Abstract
Immunohistochemistry (IHC) is an important diagnostic tool in histopathology. Dermatopathology is a rapidly developing subspecialty of histopathology. Although IHC is not widely used in routine dermatopathology practice, its application is gradually increasing. IHC is used to differentiate two conditions with similar morphology, to confirm a diagnosis as well as to assess prognosis. It is more commonly used for neoplastic conditions like melanocytic, hematolymphoid, and spindle cell tumors, although uses can be very wide. Although IHC can aid in diagnosis, sometimes interpretation can be difficult as there may be overlapping findings. Thus, IHC should not be interpreted in isolation and should be done in the context of clinical and histological findings. In this review, we have discussed the uses of various immunohistochemical markers in dermatopathology in the light of current literature and their clinical relevance.
Keywords: Benign spindle cell tumor, immunohistochemistry, lymphoma, melanoma
Introduction
Dermatopathology is a rapidly developing subspecialty of histopathology. It deals with various benign as well as neoplastic conditions. The role of dermatopathologists is not only restricted to provide the most accurate diagnosis, but also to provide additional relevant prognostic information. There is limited role of immunohistochemistry (IHC) in routine dermatopathology practice; however, recently, there has been an increased application of IHC in this field. Although IHC is more frequently used in neoplastic conditions, it is beneficial in certain non-neoplastic conditions as well. In this review, we shall discuss in brief the technique of IHC and its various applications in dermatopathology and clinical relevance in the light of current literature. As this is a vast and rapidly expanding subject, detail discussion about all the entities is beyond the scope of this review. We shall focus on hematolymphoid neoplasms, melanocytic tumors, histiocytic lesions, mesenchymal neoplasms, adnexal tumors, cutaneous metastasis, and different infectious conditions as there is more widespread use of IHC in these fields.
IHC Technique
IHC is performed using formalin-fixed, paraffin-embedded tissue. Usually 4–5-micron thick section is obtained, preferably on a polylysine-coated slide and the section should be fixed. Then deparaffinization is done by washing the slide in xylene and then followed by decreasing concentration of ethanol (100%, then 95%, then 70%, then 50%), and finally washed in cold tap water. Deparaffinization should be adequate for good IHC results. It is followed by endogenous blocking using 0.5% hydrogen peroxide in methanol. Then antigen retrieval is done. There are many techniques for antigen retrieval, however, the most commonly used methods are heat induced epitope retrieval method (using pressure cooker or microwave) and enzymatic method (usually trypsin), depending on the available facility. Either citrate buffer at pH 6 or Tris–EDTA buffer at pH 9 is used for this purpose. The choice of buffer depends on the target antigen. Following antigen retrieval, primary antibody is applied. The dilution and duration of staining depends on the antibody. Whenever a new antibody is standardized, multiple dilutions with different duration should be attempted to determine the best combination. Then the slide is washed and secondary antibody is applied. One should be careful that slides should not get dried up during any stage of staining. Incubation with primary and secondary antibodies is preferably done in a moist chamber to avoid drying. After incubation with the secondary antibody, the slide is washed and substrate is added. It is then washed, followed by nuclear staining with hematoxylin, clearing, drying, and mounting. With each slide, a positive control should be applied to ascertain that there is no false negative result. At least one positive and one negative control should be applied in one batch for individual antibody. The results should be interpreted in terms of expression (positive or negative), pattern of positivity (nuclear, cytoplasmic, or membranous), intensity (weak or strong), and extent (focal or diffuse).
Hematolymphoid Tumors
Skin is commonly affected by various hematolymphoid neoplasms. Various hematological malignancies involving skin include cutaneous T and B cell lymphomas, leukemic infiltrate, and mast cell neoplasms. There is widespread use of IHC in the diagnostic work up of cutaneous hematolymphoid neoplasms. In addition to diagnosis, IHC is also helpful in determining the prognosis of various cutaneous hematolymphoid neoplasms.
The basic panel of antibodies includes B cell markers (CD20 [membranous], CD79a [membranous]), and T cell markers (CD2, CD3, CD4, CD5, CD7 and CD8—all membranous and cytoplasmic). Among T cell markers, CD2, CD3, CD5, and CD7 are pan T cell markers, whereas CD4 and CD8 are differentially expressed by certain subsets of T lymphocytes. The basic panel of antibodies used in each case is guided by the histological findings.
Cutaneous T cell lymphoma
Cutaneous T cell lymphomas (CTCLs) are much more common than cutaneous B cell lymphomas (CBCL). In a suspected case of CTCL, multiple T cell markers should be used as loss of surface markers is well-known in T cell lymphomas. Mycosis fungoides (MF) is the commonest form of CTCL. Tumor cells of MF usually show strong CD3 positivity and loss of CD7. IHC can help to detect epidermotropism, which may be very subtle and not very evident in certain cases. Diagnosis of MF is challenging for a dermatopathologist, as it should be differentiated from its clinical and histological benign mimickers. Demonstrating T cell clonality is the best way to prove the neoplastic nature of T cells; however, this is technically difficult, expensive, and not widely available.[1] IHC may be helpful in such a situation. MF tends to lose T cell markers, most commonly CD7, followed by CD5, which may indicate the neoplastic nature of these cells [Figure 1a–d].[2] However, this is not exclusive to MF as CD7 loss has been described in many reactive dermatoses as well.[3] These cells are usually CD4+/CD8-, however, various other combinations may be observed such as CD4+/CD8+ and CD4-/CD8+.[4,5] Hypopigmented MF is a subset of MF that usually occurs in young adults. The atypical lymphoid cells in hypopigmented MF show a different immunophenotype, that is, CD4-/CD8+.[6] Thus, the combination of IHC findings should be interpreted in the context of clinical and histological findings.
Figure 1.
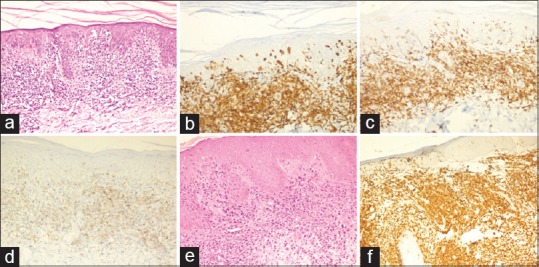
(a) Photomicrograph showing mycosis fungoides with extensive epidermotropism (HE, ×100). The atypical lymphocytes in upper dermis and within epidermis show diffuse positivity for (b) CD3 (×200) and (c) CD4 (×200). (d) These cells show loss of CD7 (×200). (e) A case of cutaneous anaplastic large cell lymphoma showing numerous large atpical lymphoid cells in the dermis (HE, ×200). (f) The tumor cells show strong and diffuse CD30 expression (×200)
CD30 positive lymphoproliferative disorders
The spectrum includes anaplastic large cell lymphoma (ALCL) and lymphomatoid papulosis (LyP).[7,8,9] ALCL is a high grade T cell lymphoma, composed of large, bizarre atypical lymphoid cells. These cells variably express pan T cell markers, although CD3 and CD5 expressions are often lost.[10,11,12] Application of multiple T cell markers (CD2, CD3, CD5, and CD7) may establish their T cell nature. However, these tumor cells express CD30 (membranous), which is the diagnostic hallmark [Figure 1e and f]. Anaplastic lymphoma kinase 1 (ALK1) is a prognostic marker in ALCL. It can show cytoplasmic or membranous and cytoplasmic positivity, depending on the ALK gene fusion partner. ALK1 expression indicates a better prognosis. Primary cutaneous ALCLs are usually ALK1 negative. ALK1 and epithelial membrane antigen (EMA) positivity in ALCL indicates secondary cutaneous involvement by a systemic ALCL.[11,12,13]
Another entity in this group is LyP. It has a different clinical profile from ALCL and shows indolent behavior. LyP shows proliferation of atypical lymphoid cells in the dermis, however, the atypical cells are smaller and fewer compared to ALCL. These cells also express CD30 in addition to pan T cell markers, however, they are essentially negative for ALK1 and EMA.[9] It should be remembered that scattered atypical CD30 positive cells may be found in many reactive dermatoses and infective conditions.[14] CD30 positivity has also been described in MF and is associated with poor outcome.[9] Thus, isolated CD30 positivity does not confirm the diagnosis of ALCL or LyP, hence, IHC should be interpreted in the light of clinical and morphological findings.
