Significance
Baicalin is a major flavonoid component from the herbal medicine Scutellaria baicalensis that has been shown to have an antisteatosis effect. Through quantitative chemoproteomic profiling, we discovered that baicalin acts as a natural allosteric activator of carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme of fatty acid β-oxidation (FAO). By directly binding to CPT1 and activating its activity to accelerate fatty acid degradation, baicalin can significantly ameliorate symptoms associated with hepatic steatosis and reduce diet-induced obesity (DIO). Our study provides an example of a natural product agonist for CPT1. The results provide mechanistic insights to explain the bioactivity of baicalin in reducing lipid accumulation and introduce exciting opportunities for developing novel flavonoid-based FAO activators for pharmacologically treating DIO and associated metabolic disorders.
Keywords: baicalin, CPT1, obesity, chemical proteomics, steatosis
Abstract
Obesity and related metabolic diseases are becoming worldwide epidemics that lead to increased death rates and heavy health care costs. Effective treatment options have not been found yet. Here, based on the observation that baicalin, a flavonoid from the herbal medicine Scutellaria baicalensis, has unique antisteatosis activity, we performed quantitative chemoproteomic profiling and identified carnitine palmitoyltransferase 1 (CPT1), the controlling enzyme for fatty acid oxidation, as the key target of baicalin. The flavonoid directly activated hepatic CPT1 with isoform selectivity to accelerate the lipid influx into mitochondria for oxidation. Chronic treatment of baicalin ameliorated diet-induced obesity (DIO) and hepatic steatosis and led to systemic improvement of other metabolic disorders. Disruption of the predicted binding site of baicalin on CPT1 completely abolished the beneficial effect of the flavonoid. Our discovery of baicalin as an allosteric CPT1 activator opens new opportunities for pharmacological treatment of DIO and associated sequelae.
In modern society, about one-third of the adult population worldwide are overweight (1), who consequently have high risk factors for developing obesity-associated metabolic diseases, including nonalcoholic fatty liver disease (2), type 2 diabetes mellitus (3), and cardiovascular disease (4). While the cause and pathogenesis of obesity are complex and multifactorial, unbalanced diets have been postulated as a major culprit to disturb regular energy metabolisms and induce excessive lipid accumulation in multiple organs (5, 6). Attenuating the lipid accumulation could therefore be an effective solution to ameliorate obesity and associated metabolic disorders. Current approaches include eating a healthier diet (7), suppressing food intake physically and therapeutically (8), removing excessive body fat surgically, and inhibiting lipid biogenesis (9). As lipid accumulation results from an imbalance in lipid metabolism, it should also be possible to ameliorate the disorder by boosting the lipid consumption.
The major route for lipid expenditure is through mitochondrial fatty acid β-oxidation (FAO), an essential process in which free fatty acids are esterified with CoA, transported into the mitochondria matrix, and oxidized to generate acetyl-CoAs (10). The transport of long-chain acyl-CoA esters into the mitochondria matrix is mediated by the carnitine palmitoyltransferase (CPT) system that consists of three proteins: CPT1, acylcarnitine translocase, and CPT2 (11). CPT1 is anchored on the mitochondrial outer membrane and is responsible for converting acyl-CoAs into acylcarnitines, which are shuttled across the mitochondria membranes by the translocase and converted back to acyl-CoAs by CPT2 inside mitochondria before entering β-oxidation (12). CPT1 is considered as the rate-limiting enzyme for FAO, and its inhibition by malonyl-CoA, the first committed intermediate for lipogenesis, serves as the key regulatory mechanism to maintain the balance of fatty acid metabolism (13). Genetic suppression of acetyl-CoA carboxylases (ACCs) to reduce the production of malonyl-CoA was shown to reverse diet-induced hepatic steatosis and insulin resistance (14), and overexpression of an active but malonyl-CoA–insensitive mutant of CPT1 was able to ameliorate insulin resistance in mice (15), both of which support the rationale of activating liver FAO for treating obesity-associated metabolic disorders.
Pharmacologically, a synthetic inhibitor of fatty acid synthase, C75, has been reported to activate CPT1 to increase peripheral energy utilization and fatty acid oxidation in a diet-induced obesity (DIO) model (16); however, its mechanism of activation is under debate because it was later discovered that the C75-CoA adduct acts as a potent inhibitor of CPT1 both in vitro and in vivo (17–19). A couple of natural flavonoids with antiobesity and antidiabetic effects have been shown to increase fatty acid oxidation by boosting the transcription of CPT1 (20); however, direct agonists of CPT1 have not been reported yet. Therefore, the search for novel compounds that can activate CPT1 and promote FAO to attenuate lipid accumulation and metabolic disorders is of substantial interest.
The flavonoid family contains more than 5,000 naturally occurring members from plants and fungi with diversified structures and bioactivity (21). Many of these polyphenolic compounds have been found to prevent hepatic steatosis (22), insulin sensitivity (23), and other metabolic syndromes (24, 25). Furthermore, several flavonoids have been reported to improve lipid metabolism, mainly through regulating lipogenic pathways (26). Scutellaria baicalensis is one of the fundamental herbs that has a long history of usage in traditional Chinese medicine (27), and its raw extracts have been reported to have a strong lipid-reducing effect (28). In particular, one of its major flavonoid components, baicalin (Fig. 1A), was shown to reduce diet-induced hepatic steatosis in rodents (29, 30); however, the mechanism of its action remains elusive.
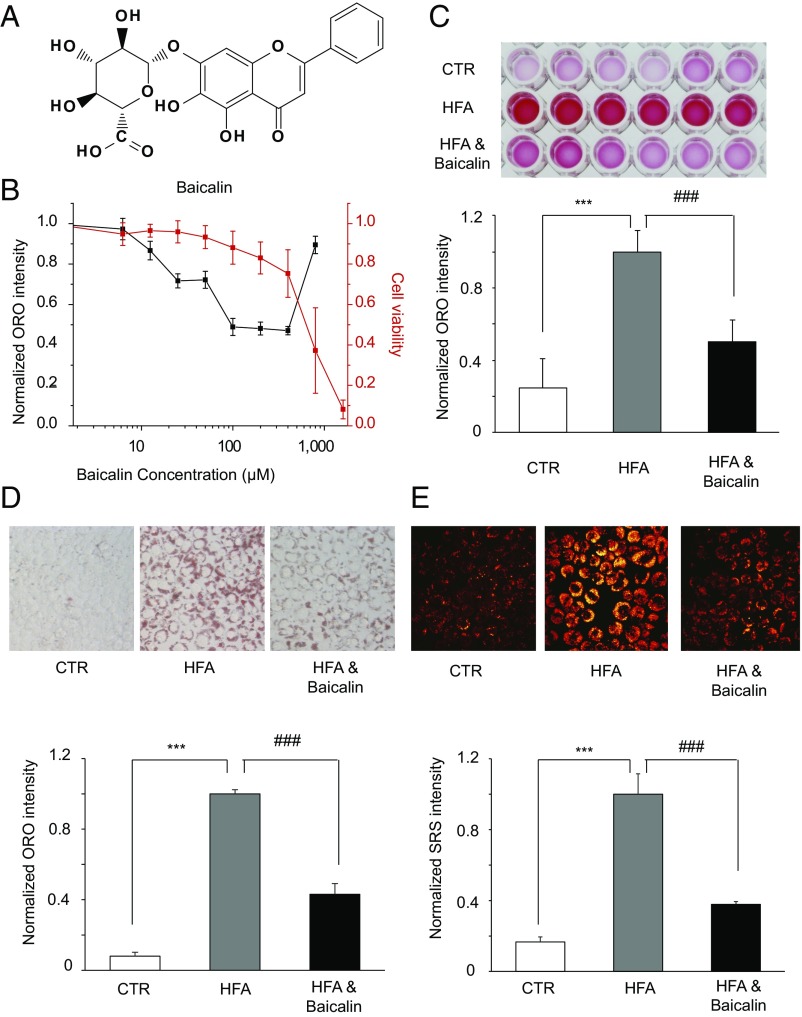
Fig. 1.
