Significance
Interactive electric signaling between mormyrid weakly electric fish by means of echoing a conspecific’s signals has been discussed both in the context of jamming avoidance during active electrolocation and as a strategy for electrocommunication. By confronting individuals of the weakly electric fish Mormyrus rume proboscirostris with a mobile robot that responds dynamically to the signaling activity of the fish, we show that interactive signaling, in turn, leads to increased synchronization of communication signals by the receiving animals. Synchronization of electric signaling occurred most frequently in approach configurations, suggesting that echoing may be employed specifically to address conspecific individuals and to initiate mutual allocation of social attention during electrocommunication.
Keywords: electrocommunication, ethorobotics, interactive signaling, social attention
Abstract
Mormyrid weakly electric fish produce electric organ discharges (EODs) for active electrolocation and electrocommunication. These pulses are emitted with variable interdischarge intervals (IDIs) resulting in temporal discharge patterns and interactive signaling episodes with nearby conspecifics. However, unequivocal assignment of interactive signaling to a specific behavioral context has proven to be challenging. Using an ethorobotical approach, we confronted single individuals of weakly electric Mormyrus rume proboscirostris with a mobile fish robot capable of interacting both physically, on arbitrary trajectories, as well as electrically, by generating echo responses through playback of species-specific EODs, thus synchronizing signals with the fish. Interactive signaling by the fish was more pronounced in response to a dynamic echo playback generated by the robot than in response to playback of static random IDI sequences. Such synchronizations were particularly strong at a distance corresponding to the outer limit of active electrolocation, and when fish oriented toward the fish replica. We therefore argue that interactive signaling through echoing of a conspecific’s EODs provides a simple mechanism by which weakly electric fish can specifically address nearby individuals during electrocommunication. Echoing may thus enable mormyrids to mutually allocate social attention and constitute a foundation for complex social behavior and relatively advanced cognitive abilities in a basal vertebrate lineage.
Mormyrid weakly electric fish produce series of electric organ discharges (EODs) for active electrolocation of their environment (1) and electrocommunication with nearby conspecifics (2). Interdischarge intervals (IDIs) between EODs are variable and can be modified to spontaneously improve the temporal resolution during active sensing (3) and to encode signaling patterns into discharge sequences that are associated with characteristic behavior patterns serving in intraspecific communication (4).
Apart from spontaneous changes in discharge frequency and temporal patterning, mormyrids can also produce interactive IDI sequences. By responding to a conspecific’s EOD with a preferred latency of only a few milliseconds, they generate so-called echo responses, which, if mutually engaged in by two individuals over a coherent time period, lead to episodes of time-locked signaling sequences that constitute electrical discharge synchronization between individuals (5–7). Although echoing is a behavior consistently observed across mormyrid species, the underlying neural pathways are unresolved, and its behavioral significance remains speculative. Echo responses have been interpreted as jamming avoidance behavior during active electrolocation (8, 9) and as a communication strategy (5, 10, 11), possibly by enhancing group integration and affirmative interactions.
Systematic investigation of the implications of echoing for communication is impeded by the difficulty to assign EODs to the respective sender in experiments involving more than a single freely moving fish (but see refs. 12 and 13), as well as by the lack of control over the behavior of the fish that invokes echo responses from a conspecific. We solved both problems by using a freely moving robotic fish capable of emitting either predefined or dynamic sequences of playback EODs in an interactive behavioral experiment with single individuals of the weakly electric fish Mormyrus rume proboscirostris.
Robotic fish have been successfully employed to investigate the features determining attraction between individual fish (14–16), as well as collective decision making and internal dynamics in groups of fish in shoals (17–22). Similar experiments have demonstrated that mormyrids are attracted to a mobile fish replica playing back electric signals (23, 24). Electrical playback signals are a convenient way to experimentally control signaling properties for waveform, IDI sequence, and latency relationships. This allows assigning signaling attributes to behavioral contexts and to uncover their significance for communication (9, 25, 26).
Here, we investigate the effect of interactive electric signaling on attraction and social interactions with a mobile robot that can respond to EODs of live fish and propose a social function of echoing based on two observations: Firstly, fish responded with an increase of interactive signaling to artificial echo responses, indicating that interactivity has some intrinsic communicative value as a signal. Secondly, discharge synchronizations were associated with following-behavior and approach configurations, suggesting that echoing represents a way to address another individual electrically. Our results suggest that discharge synchronizations between mormyrid weakly electric fish constitute a strategy for mutual allocation of social attention, thus providing a relatively simple electromotor basis for the development of complex behavioral interactions and social coordination not frequently observed at the taxonomic level of fish.
Results
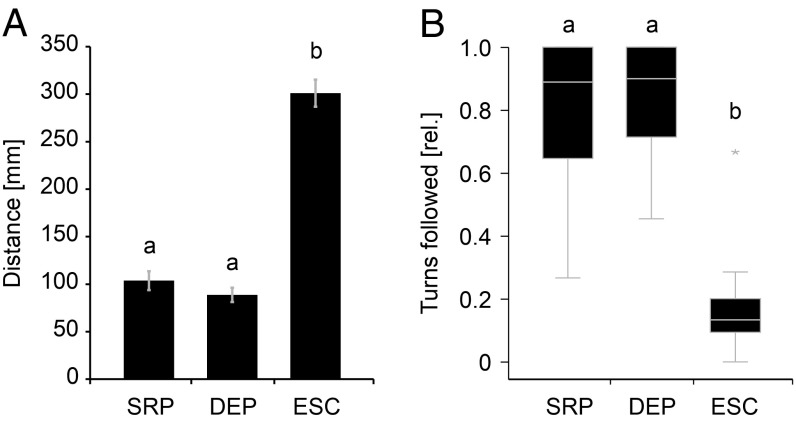
All animals were highly attracted by the robotic fish and showed interactions both by following its trajectories, as well as by synchronizing their EODs to the playback sequences through echo responses to the playback EODs. Attraction was strong when the robot emitted EODs either as a static random playback (SRP) or as a dynamic echo playback (DEP), but not in response to an electrically silent control condition (ESC). The attractiveness of the moving robot was quantified by the fish’s distance to the robot (Fig. 1A), the accuracy with which it followed the robot’s swimming trajectory (Fig. 1B), and its propensity to give up wall-following behavior (SI Appendix, Figs. S1 and S2). Electrical playback led to a significant decrease in distance between robot and fish compared with ESC [repeated-measures ANOVA: F(2,44) = 144.44; P < 0.001], indicating that electric signaling was the main feature of attraction. The relative amount of the robot’s turns that were followed by the fish confirmed this pattern [Friedman test: χ2(2) = 34.80; P < 0.001]. While electrical playback was crucial to attract individual fish to the moving robot, playback type did not influence spatial interactions. However, with regard to electric signaling, different playback types had a significant effect on the fish.