Another type of CTCL that often poses diagnostic problem is subcutaneous panniculitis like T cell lymphoma (SPTCL). It should be differentiated from other entities presenting with panniculitis. The atypical lymphoid T cells are CD8+, which show abT cell receptor rearrangement.[2] These cells show immunopositivity for perforin, granzyme B, and TIA1.[15,16] The rimming of adipocytes by CD8+ atypical lymphoid cells with presence of neutrophilic collection within histiocytes (bean bag appearance) provide the diagnostic clue [Figure 2]. However, IHC is not diagnostic for SPTCL. Other conditions presenting as panniculitis like lupus panniculitis can show overlapping immunohistochemical features. Demonstration of clonality by T cell receptor analysis using molecular techniques establishes the diagnosis.
Figure 2.
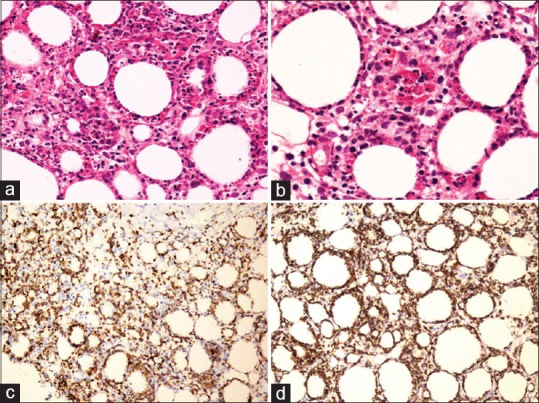
A case of subcutaneous panniculitis like T cell lymphoma. (a) Low power photomicrograph shows dense cellular infiltrate in the subcutaneous fat, composed of atypical lymphoid cells encircling the adipocytes (HE, ×200). (b) There are many apoptotic bodies and macrophages containing neutrophils and nuclear debris (HE, ×400). The atypical lymphoid cells show positivity for (c) CD3 (immunohistochemistry, ×100) and (d) CD8 (immunohistochemistry, ×100)
Cutaneous B cell lymphomas
B cell lymphomas are much less common than CTCLs. Among the B cell non-Hodgkin's lymphomas (NHL), low grade B cell lymphomas are more common than high-grade B cell NHL. CD20 and CD79a are the two most common markers used to label B cells. CD79a is expressed by both precursor as well as mature B cells, whereas CD20 is the marker of mature B cells.[17] However, certain percentage of B cell acute lymphoblastic lymphomas (ALL) and majority of plasmablastic lymphomas do not express either of these markers. PAX5, a B cell transcription factor, is expressed by B cells in all stages of maturation and is very useful to detect B cell ALL infiltrating the skin.[18] PAX5 shows nuclear staining whereas CD20 and CD79a are characterized by membranous staining pattern. Other uncommon markers such as BOB1, Oct2, and PU1 can also be used to identify B cells.
Among the low grade CBCL of the skin, follicular center lymphoma and marginal zone lymphomas are the commonest variants, whereas mantle cell lymphoma and chronic lymphocytic leukemia (CLL) affect the skin less commonly. The normal follicular center lymphoid cells are negative for Bcl-2. Presence of Bcl-2 in follicular center cells is indicative of marginal zone lymphoma.[19,20,21] Primary cutaneous follicular center lymphoma cells usually express follicular center markers such as Bcl-6 and CD10. Bcl-2 expression is rare in primary cutaneous follicular center lymphomas, its expression indicates secondary cutaneous involvement by systemic follicular lymphoma.[22,23] Cyclin D1 and SOX-11 are specific markers for mantle cell lymphoma and are characterized by nuclear positivity.[24] CLL cells show expression of CD5 and CD23 [Table 1].
Table 1.
Immunohistochemical differential diagnosis of low grade cutaneous B cell lymphoma with a follicular growth pattern

Very rarely high-grade B cell lymphomas can infiltrate the skin. The most common high-grade B cell lymphoma involving the skin is diffuse large B cell lymphoma (DLBCL). Usually DLBCL involves the skin as a part of systemic involvement. However, DLBCL can primarily involve the skin as well. DLBCL, leg type is a variant of DLBCL that primarily involves the skin of the lower extremity. Other high-grade B cell lymphomas involving the skin include plasmablastic lymphoma, Burkitt's lymphoma, and intravascular DLBCL. DLBCL usually express the pan B cell markers and BCL-2. Majority of the DLBCL involving skin show activated B cell phenotype and express MUM1 and negative for CD10 and Bcl-6, although other combinations can exist. IHC cannot differentiate between a primary cutaneous versus systemic DLBCL. Plasmablastic lymphomas are usually negative or weakly positive for CD20 and CD45, however, they usually express CD38, CD138, and MUM1. Burkitt's lymphoma also expresses pan B cell markers and CD10, but they are usually negative for Bcl-2. It is characterized by very high proliferation rate (Ki-67 labeling index is near 100%).
