Significance
Competition for mates is often intense. The resulting selection can have pervasive effects across the genome, potentially affecting components of nonsexual fitness. There is controversy over whether these effects on nonsexual fitness occur and, if so, their direction. Past studies have yielded variable results but without providing insight into why outcomes vary. Here, we show that when mate competition occurs in an environment in which male harassment is weak, there are substantial benefits in terms of the rate of adaptation to novel larval conditions as well as the purging of inbreeding depression. In contrast, these benefits are absent when mate competition occurs in an environment in which male harassment is strong.
Keywords: adaptation, purging, sexual conflict, sexual selection, inbreeding depression
Abstract
Competition for mates can be a major source of selection, not just on secondary sexual traits but across the genome. Mate competition strengthens selection on males via sexual selection, which typically favors healthy, vigorous individuals and, thus, all genetic variants that increase overall quality. However, recent studies suggest another major effect of mate competition that could influence genome-wide selection: Sexual harassment by males can drastically weaken selection on quality in females. Because of these conflicting effects, the net effect of mate competition is uncertain, although perhaps not entirely unpredictable. We propose that the environment in which mate competition occurs mediates the importance of sexual selection relative to sexual conflict and, hence, the net effect of mate competition on nonsexual fitness. To test this, we performed experimental evolution with 63 fruit fly populations adapting to novel larval conditions where each population was maintained with or without mate competition. In half the populations with mate competition, adults interacted in simple, high-density environments. In the remainder, adults interacted in more spatially complex environments in which male-induced harm is reduced. Populations evolving with mate competition in the complex environment adapted faster to novel larval environments than did populations evolving without mate competition or with mate competition in the simple environment. Moreover, mate competition in the complex environment caused a substantial reduction in inbreeding depression for egg-to-adult viability relative to the other two mating treatments. These results demonstrate that the mating environment has a substantial and predictable effect on nonsexual fitness through adaptation and purging.
Darwin (1) noted that mate competition can generate strong selection on males such that “… sexual selection will give its aid to ordinary selection, by assuring to the most vigorous and best adapted males the greatest number of offspring.” Most genes are likely to affect male reproductive success because a male must be healthy to succeed in the competition for fertilizations and most genes have some effect on the overall health of an individual (2, 3). This leads to a general expectation that mate competition will be an important source of selection genome-wide and will typically reinforce natural selection on most alleles. Sexually antagonistic alleles (4) are an important exception, but these appear to be rare among new mutations. Experiments show that most new mutations are selected in the same direction in the two sexes and that selection is typically stronger through males, likely because of sexual selection (5–7). However, mate competition can do more than create sexual selection on males; it can also substantially alter natural selection on females.
In the last 25 years, it has become clear that, in many taxa, sexual conflict over mating interactions is at least as important as traditional forms of sexual selection (8). As a by-product of sexual selection to outcompete their rivals, traits can evolve in males that inadvertently harm females, but not all females may be equally affected. A recent study with Drosophila showed that males, when given the choice, preferentially direct their sexual attention, and thus harm, toward the best (most fecund) females (9). The intrinsic fitness difference between high- and low-quality females can be dramatically reduced or eliminated because of the attention shifted away from the latter and directed toward former. Thus, biased male harm can weaken natural selection through females, possibly genome-wide. Two recent evolution experiments implicated this as the reason that evolutionary responses were not more adaptive in populations with mate competition than those without (10, 11).
Competition for mates allows for sexual selection, which strengthens selection through males, as well as interlocus sexual conflict, which may weaken selection through females. Each of these effects can vary in strength. Under some environmental conditions, females experience substantial harassment and the effects of harm can thus be large, providing scope for major changes to selection on females via biased male harm. Similarly, some types of environments may impose a stronger selective sieve on the genetic quality of males that achieve the greatest mating success. The physical environment is therefore likely to play a key role in mediating the relative importance of sexual selection and sexual conflict and, thus, whether mate competition helps or hinders the improvement of nonsexual fitness.
In the last few decades, laboratory experiments with insects have strongly shaped views on the evolutionary consequences of mate competition (12–18); work with Drosophila has been particularly influential, highlighting a major role for sexual conflict (19–21). Results from these studies are mixed; in several cases mate competition did not improve nonsexual fitness (10, 22–24). Little attention has been given to understanding this variation in outcomes. The vast majority of this work with Drosophila has been performed with mate competition occurring under “standard laboratory conditions,” which involve small, high-density containers with low spatial complexity. While this may be representative of some natural settings, other environments will have lower density and greater spatial structure. Such environmental differences are important because they can alter both sexual selection and sexual conflict (25). In larger, more spatially complex environments, it is more challenging for males to find mates, so sexual selection operates more strongly on many mutations (26). Further, in larger, more complex environments, females can evade males, reducing harm overall as well as the opportunity for males to bias harm (27). For these reasons, we predict that the benefits of mate competition on the genetic improvement of nonsexual fitness are likely to be more evident if mate competition occurs in a larger, more complex environment than in a small simple environment.