Fig. 1.
Influence of the robotic fish on following behavior during different types of electrical playback. (A) Mean distance (±SEM) between the snout of the fish and the closest point of the robot. (B) Relative amount of robot turns that were followed by the fish. Categories not sharing a common superscript letter differ significantly based on Bonferroni adjusted P values, n = 23. DEP, dynamic echo playback; ESC, electrically silent control; SRP, static random playback; rel., relative.
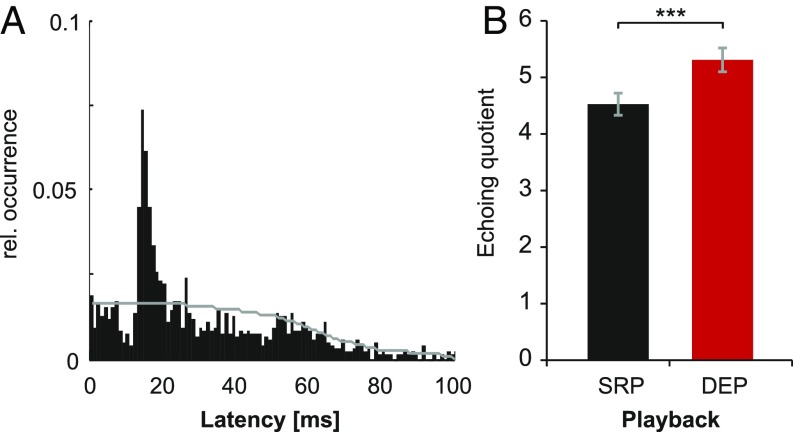
Echo responses represent an interactive signaling strategy and occur when a fish responds with an EOD at a preferred latency to the EOD of another fish more often than would be expected by chance. All fish produced echo responses with preferred latencies ranging from 15 to 19 ms both to SRP and DEP. Exemplary results of an individual fish are shown in Fig. 2A. The relative amount of echo responses produced by each fish in response to SRP and DEP was quantified by calculating an echoing quotient, which allows assessing how many times more often than expected a preferred latency occurs during echoing (6). DEP evoked more echoing responses than SRP [paired-samples t test: t(22) = −5.38; P < 0.001], suggesting that echo responses induce echoing by fish who receive echoes to their EODs (Fig. 2B).
Fig. 2.
Echo responses to static and interactive electrical playback. (A) Exemplary depiction of the relative occurrence of EOD-response latencies of fish 9 during SRP. The horizontal line represents the expected distribution of response latencies. The observed latencies at the mode of the distribution exceed the expected distribution. Bin size: 1 ms. (B) Echoing quotient, i.e., the ratio of observed to expected latencies at the mode of the latency distribution (mean ± SEM). This proportion was higher in response to DEP for almost all of the n = 23 fish compared with SRP. ***P < 0.001.
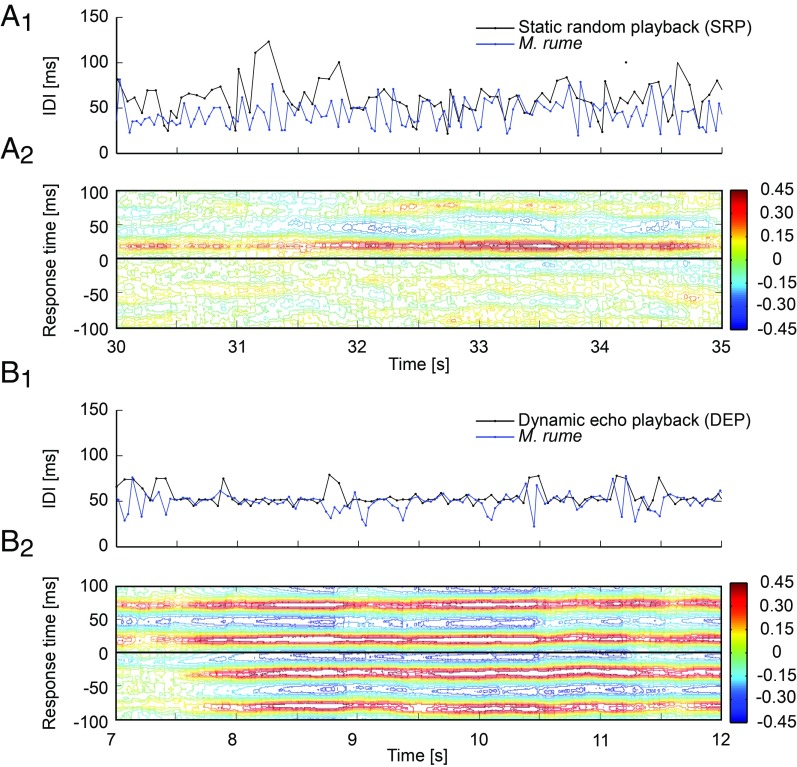
Electric signaling responses were additionally analyzed as time series to characterize temporal aspects of their interactions with the different playbacks. Fig. 3 depicts sections from trials with SRP (A) and DEP (B). The Upper panels show plots of overlaid IDI sequences of the respective playback and the responding fish. Adaptive cross-correlations calculated between each of the two sequences are shown in the Lower panels.
Fig. 3.