Leukemia cutis
Skin is commonly affected by leukemia, both of lymphoid as well as myeloid lineage. On morphological examination, they resemble other small blue round cell tumors such as high-grade NHL. IHC is essential to establish their diagnosis. The lymphoid leukemic cells express either CD3 or CD20, depending on their lineage of differentiation. Nuclear expression of terminal deoxynucleotidyl transferase (TDT) indicates their immature nature. CD34 is another marker of immaturity, although it has poor sensitivity.[25] Precursor B cell acute lymphoblastic leukemia may not express CD20, which is a mature B cell marker. Instead, these cells express PAX5, which is expressed by B cells even in their early stages of maturation. Myeloid sarcoma (MS) is defined as extramyeloid soft tissue involvement by acute myeloid leukemia (AML). MS often presents as a cutaneous nodule. It is usually detected following diagnosis of AML. However, rarely, MS can be the initial presenting feature of AML. These cases are difficult to diagnose, as there is no preceding history of AML. These tumor cells usually show variable expression of myeloperoxidase (MPO) and other myeloid markers like CD117.[26] CD68 and lysozyme are also sensitive markers to detect MS, although less specific.[26,27]
IHC for mast cells
CD117 and tryptase are common markers to detect neoplastic mast cells in cutaneous mastocytosis and other mast cell neoplasms.[28,29]
Melanocytic Tumors
There are various types of melanocytic tumors of skin, ranging from various types of benign nevus to malignant melanoma. IHC plays an important role in the management of cutaneous melanocytic tumors. In most cases, the diagnosis is straightforward. Presence of melanin pigment indicates melanocytic differentiation, and most cases do not require IHC. However, in difficult cases, IHC helps to clinch the diagnosis. Melanoma is a great mimicker and can mimic a wide variety of tumors. Pigment production can be sparse or absent in some cases, and such cases can be confused with poorly-differentiated carcinoma and lymphoma. Immunohistochemical markers for melanocytic differentiation, such as melan A, MART-1 (melanoma antigen recognized by T cell 1), HMB 45 (human melanoma black 45) (all three cytoplasmic), and S-100 (nuclear) are often positive in melanocytic tumors, whereas they are negative in epithelial or mesenchymal tumors.[30,31,32] Among these, S-100 is most sensitive and shows almost universal positivity in melanocytic tumors.[33,34] Melan A and HMB45 are more specific for melanocytic differentiation but lack sensitivity. HMB-45 has an additional benefit of differentiating benign from malignant melanocytic lesions. The deeper part of nevus shows reduced HMB-45 expression, whereas melanoma shows uniform intensity of positivity.[35] It should be remembered that some variants of malignant melanoma, such as desmoplastic melanomas, are almost always negative for Melan A and HMB45 but show S-100 expression. Thus, it is advisable to use a combination of melanocytic markers in diagnostically challenging cases. SOX-10 is a member of the Sry HMG box (Sox) family of transcription factors. It is a recently described marker for melanocytic tumors and shows strong and diffuse nuclear positivity in melanocytic tumors. It is also useful to diagnose desmoplastic melanoma and differentiate it from other spindle cell tumors and scars.[36,37] Like other melanocytic markers, this also should be combined with other markers and not used in isolation.
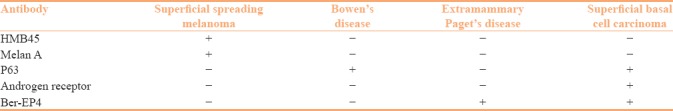
Superficial spreading melanoma is a common subtype of melanoma. It shows intraepidermal spread of tumor cells (radial growth phase) without a vertical growth phase. It should be differentiated from other intraepidermal malignancies such as Bowen's disease (BD), superficial basal cell carcinoma (BCC), extra mammary Paget's disease (EMPD), and Paget's disease of nipple. A wide panel of IHC can be helpful in such situations [Figure 3]. Superficial spreading melanoma shows positivity for Melan A, HMB 45, and S-100, whereas it is negative for pan cytokeratin, low molecular weight cytokeratin, carcinoembryonic antigen, and p63. p63 positivity is seen in BD and BCC, but it is negative in EMPD and melanoma.[38,39] Ber-EP4 is a sensitive marker for EMPD and BCC, but is not expressed in BD and superficial spreading melanoma.[40,41] Mammary Paget's disease cells express low molecular weight cytokeratin such as CK7 and Her-2, but they are negative for high molecular weight cytokeratin (HMWCK) and p63. Thus, a panel of IHC markers including Ber-EP4, p63, and melan A is useful to differentiate these conditions [Table 2].
Figure 3.
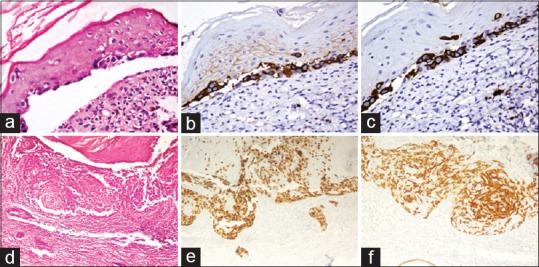
Upper panel: Paget's disease of nipple. (a) Few atypical cells seen along dermoepidermal junction (HE, ×200). These atypical cells show strong expression of (b) cytokeratin 7 (×200), and (c) Her2 neu (×200). Lower panel: superficial spreading melanoma. (d) There is spreading of atypical cells along the epidermis without vertical phase of growth (HE, ×100). (e) The atypical cells show diffuse expression of HMB45 (×100). (f) Cytokeratin 5/6 stains the keratinocytes, but the atypical cells are negative (×100)
Table 2.
Panel of immunohistochemistry used for intraepidermal neoplasms
IHC is usually not required for diagnosis of benign melanocytic nevi. The finding of pigmented nevus cells in different stages of maturation is usually diagnostic. However, some cases predominantly show spindle cell morphology, and they need to be differentiated from other benign spindle cell neoplasms. Immunohistochemical expression of melanocytic markers helps to make an accurate diagnosis. Some cases of nevoid melanomas need to be differentiated from nevi, like spitz nevus, which shows transepidermal migration and pagetoid spread. Proliferation markers may play an important role in such situations. Melanomas show much higher proliferation index compared to nevus. Although there is no accepted cutoff value, the average Ki-67 proliferation index in spitz nevus is usually <2%, whereas in melanomas the proliferation index is 10% or more.[42]
Prognostic and predictive markers in melanoma
Melanoma is characterized by activation of RAS–RAF–MAP kinase pathway. BRAF mutation is considered to be the initiating event in 50–60% of melanoma cases. Detection of BRAF mutation may be of clinical importance as currently BRAF inhibitors like vemurafenib are undergoing clinical trials for treatment of advanced stage melanoma. The commonest BRAF mutation described in melanoma is BRAF V600E. The gold standard for detecting BRAF mutation is gene sequencing. However, currently antibody (VE1 clone) is available commercially to detect this particular mutation, and shows high sensitivity and specificity.[43,44] This is extremely helpful as other point mutations in BRAF gene are exceedingly rare. Tumor infiltrating lymphocytes (TILs) play an important role in tumor immunosurveillance. T lymphocytes infiltrating the tumor can inhibit its growth. TIL response in melanoma can be of three types – nil, nonbrisk, and brisk. Programmed death ligand 1 (PD-L1) expressed by tumor cells can engage the PD receptors on T cells and inactivate them. PD1 inhibitors like pembralizumab have been introduced as a treatment option for metastatic melanoma as a part of immunotherapy. Use of this drug needs prior detection of PDL1 expression by tumor cells and TILs as it predicts treatment response.[45,46,47] IHC plays an important role in detecting PDL1 expression. The staining pattern and the diagnostic criteria are different with different clones.
Spindle Cell Neoplasms of Skin
Spindle cell neoplasms of the skin include a wide variety of tumors, ranging from benign to malignant. The common spindle cell tumors of skin are predominantly benign and include dermatofibroma, leiomyoma, benign nerve sheath tumors, dermatofibrosarcoma protuberans (DFSP), among others. Although some cases are easy to diagnose, they usually pose significant diagnostic challenge. IHC plays a central role in arriving at a correct diagnosis. The various immunohistochemical markers used to diagnose the spindle cell neoplasms of skin include vimentin (cytoplasmic), CD34 (cytoplasmic), CD68 (membranous and cytoplasmic), factor XIII (cytoplasmic), smooth muscle actin (SMA, cytoplasmic), muscle specific actin (MSA, cytoplasmic), S-100 (nuclear), etc., among others. Dermatofibroma is one of the most common benign spindle cell tumors of skin included in the group of fibrohistiocytic tumors showing classical histological appearance. Sometimes, dermatofibroma shows increased cellularity and extends into subcutaneous fat. These cases should be differentiated from DFSP, which shows intermediate malignant potential. Dermatofibroma is positive for vimentin, factor XIIIa and shows patchy CD68 expression, whereas it is negative for CD34. On the other hand, DFSP shows diffuse expression of CD34. It should be remembered that cellular dermatofibroma can show patchy CD34 positivity at the periphery of the tumor [Figure 4a–d].[48,49]
Figure 4.