Baicalin reduces cellular lipid accumulations. (A) Structure of baicalin. (B) Evaluation of the dose-dependent lipid-reducing effects and cytotoxicity of baicalin in HeLa cells using ORO staining and the thiazolyl blue tetrazolium bromide assay (n = 6). (C) Images (Top) and quantitation (Bottom) of the extracted ORO dyes from the stained HeLa cells that were treated with DMSO [control (CTR)], a high concentration of fatty acids alone (HFA), and a high concentration of HFA together with baicalin (n = 6). (D) Images (Top, 200× magnification) and quantitation (Bottom) of ORO staining of HeLa cells that were treated with DMSO (CTR), HFA alone, and HFA together with baicalin (n = 3). (E) Images (Top, 240× magnification) and quantitation (Bottom) of SRS microscopy analysis of HeLa cells treated with DMSO (CTR), a high concentration of fatty acids alone (HFA), and together with baicalin (n = 3). The cells were treated with 1 mM free fatty acids for 24 h to induce accumulation of lipid droplets and treated with 100 μM baicalin for another 24 h before being assayed. Both *** and ### represent P < 0.001.
In the current work, we implemented a quantitative chemical proteomic strategy to globally profile protein–baicalin interactions and identified the liver isoform of CPT1 (CPT1A) as one of the key targets of baicalin. The flavonoid directly activated CPT1A to accelerate the influx rate of long-chain acyl-CoAs into mitochondria for β-oxidation. Structural modeling predicted an allosteric binding site on CPT1, which, upon disruption, abolished the activation of baicalin. Chronic treatment of baicalin effectively ameliorated DIO and associated symptoms in mice, and the beneficial effect was critically dependent on the activation of hepatic CPT1. Our work reports the discovery of a natural activator for this rate-limiting enzyme of liver FAO and justifies the rationale of activating FAO as an effective approach to prevent obesity and hepatic steatosis. The results not only provide mechanistic insights to explain the bioactivity of baicalin in reducing lipid accumulation but also open exciting opportunities of developing novel flavonoid-based FAO activators for pharmacological treatment of DIO and associated metabolic disorders.
Results
Baicalin Can Lower the Lipid Accumulation in High-Fat–Treated Cells.
To facilitate our quantitative chemical proteomic study to identify the targets of baicalin, we first evaluated the ability of baicalin to lower the lipid accumulation in a mammalian cell line. HeLa cells were treated with 1 mM oleic acid and palmitic acid (2:1 molar ratio) to induce deposition of lipid droplets. These cells were then treated with a series of concentrations of baicalin for 24 h in presence of the excessive free fatty acids. The cells were stained by Oil Red O (ORO), and the extent of lipid accumulation was quantified by the intensity of DMSO-extracted ORO dye. Baicalin is able to significantly lower the cellular accumulation of lipid droplets at as low as 12.5 μM and maintains its strong lipid-reducing effect at up to 400 μM when ∼80% of the cells are still viable (Fig. 1B). Baicalin treatment at 100 μM can already reduce the amount of cellular lipids by ∼50% (Fig. 1C). Similar results were obtained when ORO-stained cells were directly imaged and quantified (Fig. 1D and SI Appendix, Fig. S1A). Furthermore, we monitored the lipid content in these cells by stimulated Raman scattering (SRS) microscopy (31). A dramatic decrease of SRS signals in the baicalin-treated cells confirmed baicalin’s activity in reducing lipid accumulations in HeLa cells (Fig. 1E and SI Appendix, Fig. S1B). In addition, we isolated hepatocytes from mouse liver and confirmed with ORO staining that baicalin retains similar activity in lowering lipid accumulation in primary cells (SI Appendix, Fig. S2A).
Target Profiling of Baicalin by SILAC-ABPP.
We next employed activity-based protein profiling (ABPP) to identify the protein targets that directly interact with baicalin in proteomes. The ABPP-based chemical proteomic strategy has enabled discovery of functionally unannotated enzymes and deconvolution of protein targets of bioactive small molecules (32, 33). In particular, its combination with stable isotope labeling by amino acids in cell culture (SILAC) has allowed unambiguous identification of protein targets of lipid binding and modifications directly in native biological systems (34, 35). Given that baicalin does not contain any obvious reactive moiety that can modify proteins covalently, we designed and synthesized a photoaffinity baicalin probe that contains a benzophenone photo–cross-linking group and an alkynyl reporter group (Fig. 2A and SI Appendix, Fig. S3A). Both functional groups were introduced at the carboxylic acid group using a recently developed “photo-click” reaction (36). Despite considerable structural derivatizations, the baicalin probe was able to lower the lipid accumulation as effectively as the native compound (Fig. 2B). When the baicalin probe (200 μM) was used to label the proteome in an in-gel fluorescence assay, cotreatment of the native baicalin compound (400 μM) was able to compete the probe labeling (SI Appendix, Fig. S3B), supporting that the probe binds to similar target proteins as baicalin does.
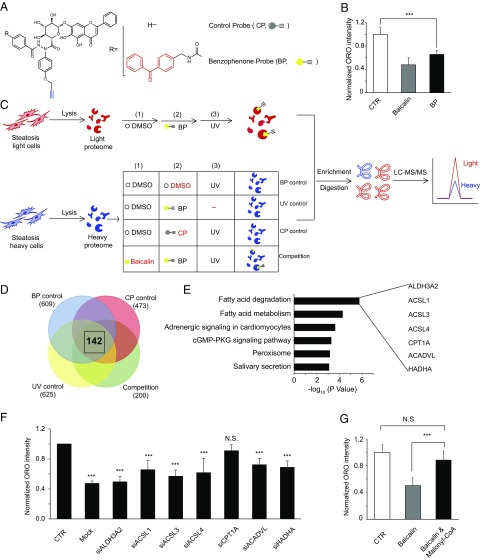
Fig. 2.