Synchronization of electrical discharge activity. Discharge synchronization of M. rume with SRP (A) and DEP (B). (A1 and B1) IDI sequences of playback (black) and fish (blue) over time (see SI Appendix, Figs. S3 and S4 for complete sequences). (A2 and B2) Cross-correlation diagrams calculated for each pair of IDI sequences displayed in A1 and B1. Correlation coefficients of the fish’s signals with the playback signals are color coded for response times of ±100 ms. High correlation coefficients at positive response times represent synchronization of the animal’s signaling behavior to the playback, whereas high correlation at negative response times represents synchronization of the playback with the signaling behavior of the fish. This can occur only randomly with SRP. High correlation coefficients at negative response times in B2 reflect the consistent synchronization of DEP with the signaling behavior of the fish.
The cross-correlation analysis in Fig. 3A reveals consistent discharge synchronization to SRP, with response times corresponding to the preferred latency of the echoing fish. Maximum correlations frequently exceed the 0.3 correlation threshold, indicative of relatively strong synchronization (see SI Appendix, Figs. S3 and S4 for illustrations). IDI sequences during interactions with DEP were more regular, and the cross-correlation analysis shows synchronization at response times corresponding to the echo latency. DEP was designed to respond to EODs of live fish with an echo latency of 21 ms. Hence, high correlation coefficients at these (negative) response times are visible in the cross-correlation diagram (Fig. 3B).
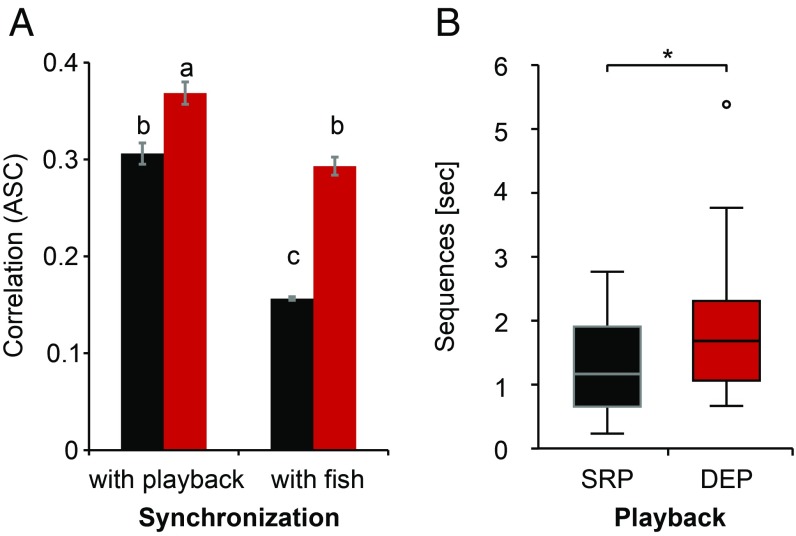
To quantify discharge synchronizations between fish and robot, the maximum correlation coefficient was averaged over time for both positive and negative response times separately (Fig. 4A). We called this metric average synchronization coefficient (ASC). For comparison, we computed two ASC baselines for randomly occurring correlations, representing no synchronization, by calculating the same metric for cross-correlations of EOD sequences of the fish during the ESC condition with SRP, and DEP, respectively. The ASC of live fish differed statistically highly significantly between the conditions [repeated-measures ANOVA with Greenhouse–Geisser correction: F(2.152, 47.349) = 192.00; P < 0.001; ε = 0.31]. Interactive playback triggered more interactive signaling responses by the fish. Synchronization of M. rume with SRP resulted in ASC values of 0.31 ± 0.011 (mean ± SEM). The ASC of SRP with live fish was significantly lower (0.16 ± 0.002), but statistically indifferent to both baselines (baseline 1: 0.16 ± 0.002, baseline 2: 0.16 ± 0.002). Responses of M. rume to DEP resulted in significantly higher discharge synchronizations (ASC: 0.37 ± 0.012; mean ± SEM) than during SRP, confirming our results for the echo quotient. The respective synchronization response of DEP to the signals of M. rume was with 0.29 ± 0.009 (mean ± SEM) statistically indifferent to the fish’s response to SRP, thus confirming the comparability of DEP with the interactive signaling behavior of live fish. The independent control responses of fish to DEP were statistically indifferent to those obtained for SRP controls (ASCs: 0.17 ± 0.002). Statistical differences in synchronization responses to the playbacks were therefore not caused by general differences between the two playback types.
Fig. 4.
Synchronization of electrical discharge sequences. (A) Bars represent the mean (±SEM) of the averaged maximum cross-correlation values (ASC) for experiments with n = 23 fish during the presentation of SRP (black) and DEP (red). Comparisons were made for synchronizations of the electrical discharges of M. rume with the playback and synchronization of the playback with the signals of the fish. Categories without a common superscript letter differ significantly based on Bonferroni-adjusted P values. (B) Duration of episodes with relatively strong synchronization. Box plots comparing sequences where n = 23 fish synchronized their discharges to the IDI sequences of SRP (black) and DEP (red) with cross-correlation coefficients ≥0.3. DEP led to a significantly longer duration of synchronization events than SRP. *P < 0.05.
We further analyzed temporal aspects of synchronization by quantifying the duration of sequences with correlation coefficients ≥0.3. Median sequence lengths of episodes with high correlation at a relative cumulative sum of 0.75 were longer in response to DEP compared with SRP (Wilcoxon signed-rank test: Z = 2.42; P = 0.016; Fig. 4B).