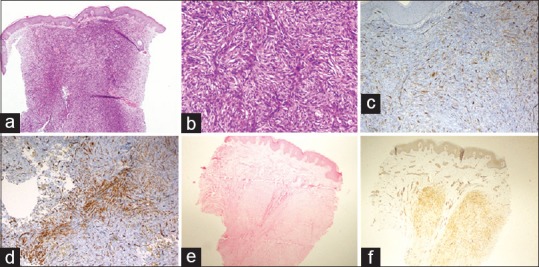
(a) A case of cellular dermatofibroma showing abutting of epidermis (HE, ×40). (b) This case shows storiform arrangement of spindle cells (HE, ×100). (c) CD34 immunostain highlights the dermal capillaries, but the tumor cells are negative (×100). (d) At the deeper portion, cellular dermatofibroma shows patchy CD34 expression (×200). (e) A case of dermal leiomyoma (HE, ×40). (f) The tumor cells show diffuse smooth muscle actin expression (×40)
Leiomyoma is another common spindle cell tumor of skin. It is composed of benign smooth muscle tumors arranged in fascicles without any significant atypia, mitosis, or necrosis. The tumor cells express SMA, MSA, desmin, and caldesmon [Figure 4e and f].[50] Benign nerve sheath tumors of the skin include schwannoma and neurofibroma. Schwannoma shows diffuse expression of S-100, whereas S-100 expression in neurofibroma is patchy. Nodular fasciitis is another commonly encountered spindle cell tumor. Although it arises from the subcutaneous tissue, it can infiltrate the dermis. The exact cell or origin of this rare tumor is not known, but it has been proposed that they arise from the fibroblasts present in subcutaneous tissue or deep fascia. In addition to proliferating fibroblasts and myofibroblasts, it can show presence of myxoid matrix, giant cells, and variable mitosis. The tumor cells show variable expression of SMA and MSA, thus can be confused with leiomyoma. However, nodular fasciitis is consistently negative for desmin and caldesmon unlike the latter. In a small biopsy, nodular fasciitis needs to be differentiated from fibromatosis. Fibromatosis, in addition to variable SMA expression, shows diffuse nuclear beta catenin positivity unlike nodular fasciitis. Spindle cell lipoma (SCL) is a benign soft tissue tumor, composed of spindle cells, admixed with adipocytes and collagen. This is often confused with diffuse neurofibroma. SCL cells express CD34, but they are negative for S-100.[51] Thus, a panel of immunostains should be applied to categorize the spindle cell lesions of skin, which is primarily guided by the morphology [Table 3].
Table 3.
Immunohistochemistry panel for spindle cell tumors of skin
Histiocytic Lesions
Many histiocytic disorders involve the skin. The different histiocytic lesions involving the skin include Langerhans cell histiocytosis (LCH), juvenile xanthogranuloma, reticulohistiocytoma, xanthomas, Rosai–Dorfman (RD) disease, and various monocytic leukemias. Although these lesions have characteristic cytomorphology, IHC plays important role in their precise diagnosis. Various antibodies commonly used for histiocytic lesions include CD68 (membranous and cytoplasmic), S-100 (nuclear), CD1a (cytoplasmic), Langerin (membranous and cytoplasmic), CD14 (cytoplasmic), fascin (cytoplasmic), factor XIII (cytoplasmic), etc., among others. CD14 is a marker of monocytic/macrophage lineage. Fascin is a reliable marker for dendritic cells. LCH is a neoplastic histiocytic disorder, commonly affecting children. It is a multisystem disease and skin involvement is fairly common. The neoplastic cells express CD1a, S-100, and Langerin with variable CD68 positivity.[52,53] CD1a and Langerin show cytoplasmic positivity whereas S-100 expression is both nuclear and cytoplasmic [Figure 5a–c]. BRAF V600E mutation has been detected in certain percentage of LCH cases. This mutation is more common in cutaneous LCH (50–55%) than pulmonary LCH (30–37%). IHC for BRAF V600E (VE1 clone) is useful to detect this mutation. When present, it denotes an aggressive behavior and treatment refractoriness.[54,55] Juvenile xanthogranuloma (JXG) and reticulohistiocytomas are other histiocytic disorders commonly affecting skin. The tumor cells express CD68, alpha1 antitrypsin, alpha 1 antichymotrypsin, and factor XIII, but they are negative for LCH markers like CD1a, S-100, and Langerin [Figure 5d–f].[56,57] Factor XIII expression in JXG is strong and diffuse, which helps to differentiate it from other histologic mimickers.[58] Fascin positivity is seen in JXG, reticulohistiocytoma, and RD disease, but not in LCH [Table 4].
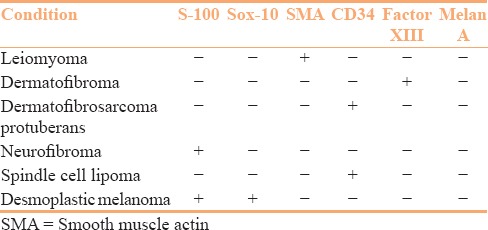
Figure 5.
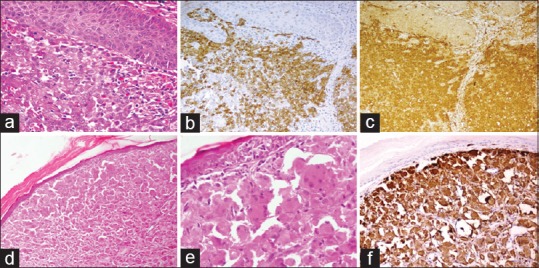
Upper panel: Langerhans cell histiocytosis. (a) Atypical histiocytes with grooved nuclei present in the dermis. Background shows many eosinophils (HE, ×200). The tumor cells diffusely express (b) CD1a (×100) and (c) S-100 (×100). Lower panel: Reticulohistiocytoma. (d) Presence of sheets of atypical histiocytes in the dermis (HE, ×100). (e) The tumor cells have abundant eosinophilic cytoplasm (HE, ×200). (f) Tumor cells show diffuse CD68 expression (×100)
Table 4.
Immunohistochemical differential diagnosis of cutaneous histiocytic disorder

Vascular Lesions of Skin
Vascular disorders commonly affect the skin, ranging from non-neoplastic reactive angiomatosis, benign vascular tumors such as hemangiomas, intermediate grade vascular tumors such as Kaposi's sarcoma (KS) to malignant vascular neoplasm including angiosarcoma. Vascular lesions usually do not need IHC for diagnosis, however, IHC is useful in difficult cases. Commonly used endothelial markers include CD31 (cytoplasmic), CD34 (cytoplasmic), factor VIII (cytoplasmic), ERG (nuclear), and FLI-1 (nuclear). Hemangiomas show diffuse positivity for vascular markers. Lymphangiomas can affect skin and should be differentiated from hemangioma. Lymphangiomas express lymphatic marker D2-40 (cytoplasmic), but they are usually negative for other endothelial markers.
