in MET clones and phosphorylation of ERBB3 in YES1 clones, which was confirmed by immunoblot analysis (SI Appendix, Fig. S1). Taken together, these findings confirm that the transposon insertions in YES1 and MET resulted in high levels of the corresponding proteins; phosphorylation of these two kinases and their associated proteins is consistent with activation of YES1 and MET kinases in their respective clones.
Clones with Activating Insertions in YES1 Are Resistant to All Three Generations of EGFR TKIs but Are Resensitized upon Inhibition of YES1.
We next determined if the YES1 and MET clones were resistant to all three generations of EGFR inhibitors and if the resistance was dependent on functional activity of YES1 and MET, respectively. Because only the second-generation EGFR inhibitor afatinib was used in the transposon mutagenesis discovery screen, we tested the sensitivity of the clones to the first-generation TKI erlotinib and the third-generation TKI osimertinib. Cell viability assays showed that all six clones were resistant to all three generations of EGFR inhibitors (Fig. 2A and SI Appendix, Fig. S2A). To block the kinase activities of YES1 and MET, we used the SFK inhibitors dasatinib and saracatinib and the MET inhibitor crizotinib, respectively. YES1 clones were sensitive to the addition of dasatinib or saracatinib to afatinib but not to the combination of crizotinib with afatinib (Fig. 2B). Conversely, MET clones were sensitive to the addition of crizotinib to afatinib but not to the pairing of dasatinib or saracatinib with afatinib (SI Appendix, Fig. S2B). YES1 clones were also sensitive to the combination of either SFK inhibitor with osimertinib (Fig. 2C). Phosphorylation of serine-threonine kinase AKT and extracellular signal-regulated kinases (ERK) was observed in both YES1 and MET clones and was blocked by inhibiting SFKs or MET in addition to EGFR (Fig. 2D). Modest phosphorylation of EGFR, likely caused by kinases other than EGFR, was also abrogated by the addition of the SFK and MET inhibitors. Removal of afatinib from the growth medium for 72 h restored high levels of phosphorylated EGFR in YES1 clones, indicating that the intrinsic kinase activity of EGFR remained intact in these clones (SI Appendix, Fig. S2C).
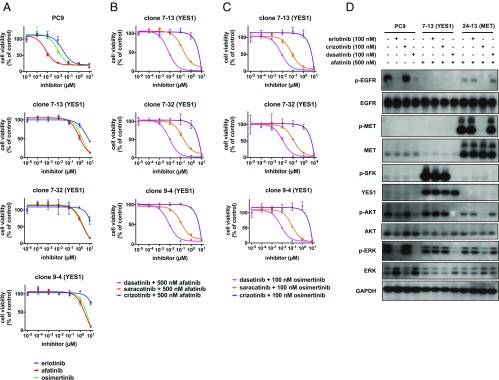
Fig. 2.
YES1 clones are resistant to EGFR inhibitors from all three generations but sensitive when YES1 is inhibited. (A–C) PC9 cells and YES1 clones were seeded in 96-well plates and treated with EGFR inhibitors or the indicated inhibitors in combination with 500 nM afatinib or 100 nM osimertinib for 96 h. Cell viability was assayed as described in Materials and Methods. Data are expressed as a percentage of the value for cells treated with a vehicle control and are means of triplicates. The experiments were performed three times with similar results. (D) Lysates from PC9 cells, clone 7-13 (YES1), and clone 24-13 (MET) treated with the indicated inhibitors for 60 min were subjected to immunoblot analysis with antibodies against the indicated proteins.