To test this, we compare evolution in populations with enforced monogamy (“mate competition absent,” MCabsent) to two versions of evolution with mate competition occurring in either spatially simple or complex environments (“mate competition simple” and “complex,” MCsimple and MCcomplex, respectively). Mate competition occurs in a standard high-density fly vial in the MCsimple treatment, but in a larger cage that includes multiple food patches and additional spatial structure in the MCcomplex treatment (27). While there may be small differences in abiotic selection due to the physical differences between the MCsimple and MCcomlex mating environments, we know from a previous study that male–female interactions differ between these specific environments in a manner that alters biotic selection, generating a strong a priori expectation. In particular, in the complex environment, harassment rates are lower and less biased, female fitness is less affected by males, and the opportunity for male choice (i.e., biased harm) no longer weakens selection in females (27). In the current study, we have 63 evolving fly populations, divided equally into three “larval adaptation” sets. Each set was maintained in a different but novel larval rearing environment to which it could adapt [set 1 cornstarch-based larval medium (rather than cornmeal) and a 2- to 4-h heat shock (37 °C) to 3-d-old larvae); set 2: elevated ethanol in the larval medium with a 2- to 4-h cold shock (4 °C) to 3-d-old larvae; set 3: elevated salinity of larval medium with constantly elevated rearing temperature (28 °C)]. Within each larval adaptation set, the 21 populations were divided evenly among the three mating treatments (MCabsent, MCsimple, MCcomplex) that differed in the mating environment, while all populations within a set experienced similar larval rearing conditions (Materials and Methods). Our goal is to test how mate competition, and the environment in which it occurs, affects the genetic improvement of nonsexual fitness. To do so, we assess the effects of mating treatment both with respect to adaptation and purging. By having independent sets of populations adapting to three different novel larval environments, we gain insight as to whether any observed effect of mating treatment is specific to adaptation to one particular environment or represents a more general result.
Results and Discussion
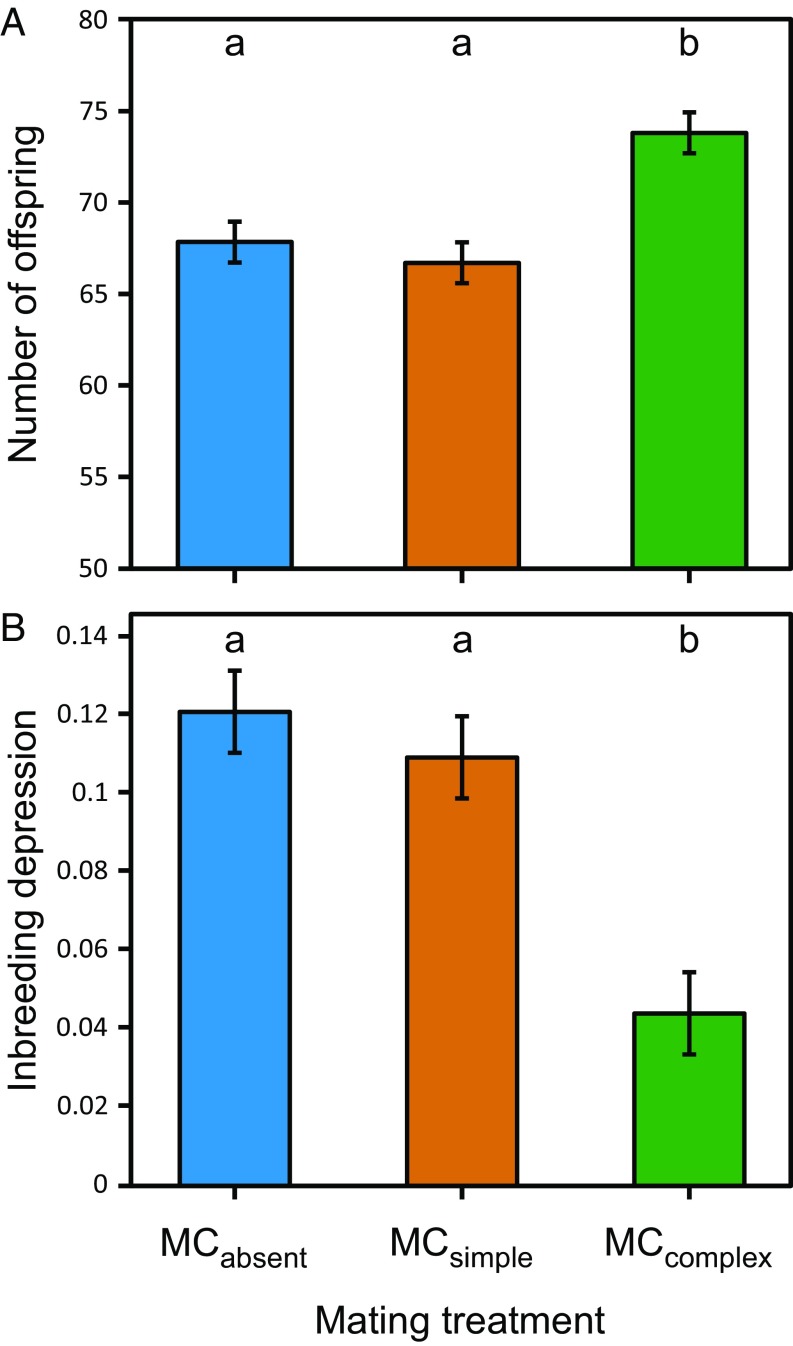
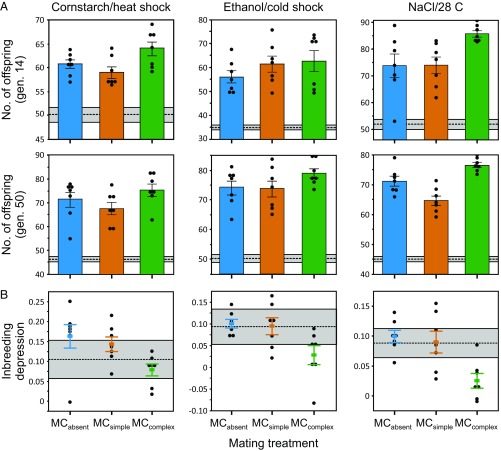