Quantitative chemoproteomic profiling identified CPT1A as a target of baicalin. (A) Structure of the baicalin probes with [benzophenone probe (BP)] or without [control probe (CP)] the benzophenone photo–cross-linking group (colored red). The alkyne reporter group is colored blue. (B) Evaluation of the ability of the baicalin BP probe to reduce lipid accumulation in HeLa cells. (C) Overall scheme of the SILAC-ABPP experiments to identify baicalin targets in proteomes. Four sets of experiments were performed, including “BP-control,” “UV-control,” “CP-control,” and “Competition” experiments. (D) Venn diagram showing the number of proteins with an averaged SILAC ratio ≥4.0 identified in each set of SILAC-ABPP experiments (shown in parentheses), highlighting a total of 142 proteins commonly identified from all four sets of SILAC-ABPP experiments. (E) Gene ontology analysis of the 142 protein targets identified by SILAC-ABPP reveals their association with fatty acid degradation and metabolism. The names of the seven proteins specifically assigned to the functional cluster of “Fatty acid degradation” are shown. (F) Knockdown of each of the seven enzymes by siRNA abolished the lipid-reducing effect of baicalin to various extents in HeLa cells. Black asterisks denote the comparison to lipid-fed cells treated with DMSO and an empty vector [control (CTR)]. “Mock” denotes lipid-fed cells treated with baicalin and an empty vector. Cells were assayed after treatment with 100 μM baicalin for 24 h. (G) Cotreatment of malonyl-CoA, a known CPT1A inhibitor, abolished the effect of baicalin in reducing lipid accumulation in HeLa cells. N.S., not significant. For all data, ***P < 0.001 (n = 6).
To identify these target proteins, we performed a series of SILAC-ABPP experiments using the baicalin probe with or without competition from the native baicalin compound (Fig. 2C). Briefly, proteomes lysed from the “light” and “heavy” HeLa cells treated with excessive fatty acids were first incubated for 15 min with DMSO or baicalin (1 mM), respectively, and labeled with the baicalin probe (200 μM) via UV-induced photo–cross-linking. The probe-labeled light and heavy proteomes were mixed, conjugated with azide-biotin, enriched on streptavidin beads, and finally subjected to trypsin digestion. The digested peptides were analyzed by liquid chromatography tandem mass spectrometry to identify proteins that are photocaptured by the baicalin probe. The quantified SILAC ratio (light vs. heavy) for each protein reflects the extent of competition by the native baicalin compound, with a larger ratio corresponding to greater competition. In addition to the “Competition” experiment, we performed three sets of control experiments (benzophenone probe “BP-control,” control probe “CP-control,” and “UV-control”) to eliminate false-positive targets resulting from indirect and/or nonspecific binding to the probe (Fig. 2C and SI Appendix, SI Materials and Methods). Three replicates were performed for each of the control and competition experiments, and all of the proteins quantified are listed in Dataset S1. After applying a cutoff of 4.0 for the averaged SILAC ratio in all of the control and competition experiments, we collectively identified 142 proteins as potential targets that are specifically bound to baicalin (Fig. 2D and Dataset S2).
Baicalin Targets Key Enzymes in Fatty Acid Oxidation.
Gene ontology analysis (37) of the pathways associated with these 142 proteins revealed a top-ranking functional cluster of “fatty acid degradation,” which includes seven enzymes (Fig. 2E). Since this specific functional cluster of enzymes is highly relevant to the observed phenotype of reduced lipid accumulation, we continued to investigate these enzymes’ potential roles in mediating baicalin’s lipid-reducing effect. We used RNA interference (RNAi) to transiently knock down each of these enzymes (SI Appendix, Fig. S4) and observed that knockdown of CPT1A, the rate-limiting enzyme directly involved in FAO, completely abolished the effect of baicalin in reducing lipid accumulation (Fig. 2F). Consistently, cotreatment with malonyl-CoA, an endogenous CPT1A inhibitor, also significantly impaired the effect of baicalin (Fig. 2G). These results collectively suggested that the CPT1A-controlled FAO is responsible for the lipid-reducing effect of baicalin. Since abnormal lipid accumulation results from an imbalance of lipid metabolism and excessive lipid biogenesis and/or insufficient lipid consumption, we also performed the transcriptome analysis of the baicalin-treated cells and found no significant changes at the transcriptional level for those enzymes associated with various lipid metabolic pathways, including CPT1A (SI Appendix, Fig. S5 and Dataset S3). Thus, we hypothesized that baicalin might exert its lipid-reducing effect by directly activating CPT1A to accelerate lipid consumption in FAO.
Direct Activation of CPT1A by Baicalin.
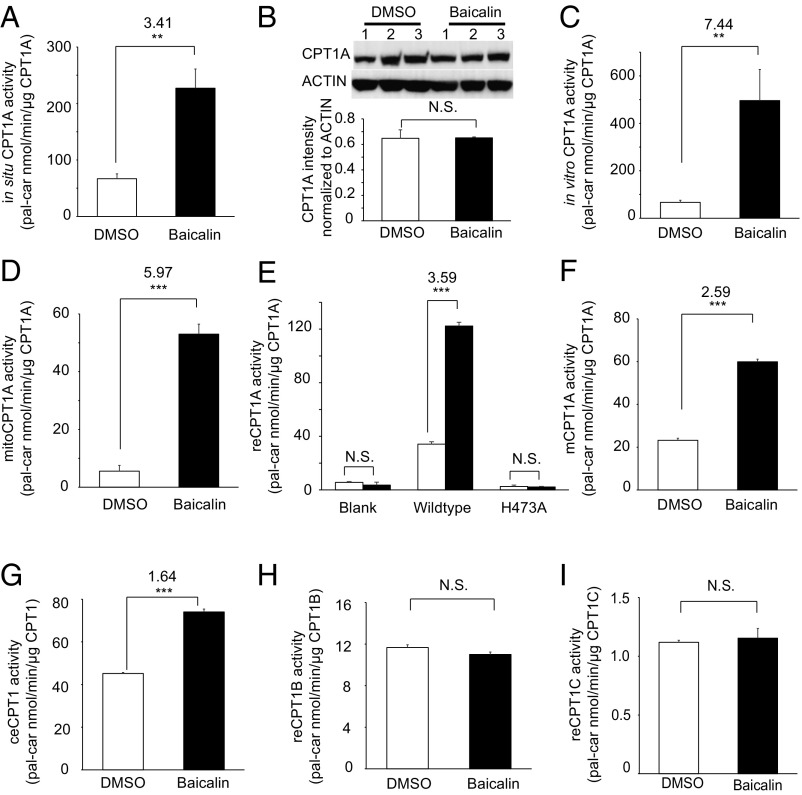
To test whether baicalin can activate CPT1A directly, we first established a quantitative enzymatic assay to measure CPT1A activity, which uses the [D9]-carnitine and palmitoyl-CoA as the substrates and detects the production of [D9]-palmitoyl-carnitine (SI Appendix, SI Materials and Methods). In this assay, lysates from the baicalin-treated HeLa cells (100 μM, 24 h) showed a threefold increase in CPT1A activity compared with that of the DMSO-treated cells (Fig. 3A). Immunoblotting analysis confirmed that baicalin treatment did not perturb the protein level of CPT1A compared with the untreated cells (Fig. 3B). Direct addition of baicalin (100 μM) to the cell lysates resulted in an approximately sevenfold increase of CPT1A activity (Fig. 3C). Furthermore, treatment of the isolated mitochondria extracts with 100 μM baicalin resulted in a similar stimulation of CPT1A activity (Fig. 3D), which is consistent with the mitochondrial location of CPT1A. Similar CPT1A-activating effects can also be observed in primary hepatocytes (SI Appendix, Fig. S2B). We overexpressed CPT1A recombinantly in Escherichia coli (SI Appendix, Fig. S6) but could not obtain the active enzyme in the purified form due to protein aggregation. Nevertheless, we observed a greater than threefold increase in CPT1A activity from the E. coli lysates treated with 100 μM baicalin, whereas the background CPT1A activities were negligible (Fig. 3E). The activation of CPT1A by baicalin was dose-dependent, as we observed a >60-fold activation when the lysates were treated with up to 800 μM baicalin (SI Appendix, Fig. S7). We also recombinantly overexpressed CPT1A of Mus musculus and CPT1 of Caenorhabditis elegans and demonstrated that baicalin is able to stimulate these orthologs as well, although to a lesser extent (Fig. 3 F and G). Interestingly, baicalin did not activate CPT1B and CPT1C, the other two human isoforms of CPT1 (Fig. 3 H and I). These data collectively demonstrated that baicalin activates CPT1A directly with specific isoform selectivity.