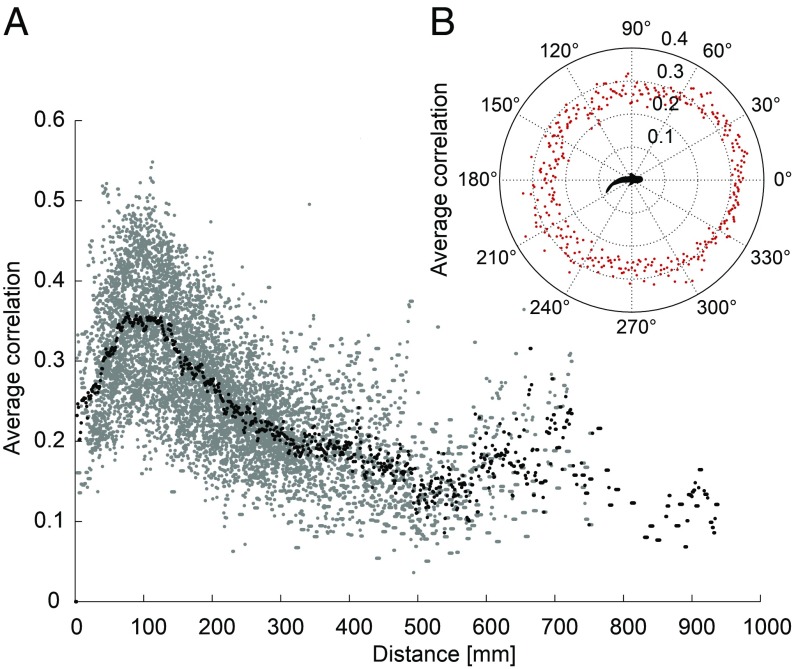
Simultaneous recording of electric signaling and motor responses of the fish allowed associating both behaviors at any given time of the experiments. Tracking data were used to analyze linear distances as well as directional relationships between robot and fish with regard to discharge synchronization of M. rume with SRP and to establish the constellations they most frequently occurred in. Averaging maximal correlation values across the distances observed during all trials with SRP revealed that fish synchronized most strongly at distances of around 100 mm (Fig. 5A). This corresponds to the range of active electrolocation, i.e., the maximum distance up to which fish can detect objects by using their active electric sense (1).
Fig. 5.
Electrical discharge synchronization and spatial interactions. (A) Cross-correlation coefficients of the IDI sequences of M. rume with SRP for n = 23 animals plotted against the distance between robot and fish observed at the respective time (gray dots). Average values per distance (black dots) show that synchronization was strongest at ∼100 mm. Bin size: 1 mm. (B) Polar plot of the average cross-correlation coefficients per 1° of the angle representing the robot’s bearings in the fish’s egocentric coordinate system. The distance from the center of the plot represents the correlation coefficient.
The intensity of electrical discharge synchronization was also affected by directional interrelations between the moving robot and the fish. When made independent of the total frequency by averaging correlation coefficients into bins of 1° (Fig. 5B), the magnitude of synchronization engaged in by M. rume correlated with the replica’s position relative to the perspective of the fish (ρp = 0.64; P < 0.001; Fig. 5B). Additionally, correlations were highest when the fish was positioned behind the replica, at about 180° relative to its coordinates (ρp = 0.43; P < 0.001; SI Appendix, Fig. S5A) and when the difference in orientation between replica and fish was smallest (ρp = 0.68; P < 0.001; SI Appendix, Fig. S5B). This shows that animals synchronized their discharge activity most intensely when they swam toward the replica and approached it from behind with similar orientation. These observations are consistent with a situation where two fish follow each other swimming in the same direction and engage in synchronization of their electric discharges.
Discussion
Determining the key stimuli triggering the release of social behaviors lies at the heart of behavioral biology (27) and is a crucial prerequisite for using robots to investigate animal behavior (28). Ethorobotical experiments with various fish species have shown that mainly visual and hydrodynamic cues mediate interactions between live fish and robotic replicas (14–16, 20, 29). In mormyrids, the importance of electric signaling for mediating social behaviors is well established (30–32), and playback studies suggest that electric signals are key stimuli, which allow using mobile fish dummies as proxies for conspecifics when investigating electrocommunication (23, 24). We investigated locomotor and electromotor responses of individual M. rume to a mobile robot, which emitted playback EODs either as a static random IDI sequence (SRP) or in a dynamically interacting echo paradigm (DEP). Electric playback reliably induced social interactions and following behavior in all fish. However, in contrast to the fish’s electromotor responses, attraction was not influenced by playback type. At the level of electric signaling interactions, however, the fish’s response depended on whether the playback sequence was static or dynamic, i.e., whether the robot responded to the electromotor behavior of the fish. Both playback types elicited echo responses, but the relative amount of echoing was higher in response to DEP, which resulted in a higher degree of discharge synchronization sustained over longer periods of time. Differences in interactive signaling by the fish did not depend on general differences between the playback types, which was demonstrated using independently recorded sequences as a control. The higher amount of interactive signaling by M. rume seems therefore to result from the interactivity of DEP, meaning that an animal that receives echoes reacts by responding with more echoes of its own. This in turn leads to more intense discharge synchronization between individuals, supporting the notion that echoing serves an important function during electrocommunication in mormyrids.
Echoing has been proposed to serve a jamming avoidance function during active electrolocation (8, 9), a notion contested by the observation that object discrimination in Gnathonemus petersii was not impaired during jamming (33). However, jamming avoidance also occurs in other active sensory systems such as active electrolocation in gymnotiform weakly electric fish (34, 35) and echolocation in bats (36). The necessity for a jamming avoidance strategy is apparent in gymnotiforms because they lack a reafferent neuronal pathway, which enables mormyrids to distinguish between self- and nonself-generated EODs (37). Nevertheless, jamming avoidance has been linked also in gymnotiforms to social communication (34, 38), and the two functions need not be mutually exclusive in mormyrids either. Effective communication may even require jamming avoidance mechanisms because they can provide senders with a strategy to emphasize their signals. Such strategic signaling adjustments have for instance been described in calling insects (39), frogs (40), and songbirds (41).
Gymnotiform Gymnotus carapo exhibit a strongly reduced sensitivity for electric signals directly after they generate an EOD (42). In an interactive electrical playback protocol, G. carapo preferentially discharged within the time window during which the receptors of the conspecific represented by the playback would have been more sensitive to the signals of the fish, which may enable these animals to adjust their EODs with respect to those of a conspecific, depending on an intent to communicate (43). Similarly, the corollary discharge, generated in the mormyrid brain each time an EOD is initiated, results in inhibitory postsynaptic potentials of up to 10 ms that are measurable in the nucleus of the electrosensory lateral line lobe (nELL) (37). The nELL receives afferent input from knollenorgans, which are electroreceptor organs specialized for communication (44). The echo response might thereby assure that a sender places its EOD at the end of this refractory period, but before the next EOD of the receiver is initiated. The EOD will thus be detected by the receiver and may signal social intentions of the sender. If the receiver responds by echoing the sender’s signals, this may eventually lead to mutual allocation of social attention through discharge synchronization in a variety of behavioral contexts.