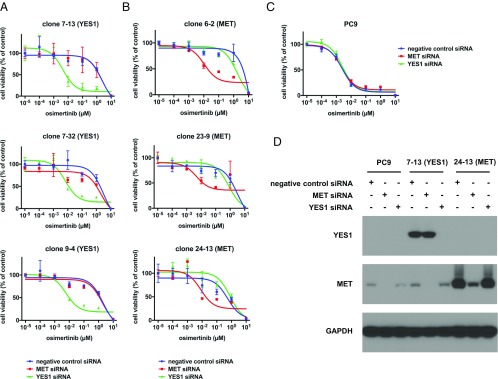
Because dasatinib and saracatinib have activity against kinases other than YES1, we specifically reduced YES1 levels by siRNA-mediated knockdown and assessed the effect on the viability of YES1 clones. In addition, since the FLAURA study recently showed superior efficacy of osimertinib to that of standard EGFR TKIs in the first-line treatment of EGFR mutation-positive advanced NSCLC, we chose osimertinib to combine with siRNA-mediated knockdown of YES1 (33). As shown in Fig. 3A, the YES1-specific siRNA, but not the negative control or MET-specific siRNA, sensitized YES1 clones to treatment with osimertinib. In contrast, the MET-specific siRNA, but not the negative control or YES1-specific siRNA, sensitized MET clones to treatment with osimertinib (Fig. 3B). Neither the YES1-specific siRNA nor MET-specific siRNA increased the sensitivity of parental PC9 cells to treatment with osimertinib (Fig. 3C). These results are consistent with YES1 as the key target of SFK inhibitors in YES1 clones and confirm that YES1 is required to mediate the resistance of these clones to EGFR inhibitors.
Fig. 3.
YES1 clones are resistant to osimertinib but are resensitized by siRNA-mediated knockdown of YES1. (A) YES1 clones, (B) MET clones, and (C) PC9 cells were transfected with negative control, MET-specific, and YES1–specific siRNAs at a final concentration of 10 nM. After 24 h, cells were trypsinized and seeded in 96-well plates at a density of 5,000 cells per well with the indicated concentrations of osimertinib for 72 h followed by measurement of cell viability. Experiments were performed three times with similar results. (D) Immunoblot analysis with YES1, MET, and GAPDH antibodies was performed on lysates prepared from PC9 cells, clone 7-13, and clone 24-13 72 h after transfection with the indicated siRNAs.
YES1 Is Amplified in Clinical Cases of Acquired Resistance to EGFR Inhibitors.
To search for clinical evidence of a role for YES1 in acquired resistance to EGFR inhibition, we examined clinical genomic sequencing data generated with the Memorial Sloan Kettering (MSK)-Integrated Mutational Profiling of Actionable Cancer Targets (IMPACT) panel from 136 patients whose EGFR-mutant lung adenocarcinomas progressed on EGFR inhibitors (34). This dataset included 128 post-erlotinib, 6 post-afatinib, and 2 post-dacomitinib cases of acquired resistance to EGFR inhibition. Amplification of YES1 was identified in 3 out of 66 T790M-negative cases and 1 out of 70 T790M-positive cases. None of the 136 cases had detectable amplification of SRC or FYN, the two other SFKs included in the MSK-IMPACT panel assay. The MSK-IMPACT fold changes (normalized log2 transformed fold changes of coverage of tumor versus normal) and FACETS (Fraction and Allele-Specific Copy Number Estimates from Tumor Sequencing) integer values (allele-specific copy numbers corrected for tumor purity, ploidy, and clonal heterogeneity) for YES1 are listed in Table 2, and all of the copy number profiles are shown in Fig. 4A and SI Appendix, Fig. S3. The responses to the indicated EGFR TKIs in the first four cases with amplification of YES1 ranged from ∼5 mo (patient 1) to ∼30 mo (patient 2). Additionally, separate from this cohort of 136 consecutive patients with MSK-IMPACT data on their EGFR-mutant lung adenocarcinomas with acquired resistance to EGFR inhibitors, we recently detected a striking level of acquired YES1 amplification in a fifth EGFR-mutant case, a T790M-negative case of progression of disease on erlotinib and maintenance pemetrexed. Previous treatment regimens in this patient also included the use of carboplatin, bevacizumab, and localized radiation therapy to the site of progression. The progression sample also harbored a missense mutation of unclear significance in YES1, Q322H, that appeared to be amplified copies of the gene based on its variant allele fraction (patient 5, Fig. 4A and Table 2). A prior post-TKI sample 2 y earlier did not show YES1 amplification.
Table 2.