KS is a vascular tumor with intermediate malignant potential. It commonly affects immunocompromized patients. It shows spindle cell proliferation with variable atypia, mitosis, and extravasation of RBCs. The tumor cells express the vascular markers. In addition, Kaposi's sarcoma cells express HHV-8, which confirms the diagnosis.[59,60] Commercially available antibody directed against HHV-8 latent nuclear antigen 1 shows high sensitivity (70–100%) to diagnose KS. Epithelioid hemangioendothelioma is another vascular tumor with intermediate malignant potential. The tumor cells are epithelioid, with presence of intracytoplasmic lumina. They usually express vascular markers, although CD34 expression can be patchy or even absent.[61,62] Certain proportion of tumor cells express cytokeratin, which is an epithelial marker. Angiosarcoma is a malignant vascular tumor. It can commonly affect skin, especially in the scalp region. The morphology of angiosarcoma is extremely variable and can be deceptive. Some cases can be bland and mimic hemangioma, while some cases show very bizarre morphology, resembling pleomorphic sarcoma. The tumor cells usually express vascular markers, however, CD34 expression can be lost in some cases. Epithelioid variant of angiosarcoma can express cytokeratin. Thus, expression of cytokeratin does not rule out a vascular tumor. Poorly differentiated angiosarcoma should be differentiated from other malignant spindle cell tumors of skin, namely, spindle cell melanoma and sarcomatoid squamous cell carcinoma (SCC). Spindle cell melanoma expresses melanocytic markers. Sarcomatoid SCC often shows vimentin positivity and negativity for cytokeratin. However, they usually show nuclear p63 expression, which can distinguish sarcomatoid SCC from angiosarcoma.[63]
Glomus tumor and myopericytomas are rare tumors derived from perivascular specialized smooth muscle cells. Although most cases do not need IHC for their diagnosis, some cases may show atypical morphology and require IHC. These tumors express SMA, MSA, and h-Caldesmon, but are usually negative or focal positive for desmin. They are usually negative for endothelial markers (CD31 and CD34) and other epithelial markers.
Cutaneous Adnexal Tumors
Adnexal tumors are one of the commonest groups of skin neoplasms. There are many variants of adnexal tumors. Most of the adnexal tumors can be diagnosed on histology alone and IHC is generally not required. The immunohistological characteristic of individual adnexal tumors is beyond the scope of this review. Here, we have discussed only the challenging situations where IHC can aid in diagnosis.
Squamous cell carcinoma versus sebaceous carcinoma
Sebaceous carcinoma (SC) and squamous cell carcinoma SCC show similar pattern of growth and similar cytological features. Sometimes it is extremely difficult to distinguish these two conditions based on histology. Both SCC and SC show positivity for p63 and high molecular weight cytokeratin (HMWCK). SC expresses androgen receptor (AR) and adipophilin, whereas SCC does not express these markers. The expression of AR in SC is variable, whereas adipophilin is expressed more consistently in SC.[64,65]
Basal cell carcinoma versus tumor with hair follicular differentiation
BCC shows characteristic histomorphology and is an easy diagnosis in most cases. However, sometimes it closely mimics tumors with hair follicular differentiation like trichoblastoma and trichoepithelioma. Although presence of epidermal origin, clefting artifact, peripheral palisading of tumor cells favor a diagnosis of BCC, sometimes the distinction is difficult. Bcl-2 is expressed in trichoblastic carcinoma, but it is negative in BCC. Tumor cells in BCC express CD10, but CD10 highlights the peritumoral stroma in trichoblastic carcinoma.[66,67] Androgen receptor is at least focally expressed in BCC (60–70%) but it is usually not expressed by tumors with hair follicular differentiation.[68]
Primary versus metastatic carcinoma of skin
Metastatic carcinomas to skin often mimic primary cutaneous malignancies. Sometimes the distinction between these two conditions is difficult, though the distinction is necessary for patient management. The tumor cells in primary cutaneous adnexal tumors usually express cytokeratin (CK) 5/6 and p63, but they are usually negative in metastatic adenocarcinomas. Although some cases of metastatic adenocarcinoma express focal CK 5/6, expression of p63 is very uncommon in metastatic carcinomas. Primary cutaneous mucinous carcinomas often express CK 7, estrogen receptor (ER), progesterone receptor (PR), GATA3, and gross cystic disease fluid protein (GCDFD) [Figure 6]. This immunoprofile is similar to mucinous carcinoma of breast, and it is virtually impossible to differentiate these two entities by morphology and IHC.[69,70] However, it has been reported that primary mucinous carcinoma of skin expresses p16 focally in 40–60% cases, whereas it is not expressed in metastatic mucinous carcinoma to skin.[71] Thus, metastatic mucinous carcinoma from breast should be excluded by history, clinical examination, and other investigations before diagnosing a primary mucinous carcinoma of skin.
Figure 6.
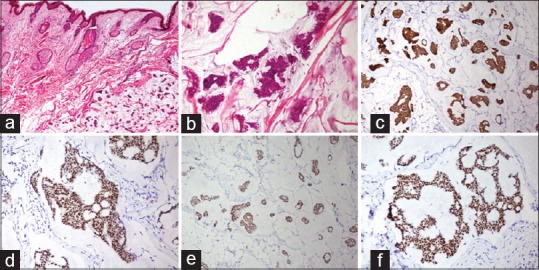
Primary mucinous carcinoma of the skin. (a) Tumor is present in the dermis with cells floating in abundant extracellular mucin (HE, ×40). (b) Small nests of tumor cells with hyperchromatic nuclei (HE, ×200). The tumor cells show (c) cytokeratin 7 (×100), (d) estrogen receptor (×200), (e) progesterone receptor (×100), and (f) GATA3 (×200)
To detect the primary site in case of metastatic carcinoma
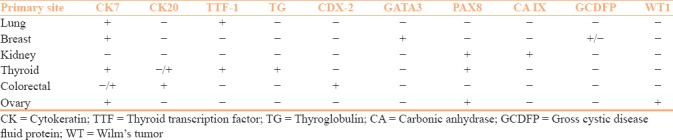
Skin is one of the most common sites of metastasis. Tumors from lung and breast are the most common source of cutaneous metastasis in males and females, respectively, although virtually tumors from all the organs can metastasize to skin. Recognition of the primary site is essential for management purpose as in many cases cutaneous metastasis is the first presenting symptom. Although the morphology of the tumor gives some clue about the possible site of origin, it can be misleading. IHC provides vital role for this purpose. The panel of antibodies to determine the primary site has been discussed in Table 5. Various combinations of cytokeratins (CKs) and transcription factors are used for this purpose. Isolated CK7 positivity indicates origin from respiratory tract, breast, upper gastrointestinal system, pancreatico-biliary system, and genitourinary system whereas isolated CK20 positivity indicates possible origin from the large intestine. Tumors arising from pancreas, urinary bladder can express both CK7 and CK20. Thyroid transcription factor-1 (TTF-1) is expressed in tumors arising from thyroid and lung. GATA3 is a reliable marker for tumors arising from breast and urinary bladder. PAX8 is another transcription factor that is expressed in tumors of thyroid, kidney, and ovarian origin. The CKs show cytoplasmic expression whereas the transcription factors (TTF-1, GATA3, and PAX8) show nuclear positivity. It should be remembered that one single marker is not sufficient to determine the primary site as there can be significant overlap and panel of antibodies should be applied for this purpose.