Within each larval adaptation set, we examine adaptation by measuring egg-to-adult survivorship in the appropriate novel larval environment at generations 14 and 50. Egg-to-adult viability is a major component of nonsexual fitness that is both directly affected by the novel larval environment and is fully comparable across mating treatments. In all three sets, there is clear evidence of adaptation: When tested in their respective novel larval environments, populations from all three mating treatments have substantially higher viability than the ancestral population (i.e., average survivorship in each treatment is greater than the ancestor by at least 1.40-fold in generation 14 and 1.46-fold in generation 50). However, our key interest concerns variation among the mating treatments in the degree of adaptation. Analyzing across generations and adaptation sets, there is significant variation among mating treatments (F2,108 = 13.69, P < 0.0001; SI Appendix, Table S1); adaptation in the treatment where mate competition occurs in the complex environment is greater than the other two treatments, which do not differ significantly from one another (MCcomplex > MCabsent, MCsimple; Fig. 1A). This pattern is present when examined separately by adaptation set and generation; in all cases, the point estimate of mean viability in MCcomplex is higher than the other two treatments, although the effect is small in some cases (Fig. 2A). Averaging across adaptation sets, the amount of adaptation at generation 14 (measured as viability of evolved populations − viability of ancestor) is 1.40-fold greater in MCcomplex than MCabsent but only 1.07-fold greater in MCsimple than MCabsent. At generation 50, adaptation is 1.19-fold greater in MCcomplex than MCabsent, whereas adaptation in MCsimple is 0.89-fold (i.e., less than) that of MCabsent.
Fig. 1.
Quantifying adaptation (A) and inbreeding depression (B) in the 63 replicate experimental populations from all three adaptation sets evolving under the three different mating treatments. Values are least square means (±1 SE) for the different mating treatments, treating populations as replicates. Adaptation was measured as egg-to-adult survivorship (quantified as the number of focal adult offspring emerging from a vial) in two competitive assays in the appropriate novel larval environment during generations 14 and 50. Inbreeding depression for egg-to-adult survivorship in the ancestral larval environment was calculated as 1 − (Winbred/Woutbred), where W is the average number of emerged focal flies (inbred or outbred) across the replicate measures for a single population). Treatments with different letters indicate values that are significantly different at α < 0.05 using Tukey post hoc test.
Fig. 2.
Quantifying adaptation (A) and inbreeding depression (B) in the 63 replicate experimental populations evolving under the three different mating treatments separately by adaptation set (columns) and generation (rows; adaptation only). Adaptation and inbreeding depression were quantified as in Fig. 1. Filled circles indicate values for individual populations; means (±1 SE) are also shown for the different mating treatments. Horizontal dashed lines indicate the value for the ancestral population in each assay, with the gray shading indicating ±1 SE.