Clinical and molecular features of cases of acquired resistance to EGFR or ALK inhibitors with amplification of YES1
| YES1 gain by MSK-IMPACT | ||||||||||
| Patient ID | Age | Sex | Driver alteration | Pre-biopsy therapies | Somatic mutations | Copy number alterations | FACETS copy number | Fold change | Confirmation of YES1 amp by FISH | Pre-TKI YES1 amp by MSK-IMPACT |
| 1 | 66 | F | EGFR L858R | erlotinib | EGFR V689M, IDH1 R132G, TP53 P36Rfs*7, FGFR R399C, AR G578* | YES1 amp | 7 | 2.2† | N/A | Absent |
| 2 | 60 | F | EGFR L858R | erlotinib, carboplatin + pemetrexed | TP53 G245D, SMARCA4 G1232V, BRCA2 Q3036E, TERT S796Y | YES1 amp, AKT2 amp, AXL amp | 5 | 1.6‡ | Yes§ | N/A |
| 3 | 82 | F | EGFR L747-A750del | erlotinib, pemetrexed, afatinib | PIK3CA N345H, TP53 A138V, RB1 X313_splice | YES1 amp, EGFR amp, GNAS amp, PRDM1 del | 6 | 1.9‡ | N/A | N/A |
| 4 | 69 | M | EGFR L858R | erlotinib, afatinib + cetuximab, erlotinib+ pemetrexed + bevacizumab | EGFR T790M, TP53 R213L, SP2-NF1 fusion¶, ZFHX3 F2097V, POLD1-MYH14 fusion, RAF1 R73Q, SOX17 R125H | YES1 amp, EGFR amp, PRDM1 del, NPM1 amp, CRLF2 amp, CEBPA amp | >10 | 4.0‡ | Yes# | Absent (pre-afatinib) |
| 5 | 60 | M | EGFR E746_T751delinsVA | erlotinib, carboplatin + pemetrexed + bevacizumab + erlotinib, radiation therapy | ARAF R297Q, SMAD4 D493Y, PBRM1 V1139Lfs*16, ARID2 Q455*, YES1 Q322H | YES1 amp, CDKN2A del, CDKN2B del, CRLF2 amp | 51 | 14.6† | N/A | Absent from previous post-TKI sample |
| 6 | 58 | F | EML4-ALK fusion | crizotinib, ceritinib | EP300 Q1874*, EP300 S1730F | YES1 amp, CDK4 amp, MDM2 amp | 4 | 5.2‡ | N/A | N/A |
| 7 | 45 | F | HIP1-ALK fusion | erlotinib, pemetrexed + bevacizumab, gemcitabine + vinorelbine, abraxane, crizotinib | CDKN2A R80*, ARID2 R80Efs*10 | YES1 amp, MDM2 amp | >10 | 12.1‡ | N/A | Absent |
The FACETS integer values are allele-specific copy numbers corrected for tumor purity, ploidy, and clonal heterogeneity. The MSK-IMPACT fold changes are normalized log2 transformed fold changes of coverage of tumor versus normal. N/A indicates not available. Boldface type indicates alteration was not detected pre-TKI in the three patients with available pre-TKI samples (for patient 4, not detected in the post-erlotinib/pre-afatinib sample).
See copy number plots in Fig. 3.
See copy number plots in SI Appendix, Fig. S3.
FISH ratio for YES1 gain was 2.6-fold.
Predicted to cause truncation of NF1 at exon 48.
Increased YES1 signals compared with the chromosome 18 centromere probe were clumped, precluding an accurate count.
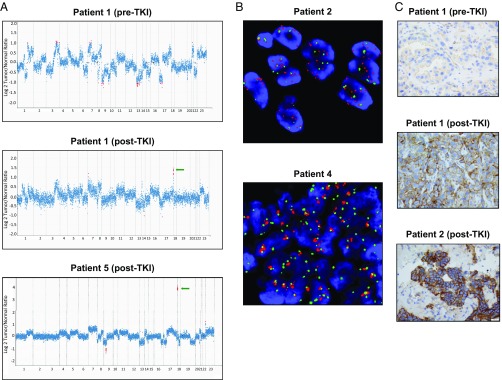
Fig. 4.
Amplification of YES1 in tumor samples from patients with acquired resistance to EGFR inhibitors. (A) Copy number plots for tumor samples from patients 1 and 5. Each dot represents a target region in the MSK-IMPACT targeted capture assay. Red dots are target regions exceeding a fold change cutoff of twofold. The log ratios (y axis) comparing tumor versus normal coverage values are calculated across all targeted regions (x axis). Green arrows indicate focal amplification of YES1 (11 coding exons targeted). (B) YES1 FISH for post-TKI tumor specimens from patients 2 and 4. YES1 (red) and CEP18 (green). For patient 2, the FISH ratio for YES1 gain was 2.6 fold. For patient 4, increased YES1 signals were clumped, precluding an accurate count. (C) Immunohistochemistry for YES1 on tumor samples from patients 1 and 2. The clinical and molecular features of these patients are summarized in Table 2.