The sensory perception of mormyrids also benefits from echoing because it ensures compatibility of active and passive sensing during social interactions. Mormyrids were shown to use the information provided by knollenorgans to approach a dipole source representing a conspecific from outside the range of active electrolocation (45). Thus, a conspecific’s EODs also provide spatial information during social interactions (46). Since afferent information from knollenorgans is inhibited in the nELL during EOD production (37), echoing ensures that active electrolocation does not impair passive sensing performance in social contexts. Echoing will consequently be perceived by the individual that is approached and could have ritualized into a signaling display during which the approached individual echoes as well and thereby acknowledges its detection. In the current study, synchronization was strongest at the outer range of active electrolocation at around 10 cm (1), a distance where passive sensing may be the most reliable source of information about conspecifics during nocturnal activity.
The echo response has been described in several mormyrid species and in behavioral contexts as diverse as agonistic encounters, foraging, and resting (5, 6, 11, 13, 47). This suggests that it may serve a more general signaling purpose, which is not necessarily linked to an activity-dependent behavioral context. Field reports from predatory Mormyrops anguilloides have demonstrated that these mormyrids gather in relatively stable groups and hunt in packs for small cichlids. Based on these observations, Arnegard and Carlson (10) hypothesized that mutual synchronization of discharge bursts through echoing allows “mutual acknowledgement of recognition” between individuals of the group.
Collective, coordinated, and collaborative hunting strategies of varying complexity have been documented for several fish species, and it becomes increasingly evident that such abilities are not unique features of mammalian predators (48). The involvement of echoing in the nocturnal hunting behavior of M. anguilloides has interesting implications, because it may enable mormyrids to perform social behaviors otherwise restricted to animals with more advanced cognitive abilities. One such ability is the capacity for vocal imitation, which is rare among nonhuman animals (49). It is, however, used by parrots and dolphins, which were shown to use learned vocal labels to address specific individuals by imitating their calls (50, 51). The mormyrid echo response allows spontaneous matching of signaling sequences with high temporal precision and may thus enable mormyrids to address other individuals of a group without the need for a capacity of imitation or learning. Jamming avoidance in the knollenorgan pathway could in these situations facilitate undisturbed mutual identification of individuals based on differences in EOD waveform (25), or the exchange of dominance-related waveform information between unfamiliar individuals to determine hierarchy ranks without fighting (52, 53). The echo response, as a simple mechanism providing the capability to synchronize electric signals with conspecifics, combined with the ability of mormyrids to recognize individuals based on the waveform of their EOD, may have served as a foundation for the evolution of complex social interactions and cognitive capacities not frequently observed at the taxonomic level of fish (54).
Materials and Methods
Animals.
A total of 23 M. rume proboscirostris were kept in 50- to 200-L tanks at temperatures of ∼25 °C, water conductivity of ∼100 µS⋅cm−1 and a light/dark cycle of 12/12 h. Animals were bred in captivity and measured 6.4–11.4 cm in standard length. In each tank, two or more fish were confined to single compartments providing a shelter. Compartments were separated by water permeable barriers that prevented physical contact but allowed electrocommunication. Food was provided in the form of defrosted chironomid larvae. All experiments were carried out in accordance with the guidelines of German law, with the animal welfare regulations of the University of Bonn, and with the Guidelines for the treatment of animals in behavioural research and teaching (55).
Setup.
Experiments were performed in a 120 × 100 × 20 cm3 tank, which was mounted on a support frame leaving the base area accessible from below (SI Appendix, Fig. S6). The water in the tank was of 26 ± 1 °C and had a conductivity of 100 ± 5 µS⋅cm−1 during experiments. The water level was kept at 15 cm. The frame supported a second plane below the tank, on which a wheeled robot (56) (SI Appendix, Fig. S7A) could be manually controlled by a personal computer to move on arbitrary trajectories via a wireless connection. A fish replica (SI Appendix, Fig. S7B) was made from an 8-cm fishing lure mounted on a base plate with a magnet underneath. With this magnet, the replica inside the tank was coupled to the mobile robot underneath the tank and could thus reproduce its trajectories.
The replica was fitted with a pair of carbon electrodes inserted at the front and the rear end, and with a pair of silver electrodes along the longitudinal axis. A multielectrode array consisting of five pairs of carbon electrodes recorded all electrical activity in the tank. Signals were recorded differentially (Brownlee Precision Model 440), digitized (CED Power 1401; Cambridge Electronic Design), and recorded to disc using Spike2 software (version 5.21; Cambridge Electronic Design). All behavior in the tank was simultaneously video recorded at 15 fps using an infrared sensitive camera (DMK 23FM021 FireWire camera with Vari Focal T4Z2813CS-IR CCTV lens; The Imaging Source) and Spike2 video recorder. Illumination was provided indirectly using a LED floodlight resulting in 1.5 lx of visible light intensity (Light ProbeMeter, 403 125; Extech Instruments). Camera vision was enhanced by additional illumination with infrared spotlights (850 nm, IR Illuminator Model SA1-60-C-IR; Itakka).
The silver electrodes of the robot were used to record signals of the fish when it came into close range. These signals were amplified differentially using a custom-built trigger box (University of Regensburg, Regensburg, Germany) which generated a transistor–transistor logic (TTL) pulse for each signal exceeding a threshold. The TTL output of the trigger box was used to generate DEP in real-time via the Spike2 sequencer (see below). The robot’s carbon electrodes were used for playback generation and were connected to a stimulus isolator (model 2200; A-M Systems, Inc.) as a power supply. Playback signals were output via the Spike2 sequencer, converted digital to analog using the CED 1401 and attenuated (dB-attenuator; University of Regensburg) to match the signal characteristics of a medium-sized fish (23). The key components of the setup are illustrated in SI Appendix, Fig. S6.
Playbacks.