Table 5.
Immunohistochemical differentiation of cutaneous metastasis
IHC for Infectious Diseases
IHC has limited role in the diagnosis of infections of skin. Viral infections are common, but most are self-limiting. Human papilloma virus (HPV) commonly affects skin and causes verruca vulgaris, condyloma accuminata, and other pre-neoplastic conditions like Bowenoid papulosis. Among these, Bowenoid papulosis is caused by high-risk HPV whereas others are associated with low-risk HPV infection. These lesions can show considerable histological overlap. There is currently no antibody available to detect high-risk HPV infection. However, p16 acts as a surrogate marker of high-risk HPV infection. p16 and Ki-67 proliferation indexes play important role in the management of lower anogenital squamous lesions. Diffuse p16 positivity and higher proliferation indexes indicate HPV induced pre-neoplastic condition over reactive changes.[72] Human herpes virus (HHV) 8 is another virus, which is responsible for KS. Although KS has characteristic morphological features, nuclear staining for HHV-8 confirms the diagnosis in challenging cases. Cutaneous cytomegalovirus (CMV) infection can be seen in the immunocompromized patients. Clinically, cutaneous CMV infection resembles many other conditions and the diagnosis is challenging. IHC plays a useful role to detect the viral inclusions. Antibodies directed against various CMV antigens (glycoprotein b, GP55) show good sensitivity.
Thus, we present here the commonly applied IHCs in dermatopathology and their uses in the management. It should be remembered that choice of antibodies is primarily guided by the morphology and IHC should be used judiciously. In most of the cases, a panel of antibodies is more helpful as there may be overlapping of staining pattern. Results of IHC should be interpreted with caution, and should be correlated with clinical and histological findings before reaching to a final impression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cerroni L, Kerl H. Diagnostic immunohistology: Cutaneous lymphomas and pseudolymphomas. Semin Cutan Med Surg. 1999;18:64–70. doi: 10.1016/s1085-5629(99)80010-8. [DOI] [PubMed] [Google Scholar]
- 2.Robson A. Immunocytochemistry and the diagnosis of cutaneous lymphoma. Histopathology. 2010;56:71–90. doi: 10.1111/j.1365-2559.2009.03457.x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy M, Fullen D, Carlson JA. Low CD7 expression in benign and malignant cutaneous lymphocytic infiltrates: Experience with an antibody reactive with paraffin-embedded tissue. Am J Dermatopathol. 2002;24:6–16. doi: 10.1097/00000372-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Nuckols JD, Shea CR, Horenstein MG, Burchette JL, Prieto VG. Quantitation of intraepidermal T-cell subsets in formalin-fixed, paraffin-embedded tissue helps in the diagnosis of mycosis fungoides. J Cutan Pathol. 1999;26:169–75. doi: 10.1111/j.1600-0560.1999.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 5.Harvell JD, Nowfar-Rad M, Sundram U. An immunohistochemical study of CD4, CD8, TIA-1 and CD56 subsets in inflammatory skin disease. J Cutan Pathol. 2003;30:108–13. doi: 10.1034/j.1600-0560.2002.00038.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodney IJ, Kindred C, Angra K, Qutub ON, Villanueva AR, Halder RM. Hypopigmented mycosis fungoides: Aretrospective clinicohistopathologic study. J Eur Acad Dermatol Venereol. 2017;31:808–14. doi: 10.1111/jdv.13843. [DOI] [PubMed] [Google Scholar]
- 7.Plaza JA, Feldman AL, Magro C. Cutaneous CD30-positive lymphoproliferative disorders with CD8 expression: Aclinicopathologic study of 21 cases. J Cutan Pathol. 2013;40:236–47. doi: 10.1111/cup.12047. [DOI] [PubMed] [Google Scholar]
- 8.Kempf W, Kazakov DV, Scharer L, Rutten A, Mentzel T, Paredes BE, et al. Angioinvasive lymphomatoid papulosis: Anew variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1–13. doi: 10.1097/PAS.0b013e3182648596. [DOI] [PubMed] [Google Scholar]
- 9.Kempf W. Cutaneous CD30-Positive Lymphoproliferative Disorders. Surg Pathol Clin. 2014;7:203–28. doi: 10.1016/j.path.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Massone C, Cerroni L. Phenotypic variability in primary cutaneous anaplastic large T-cell lymphoma: Astudy on 35 patients. Am J Dermatopathol. 2014;36:153–7. doi: 10.1097/DAD.0b013e3182a5683a. [DOI] [PubMed] [Google Scholar]
- 11.Papalas JA, Kulbacki E, Wang E. Anaplastic lymphoma kinase (ALK1) immunohistochemistry in diagnostic dermatopathology; an update. Am J Dermatopathol. 2013;35:403–8. doi: 10.1097/DAD.0b013e31823d2943. quiz 9-11. [DOI] [PubMed] [Google Scholar]
- 12.Kwatra KS, Paul PAM, Calton N, John JM, Cotelingam JD. Systemic and primary cutaneous anaplastic large cell lymphoma: Clinical features, morphological spectrum, and immunohistochemical profile. South Asian J Cancer. 2017;6:129–31. doi: 10.4103/2278-330X.214575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein H, Foss HD, Durkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: Areview of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–95. [PubMed] [Google Scholar]
- 14.Cepeda LT, Pieretti M, Chapman SF, Horenstein MG. CD30-positive atypical lymphoid cells in common non-neoplastic cutaneous infiltrates rich in neutrophils and eosinophils. Am J Surg Pathol. 2003;27:912–8. doi: 10.1097/00000478-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: Redefinition of diagnostic criteria in the recent World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009;133:303–8. doi: 10.5858/133.2.303. [DOI] [PubMed] [Google Scholar]
- 16.Kong YY, Dai B, Kong JC, Zhou XY, Lu HF, Shen L, et al. Subcutaneous panniculitis-like T-cell lymphoma: Aclinicopathologic, immunophenotypic, and molecular study of 22 Asian cases according to WHO-EORTC classification. Am J Surg Pathol. 2008;32:1495–502. doi: 10.1097/PAS.0b013e31817a9081. [DOI] [PubMed] [Google Scholar]
- 17.Ferringer T. Immunohistochemistry in dermatopathology. Arch Pathol Lab Med. 2015;139:83–105. doi: 10.5858/arpa.2014-0075-RA. [DOI] [PubMed] [Google Scholar]
- 18.Hoang MP, Mahalingam M, Selim MA. Immunohistochemistry in the diagnosis of cutaneous neoplasms. Future Oncol. 2010;6:93–109. doi: 10.2217/fon.09.143. [DOI] [PubMed] [Google Scholar]