Although the MSK-IMPACT assay is not designed to enable a formal analysis of minimal regions of gain or loss, additional focality data were available based on the assay results. YES1 is the most telomeric gene on 18p included in the MSK-IMPACT assay, extending from position 724,421 to 756,830. In all seven cases in Table 2, all YES1 exons showed an increase in copy number. The next set of probes immediately centromeric to YES1 are intergenic tiling probes extending from 2,224,682 to 38,530,030. The next closest gene in the assay panel is PIK3C3 starting at 39,535,254; none of the seven cases showed coamplification of PIK3C3. In two of the seven cases in Table 2 (patients 2 and 3), the YES1 gains included some of the tiling probes on the centromeric side, with the furthest being 34,882,991 in the former case. In the remaining five cases, amplification was only detected with the YES1 probes.
Amplification of YES1 was confirmed by fluorescence in situ hybridization (FISH) for two cases with sufficient material for analysis (Fig. 4B). Immunohistochemical staining for YES1 in post-TKI samples from patients 1 and 2 showed prominent labeling of lung adenocarcinoma cells which, moreover, was absent in the pre-TKI specimen available from patient 1 (Fig. 4C). A previously known mechanism of resistance was found in only one out of the four samples containing amplified YES1, namely, the EGFR T790M mutation, but with EGFR mutation allele frequencies (L858R 0.77 and T790M 0.16) that were consistent with intratumoral heterogeneity, raising the possibility that the T790M EGFR allele and the amplified YES1 allele were in separate subpopulations, as has been described in other instances of multiple concurrent resistance mechanisms (7, 8, 35, 36). In addition, amplification of YES1 was not detected in pre-TKI samples that were available for patients 1 and 4, confirming that it had emerged during treatment. Review of the MSK-IMPACT data in other molecular subsets of lung adenocarcinoma revealed YES1 amplification in 2 out of 17 ALK fusion-driven lung adenocarcinomas that had acquired resistance to ALK TKIs. These two cases did not show evidence of other changes, such as secondary mutations in ALK that have previously been found in such tumors that developed resistance to ALK inhibitors (Table 2). In one of these cases, the pre-TKI sample was available and showed no YES1 amplification.
To assess the occurrence of YES1 amplification generally in lung adenocarcinomas, not just those with acquired resistance to EGFR and ALK inhibitors, we reviewed all 2,466 lung adenocarcinomas in a more recent version of the MSK-IMPACT patient database (data freeze: August 31, 2017). In addition to the previously described four EGFR-mutant and two ALK-rearranged lung adenocarcinomas, we found 13 more cases with an amplified YES1 locus, including two EGFR-mutant tumors pre-TKI treatment, three tumors with a KRAS mutation, one pre-TKI tumor with a MET mutation causing exon 14 skipping, and eight tumors without a known driver mutation. These data indicate that YES1 amplification is rarely detected before targeted therapy for EGFR-mutant and ALK-rearranged lung adenocarcinomas and is not commonly found in lung adenocarcinomas in general.
Discussion
The present approach of integrating transposon mutagenesis screening data in lung adenocarcinoma cell lines with clinical genomic sequencing data from patient tumor specimens identified both established and previously uncharacterized mechanisms of resistance to EGFR inhibition. The most prominent candidate genes from the screen for resistance to afatinib in PC9 cells were MET and YES1. Although our screen with afatinib was initiated well before the FDA approval of the third-generation EGFR TKI osimertinib, it is important to note that clones with activating transposon insertions in these genes were resistant to erlotinib, afatinib, and osimertinib, representing all three generations of FDA-approved EGFR TKIs.