Fig. 3.
Baicalin directly activates the enzymatic activity of CPT1A. (A) Treatment of live HeLa cells with 100 μM baicalin resulted in a greater than threefold increase in CPT1A activity. (B) Immunoblots of CPT1A (Top) and quantification of its protein abundance (Bottom) in HeLa cells with or without baicalin treatment. (C) Treatment of crude HeLa cell lysates with 100 μM baicalin resulted in a greater than sevenfold increase in CPT1A activity. (D) Treatment of isolated mitochondrial extracts with 100 μM baicalin resulted in a greater than fivefold increase in CPT1A activity (mitoCPT1A). (E) Treatment of crude lysates from E. coli overexpressing wild-type CPT1A with 100 μM baicalin resulted in a greater than threefold increase in recombinant CPT1A activity (reCPT1A). The lysates from E. coli overexpressing an empty vector or the catalytic dead mutant H473A of CPT1A showed minimum background CPT1A activity. (F) Treatment of crude lysates from E. coli overexpressing CPT1A of M. musculus (mCPT1A) with 100 μM baicalin increased CPT1A activity. (G) Treatment of crude lysates from E. coli overexpressing CPT1 of C. elegans (ceCPT) with 100 μM baicalin increased CPT1A activity. (H) Baicalin does not activate the recombinant CPT1B. (I) Baicalin does not activate the recombinant CPT1C. N.S., not significant; pal-car, palmitoyl-carnitine. For all data, **P < 0.01; ***P < 0.001 (n = 3).
Structural Model for the CPT1A Activation by Baicalin.
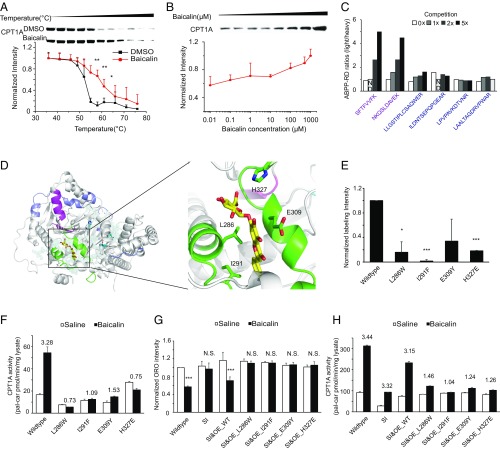
Biophysical analysis of the interaction between CPT1A and baicalin can provide more insights on the mechanism of enzyme activation by the flavonoid. Since we could not obtain purified CPT1A with enzymatic activity, we resorted to other complementary methods to analyze the protein–flavonoid interaction directly in cell lysates. Both temperature- and dose-dependent cellular thermal shift assays (38) demonstrated that baicalin affects the thermal stability of CPT1A but not the actin control (Fig. 4 A and B and SI Appendix, Fig. S8), supporting that there is a direct interaction between CPT1A and baicalin. We next used the baicalin probe to map its potential binding site on CPT1A by a competitive ABPP reductive dimethylation experiment (39) (SI Appendix, SI Materials and Methods and Fig. S9A). Based on the CPT1A peptides whose probe labeling was sensitive to baicalin treatment or not (Fig. 4C and SI Appendix, Fig. S9B), we used the Rosetta software suite (40) to construct a model of the CPT1A–baicalin complex by homology modeling and ligand docking (Fig. 4D). Guided by the docking model, we designed four mutations in CPT1A at the complex interface (L286W, I291F, E309Y, and H327E) that are predicted to maintain the structural integrity of CPT1A but disrupt the binding of baicalin (SI Appendix, Fig. S10A). All of the four disruptive mutants were confirmed with much reduced labeling by the baicalin probe (Fig. 4E and SI Appendix, Fig. S10B). We tested their enzyme activity in response to baicalin treatment in vitro and found that all of them significantly lost the activation by baicalin (Fig. 4F). To further verify the role of CPT1 in mediating the lipid-reducing activity of baicalin, we overexpressed either wild-type CPT1A or each of the four mutants in HeLa cells after knocking down endogenous CPT1A expression by RNAi (SI Appendix, Fig. S11A) and evaluated the lipid-reducing activity of baicalin on them. As expected, only overexpression of wild-type CPT1A, but not any of the mutants, could rescue the phenotypic reduction of lipid accumulation in cells by baicalin (Fig. 4G), and the response is well correlated with the overall CPT1A enzymatic activity upon baicalin treatment (Fig. 4H).
Fig. 4.