Two types of electrical playback sequences were generated using a prerecorded template EOD that was averaged from 50 EODs of an M. rume recorded head to tail (high pass: 1 Hz) and digitized at a sampling rate of 50 kHz. SRP was generated using a custom-written Matlab script (version R2013b; The MathWorks, Inc.), which concatenated template EODs to 15-s sequences. IDIs were randomly selected within two SDs around the mean (67 ms) of a distribution with a mode of 60 ms that was obtained from a similar experiment (24) and contained a total of 17,644 IDIs. SRP sequences were repeated three times to obtain a 45-s stimulus protocol and a new sequence was designed for every trial. DEP was generated by programming the Spike2 sequencer to produce playback signals at intervals greater than 60 ms in the absence of a trigger signal, but respond with a latency of 21 ms to the detection of a fish’s EOD. A refractory period prevented the program from echoing to its own signals. DEP generation is illustrated in SI Appendix, Fig. S8.
Procedures.
Individual fish were taken from the holding tanks and placed into a 22 × 14 cm2 opaque start box inside the experimental tank. The robot was then moved on random trajectories for 3 min to habituate the fish to any disturbances associated with the robot’s movements. Fish were then released from the start box and confronted with the robot in three consecutive trials featuring either SRP, DEP, or no playback as a control (ESC). The order of these conditions was pseudorandomized. The robot was moved by the experimenter on arbitrary trajectories designed to approach the fish and entice it to follow into the open area of the tank. Each presentation started with a 10-s period without playback, followed by three 15-s episodes during which the respective condition was repeated. This resulted in a total of 55 s of recorded data. In additional control experiments, the robot was removed after the habituation period, and the behavior of the fish was recorded according to the time points defined for playback presentation. This control was performed with all fish in a separate session on a nonconsecutive day. Half of the animals were subjected to this control in the first session, while the other half were first confronted with the robot.
Video Analysis.
Videos were rectified to compensate for radial distortion, and tracking was performed using Ctrax (Caltech Multiple Walking Fly Tracker, version 5.0, ref. 57) and the FixErrors graphical user interface provided for Matlab. The center distance between test fish and replica, their difference in orientation, as well as the relative position of the fish, which was defined by the angular difference of the robot’s orientation and the connecting line between the centers of the robot and the fish, were calculated using the BehavioralMicroarray toolbox provided with Ctrax. Distances from the snout of the fish to the closest wall of the tank, as well as the closest distance to the robot, were manually assessed every 3 s using ImageJ (version 1.46r, NIH). The resulting 15 values were averaged to obtain 1 value per fish for statistical analysis. The number of turns performed by the robot was counted manually from video recordings, and the proportion of turns that were followed by the fish was calculated for each condition. Videos were renamed and randomized to leave the experimenter blind to the test condition.
Signal Analysis.
Waveform data were transformed into time series marking the occurrence of all EODs, which were then assigned to either the playback or the fish (24). Echo responses displayed by the fish were analyzed by using the EOD sequence of the playback as a reference and calculating the latencies with which the fish generated EODs in response to the stimulus until the occurrence of the following playback EOD. The latency distribution that would be expected if both IDI sequences were independent time series was obtained by inverting the relative cumulative histogram of the stimulus IDI distribution. To check for echo responses of the playback to the fish’s signals, the sequences used as stimulus and response signals were reversed. Echo responses were quantified according to ref. 6 by calculating the ratio of observed latencies at the mode of the latency distribution to the amount expected at that latency, if assuming no dependency between the IDI sequences of playback and the tested fish.
Adaptive cross-correlations for a response window of ±100 ms were calculated between two IDI sequences each, using the playback signals as reference values (see ref. 7 for details). The IDI sequences of playback and fish were thereby transformed into high-resolution time series comprising one value per millisecond. The time course of temporal synchronization between the IDI sequences of M. rume and the playback could thus be quantified via correlation coefficients. For each of the high-resolution time points, the maximum correlation value within the 100-ms response frame was extracted from the matrix of correlation coefficients (see SI Appendix, Figs. S3 and S4 for an illustration). This was done separately for correlations of the fish’s signals with the playback, as well as for correlations of the playback with the IDI sequence of the fish. The average of these maximum values over the 45-s period (ASC) was calculated for each trial and used for statistical analysis. To control for randomly occurring correlations, cross-correlation analysis was performed for both playbacks by using the IDI sequence the fish had emitted independently of the playback during ESC in the same session.
Values of maximum correlation from the high-resolution time series were averaged to obtain single values matched to the corresponding video frames (24). For both SRP and DEP, sequences of successive frames during which the assigned value of correlation of the fish’s signaling response was ≥0.3 were quantified to calculate the duration of synchronization episodes. Relative cumulative histograms were used to determine the duration of synchronization sequences at a proportion on 0.75 for statistical comparison of the effects of SRP and DEP on the duration the fish synchronized their signals to the respective playback. Simultaneous tracking and waveform data were used to associate the linear and directional relationships between fish and replica to the amount of discharge synchronizations at a given time defined by the frame rate of video recording. This analysis was only performed for SRP.
Statistics.
Statistical analyses were performed using IBM SPSS (version 22.0; IBM Corp.). Normality of data were assessed by Shapiro–Wilk’s test and parametric or nonparametric tests for repeated measurements were used accordingly. Circular–linear correlations between the magnitude of EOD synchronization and the angular relationships of fish and replica were calculated using the CircStat toolbox for Matlab (58).
Supplementary Material
Acknowledgments
This study was supported by the German Research Foundation (DFG Graduiertenkolleg Bionik GR1572).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801283115/-/DCSupplemental.