Review of the MSK-IMPACT patient database revealed post-TKI amplification of YES1 in five EGFR-mutant and two ALK-rearranged lung adenocarcinomas with acquired resistance to targeted therapy. Only one of these seven post-TKI samples harbored another known resistance-conferring alteration, specifically a concurrent EGFR T790M mutation. The presence of a concurrent EGFR T790M in one case is not unexpected, because biopsy samples in this clinical setting can show more than one resistance mechanism, presumably due to intratumoral heterogeneity. Amplification of YES1 was not detected in pre-TKI samples that were available for two of the EGFR-mutant cases and one of the ALK-rearranged cases, confirming its acquired nature.
Previous laboratory studies support amplification of YES1 as a mediator of resistance to inhibitors of ERBB family members. Ichihara et al. (18) found that amplification of YES1 mediated resistance in one out of five PC9-derived cell lines that were rendered resistant in culture to osimertinib through gradual dose escalation. Amplification of YES1 has also been shown to mediate resistance to trastuzumab and lapatinib in drug-resistant models that were generated from an ERBB2-amplified breast cancer cell line (37). In addition, an initial ORF-based screen for genetic modifiers of EGFR dependence in PC9 cells identified eight of the nine SFK genes as potentially reducing sensitivity to erlotinib (19). YES1 was the only one of the eight SFK genes to fail subsequent validation assays, but its functional validation may have been hampered by the markedly lower level of YES1 protein achievable experimentally in comparison with the other seven SFKs. Similarly, despite utilizing multiple vectors featuring either constitutive or tetracycline-regulated promoters, we have also been unable to achieve robust ectopic expression of YES1 in PC9 cells. Given these technical limitations, further functional studies in PC9 cells will likely require approaches to up-regulate YES1 expression from its endogenous locus.
Treatment with SFK inhibitors, such as dasatinib, has previously been investigated in the setting of acquired resistance to EGFR TKIs. Johnson et al. (38) did not observe activity for the combination of erlotinib and dasatinib in 12 patients with EGFR-mutant lung adenocarcinoma with acquired resistance to erlotinib or gefitinib, of which 8 were positive for T790M before initiation of this combination therapy. However, the copy number status of YES1 was not determined in this trial, and the likelihood that one of the four T790M-negative patients in this trial had YES1 amplification as a resistance mechanism is statistically very low. Our findings justify consideration of treatment with combined EGFR and SFK inhibition in the subset of cases of acquired resistance to EGFR TKIs that harbor amplification of YES1. Our results also suggest that this mechanism might contribute to resistance to ALK TKIs. In this context, it is notable that a pharmacogenomic screen of cell lines derived from ALK TKI-resistant ALK fusion-positive lung cancer biopsies recently identified several cell lines that exhibited up-regulated SRC activity upon ALK inhibition and sensitivity to dual ALK and SRC inhibition (39). Although the mechanism of SRC regulation by ALK signaling remains unclear, these data suggest an important role for signaling downstream of SFKs in a subset of ALK TKI-resistant ALK fusion-positive lung cancers.
We have shown that transposon mutagenesis screening can facilitate identification of clinically relevant target-independent mechanisms of resistance to EGFR inhibition. This approach can be rapidly reimplemented to screen in vitro for resistance to additional drugs or drug combinations. By incorporating additional EGFR TKIs (e.g., osimertinib); other EGFR-mutant cell lines; and concurrent inhibition of EGFR, MET, and YES1 in PC9 cells, reapplication of transposon mutagenesis has the potential to clarify the contributions of other candidate genes identified in our screen to resistance to EGFR inhibition and to uncover additional mediators of resistance to EGFR inhibitors. We anticipate that other targeted therapies in lung adenocarcinomas will be amenable to this approach to identifying novel mechanisms of resistance.
Materials and Methods
Cell Culture and Inhibitors.
PC9 cells were obtained from the Varmus laboratory and have been maintained in the Ladanyi laboratory since 2010. Cells were cultured in RPMI-1640 medium supplemented with 10% FBS (Atlanta Biologicals) and 100 U/mL penicillin/100 μg/mL streptomycin (Gemini Bio-Products). Cells were grown in a humidified incubator with 5% CO2 at 37 °C. Afatinib-resistant clones were maintained in growth medium with 500 nM afatinib. Afatinib, erlotinib, osimertinib, dasatinib, saracatinib, and crizotinib were obtained from Selleck Chemicals.
Transposon Mutagenesis.