Although alleles with beneficial effects specific to a given larval rearing environment likely underlie much of the observed adaptation, fitness in all populations will also be affected by the presence of unconditionally deleterious alleles segregating at mutation-selection-drift equilibrium. When selection is stronger, these deleterious alleles should be rarer. In an experiment such as ours, it is extremely difficult to identify which genetic variants have detectable deleterious effects. However, useful inferences about the genetic load of deleterious mutations can be made from studying inbreeding depression (28). In comparison with a population that experiences a smaller magnitude of inbreeding depression, a population with greater inbreeding depression harbors a higher frequency of deleterious alleles, presumably due to weaker selection. We measured inbreeding depression for egg-to-adult survivorship in the ancestral larval environment, so these measures of inbreeding depression likely reflect the effects of unconditionally deleterious alleles. We find significant variation among the mating treatments in inbreeding depression (F2,54 = 15.64, P < 0.0001; SI Appendix, Table S2). In all three larval adaptation sets, inbreeding depression in the MCabsent and MCsimple treatments are similar, while that in the MCcomplex treatment is significantly lower (Fig. 1B). This pattern is also present when examined separately by adaptation set (Fig. 2B). Averaging across larval adaptation sets, inbreeding depression is 36% as large in MCcomplex as in MCabsent, whereas it is 90% as large in MCsimple as in MCabsent. Low inbreeding depression can occur either because both outbred and inbred offspring are reasonably fit or because both offspring types are unfit. In all three larval adaptation sets, the MCcomplex populations have low inbreeding depression because both outbred and inbred offspring are fit (SI Appendix, Fig. S1). This suggests that purging of deleterious alleles has been more efficient in the MCcomplex treatment than in the other mating treatments.
Several recent studies have reported that mate competition hinders the improvement of nonsexual fitness. We replicate these results with respect to adaptation to three distinct novel larval environments, as well as purging, for populations evolving in simple mating environments. Crucially, we also show that this result changes dramatically and predictably in a slightly more complex—and arguably more realistic—mating environment, with mate competition now promoting more rapid adaptation and more efficient purging. Such environmental effects on the relative importance of sexual conflict and sexual selection are consistent with predictions from mechanistic studies in Drosophila melanogaster (26, 27). Our results are supported by experiments with other fly populations examining the purging of large-effect marker mutations (29, 30) in similar mating environments.
The idea that genome-wide consequences of mate competition can vary predictably with environment is appealing and may be general. Sexual conflict, for instance, has been shown in a variety of taxa to vary with population density, sex ratio, the availability of refuges, resource levels, predation risk, and other factors (31–34), which, in turn, are likely to vary with the environment. Likewise, the intensity of sexual selection on traits likely reflecting male quality has been shown to vary with the environment (35–37). However, environment may have little effect in some systems. For example, in some taxa, females have more control over sexual interactions and, thus, are unlikely to suffer male harm (38) regardless of environmental conditions. Nonetheless, our work demonstrates that the environment can play a key, and even predictable, role in determining the effect of mate competition on nonsexual fitness in some systems and points to the need for caution in interpreting results from simple laboratory environments.
Materials and Methods
Creation and Maintenance of Experimental Populations.
The source for our experimental populations were ∼2,000 D. melanogaster collected in the Similkameen Valley, British Columbia, in 2005. This population was originally maintained in a constant environment (cornmeal-yeast media, 25 °C, 12 h light:12 h dark photoperiod, 50% relative humidity) at 2,000–4,000 adults per generation. For at least 4 years before the start of this experiment, stock maintenance has involved discrete, nonoverlapping 2-wk-long generations. Throughout the evolution experiment described below, a copy of the stock population (∼2,000 flies) was maintained under its original conditions (i.e., 2-wk generation time on cornmeal-yeast–based larval food, maintained at 25 °C in bottles). We refer to this copy as the “ancestor.”
In September 2014, separate samples of flies from the stock population were collected to form replicate experimental populations. Specifically, 21 independent populations per week over 3 wk (63 populations in total) were established; 140 males and 140 females were sampled to found each population. As described below, experimental populations were maintained on a 3-wk life cycle. All 21 populations from each establishment week were assigned to the same “larval adaptation” set (i.e., novel larval rearing conditions). Corresponding to each of the 3 wk of population establishment, there were larval adaptation sets involving: (i) larval medium based on cornstarch (rather than cornmeal) and a 2-h heat shock (37 °C) to 3-d-old larva; (ii); standard cornmeal larval medium supplemented with 10% ethanol and a 2-h cold shock (4 °C) to 3-d-old larva; and (iii) standard cornmeal larval medium supplemented with 5% salt and constant exposure to 28 °C (rather than the standard 25 °C). To ensure continuing directional selection on these populations, we increased the salt concentration to 6% (set III) and the duration of heat (set I) and cold shock (set II) to 4 h after the sixth generation.