References
- 1.von der Emde G. Active electrolocation of objects in weakly electric fish. J Exp Biol. 1999;202:1205–1215. doi: 10.1242/jeb.202.10.1205. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins CD. Neuroethology of electric communication. Annu Rev Neurosci. 1988;11:497–535. doi: 10.1146/annurev.ne.11.030188.002433. [DOI] [PubMed] [Google Scholar]
- 3.Post N, von der Emde G. The “novelty response” in an electric fish: Response properties and habituation. Physiol Behav. 1999;68:115–128. doi: 10.1016/s0031-9384(99)00153-5. [DOI] [PubMed] [Google Scholar]
- 4.Carlson BA, Hopkins CD. Stereotyped temporal patterns in electrical communication. Anim Behav. 2004;68:867–878. [Google Scholar]
- 5.Russell CJ, Myers JP, Bell CC. The echo response in Gnathonemus petersii (Mormyridae) J Comp Physiol A. 1974;92:181–200. [Google Scholar]
- 6.Kramer B. Electric organ discharge interaction during interspecific agonistic behaviour in freely swimming mormyrid fish. J Comp Physiol A. 1974;93:203–235. [Google Scholar]
- 7.Gebhardt K, Alt W, von der Emde G. Electric discharge patterns in group-living weakly electric fish, Mormyrus rume (Mormyridae, Teleostei) Behaviour. 2012;149:623–644. [Google Scholar]
- 8.Heiligenberg W. Electrolocation and jamming avoidance in the mormyrid fish Brienomyrus. J Comp Physiol A. 1976;109:357–372. [Google Scholar]
- 9.Schuster S. Count and spark? The echo response of the weakly electric fish Gnathonemus petersii to series of pulses. J Exp Biol. 2001;204:1401–1412. doi: 10.1242/jeb.204.8.1401. [DOI] [PubMed] [Google Scholar]
- 10.Arnegard ME, Carlson BA. Electric organ discharge patterns during group hunting by a mormyrid fish. Proc Biol Sci. 2005;272:1305–1314. doi: 10.1098/rspb.2005.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebhardt K, Böhme M, von der Emde G. Electrocommunication behaviour during social interactions in two species of pulse-type weakly electric fishes (Mormyridae) J Fish Biol. 2012;81:2235–2254. doi: 10.1111/j.1095-8649.2012.03448.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong RY, Hopkins CD. Electrical and behavioral courtship displays in the mormyrid fish Brienomyrus brachyistius. J Exp Biol. 2007;210:2244–2252. doi: 10.1242/jeb.003509. [DOI] [PubMed] [Google Scholar]
- 13.Bell CC, Myers JP, Russell CJ. Electric organ discharge patterns during dominance related behavioral displays in Gnathonemus petersii (Mormyridae) J Comp Physiol A. 1974;92:201–228. [Google Scholar]
- 14.Landgraf T, et al. RoboFish: Increased acceptance of interactive robotic fish with realistic eyes and natural motion patterns by live Trinidadian guppies. Bioinspir Biomim. 2016;11:015001. doi: 10.1088/1748-3190/11/1/015001. [DOI] [PubMed] [Google Scholar]
- 15.Marras S, Porfiri M. Fish and robots swimming together: Attraction towards the robot demands biomimetic locomotion. J R Soc Interface. 2012;9:1856–1868. doi: 10.1098/rsif.2012.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polverino G, Phamduy P, Porfiri M. Fish and robots swimming together in a water tunnel: Robot color and tail-beat frequency influence fish behavior. PLoS One. 2013;8:e77589. doi: 10.1371/journal.pone.0077589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butail S, Bartolini T, Porfiri M. Collective response of zebrafish shoals to a free-swimming robotic fish. PLoS One. 2013;8:e76123. doi: 10.1371/journal.pone.0076123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faria J, et al. A novel method for investigating the collective behaviour of fish: Introducing ‘Robofish’. Behav Ecol Sociobiol. 2010;64:1211–1218. [Google Scholar]
- 19.Landgraf T, et al. 2014. Blending in with the shoal: Robotic fish swarms for investigating strategies of group formation in guppies. Biomimetic and Biohybrid Systems, Lecture Notes in Computer Science, eds Duff A, Lepora NF, Mura A, Prescott TJ, Verschure PFMJ (Springer International Publishing, Cham, Switzerland), Vol 8608, pp 178–189.
- 20.Kruusmaa M, Rieucau G, Montoya JCC, Markna R, Handegard NO. Collective responses of a large mackerel school depend on the size and speed of a robotic fish but not on tail motion. Bioinspir Biomim. 2016;11:056020. doi: 10.1088/1748-3190/11/5/056020. [DOI] [PubMed] [Google Scholar]
- 21.Swain DT, Couzin ID, Leonard NE. Real-time feedback-controlled robotic fish for behavioral experiments with fish schools. Proc IEEE. 2012;100:150–163. [Google Scholar]
- 22.Bonnet F, Gribovskiy A, Halloy J, Mondada F. Closed-loop interactions between a shoal of zebrafish and a group of robotic fish in a circular corridor. Swarm Intell. 2018:1–18. [Google Scholar]
- 23.Donati E, et al. Investigation of collective behaviour and electrocommunication in the weakly electric fish, Mormyrus rume, through a biomimetic robotic dummy fish. Bioinspir Biomim. 2016;11:066009. doi: 10.1088/1748-3190/11/6/066009. [DOI] [PubMed] [Google Scholar]
- 24.Worm M, Kirschbaum F, von der Emde G. Social interactions between live and artificial weakly electric fish: Electrocommunication and locomotor behavior of Mormyrus rume proboscirostris towards a mobile dummy fish. PLoS One. 2017;12:e0184622. doi: 10.1371/journal.pone.0184622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanika S, Kramer B. Intra-male variability of its communication signal in the weakly electric fish, Marcusenius macrolepidotus (South African form), and possible functions. Behaviour. 2005;142:145–166. [Google Scholar]
- 26.Kramer B. Electric and motor responses of the weakly electric fish, Gnathonemus petersii (Mormyridae), to play-back of social signals. Behav Ecol Sociobiol. 1979;6:67–79. [Google Scholar]
- 27.Tinbergen N. Social releasers and the experimental method required for their study. Wilson Bull. 1948;60:6–51. [Google Scholar]
- 28.Mondada F, et al. A general methodology for the control of mixed natural-artificial societies. In: Kernbach S, editor. Handbook of Collective Robotics: Fundamentals and Challenges. Pan Stanford Publishing; Singapore: 2013. [Google Scholar]