PC9 cells were seeded at a density of 5 × 105 cells per well in six-well plates 24 h before cotransfection with plasmids pCMV-HA-hyPBase (obtained from the Wellcome Trust Sanger Institute) and pPB-SB-CMV-puroSD (obtained from Li Chen, Schmidt Laboratory, Massachusetts General Hospital, Boston) using X-tremeGENE 9 DNA transfection reagent (Roche) according to the manufacturer’s protocol. After 48 h, cells were selected in growth medium with 0.3 µg/mL puromycin for 8 d. Surviving cells from 13 independent cotransfections were replated at a density of 2.7 × 105 cells per 15-cm plate in growth medium with 1 µM afatinib for 17–19 d. A total of 225 resistant clones were isolated using cloning discs (Scienceware) and expanded. Genomic DNA was prepared from clones using DNeasy Blood & Tissue Kits (Qiagen).
Library Preparation, Next-Generation Sequencing, and Bioinformatics Analysis for Identification of Transposon Insertion Sites.
Immunoblot and Phosphokinase Array Analyses.
Cells were washed with ice-cold PBS and lysed with RIPA buffer (Cell Signaling Technology) supplemented with protease and phosphatase inhibitors (Roche). Protein levels were quantified with Bradford dye reagent (Bio-Rad), and equal amounts were loaded for SDS/PAGE using precast Bis-Tris gels (Invitrogen), followed by transfer to polyvinylidene difluoride membranes. Membranes were blotted with the following antibodies according to the supplier’s recommendations and were all obtained from Cell Signaling Technology unless otherwise noted: phospho-EGFR Y1068 (no. 3777), EGFR E746-A750del specific (no. 2085), phospo-MET Y1234/1235 (no. 3777), MET (no. 8198), phospho-SFK (no. 6943), YES (no. 3201), phospho-YAP1 Y357 (no. ab62751; Abcam), YAP1 (no. 14704), phospho-AKT S473 (no. 4060), AKT (no. 4691), phospho-ERK T202/Y204 (no. 4370), ERK (no. 4695), phospho-ERBB3 Y1197 (no. 4561), ERBB3 (no. 12708), and GAPDH (no. 2118). Human phosphokinase (no. ARY003B; R&D Systems) and human phospho-RTK (no. ARY001B; R&D Systems) array kits were used according to the manufacturer’s protocols.
Cell Viability Assays.
Cells were seeded in 96-well plates at a density of 2,500 (PC9) to 5,000 (YES1 and MET clones) cells per well in growth medium with the indicated inhibitors. After 96 h, AlamarBlue cell viability reagent (Invitrogen) was added at a final concentration of 10% (vol/vol), and fluorescence was measured (Ex: 555 nm, Em: 585) with a SpectraMax M2 plate reader.
siRNA Experiments.
Cells were transfected with negative control, MET-specific, and YES1-specific siRNAs (Invitrogen) at a final concentration of 10 nM using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer’s protocol. After 24 h, cells were trypsinized and seeded in 96-well plates at a density of 5,000 cells per well and incubated with the indicated inhibitors for 72 h followed by measurement of cell viability.
Statistical Analysis.
Mean and SD values for cell viability assays were calculated and plotted using Prism 7 software (GraphPad Software). Copy number aberrations were identified using an in-house developed algorithm by comparing sequence coverage of targeted regions in a tumor sample relative to a standard diploid normal sample (40), as extensively validated for ERBB2 (HER2) amplification (41). Allele-specific copy number alterations were also identified using the FACETS analysis tool, which performs a joint segmentation of the copy ratios (42). For a complete list of genes included in the MSK-IMPACT panel, see table A1 in ref. 43. All analyses of MSK-IMPACT data were performed under MSKCC Institutional Review Board (IRB) protocol 12-245.
FISH Analysis.
Interphase FISH analysis on formalin-fixed paraffin-embedded (FFPE) tumor tissue was performed to evaluate YES1 gene copy number status. The probe targeting YES1 at 18p11.32 was labeled with SpectrumOrange fluorochrome (Empire Genomic), and the control probe targeting the centromere of chromosome 18 was labeled with SpectrumGreen (Abbott Molecular). Four-micrometer FFPE tissue sections were used for the FISH study, following the protocol for FFPE tissue FISH from Vysis/Abbott Molecular with minor adjustments of pepsin treatment as needed. FISH analysis and signal capture were conducted on a fluorescence microscope (Zeiss) coupled with the ISIS FISH Imaging System (Metasystems). We analyzed 100 interphase nuclei from tumor-rich areas in each specimen.