Structural modeling and physiological validation of the CPT1A–baicalin interaction. (A) Baicalin treatment (100 μM) increases the thermal stability of CPT1A in cell lysates as measured by the temperature-dependent cellular thermal shift assay (n = 3). (B) Baicalin treatment increases the thermal stability of CPT1A in cell lysates as measured by the concentration-dependent cellular thermal shift assay at 52 °C (n = 3). (C) CPT1A peptides identified by the competitive ABPP reductive dimethylation (RD) experiment with ratios sensitive (magenta) or insensitive (purple) to the increasing competition of baicalin. (D, Left) Structural overview of a CPT1A–baicalin complex model predicted based on information from the competitive ABPP-RD experiment. The protein is shown in a cartoon representation in gray, and baicalin is shown in a stick representation in yellow. The active-site residues (H473, Y589, and T602) are shown in the stick representation in cyan. The peptides with ABPP-RD ratios sensitive and insensitive to the binding of baicalin are colored magenta and purple, respectively. Residues around the predicted binding pocket are colored green. (D, Right) Zoom-in view of the predicted CPT1A–baicalin interface. Key interface residues (L286, I291, E309, and H327) in CPT1A are shown in the stick representation and are labeled by residue name and position. (E) Disruptive mutations of predicted key interface residues abolished CPT1A labeling by the baicalin photoaffinity probe (n = 2). Four disruptive mutations were predicted based the docking model of the CPT1A–baicalin complex. E. coli lysates overexpressing these mutants were labeled by the baicalin probe with or without baicalin competition and enriched by streptavidin. Immunoblotting analysis of CPT1A was performed for the input lysate before enrichment (input), for the sample after enrichment (pulldown), and for the sample after enrichment with baicalin competition (competition). The intensity of immunoblotting signals was quantified by ImageJ (NIH), and the quantitative ratio of probe labeling was calculated by subtracting the intensity from the competition lane from that of the pulldown lane, and then divided by that of the input lane. *P < 0.05; ***P < 0.001, compared with wild-type. (F) Overall activity of CPT1A upon baicalin activation in HeLa cells with siRNA knockdown of endogenous CPT1A followed by overexpression of wild-type CPT1A or each of the disruptive mutants. The fold of activation is shown above each bar (n = 3). (G) Overexpression of wild-type CPT1A but not any of the disruptive mutants in HeLa cells with siRNA knockdown of endogenous CPT1A can rescue the lipid-reducing response to baicalin treatment (100 μM treatment for 24 h, n = 3). N.S., not significant; ***P < 0.001, compared with Saline. (H) Overall activity of CPT1A upon baicalin activation in HeLa cells with siRNA knockdown of endogenous CPT1A followed by overexpression of wild-type CPT1A or each of the disruptive mutants. The fold of activation is shown above each bar (100 μM baicalin for 24 h, n = 3).
Baicalin Ameliorates DIO and Associated Metabolic Disorders in Mice.
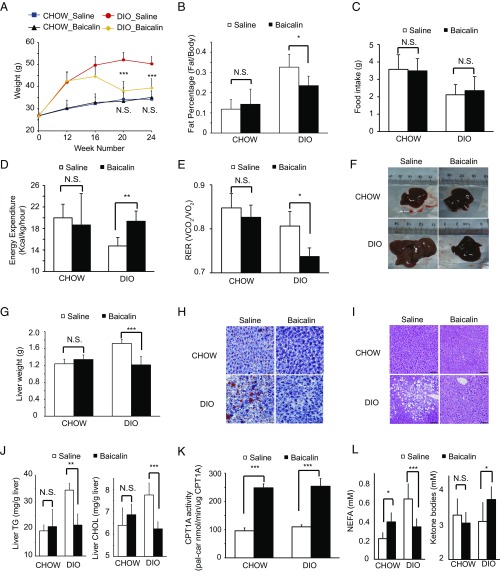
We next investigated the effect of baicalin in a DIO animal model. Mice were fed a high-fat diet for 12 wk to first induce obesity and hepatic steatosis; Under the same high fat diet, they were then administered intragastrically with baicalin (400 mg/kg daily) for another 12 wk. The dose was chosen based on a previous pharmacokinetic study that measured the final plasma concentration of baicalin as 0.8 μg/mL 6 h after intake (41). As expected, DIO mice gained significantly more body weight (42 ± 4.7 g) than those on regular chow (30 ± 1.7 g) after 12 wk (Fig. 5A). Ensuing treatment with baicalin resulted in a dramatic reduction of whole-body weight (Fig. 5A), lowered the percentage of body fat (Fig. 5B), improved insulin sensitivity, and ameliorated hyperlipidemia and hyperglycemia (SI Appendix, Fig. S12). Without affecting the amount of food intake (Fig. 5C), baicalin treatment increased energy expenditure in DIO mice (Fig. 5D) and shifted the respiratory exchange ratio (RER), suggesting that these mice use more lipid as the energy source (Fig. 5E). Notably, the RER shift occurred as early as 3 wk after baicalin treatment and preceded the divergence of body weights (SI Appendix, Fig. S13), suggesting that activated FAO is driving the improvement in the metabolic profiles of DIO mice.
Fig. 5.
Baicalin ameliorates DIO and associated metabolic disorders in mice. (A) Baicalin treatment reduced the body weights in DIO mice, but not in mice on the regular diet (CHOW). (B) Baicalin treatment decreased the percentage of body fat in DIO mice. (C) Baicalin treatment does not affect food intake in either CHOW or DIO mice. (D) Baicalin treatment increased the energy expenditure in DIO mice. (E) Baicalin treatment shifted the RER toward 0.7 in DIO mice, suggesting that more fat is used as the energy source. (F and G) Baicalin treatment reduced the liver sizes and weights in DIO mice. (H and I) Images of hematoxylin and eosin staining and ORO staining of liver tissue slices from CHOW or DIO mice with or without baicalin treatment. (Magnification: 200×.) (J) Baicalin treatment reduced the levels of hepatic triglyceride (TG) and cholesterol (CHOL) in DIO mice. (K) Baicalin treatment increased the activity of CPT1A in the livers of in both CHOW and DIO mice. (L) Baicalin treatment reduced the levels of NEFAs and increased the level of TKBs in plasma. All data were measured after daily treatment with saline or 400 mg/kg baicalin for 12 wk. N.S., not significant. *P < 0.05; **P < 0.01; ***P < 0.001 (n = 5 per group).
Analysis of the raw liver tissues showed that baicalin treatment effectively reduced the size and weight of livers (Fig. 5 F and G) as well as the accumulation of hepatic lipid (Fig. 5 H and I) in DIO mice. Furthermore, quantitative analysis of the tissue homogenates and blood samples showed that baicalin could significantly reduce the levels of hepatic triglyceride and cholesterol (Fig. 5J) and improve liver function (SI Appendix, Fig. S12B) in DIO mice. It should be noted that while baicalin could ameliorate hepatic steatosis in DIO mice, it showed no obvious adverse effects on the lean mice, such as weight reduction, loss of appetite, and hepatotoxicity (Fig. 5 A and C and SI Appendix, Fig. S12B), suggesting that it could be used safely to treat hepatic steatosis and obesity.
We measured the CPT1A activity from the homogenized liver lysates and found that baicalin treatment resulted in a greater than twofold increase in CPT1A activity (Fig. 5K) with little change on protein abundance or the level of malonyl-CoA (SI Appendix, Fig. S14). Consistent with activated FAO, the plasma level of nonesterified fatty acid (NEFA) was decreased and that of total ketone bodies (TKBs) was increased (Fig. 5L).