Within each larval adaptation set, the replicate populations were divided equally among three different mating treatments: mate competition absent (MCabsent), mate competition simple (MCsimple), and mate competition complex (MCcomplex). The MCabsent treatment involved keeping flies each generation with enforced monogamy, achieved by creating randomly chosen pairs (140 male/female pairings per population), each held in wide straws (radius = 6.35 mm; height = 88.9 mm) that had been inserted into a 3-oz Dixie cup filled with 25 mL of ancestral food (28 straws per cup). Straws were used to minimize the space requirements and maintenance costs associated with this treatment given the scale of the experiment. The food surface within each straw was supplemented with one to two yeast pellets. For the MCsimple treatment, 35 males and 35 females were placed in a standard fly vial (28.5 mm × 95 mm Drosophila culture vial filled with 10 mL of ancestral food supplemented with 30–50 dry yeast pellets). Four such vials were created for each population (totaling 140 adults of each sex) each generation. For the MCcomlex treatment, 35 males and 35 females were placed in a 1.65-L cylindrical plastic Ziploc food storage container. Four such containers, hereafter “cages,” were created for each population (totaling 140 adults of each sex) each generation. Within each cage, there were five separate food sources (three 3-oz Dixie cups containing 25 mL of ancestral media, each divided into two by a plastic divider inserted into the food, and two smaller 1-oz cups containing 7.5 mL of ancestral media) and pipe cleaners protruding from the lid into the interior. The food surface in each 3-oz Dixie cup was supplemented with 10–15 yeast pellets, and 5–10 yeast pellets were placed onto the food surface in each 1-oz cup. This cage design was chosen to reduce density and increase spatial complexity, allowing flies to be out of sight from one another and providing multiple food patches so that all females are not forced to a single locale to feed and lay eggs. The cages are pictured in figure S1 of Yun et al. (27). We have previously used these types of mating “arenas” (i.e., cages and vials) in more mechanistic studies of behavior and selection (27). For simplicity in the main text, we refer to vials and cages as “simple” and “complex” environments, respectively; however, we note that, in addition to spatial complexity, there are additional differences including fly density and the availability of food and laying sites.
In all mating treatments, adult flies were held in their respective mating arena for a total of 6 d to feed, interact, and mate. Because females lay eggs in the food during this time, developing larvae may alter adult use of the food and mating interactions, and/or simply cause the food to become “soupy” and hazardous for adults. To minimize this issue, adult flies were transferred via light CO2 anesthesia to fresh mating arenas (i.e., straw, vial, or cage) supplied with new food on the third day. After 6 d (day 20 of the maintenance protocol), we anesthetized flies in the arenas and randomly sampled 105 females from each population for egg laying. These females were evenly distributed into seven standard fly vials with three to five yeast pellets to lay eggs for 24 ± 3 h; the vials contained the appropriate novel abiotic larval medium (i.e., from set I, II, or III). After the egg-laying period, adult females were discarded. Vials were visually inspected and egg density was lowered to ∼200 eggs by scraping to reduce variation in egg densities among vials. Eleven days later, we collected the adult offspring into holding vials separated by sex (35 flies per vial) and stored them for 3 d (for logistical reasons to give a 21-d generation time) before repeating the above mating protocols for the next generation. Note that populations in the same larval adaptation set but in different mating treatments were maintained in the same manner at all stages except during the “mating phase” (days 14–19) of the life cycle. Each nonoverlapping generation lasted 3 wk (day 0/21, egg-laying mothers removed from larval vials; day 11, adults collected from larval vials and placed in holding vials; day 14, adults placed in mating environments; day 17, adults transferred to mating environments with fresh media; day 20, females collected from mating environments and placed in egg-laying vials). As noted above, larval adaptation sets I–III (each consisting of 21 populations) were offset from one another by 1 wk each.
In March 2015, an autosomal DsRed marker (39) was introgressed into a sample of the ancestral population by mating 100 DsRed males to 100 virgin ancestral females and backcrossing the F1 offspring to DsRed males once more. The DsRed marker is a dominant mutation that causes all bearers to emit red light under a fluorescent microscope. Following the backcross, 100 families were created, each consisting of a virgin red female and male. These families were inbred for at least four generations and families that produced any individuals of wild-type (i.e., nonred) phenotype during this time were discarded; those that did not produce any individuals of wild-type phenotype (around 30 families) were pooled to create a population in which the DsRed marker was likely fixed. The population was maintained on a 2-wk cycle in cornmeal-yeast media, matching the ancestral population. These marked flies served as competitors for the first viability adaptation assay (generation 14) described below.
In November 2016, another competitor population was created by crossing the brown eye recessive (bw) marker into the ancestral genetic background. We first sampled 100 bw/bw males and 100 ancestral virgin females and mated them in 10 vials containing ancestral food media. The virgin female offspring of these flies were then backcrossed with ancestral males. We allowed the offspring of the resulting backcross to mate with each other for one generation to obtain homozygous recessive bw flies. This population was maintained on a 2-wk cycle in cornmeal-yeast media; 25 °C; 12L:12D photoperiod; 50% RH, matching the ancestral stock. This competitor was used in the second viability adaptation assay (generation 50) and the inbreeding depression assay.