Immunohistochemistry.
For the immunohistochemical detection of YES1, monoclonal antibody EPR3173 (1:250; Abcam) was used. All staining procedures were performed on a Leica Bond-3 automated stainer platform. Heat-based antigen retrieval using a high-pH buffer (ER2; Leica) was employed before the actual staining. A polymer-based secondary system (Leica Refine) was used to detect the primary antibody.
Supplementary Material
Acknowledgments
We thank Li Chen and Eiki Ichihara for reagents, Venkatraman Seshan for assistance with FACETS analysis, and Mary Ann Melnick for technical assistance. This project was begun as a collaboration with K.P. when P.-D.F. was a postdoctoral fellow in the H.V. laboratory at Memorial Sloan Kettering Cancer Center (MSKCC) and was continued by P.-D.F. in the M.L. laboratory. This work was supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) Grants R01 CA120247 (to H.V. and K.P.), P01 CA129243 (to M.G.K. and M.L.), and P30 CA008748 (MSKCC); the New York State Empire Clinical Research Investigator Program (P.-D.F.); the Lung Cancer Research Foundation (P.-D.F. and M.L.); the Functional Genomics Initiative at MSKCC (P.-D.F., M.L., G.J.R. and L.W.); and the Katha Diddel Sussman & Warren Family Fund for Genome Research (P.-D.F.). C.M.L. was additionally supported by a V Foundation Scholar-in-Training Award, an American Association of Cancer Research (AACR)-Genentech Career Development Award, a Damon Runyon Clinical Investigator Award, a LUNGevity Career Development Award, and NIH/NCI R01 CA121210. K.P. was additionally supported by Yale SPORE in Lung Cancer Grant P50CA196530.
Footnotes
Conflict of interest statement: H.A.Y. has served on the advisory boards for AstraZeneca and Boehringer Ingelheim. Y.Y.J. has received consulting fees from Bristol–Myers Squibb and honoraria from Pfizer, Genentech, and Boehringer Ingelheim. J.E.C. has received consulting fees from AstraZeneca, Genentech, Bristol–Myers Squibb, and Merck. M.G.K. has served as a consultant for AstraZeneca. C.M.L. has served on the Advisory Board for Cepheid Oncology and has received consulting fees from Pfizer, Novartis, AstraZeneca, Genoptix, Sequenom, Ariad, Takeda, and Foundation Medicine. G.J.R. has received consulting fees from Roche, and Memorial Sloan Kettering Cancer Center (MSKCC) has received support from Pfizer and Roche to fund G.J.R.’s clinical research. K.P. has received research funding from AstraZeneca, Roche, Kolltan, and Symphogen; honoraria for consulting or advisory roles from AstraZeneca, Merck, Novartis, and Tocagen; and royalties from intellectual property licensed by MSKCC to Molecular MD. M.L. has received advisory board compensation from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Takeda, and Bayer, and research support from LOXO Oncology.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717782115/-/DCSupplemental.