- 29.Ruberto T, Mwaffo V, Singh S, Neri D, Porfiri M. Zebrafish response to a robotic replica in three dimensions. R Soc Open Sci. 2016;3:160505. doi: 10.1098/rsos.160505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moller P. Electric signals and schooling behavior in a weakly electric fish, Marcusenius cyprinoides L. (Mormyriformes) Science. 1976;193:697–699. doi: 10.1126/science.948747. [DOI] [PubMed] [Google Scholar]
- 31.Moller P, Serrier J, Squire A, Boudinot M. Social spacing in the mormyrid fish Gnathonemus petersii (Pisces): A multisensory approach. Anim Behav. 1982;30:641–650. [Google Scholar]
- 32.Khait V, Tahiraj E, Seemungal N, Breakstone S, Moller P. Group cohesion in juvenile weakly electric fish Mormyrus rume proboscirostris. J Fish Biol. 2009;75:490–502. doi: 10.1111/j.1095-8649.2009.02250.x. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher S, Burt de Perera T, von der Emde G. Object discrimination through active electrolocation: Shape recognition and the influence of electrical noise. J Physiol Paris. 2016;110:151–163. doi: 10.1016/j.jphysparis.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Westby GWM. Electrical communication and jamming avoidance betwen resting Gymnotus carapo. Behav Ecol Sociobiol. 1979;4:381–393. [Google Scholar]
- 35.Watanabe A, Takeda K. The change of discharge frequency by AC stimulus in a weak electric fish. J Exp Biol. 1963;40:57–66. [Google Scholar]
- 36.Takahashi E, et al. Adaptive changes in echolocation sounds by Pipistrellus abramus in response to artificial jamming sounds. J Exp Biol. 2014;217:2885–2891. doi: 10.1242/jeb.101139. [DOI] [PubMed] [Google Scholar]
- 37.Bell CC, Grant K. Corollary discharge inhibition and preservation of temporal information in a sensory nucleus of mormyrid electric fish. J Neurosci. 1989;9:1029–1044. doi: 10.1523/JNEUROSCI.09-03-01029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer B. The sexually dimorphic jamming avoidance response in the electric fish Eigenmannia (Teleostei, Gymnotiformes) J Exp Biol. 1987;130:39–62. [Google Scholar]
- 39.Murphy MA, Thompson NL, Schul J. Keeping up with the neighbor: A novel mechanism of call synchrony in Neoconocephalus ensiger katydids. J Comp Physiol A. 2016;202:225–234. doi: 10.1007/s00359-016-1068-1. [DOI] [PubMed] [Google Scholar]
- 40.Zelick R. Jamming avoidance in electric fish and frogs: Strategies of signal oscillator timing. Brain Behav Evol. 1986;28:60–69. doi: 10.1159/000118692. [DOI] [PubMed] [Google Scholar]
- 41.Benichov JI, et al. The forebrain song system mediates predictive call timing in female and male zebra finches. Curr Biol. 2016;26:309–318. doi: 10.1016/j.cub.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westby GWM. Has the latency dependent response of Gymnotus carapo to discharge-triggered stimuli a bearing on electric fish communication? J Comp Physiol A. 1975;96:307–341. [Google Scholar]
- 43.Forlim CG, Pinto RD. Automatic realistic real time stimulation/recording in weakly electric fish: Long time behavior characterization in freely swimming fish and stimuli discrimination. PLoS One. 2014;9:e84885. doi: 10.1371/journal.pone.0084885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker CA, Kohashi T, Lyons-Warren AM, Ma X, Carlson BA. Multiplexed temporal coding of electric communication signals in mormyrid fishes. J Exp Biol. 2013;216:2365–2379. doi: 10.1242/jeb.082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schluger JH, Hopkins CD. Electric fish approach stationary signal sources by following electric current lines. J Exp Biol. 1987;130:359–367. doi: 10.1242/jeb.130.1.359. [DOI] [PubMed] [Google Scholar]
- 46.Worm M, Kirschbaum F, von der Emde G. Disembodying the invisible: Electrocommunication and social interactions by passive reception of a moving playback signal. J Exp Biol. 2018;221:jeb172890. doi: 10.1242/jeb.172890. [DOI] [PubMed] [Google Scholar]
- 47.Terleph TA. The function of agonistic display behaviours in Gnathonemus petersii. J Fish Biol. 2004;64:1373–1385. [Google Scholar]
- 48.Bshary R, Gingins S, Vail AL. Social cognition in fishes. Trends Cogn Sci. 2014;18:465–471. doi: 10.1016/j.tics.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Hauser MD, Chomsky N, Fitch WT. The faculty of language: What is it, who has it, and how did it evolve? Science. 2002;298:1569–1579. doi: 10.1126/science.298.5598.1569. [DOI] [PubMed] [Google Scholar]
- 50.Balsby TJS, Momberg JV, Dabelsteen T. Vocal imitation in parrots allows addressing of specific individuals in a dynamic communication network. PLoS One. 2012;7:e49747. doi: 10.1371/journal.pone.0049747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King SL, Janik VM. Bottlenose dolphins can use learned vocal labels to address each other. Proc Natl Acad Sci USA. 2013;110:13216–13221. doi: 10.1073/pnas.1304459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlson BA, Hopkins CD, Thomas P. Androgen correlates of socially induced changes in the electric organ discharge waveform of a mormyrid fish. Horm Behav. 2000;38:177–186. doi: 10.1006/hbeh.2000.1613. [DOI] [PubMed] [Google Scholar]
- 53.Parker GA. Assessment strategy and the evolution of fighting behaviour. J Theor Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. [DOI] [PubMed] [Google Scholar]
- 54.Bshary R. Machiavellian intelligence in fishes. In: Brown C, Laland K, Krause J, editors. Fish Cognition and Behavior. 2nd Ed. Wiley-Blackwell; West Sussex, UK: 2011. pp. 277–297. [Google Scholar]
- 55.ASAB Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav. 2006;71:245–253. doi: 10.1006/anbe.1999.1349. [DOI] [PubMed] [Google Scholar]
- 56.Landgraf T, et al. A multi-agent platform for biomimetic fish. In: Prescott TJ, Lepora NF, Mura A, Verschure PFMJ, editors. Biomimetic and Biohybrid Systems: First International Conference, Living Machines 2012, Barcelona, Spain, July 9-12, 2012. Proceedings. Springer; Berlin: 2012. pp. 365–366. [Google Scholar]
- 57.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berens P. CircStat: A MATLAB toolbox for circular statistics. J Stat Softw. 2009;31:1–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.