Adaptation Assays.
At generation 14, we measured egg-to-adult viability of each population in the appropriate novel larval environment and in competition with the DsRed-marked ancestral population. We conducted this assay separately in consecutive weeks for each larval adaptation set (I–III above). On day 11 of the maintenance protocol, we sampled 300 male and 300 female adults from each population, placing sets of 10 adults of each sex into holding vials containing the ancestral media for 1 d. These flies were subsequently transferred into a new set of vials to oviposit for 1 d. Each vial contained the appropriate novel larval medium corresponding to their adaptation set (with three to five pellets of live yeast sprinkled on top) and were held under the appropriate novel temperature regime. Eleven days later, we released all emerged flies into a large (24 × 24 × 35 cm) plexiglass cage (one cage per population; ∼5,000 flies per cage). Within each cage, six Petri dishes (60 mm × 15 mm) filled with the appropriate novel abiotic media and covered in yeast paste were placed for flies to lay eggs for 15 h. An egg harvesting and pipetting protocol (40) was then used to collect and allocate ∼100 focal eggs and ∼100 DsRed competitor eggs together into replicate vials filled with the appropriate novel food. An average of 9.97 replicates per experimental population (range 9–10) was set up and the appropriate novel temperature regime was applied to each vial. After 11 d, emerging focal (wild-type) and DsRed flies were counted. We included the DsRed flies to help homogenize the competitive environment across populations, although our interest is in the survival of the focal flies. In addition, we performed the same protocol for the ancestral population (i.e., unmarked ancestor larvae against DsRed competitors); 30 replicate vials were set up at the same time as each of the three separate larval adaptation sets.
The egg-to-adult viability assay was repeated in generation 50. The protocol was the same except that we set up an average of 19.98 (range 19–21) replicates per experimental population, the competitor for this assay was the bw/bw population described above, and narrow glass vials were used instead of wide plastic vials. The ancestral population was again tested using the same protocol, and an average of 26 (range 20–30) replicates were set up at the same time as each of the three larval adaptation sets. Combining data across the two assays (i.e., generations), variation in egg-to-adult viability was analyzed using a general linear model with the average number of emerging focal (i.e., wild-type) offspring of each experimental population in a given generation as the dependent variable and adaptation set (I, II, III), mating treatment (MCabsent, MCsimple, MCcomplex), generation, and their interactions (two- and three-way) as independent variables. Results were qualitatively unchanged if significance was determined via a nonparametric permutation procedure.
Inbreeding Depression Assay.
We created replicate inbred and outbred mating pairs from each population and measured the average fitness differences of their offspring with respect to egg-to-adult viability within each population. For each larval adaptation set (I–III), we calculated the average inbreeding depression for each of the three mating treatments as the mean inbreeding depression of the seven replicate populations.
For each population, we set up 30 inbred families by forming 30 male-female virgin pairs from newly emerged adults (10–12 h after eclosion) and placed each into a separate vial containing the ancestral (cornmeal-yeast) media with three to five yeast pellets. The pairs were allowed to mate and oviposit for 72 h. After 11 d, we collected single virgin male and female (full-sib) offspring from each family and placed them into a fresh vial to mate and lay eggs for 24 h. This procedure was repeated for one more generation (for families that had viable progeny from the previous inbreeding generation) to further increase the average inbreeding. The procedure was conducted separately for each larval adaptation set (i.e., 21 populations). The families used for this assay came from generations 38, 39, and 41 for larval adaptation sets I, II, and III, respectively. For each larval adaptation set, we also attempted to create 40 inbred families from the ancestral population.
After two generations of inbreeding within families, we collected 10 flies of each sex from each family on days 11–12 and placed them in single-sex holding vials. After a 3-d holding period, we generated inbred eggs by sampling five males and five females from the same family and put them into a laying vial. Simultaneously, we generated outbred eggs by placing the remaining five females into another laying vial with five males from a separate family in a round-robin design. Each laying vial consisted of the ancestral food media supplemented with blue dye (the dye aids in counting eggs) and three to five yeast pellets. Flies were cleared after 8 h, and immediately after, we scraped the food surface to ensure all laying vials contained ∼50 eggs. To standardize resource competition among all of the laying vials, we used the egg harvesting and pipetting technique (40) from the adaptation assay to allocate ∼100 competitor bw eggs to each of the vials.
We counted and phenotyped the number of adults 12 and 15 d after the laying-vial setup; the counts across the 2 d were summed. Both inbred and outbred eggs produce adults with wild-type eye color, whereas the competitors have brown eyes. An average (range) of 23.5 (16–29) inbred replicates (i.e., vials) were obtained for each experimental population and 23.3 (15–29) outbred replicates. For the ancestor, we obtained 30 replicate inbred and 30 replicate outbred vials for all three larval adaptation sets with the single exception of 28 replicate inbred vials in set I (cornstarch/heat shock).