CPT1A Activation Is Critical for the Antiobesity Effect of Baicalin.
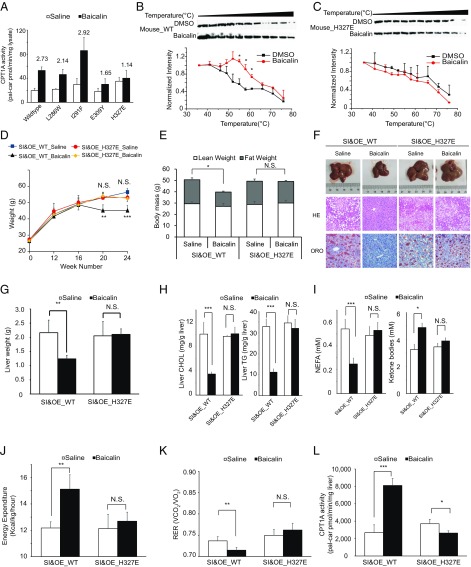
To corroborate the role of CPT1A activation in mediating the antiobesity effect of baicalin, we proceeded to test the baicalin-insensitive mutants in the DIO mouse model. We first examined the four baicalin-insensitive mutations in the mouse enzyme as they were designed based on the docking model from the human ortholog. The in vitro CPT1A assay revealed that H327E retained its resistance toward activation by baicalin (Fig. 6A). Consistently, sequence alignment of the human and mouse CPT1A showed that H327 is conserved among the four mutated sites (SI Appendix, Fig. S15). A thermal shift assay also confirmed that the H327E mutant of mouse CPT1A was no longer bound by baicalin (Fig. 6 B and C).
Fig. 6.
Antiobesity and antisteatosis effects of baicalin are dependent on its interaction with CPT1A. (A) H327E mutant of mouse CPT1A retains its resistance to activation by baicalin. The fold of activation of mouse CPT1A activity by baicalin is shown above each bar (n = 3). Baicalin treatment increases the thermal stability of recombinant mouse wild-type CPT1A (B) in E. coli lysates but not in its baicalin-resistant H327E mutant (C) as measured by the temperature-dependent cellular thermal shift assay (n = 3). *P < 0.05, compared with DMSO. Overexpression of wild-type CPT1A but not the disruptive mutant H327E in mouse liver after knockdown of endogenous hepatic CPT1A can rescue the antisteatosis response to baicalin in DIO mice, including the reduction of body weight (D), the reduced percentage of body fat (E), the reduced liver sizes and hepatic steatosis as measured by hematoxylin and eosin (HE) and ORO staining (200× magnification) (F), the reduced liver weights (G), the reduced levels of hepatic triglyceride (TG) and cholesterol (CHOL) (H), the reduced levels of NEFAs and the increased level of TKBs in plasma (I), the increased energy expenditure (J), and the shifted RER to 0.7 (K). (L) Overall of hepatic CPT1A activity in DIO mice with overexpression of wild-type CPT1A or the disruptive mutant H327E in mouse liver after knockdown of endogenous hepatic CPT1A. For D–L, all data were measured after daily treatment with saline or 400 mg/kg baicalin for 12 wk. N.S., not significant. *P < 0.05; **P < 0.01; ***P < 0.001 (n = 5 per group). pal-car, palmitoyl-carnitine; SI&OE_WT or SI&OE_H327E, overexpression of wild-type CPT1A or H327E mutant in mouse liver after knockdown of endogenous hepatic CPT1A, respectively.
Since genetic knockout of CPT1A is embryonically lethal (42), we first knocked down the hepatic CPT1A expression in DIO mice using shRNA interference and observed that all beneficial effects of baicalin in ameliorating diet-induced hepatic steatosis and obesity were effectively abolished (SI Appendix, Fig. S16). To further demonstrate that activation of CPT1A is critical for these beneficial effects, we restored the level of CPT1A in liver by overexpressing either the wild-type CPT1A or the H327E mutant via adenoassociated virus-mediated transfection (SI Appendix, SI Materials and Methods). Consistent with our observation in the cellular model, only rescue with the wild-type CPT1A, but not the baicalin-insensitive mutant H327E, was able to ameliorate the obesity-associated metabolic symptoms in DIO mice, including reduced body weight (Fig. 6D) and percentage of body fat (Fig. 6E), reduced liver size, weight and lipid deposition (Fig. 6 F and G), decreased triglyceride and cholesterol levels in the liver (Fig. 6H), decreased NEFA and increased TKB levels in the plasma (Fig. 6I), and shifted metabolic rates (Fig. 6 J and K). In addition, baicalin ameliorates insulin sensitivity, hyperlipidemia, and hyperglycemia with dependence only on wild-type CPT1A but not the baicalin-insensitive mutant H327E (SI Appendix, Fig. S17). We confirmed that the “rescuing” effect was not due to different expression levels of the wild-type and mutant CPT1A (SI Appendix, Fig. S11B), but rather correlated with the overall enzymatic activity of CPT1A upon baicalin treatment (Fig. 6L). We also measured the abundance and activity of CPT1A in other tissues, such as adipose tissue, intestine, and kidney. Although CPT1A could be activated by baicalin across all these tissues, the overall activity of hepatic CPT1A was measured as the highest one (SI Appendix, Fig. S18). These data collectively supported that a direct interaction between baicalin and hepatic CPT1A is responsible for mediating the antiobesity and antisteatosis activity of the flavonoid.
Discussion
Baicalin is a major flavonoid component from the traditional Chinese medicine S. baicalensis. Inspired by its remarkable antisteatosis activity (29, 30), we have performed a quantitative chemoproteomic profiling study to identify the protein targets of baicalin in cellular proteomes. We found that CPT1A, which is the rate-limiting enzyme in FAO, is directly bound and activated allosterically by baicalin. The CPT1A activation by the flavonoid results in acceleration of hepatic FAO and amelioration of obesity and associated metabolic disorders in DIO mice.
Several strategies have been exploited to promote mitochondrial FAO in liver via CPT1A to treat obesity and associated metabolic diseases, including using peroxisome proliferator-activated receptor agonists to boost its expression (43), reducing the malonyl-CoA level to alleviate its inhibition (44), and overexpressing a malonyl-CoA–insensitive CPT1A mutant (15). A fatty acid synthase inhibitor, C75, was reported to increase CPT1 activity and reduce DIO (16); however, its modes of action are still questionable (18). While a transient and moderate elevation of CPT1A genetically was shown to reduce hepatic triglyceride accumulation (45), our attempt to overexpress CPT1A by an adenoassociated virus with a strong liver-specific promoter resulted in the premature death of mice (SI Appendix, Fig. S19A), suggesting that excessive elevation of CPT1A by genetic means is toxic. In this regard, our discovery of baicalin as a direct natural activator of CPT1 with isoform selectivity in liver provides a unique pharmacological tool to promote mitochondrial FAO specifically in hepatocytes. Compared with transient CPT1A overexpression, baicalin treatment resulted in higher activation on CPT1A activity and more reduction in lipid accumulation without affecting cell viability in a cellular model (SI Appendix, Fig. S19B). The chronic treatment of baicalin not only ameliorated the DIO and hepatic steatosis but also improved associated metabolic syndromes, including insulin resistance, hyperglycemia, hyperlipidemia, and damaged liver functions in DIO mice. It is very gratifying to observe that baicalin treatment, despite administration at a seemingly high dose, causes few phenotypical and metabolic changes in healthy animals. Considering its long history of usage in traditional Chinese medicine practice with no major safety concerns reported, we believe that baicalin may serve as an ideal candidate drug to prevent and treat DIO and associated metabolic disorders.