- 19.Bailey EM, Ferry JA, Harris NL, Mihm MC, Jr, Jacobson JO, Duncan LM. Marginal zone lymphoma (low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type) of skin and subcutaneous tissue: A study of 15 patients. Am J Surg Pathol. 1996;20:1011–23. doi: 10.1097/00000478-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Servitje O, Gallardo F, Estrach T, Pujol RM, Blanco A, Fernandez-Sevilla A, et al. Primary cutaneous marginal zone B-cell lymphoma: Aclinical, histopathological, immunophenotypic and molecular genetic study of 22 cases. Br J Dermatol. 2002;147:1147–58. doi: 10.1046/j.1365-2133.2002.04961.x. [DOI] [PubMed] [Google Scholar]
- 21.de Leval L, Harris NL, Longtine J, Ferry JA, Duncan LM. Cutaneous b-cell lymphomas of follicular and marginal zone types: Use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732–41. doi: 10.1097/00000478-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Goodlad JR, Krajewski AS, Batstone PJ, McKay P, White JM, Benton EC, et al. Primary cutaneous follicular lymphoma: Aclinicopathologic and molecular study of 16 cases in support of a distinct entity. Am J Surg Pathol. 2002;26:733–41. doi: 10.1097/00000478-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Kim BK, Surti U, Pandya A, Cohen J, Rabkin MS, Swerdlow SH. Clinicopathologic, immunophenotypic, and molecular cytogenetic fluorescence in situ hybridization analysis of primary and secondary cutaneous follicular lymphomas. Am J Surg Pathol. 2005;29:69–82. doi: 10.1097/01.pas.0000146015.22624.c7. [DOI] [PubMed] [Google Scholar]
- 24.Yatabe Y, Suzuki R, Tobinai K, Matsuno Y, Ichinohasama R, Okamoto M, et al. Significance of cyclin D1 overexpression for the diagnosis of mantle cell lymphoma: Aclinicopathologic comparison of cyclin D1-positive MCL and cyclin D1-negative MCL-like B-cell lymphoma. Blood. 2000;95:2253–61. [PubMed] [Google Scholar]
- 25.Greaves MF, Brown J, Molgaard HV, Spurr NK, Robertson D, Delia D, et al. Molecular features of CD34: Ahemopoietic progenitor cell-associated molecule. Leukemia. 1992;6(Suppl 1):31–6. [PubMed] [Google Scholar]
- 26.Cibull TL, Thomas AB, O’Malley DP, Billings SD. Myeloid leukemia cutis: Ahistologic and immunohistochemical review. J Cutan Pathol. 2008;35:180–5. doi: 10.1111/j.1600-0560.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 27.Pulford KA, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, et al. KP1: Anew monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989;42:414–21. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horny HP, Sillaber C, Menke D, Kaiserling E, Wehrmann M, Stehberger B, et al. Diagnostic value of immunostaining for tryptase in patients with mastocytosis. Am J Surg Pathol. 1998;22:1132–40. doi: 10.1097/00000478-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Natkunam Y, Rouse RV. Utility of paraffin section immunohistochemistry for C-KIT (CD117) in the differential diagnosis of systemic mast cell disease involving the bone marrow. Am J Surg Pathol. 2000;24:81–91. doi: 10.1097/00000478-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Viray H, Bradley WR, Schalper KA, Rimm DL, Gould Rothberg BE. Marginal and joint distributions of S100, HMB-45, and Melan-A across a large series of cutaneous melanomas. Arch Pathol Lab Med. 2013;137:1063–73. doi: 10.5858/arpa.2012-0284-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein D, Leininger J, Hamby C, Safai B. Diagnostic and prognostic biomarkers in melanoma. J Clin Aesthet Dermatol. 2014;7:13–24. [PMC free article] [PubMed] [Google Scholar]
- 32.Nybakken GE, Sargen M, Abraham R, Zhang PJ, Ming M, Xu X. MITF accurately highlights epidermal melanocytes in atypical intraepidermal melanocytic proliferations. Am J Dermatopathol. 2013;35:25–9. doi: 10.1097/DAD.0b013e31825666c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palla B, Su A, Binder S, Dry S. SOX10 expression distinguishes desmoplastic melanoma from its histologic mimics. Am J Dermatopathol. 2013;35:576–81. doi: 10.1097/DAD.0b013e31827a0b98. [DOI] [PubMed] [Google Scholar]
- 34.Miller DD, Emley A, Yang S, Richards JE, Lee JE, Deng A, et al. Mixed versus pure variants of desmoplastic melanoma: Agenetic and immunohistochemical appraisal. Mod Pathol. 2012;25:505–15. doi: 10.1038/modpathol.2011.196. [DOI] [PubMed] [Google Scholar]
- 35.Rothberg BE, Moeder CB, Kluger H, Halaban R, Elder DE, Murphy GF, et al. Nuclear to non-nuclear Pmel17/gp100 expression (HMB45 staining) as a discriminator between benign and malignant melanocytic lesions. Mod Pathol. 2008;21:1121–9. doi: 10.1038/modpathol.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos-Herberth FI, Karamchandani J, Kim J, Dadras SS. SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar. J Cutan Pathol. 2010;37:944–52. doi: 10.1111/j.1600-0560.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 37.Nonaka D, Chiriboga L, Rubin BP. Sox10: Apan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32:1291–8. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 38.Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517–23. doi: 10.1034/j.1600-0560.2002.290902.x. [DOI] [PubMed] [Google Scholar]
- 39.Memezawa A, Okuyama R, Tagami H, Aiba S. p63 constitutes a useful histochemical marker for differentiation of pagetoid Bowen's disease from extramammary Paget's disease. Acta Derm Venereol. 2008;88:619–20. doi: 10.2340/00015555-0512. [DOI] [PubMed] [Google Scholar]
- 40.Hilliard NJ, Huang C, Andea A. Pigmented extramammary Paget's disease of the axilla mimicking melanoma: Case report and review of the literature. J Cutan Pathol. 2009;36:995–1000. doi: 10.1111/j.1600-0560.2009.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sellheyer K, Krahl D. Ber-EP4 enhances the differential diagnostic accuracy of cytokeratin 7 in pagetoid cutaneous neoplasms. J Cutan Pathol. 2008;35:366–72. doi: 10.1111/j.1600-0560.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 42.Vollmer RT. Use of Bayes rule and MIB-1 proliferation index to discriminate Spitz nevus from malignant melanoma. Am J Clin Pathol. 2004;122:499–505. doi: 10.1309/MFFF-06D5-CYXR-2F8T. [DOI] [PubMed] [Google Scholar]
- 43.Pearlstein MV, Zedek DC, Ollila DW, Treece A, Gulley ML, Groben PA, et al. Validation of the VE1 immunostain for the BRAF V600E mutation in melanoma. J Cutan Pathol. 2014;41:724–32. doi: 10.1111/cup.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Just PA, Audebourg A, Pasmant E, Clauser E, Carlotti A, Laurent S, et al. Immunohistochemistry versus next-generation sequencing for the routine detection of BRAF V600E mutation in melanomas. Hum Pathol. 2014;45:1983–4. doi: 10.1016/j.humpath.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Massi D, Brusa D, Merelli B, Ciano M, Audrito V, Serra S, et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol. 2014;25:2433–42. doi: 10.1093/annonc/mdu452. [DOI] [PubMed] [Google Scholar]