We calculated inbreeding depression for each population as 1 − (Winbred/Woutbred), where W (absolute fitness) is the average number of emerged focal flies (inbred or outbred) across the replicate measures for a single population. SEs for the inbreeding depression of experimental populations (i.e., Figs. 1 and 2) are parametric based on among-population variation. The ancestral population is unreplicated at the population level however, so the SE of its inbreeding depression (Fig. 2) was estimated via a bootstrap procedure with 10,000 bootstrap samples of the vial replicates. In each bootstrap sample, replicate inbred and outbred vials were separately sampled with replacement and then Winbred, Woutbred, and inbreeding depression, were then calculated. Variation in inbreeding depression was analyzed using a general linear model with the inbreeding depression of each experimental population as the dependent variable and adaptation set (I, II, III), mating treatment (MCabsent, MCsimple, MCcomplex), and their interaction as independent variables. Results were qualitatively unchanged if significance was determined via a nonparametric permutation procedure.
Data Availability.
Data will be made available on Dryad following publication.
Supplementary Material
Acknowledgments
We thank Malak Bayoumi and Seon Yang for help in the laboratory. Funding was provided by grants from the Natural Sciences and Engineering Research Council of Canada (to A.F.A. and H.D.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data have been deposited in the Dryad Repository (doi: 10.5061/dryad.837d96j).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805435115/-/DCSupplemental.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. John Murray; London: 1859. [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc B. 1996;263:1415–1421. [Google Scholar]
- 3.Whitlock MC, Agrawal AF. Purging the genome with sexual selection: Reducing mutation load through selection on males. Evolution. 2009;63:569–582. doi: 10.1111/j.1558-5646.2008.00558.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonduriansky R, Chenoweth SF. Intralocus sexual conflict. Trends Ecol Evol. 2009;24:280–288. doi: 10.1016/j.tree.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Mallet MA, Bouchard JM, Kimber CM, Chippindale AK. Experimental mutation-accumulation on the X chromosome of Drosophila melanogaster reveals stronger selection on males than females. BMC Evol Biol. 2011;11:156. doi: 10.1186/1471-2148-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharp NP, Agrawal AF. Male-biased fitness effects of spontaneous mutations in Drosophila melanogaster. Evolution. 2013;67:1189–1195. doi: 10.1111/j.1558-5646.2012.01834.x. [DOI] [PubMed] [Google Scholar]
- 7.Grieshop K, Stångberg J, Martinossi-Allibert I, Arnqvist G, Berger D. Strong sexual selection in males against a mutation load that reduces offspring production in seed beetles. J Evol Biol. 2016;29:1201–1210. doi: 10.1111/jeb.12862. [DOI] [PubMed] [Google Scholar]
- 8.Arnqvist G, Rowe L. Sexual Conflict. Princeton Univ Press; Princeton: 2005. p. 330. [Google Scholar]
- 9.Long TA, Pischedda A, Stewart AD, Rice WR. A cost of sexual attractiveness to high-fitness females. PLoS Biol. 2009;7:e1000254. doi: 10.1371/journal.pbio.1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbuthnott D, Rundle HD. Sexual selection is ineffectual or inhibits the purging of deleterious mutations in Drosophila melanogaster. Evolution. 2012;66:2127–2137. doi: 10.1111/j.1558-5646.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- 11.Chenoweth SF, Appleton NC, Allen SL, Rundle HD. Genomic evidence that sexual selection impedes adaptation to a novel environment. Curr Biol. 2015;25:1860–1866. doi: 10.1016/j.cub.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Lumley AJ, et al. Sexual selection protects against extinction. Nature. 2015;522:470–473. doi: 10.1038/nature14419. [DOI] [PubMed] [Google Scholar]
- 13.Radwan J. Effectiveness of sexual selection in removing mutations induced with ionizing radiation. Ecol Lett. 2004;7:1149–1154. [Google Scholar]
- 14.Radwan J, Unrug J, Snigórska K, Gawrońska K. Effectiveness of sexual selection in preventing fitness deterioration in bulb mite populations under relaxed natural selection. J Evol Biol. 2004;17:94–99. doi: 10.1046/j.1420-9101.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 15.Fricke C, Arnqvist G. Rapid adaptation to a novel host in a seed beetle (Callosobruchus maculatus): The role of sexual selection. Evolution. 2007;61:440–454. doi: 10.1111/j.1558-5646.2007.00038.x. [DOI] [PubMed] [Google Scholar]
- 16.Power DJ, Holman L. Assessing the alignment of sexual and natural selection using radiomutagenized seed beetles. J Evol Biol. 2015;28:1039–1048. doi: 10.1111/jeb.12625. [DOI] [PubMed] [Google Scholar]