Baicalin belongs to the family of flavonoids with more than 5,000 members, and these structurally diversified natural products ubiquitously exist in plants and fungi, some of which have also been reported with unique hepatoprotecting activities (26). It remains to be explored whether the CPT1A-activating activity of baicalin is shared by other naturally occurring flavonoids with even higher potency and better selectivity. Baicalein is another major flavonoid from S. baicalensis, and it can be converted from baicalin by deglycosylation. Our preliminary tests suggested that baicalein could effectively compete the photolabeling of CPT1A by the baicalin probe and it has similar CPT1A-activating activity as well. However, the deglycosylation resulted in increased cytotoxicity, which, in turn, significantly dampened the lipid-reducing effect of baicalein (SI Appendix, Fig. S20). It is therefore important to pay close attention to the cytotoxicity profiles when screening more flavonoids in the future.
Due to its poor bioavailability, a relatively high (but safe) dose of baicalin was applied in our animal studies, and based on the previous pharmacokinetics analysis, its plasma steady-state concentration is predicted at 0.8 μg/mL after an oral dose of 400 mg/kg (41). It is therefore conceivable that optimization of the structure/activity relationship of baicalin by medicinal chemistry, together with more detailed structural insights of CPT1–baicalin interaction, may yield new synthetic antiobesity drugs with improved pharmacodynamics and pharmacokinetics. In this regard, we have preliminarily explored the activity of an acetylated derivative, Ac-baicalin, which is predicted to have improved bioavailability. The results showed that structural modification of the flavonoid core by acetylation disrupted the binding of CPT1A and that loss of CPT1A activation abolished the lipid-reducing effect of Ac-baicalin (SI Appendix, Fig. S20).
We exploited an ABPP-based chemical proteomic strategy to identify the protein targets of baicalin. The bulky modification on the baicalin structure may hinder the identification of some of its genuine targets; however, the unchanged lipid-reducing activity justified its use in profiling and led to the identification of the interaction of CPT1A and baicalin. Recently, baicalein was also found to have antiinfection properties against Salmonella typhimurium, and an elegantly conducted ABPP study revealed that the type III secretion system was targeted (46). These studies demonstrated that chemical proteomics have become a valuable tool to discover the protein targets of a small molecule with specific bioactivity, which will provide important clues to guide functional elucidation of its mechanism of action and open new windows for drug development.
Several studies have previously attempted to explore the mechanisms of the antiobesity and antisteatosis activity of baicalin (29, 30, 47), and although multiple targets and pathways were observed with transcriptional and posttranslational perturbations, no defined mode of action was confirmed. It was observed that the AMP-activated protein kinases and ACC pathways were activated and inhibited, respectively, upon baicalin treatment (29, 30), and the implied activation of FAO fits our discovery of baicalin as a direct allosteric activator of CPT1A. In addition, our chemoproteomic profiling has identified other proteins that potentially interact with baicalin. It remains to be investigated whether they are also involved in mediating the antiobesity as well as other aspects of baicalin’s bioactivity. Furthermore, we performed transcriptome analysis of HeLa cells upon baicalin treatment (SI Appendix, Fig. S5 and Dataset S3), and although no major lipid metabolism pathways are significantly perturbed transcriptionally, more thorough analysis may help reveal other modes of action indirectly targeted by the flavonoid. Nevertheless, our mechanistic investigation of the CPT1A–baicalin interaction by both structural modeling and in vivo rescuing experiments provided strong evidence that the flavonoid exerts its antiobesity and antisteatosis activity mainly through activating this rate-limiting enzyme in FAO.
Our structural modeling combined with quantitative mass spectrometry identified a binding pocket of baicalin on CPT1A away from the enzyme’s active site, suggesting a mechanism of allosteric activation. However, failure to obtain the purified enzyme with full activity precluded us from characterizing this interaction using other biophysical methods. It is intriguing to observe that endogenous and recombinant CPT1A responded differently to baicalin activation (Fig. 3 A–D), and this may suggest a potential role of posttranslational modification involved in regulating the enzyme’s activity. Equally interesting is the isoform selectivity of baicalin on CPT1 (Fig. 3 H and I). Sequence alignment reveals some divergence among CPT1 isoforms (SI Appendix, Fig. S15), but the structural basis for the selectivity remains to be investigated. Collectively, a crystal structure of the CPT1A-baicalin complex will be well warranted to ultimately unveil the detailed mechanism of action by the flavonoid and enable structure-based design of novel regulators for this critical metabolic enzyme.
Materials and Methods
Details are provided in SI Appendix, SI Materials and Methods, including detailed methods for cell culture, RNAi, thiazolyl blue tetrazolium bromide assay, ORO staining assay, SRS microscopy, probe synthesis, in-gel fluorescence analysis, quantitative chemoproteomic profiling, cloning and expression of CPT1, CPT1 activity assay, animal works, histological analysis, metabolic rate measurements, computational modeling, and thermal shift assay. All animal experiments were performed in accordance with guidelines approved by the Institutional Animal Care and Use Committee of Peking University accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Supplementary Material
Acknowledgments
We thank the Center for Experimental Animals at Peking University for supporting the animal work and the Computing Platform of the Center for Life Science for supporting the proteomic data analysis. We thank Prof. Wanzhu Jin, Prof. Zhen Yang, Prof. Min Ye, and members of C.W. laboratory for insightful discussions. This work was supported by the Ministry of Science and Technology of China (Grant 2016YFA0501500), the National Natural Science Foundation of China (Grants 81490740, 21472008, and 21521003), and a “1000 Talents Plan” Young Investigator Award (to C.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801745115/-/DCSupplemental.