References
- 1.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 2.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu SG, et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget. 2016;7:12404–12413. doi: 10.18632/oncotarget.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campo M, et al. Acquired resistance to first-line afatinib and the challenges of prearranged progression biopsies. J Thorac Oncol. 2016;11:2022–2026. doi: 10.1016/j.jtho.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Thress KS, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21:560–562. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu HA, et al. Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: Emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol. 2015;1:982–984. doi: 10.1001/jamaoncol.2015.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu HA, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman JA, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116:2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogino A, et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res. 2007;67:7807–7814. doi: 10.1158/0008-5472.CAN-07-0681. [DOI] [PubMed] [Google Scholar]
- 13.Chmielecki J, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Astsaturov I, et al. Synthetic lethal screen of an EGFR-centered network to improve targeted therapies. Sci Signal. 2010;3:ra67. doi: 10.1126/scisignal.2001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bruin EC, et al. Reduced NF1 expression confers resistance to EGFR inhibition in lung cancer. Cancer Discov. 2014;4:606–619. doi: 10.1158/2159-8290.CD-13-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao S, et al. A genetic interaction analysis identifies cancer drivers that modify EGFR dependency. Genes Dev. 2017;31:184–196. doi: 10.1101/gad.291948.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bivona TG, et al. FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichihara E, et al. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer. Cancer Res. 2017;77:2990–3000. doi: 10.1158/0008-5472.CAN-16-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharifnia T, et al. Genetic modifiers of EGFR dependence in non-small cell lung cancer. Proc Natl Acad Sci USA. 2014;111:18661–18666. doi: 10.1073/pnas.1412228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krall EB, et al. KEAP1 loss modulates sensitivity to kinase targeted therapy in lung cancer. eLife. 2017;6:e18970. doi: 10.7554/eLife.18970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeNicola GM, Karreth FA, Adams DJ, Wong CC. The utility of transposon mutagenesis for cancer studies in the era of genome editing. Genome Biol. 2015;16:229. doi: 10.1186/s13059-015-0794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, et al. Transposon activation mutagenesis as a screening tool for identifying resistance to cancer therapeutics. BMC Cancer. 2013;13:93. doi: 10.1186/1471-2407-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandzic T, et al. Transposon mutagenesis reveals fludarabine resistance mechanisms in chronic lymphocytic leukemia. Clin Cancer Res. 2016;22:6217–6227. doi: 10.1158/1078-0432.CCR-15-2903. [DOI] [PubMed] [Google Scholar]
- 24.Pettitt SJ, et al. A genetic screen using the PiggyBac transposon in haploid cells identifies Parp1 as a mediator of olaparib toxicity. PLoS One. 2013;8:e61520. doi: 10.1371/journal.pone.0061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapeau EA, et al. Resistance mechanisms to TP53-MDM2 inhibition identified by in vivo piggyBac transposon mutagenesis screen in an Arf-/- mouse model. Proc Natl Acad Sci USA. 2017;114:3151–3156. doi: 10.1073/pnas.1620262114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perna D, et al. BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. Proc Natl Acad Sci USA. 2015;112:E536–E545. doi: 10.1073/pnas.1418163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J, et al. Identification of PLX4032-resistance mechanisms and implications for novel RAF inhibitors. Pigment Cell Melanoma Res. 2014;27:253–262. doi: 10.1111/pcmr.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yusa K, Zhou L, Li MA, Bradley A, Craig NL. A hyperactive piggyBac transposase for mammalian applications. Proc Natl Acad Sci USA. 2011;108:1531–1536. doi: 10.1073/pnas.1008322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langridge GC, et al. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 2009;19:2308–2316. doi: 10.1101/gr.097097.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell JD, et al. Cancer Genome Atlas Research Network Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takezawa K, et al. HER2 amplification: A potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2:922–933. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soria JC, et al. FLAURA Investigators Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 34. Yu H, et al., Concurrent alterations in EGFR-mutant lung cancers associated with resistance to EGFR kinase inhibitors and characterization of MTOR as a mediator of resistance. Clin Cancer Res, 10.1158/1078-0432.CCR-17-2961. [DOI] [PMC free article] [PubMed]
- 35.Ohashi K, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci USA. 2012;109:E2127–E2133. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suda K, et al. Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin Cancer Res. 2010;16:5489–5498. doi: 10.1158/1078-0432.CCR-10-1371. [DOI] [PubMed] [Google Scholar]
- 37.Takeda T, et al. Yes1 signaling mediates the resistance to Trastuzumab/Lap atinib in breast cancer. PLoS One. 2017;12:e0171356. doi: 10.1371/journal.pone.0171356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson ML, et al. Phase II trial of dasatinib for patients with acquired resistance to treatment with the epidermal growth factor receptor tyrosine kinase inhibitors erlotinib or gefitinib. J Thorac Oncol. 2011;6:1128–1131. doi: 10.1097/JTO.0b013e3182161508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crystal AS, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346:1480–1486. doi: 10.1126/science.1254721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng DT, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for Solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross DS, et al. Next-generation assessment of human epidermal growth factor receptor 2 (ERBB2) amplification status: Clinical validation in the context of a hybrid capture-based, comprehensive solid tumor genomic profiling assay. J Mol Diagn. 2017;19:244–254. doi: 10.1016/j.jmoldx.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen R, Seshan VE. FACETS: Allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 2016;44:e131. doi: 10.1093/nar/gkw520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizvi H, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.