- 46.Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 47.Berghoff AS, Ricken G, Widhalm G, Rajky O, Dieckmann K, Birner P, et al. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology. 2015;66:289–99. doi: 10.1111/his.12537. [DOI] [PubMed] [Google Scholar]
- 48.Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathol. 1997;19:147–53. doi: 10.1097/00000372-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous (“high-grade”) dermatofibrosarcoma protuberans: Clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576–87. doi: 10.1097/00000478-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang JZ, Zhou J, Zhang ZC. Subcutaneous Angioleiomyoma: Clinical and Sonographic Features With Histopathologic Correlation. J Ultrasound Med. 2016;35:1669–73. doi: 10.7863/ultra.15.06056. [DOI] [PubMed] [Google Scholar]
- 51.Ko JS, Daniels B, Emanuel PO, Elson P, Khachaturov V, McKenney JK, et al. Spindle Cell Lipomas in Women: A Report of 53 Cases. Am J Surg Pathol. 2017;41:1267–74. doi: 10.1097/PAS.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 52.Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of Langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615–9. doi: 10.1097/PAS.0b013e31815b212b. [DOI] [PubMed] [Google Scholar]
- 53.El Demellawy D, Young JL, de Nanassy J, Chernetsova E, Nasr A. Langerhans cell histiocytosis: Acomprehensive review. Pathology. 2015;47:294–301. doi: 10.1097/PAT.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 54.Mehes G, Irsai G, Bedekovics J, Beke L, Fazakas F, Rozsa T, et al. Activating BRAF V600E mutation in aggressive pediatric Langerhans cell histiocytosis: Demonstration by allele-specific PCR/direct sequencing and immunohistochemistry. Am J Surg Pathol. 2014;38:1644–8. doi: 10.1097/PAS.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 55.Roden AC, Hu X, Kip S, Parrilla Castellar ER, Rumilla KM, Vrana JA, et al. BRAF V600E expression in Langerhans cell histiocytosis: Clinical and immunohistochemical study on 25 pulmonary and 54 extrapulmonary cases. Am J Surg Pathol. 2014;38:548–51. doi: 10.1097/PAS.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 56.Sandell RF, Carter JM, Folpe AL. Solitary (juvenile) xanthogranuloma: Acomprehensive immunohistochemical study emphasizing recently developed markers of histiocytic lineage. Hum Pathol. 2015;46:1390–7. doi: 10.1016/j.humpath.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 57.Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: Aclinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29:21–8. doi: 10.1097/01.pas.0000147395.01229.06. [DOI] [PubMed] [Google Scholar]
- 58.Misery L, Boucheron S, Claudy AL. Factor XIIIa expression in juvenile xanthogranuloma. Acta Derm Venereol. 1994;74:43–4. doi: 10.2340/00015555744344. [DOI] [PubMed] [Google Scholar]
- 59.Robin YM, Guillou L, Michels JJ, Coindre JM. Human herpesvirus 8 immunostaining: Asensitive and specific method for diagnosing Kaposi sarcoma in paraffin-embedded sections. Am J Clin Pathol. 2004;121:330–4. doi: 10.1309/96U1-6LRR-AN5H-WWVE. [DOI] [PubMed] [Google Scholar]
- 60.Wada DA, Perkins SL, Tripp S, Coffin CM, Florell SR. Human herpesvirus 8 and iron staining are useful in differentiating Kaposi sarcoma from interstitial granuloma annulare. Am J Clin Pathol. 2007;127:263–70. doi: 10.1309/GMH9CENH4909AWVB. [DOI] [PubMed] [Google Scholar]
- 61.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: Astudy of 80 cases. Am J Surg Pathol. 1998;22:683–97. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Ohsawa M, Naka N, Tomita Y, Kawamori D, Kanno H, Aozasa K. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75:2867–74. doi: 10.1002/1097-0142(19950615)75:12<2867::aid-cncr2820751212>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 63.Jo VY, Fletcher CD. p63 immunohistochemical staining is limited in soft tissue tumors. Am J Clin Pathol. 2011;136:762–6. doi: 10.1309/AJCPXNUC7JZSKWEU. [DOI] [PubMed] [Google Scholar]
- 64.Asadi-Amoli F, Khoshnevis F, Haeri H, Jahanzad I, Pazira R, Shahsiah R. Comparative examination of androgen receptor reactivity for differential diagnosis of sebaceous carcinoma from squamous cell and basal cell carcinoma. Am J Clin Pathol. 2010;134:22–6. doi: 10.1309/AJCP89LYTPNVOBAP. [DOI] [PubMed] [Google Scholar]
- 65.Plaza JA, Mackinnon A, Carrillo L, Prieto VG, Sangueza M, Suster S. Role of immunohistochemistry in the diagnosis of sebaceous carcinoma: Aclinicopathologic and immunohistochemical study. Am J Dermatopathol. 2015;37:809–21. doi: 10.1097/DAD.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 66.Heidarpour M, Rajabi P, Sajadi F. CD10 expression helps to differentiate basal cell carcinoma from trichoepithelioma. J Res Med Sci. 2011;16:938–44. [PMC free article] [PubMed] [Google Scholar]
- 67.Cordoba A, Guerrero D, Larrinaga B, Iglesias ME, Arrechea MA, Yanguas JI. Bcl-2 and CD10 expression in the differential diagnosis of trichoblastoma, basal cell carcinoma, and basal cell carcinoma with follicular differentiation. Int J Dermatol. 2009;48:713–7. doi: 10.1111/j.1365-4632.2009.04076.x. [DOI] [PubMed] [Google Scholar]
- 68.Izikson L, Bhan A, Zembowicz A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am J Dermatopathol. 2005;27:91–5. doi: 10.1097/01.dad.0000154392.92099.aa. [DOI] [PubMed] [Google Scholar]
- 69.Papalas JA, Proia AD. Primary mucinous carcinoma of the eyelid: Aclinicopathologic and immunohistochemical study of 4 cases and an update on recurrence rates. Arch Ophthalmol. 2010;128:1160–5. doi: 10.1001/archophthalmol.2010.177. [DOI] [PubMed] [Google Scholar]
- 70.Kwatra KS, Prabhakar BR, Jain S. Oestrogen and progesterone receptors in primary mucinous carcinoma of skin. Australas J Dermatol. 2005;46:246–9. doi: 10.1111/j.1440-0960.2005.00193.x. [DOI] [PubMed] [Google Scholar]
- 71.Levy G, Finkelstein A, McNiff JM. Immunohistochemical techniques to compare primary vs.metastatic mucinous carcinoma of the skin. J Cutan Pathol. 2010;37:411–5. doi: 10.1111/j.1600-0560.2009.01436.x. [DOI] [PubMed] [Google Scholar]
- 72.Kazlouskaya V, Shustef E, Allam SH, Lal K, Elston D. Expression of p16 protein in lesional and perilesional condyloma acuminata and bowenoid papulosis: Clinical significance and diagnostic implications. J Am Acad Dermatol. 2013;69:444–9. doi: 10.1016/j.jaad.2013.04.036. [DOI] [PubMed] [Google Scholar]