- 17.Almbro M, Simmons LW. Sexual selection can remove an experimentally induced mutation load. Evolution. 2014;68:295–300. doi: 10.1111/evo.12238. [DOI] [PubMed] [Google Scholar]
- 18.Jarzebowska M, Radwan J. Sexual selection counteracts extinction of small populations of the bulb mites. Evolution. 2010;64:1283–1289. doi: 10.1111/j.1558-5646.2009.00905.x. [DOI] [PubMed] [Google Scholar]
- 19.Rice WR. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 20.Holland B, Rice WR. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc Natl Acad Sci USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler K, Partridge L. A cost of mating in female fruit-flies. Nature. 1989;338:760–761. [Google Scholar]
- 22.Rundle HD, Chenoweth SF, Blows MW. The roles of natural and sexual selection during adaptation to a novel environment. Evolution. 2006;60:2218–2225. [PubMed] [Google Scholar]
- 23.Hollis B, Houle D. Populations with elevated mutation load do not benefit from the operation of sexual selection. J Evol Biol. 2011;24:1918–1926. doi: 10.1111/j.1420-9101.2011.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland B. Sexual selection fails to promote adaptation to a new environment. Evolution. 2002;56:721–730. doi: 10.1111/j.0014-3820.2002.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 25.Cordero C, Eberhard WG. Female choice of sexually antagonistic male adaptations: A critical review of some current research. J Evol Biol. 2003;16:1–6. doi: 10.1046/j.1420-9101.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 26.Maclellan K, Whitlock MC, Rundle HD. Sexual selection against deleterious mutations via variable male search success. Biol Lett. 2009;5:795–797. doi: 10.1098/rsbl.2009.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun L, Chen PJ, Singh A, Agrawal AF, Rundle HD. The physical environment mediates male harm and its effect on selection in females. Proc R Soc B. 2017;284:20170424. doi: 10.1098/rspb.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sinauer Associates; Sunderland, MA: 1998. p. 908. [Google Scholar]
- 29.Singh A, Agrawal AF, Rundle HD. Environmental complexity and the purging of deleterious alleles. Evolution. 2017;71:2714–2720. doi: 10.1111/evo.13334. [DOI] [PubMed] [Google Scholar]
- 30.Colpitts J, Williscroft D, Sekhon HS, Rundle HD. The purging of deleterious mutations in simple and complex mating environments. Biol Lett. 2017;13:20170518. doi: 10.1098/rsbl.2017.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karlsson K, Eroukhmanoff F, Härdling R, Svensson EI. Parallel divergence in mate guarding behaviour following colonization of a novel habitat. J Evol Biol. 2010;23:2540–2549. doi: 10.1111/j.1420-9101.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- 32.Magurran AE, Seghers BH. Sexual conflict as a consequence of ecology: Evidence from guppy, Poecilia reticulata, populations in Trinidad. Proc R Soc B. 1994;255:31–36. [Google Scholar]
- 33.Rowe L, Arnqvist G, Sih A, J Krupa J. Sexual conflict and the evolutionary ecology of mating patterns: Water striders as a model system. Trends Ecol Evol. 1994;9:289–293. doi: 10.1016/0169-5347(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 34.Gosden TP, Svensson EI. Density-dependent male mating harassment, female resistance, and male mimicry. Am Nat. 2009;173:709–721. doi: 10.1086/598491. [DOI] [PubMed] [Google Scholar]
- 35.Miller CW, Svensson EI. Sexual selection in complex environments. Annu Rev Entomol. 2014;59:427–445. doi: 10.1146/annurev-ento-011613-162044. [DOI] [PubMed] [Google Scholar]
- 36.Cockburn A, Osmond HL, Double MC. Swingin’ in the rain: Condition dependence and sexual selection in a capricious world. Proc R Soc B. 2008;275:605–612. doi: 10.1098/rspb.2007.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myhre LC, Forsgren E, Amundsen T. Effects of habitat complexity on mating behavior and mating success in a marine fish. Behav Ecol. 2013;24:553–563. [Google Scholar]
- 38.Zuk M, Garcia-Gonzalez F, Herberstein ME, Simmons LW. Model systems, taxonomic bias, and sexual selection: Beyond Drosophila. Annu Rev Entomol. 2014;59:321–338. doi: 10.1146/annurev-ento-011613-162014. [DOI] [PubMed] [Google Scholar]
- 39.Zikovitz AE, Agrawal AF. The condition dependency of fitness in males and females: The fitness consequences of juvenile diet assessed in environments differing in key adult resources. Evolution. 2013;67:2849–2860. doi: 10.1111/evo.12170. [DOI] [PubMed] [Google Scholar]
- 40.Yun L, Agrawal AF. Variation in the strength of inbreeding depression across environments: Effects of stress and density dependence. Evolution. 2014;68:3599–3606. doi: 10.1111/evo.12527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on Dryad following publication.