References
- 1.Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110–121. doi: 10.1038/nrgastro.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 3.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 4.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 6.van der Klaauw AA, Farooqi IS. The hunger genes: Pathways to obesity. Cell. 2015;161:119–132. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 7.de Wit NJ, Afman LA, Mensink M, Müller M. Phenotyping the effect of diet on non-alcoholic fatty liver disease. J Hepatol. 2012;57:1370–1373. doi: 10.1016/j.jhep.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg JM, Tymoczko JL, Stryer L, Stryer L. Biochemistry. 5th Ed Freeman; New York: 2002. [Google Scholar]
- 11.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonnefont JP, et al. Carnitine palmitoyltransferases 1 and 2: Biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes Rev. 2010;11:380–388. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- 14.Savage DB, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monsénégo J, et al. Enhancing liver mitochondrial fatty acid oxidation capacity in obese mice improves insulin sensitivity independently of hepatic steatosis. J Hepatol. 2012;56:632–639. doi: 10.1016/j.jhep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci USA. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cha SH, Hu Z, Chohnan S, Lane MD. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:14557–14562. doi: 10.1073/pnas.0507300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mera P, et al. C75 is converted to C75-CoA in the hypothalamus, where it inhibits carnitine palmitoyltransferase 1 and decreases food intake and body weight. Biochem Pharmacol. 2009;77:1084–1095. doi: 10.1016/j.bcp.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Bentebibel A, et al. Novel effect of C75 on carnitine palmitoyltransferase I activity and palmitate oxidation. Biochemistry. 2006;45:4339–4350. doi: 10.1021/bi052186q. [DOI] [PubMed] [Google Scholar]
- 20.Shin ES, et al. Positive regulation of hepatic carnitine palmitoyl transferase 1A (CPT1A) activities by soy isoflavones and L-carnitine. Eur J Nutr. 2006;45:159–164. doi: 10.1007/s00394-005-0576-5. [DOI] [PubMed] [Google Scholar]
- 21.Nijveldt RJ, et al. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 22.Kwon EY, Jung UJ, Park T, Yun JW, Choi MS. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes. 2015;64:1658–1669. doi: 10.2337/db14-0631. [DOI] [PubMed] [Google Scholar]
- 23.Mulvihill EE, et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes. 2011;60:1446–1457. doi: 10.2337/db10-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assini JM, et al. Naringenin prevents cholesterol-induced systemic inflammation, metabolic dysregulation, and atherosclerosis in Ldlr−/− mice. J Lipid Res. 2013;54:711–724. doi: 10.1194/jlr.M032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pu P, et al. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch Biochem Biophys. 2012;518:61–70. doi: 10.1016/j.abb.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Kawser Hossain M, et al. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci. 2016;17:569. doi: 10.3390/ijms17040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Guo XY. [Progress in pharmacologic research on Scutellaria baicalensis] Zhong Xi Yi Jie He Za Zhi. 1989;9:698–700. Chinese. [PubMed] [Google Scholar]
- 28.Regulska-Ilow B, Biernat J, Grajeta H, Ilow R, Drzewicka M. Influence of bioflavonoids from the radix extract of Scutellaria baicalensis on the level of serum lipids, and the development of laboratory rats fed with fresh and oxidized fats. Nahrung. 2004;48:123–128. doi: 10.1002/food.200300382. [DOI] [PubMed] [Google Scholar]
- 29.Guo HX, et al. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol Sin. 2009;30:1505–1512. doi: 10.1038/aps.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xi Y, et al. Baicalin attenuates high fat diet-induced obesity and liver dysfunction: Dose-response and potential role of CaMKKbeta/AMPK/ACC pathway. Cell Physiol Biochem. 2015;35:2349–2359. doi: 10.1159/000374037. [DOI] [PubMed] [Google Scholar]
- 31.Cao C, Zhou D, Chen T, Streets AM, Huang Y. Label-free digital quantification of lipid droplets in single cells by stimulated Raman microscopy on a microfluidic platform. Anal Chem. 2016;88:4931–4939. doi: 10.1021/acs.analchem.6b00862. [DOI] [PubMed] [Google Scholar]
- 32.Niphakis MJ, Cravatt BF. Enzyme inhibitor discovery by activity-based protein profiling. Annu Rev Biochem. 2014;83:341–377. doi: 10.1146/annurev-biochem-060713-035708. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, et al. Mapping sites of aspirin-induced acetylations in live cells by quantitative acid-cleavable activity-based protein profiling (QA-ABPP) Sci Rep. 2015;5:7896. doi: 10.1038/srep07896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin BR, Wang C, Adibekian A, Tully SE, Cravatt BF. Global profiling of dynamic protein palmitoylation. Nat Methods. 2011;9:84–89. doi: 10.1038/nmeth.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hulce JJ, Cognetta AB, Niphakis MJ, Tully SE, Cravatt BF. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat Methods. 2013;10:259–264. doi: 10.1038/nmeth.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao S, et al. Photo-induced coupling reactions of tetrazoles with carboxylic acids in aqueous solution: Application in protein labelling. Chem Commun (Camb) 2016;52:4702–4705. doi: 10.1039/c5cc10445a. [DOI] [PubMed] [Google Scholar]
- 37.Ashburner M, et al. The Gene Ontology Consortium Gene ontology: Tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jafari R, et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 2014;9:2100–2122. doi: 10.1038/nprot.2014.138. [DOI] [PubMed] [Google Scholar]
- 39.Inloes JM, et al. The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc Natl Acad Sci USA. 2014;111:14924–14929. doi: 10.1073/pnas.1413706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das R, Baker D. Macromolecular modeling with rosetta. Annu Rev Biochem. 2008;77:363–382. doi: 10.1146/annurev.biochem.77.062906.171838. [DOI] [PubMed] [Google Scholar]
- 41.Cai Y, et al. Oral pharmacokinetics of baicalin, wogonoside, oroxylin A 7-O-β-d-glucuronide and their aglycones from an aqueous extract of Scutellariae Radix in the rat. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1026:124–133. doi: 10.1016/j.jchromb.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 42.Nyman LR, et al. Homozygous carnitine palmitoyltransferase 1a (liver isoform) deficiency is lethal in the mouse. Mol Genet Metab. 2005;86:179–187. doi: 10.1016/j.ymgme.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Barroso E, et al. The PPARβ/δ activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1α-Lipin 1-PPARα pathway leading to increased fatty acid oxidation. Endocrinology. 2011;152:1848–1859. doi: 10.1210/en.2010-1468. [DOI] [PubMed] [Google Scholar]
- 44.Harwood HJ, Jr, et al. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. J Biol Chem. 2003;278:37099–37111. doi: 10.1074/jbc.M304481200. [DOI] [PubMed] [Google Scholar]
- 45.Stefanovic-Racic M, et al. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab. 2008;294:E969–E977. doi: 10.1152/ajpendo.00497.2007. [DOI] [PubMed] [Google Scholar]
- 46.Tsou LK, et al. Antibacterial flavonoids from medicinal plants covalently inactivate type III protein secretion substrates. J Am Chem Soc. 2016;138:2209–2218. doi: 10.1021/jacs.5b11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang P, et al. Effect of baicalin on GLUT4 expression and glucose uptake in myotubes of rats. Life Sci. 2018;196:156–161. doi: 10.1016/j.lfs.2018.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.