Abstract
Background:
The anti-inflammatory, immunomodulatory, and anti-proliferative effects of vitamin D in pathogenesis of autoimmune diseases have been highlighted in recent years but implications of vitamin D deficiency in systemic sclerosis (SSc) remain understudied.
Objectives:
To evaluate serum vitamin D levels in SSc patients and matched controls.
Materials and Methods:
Serum vitamin D levels were estimated in 38 (M:F 5:33) patients aged 23–70 years of untreated SSc and age and gender matched healthy controls. Clinical and investigative evaluation for skin sclerosis by modified Rodnan skin score (mRSS), presence of digital ulcers, Raynaud's phenomenon, type of auto-antibodies, systemic involvement, and serum vitamin D levels were performed. Serum vitamin D levels were defined as normal (30–100 ng/ml), insufficient (10–30 ng/ml), and deficient (<10 ng/ml).
Results:
Serum vitamin D levels (median ± IQR) were 19.5 ± 77.8 ng/ml in 38 patients and 100 ± 31.3 ng/ml in controls each. Vitamin D deficiency in 13 (34.2%) and insufficiency in 10 (26.3%) patients were identified. Only 2 (5.3%) controls had vitamin D insufficiency and the difference was statistically significant (P = 0.001). An inverse relationship was observed between mRSS and serum vitamin D levels.
Conclusions:
Patients with SSc have significantly lower serum vitamin D levels than healthy controls. Serum vitamin D levels do not correlate well with age, gender, disease duration or its variants, type of auto antibodies, presence of digital ulceration, or systemic involvement but has inverse correlation with skin sclerosis. Better-designed studies will perhaps resolve issues of potential benefits of vitamin D supplementation in modification of disease activity or severity in SSc.
Keywords: Autoimmune disorders, connective tissue diseases, dysphagia, dyspnea, Raynaud's phenomenon, scleroderma
Introduction
Systemic sclerosis (SSc), an uncommon connective tissue disorder, is characterized by vascular obliteration, immune dysfunction, and excessive extracellular matrix deposition involving skin, lungs, gastrointestinal tract, heart, and kidneys. Its incidence varies from 2.3 to 10 per million population affecting females 3–6 times more than males.[1,2] Clinically, it presents as diffuse or limited cutaneous scleroderma with typical features of skin binding/thickening, Raynaud's phenomenon and visceral involvement, and serologically by distinct autoantibody subsets against nuclear antigens.[3] Genetic, infectious, hormonal, and environmental factors are often implicated in its etiopathogenesis that yet remains obscure. Although speculative, vitamin D deficiency affects both adaptive and innate immunity in various autoimmune diseases. It acts through vitamin D receptors (VDRs) present on the surface of antigen presenting cells, natural killer cells as well as B and T lymphocytes exerting multiple immunomodulating effects on both innate and adaptive immune responses. It is achieved via inhibiting Th1 cells (the main effector cells of autoimmune disorders), production of Th1 cytokines IL-2, IFN-γ, and TNF-α, and decreasing proinflammatory cytokines (IL-6, -17), and upregulating anti-inflammatory mediators (IL-4, -10). VDR signaling is recognized as a key regulator of cell proliferation, differentiation, and immunomodulation and decreased VDRs expression leads to enhanced sensitivity of fibroblasts to transforming growth factor (TGF-β), central mediator of fibroblast activation, leading to increased production of extracellular matrix.[4,5] There is a shift of immune response toward a Th2 cytokines pattern that contributes to a more profibrotic environment from direct stimulation of collagen synthesis and myofibroblast trans-differentiation. The active vitamin D hormone, 1,25(OH)2D3, can be produced in endothelial cells through activity of a specific endothelial α-hydroxylase on circulating 25(OH)D3. Thus, any endothelial dysfunction leads to increased levels of von Willebrand factor or plasminogen activator inhibitor (PAI-1) as well as other inflammatory markers such as C-reactive protein (CRP), cellular adhesion molecules (CAMs), vascular adhesion molecules, and P- or E-selectin promoting thrombus formation and tissue inflammation.[6] Based on foregoing, the Th2 cytokines and profibrotic cytokines including TGF-β synergistically favor collagen deposition and metalloproteinase inhibition by fibroblasts. Thus, T cell activation, production and release of cytokines and autoantibody production are key events leading to microvascular damage, inflammation, and fibrosis. Raynaud's phenomenon that usually predates the fibrotic process suggests that endothelial cells are perhaps the initial target for primary dysfunction. Thus, vitamin D perhaps represents a modifiable factor in the complex interplay of genetic and environmental factors favoring the onset and the clinical expression of SSc. Vitamin D deficiency in SSc patients is reported across geographical locations including Europe, Italy, France, German, and Southern Spain.[3,7,8,9,10,11,12] We studied possible correlation of serum vitamin D levels and SSc manifestations in Indian patients.
Material and Methods
Thirty-eight consecutive patients (men 5, women 33, M:F = 1:6.6) aged 23–70 years who fulfilled the ARA diagnostic criteria[13] of SSc [Table 1] were enrolled after written/informed consent from outdoor dermatology clinic between April 2015 and March 2016. The distinction between limited and diffuse cutaneous SSc was made based on criteria of LeRoy et al. [Table 1].[14] Patients <18 years of age, pregnant and lactating women, and patients who had taken vitamin D supplements within the preceding 4 weeks were excluded. The 38 controls comprised age, gender, and occupation-matched unrelated, healthy adults. Clinical details for age, gender, chief complaints, and age of onset, duration, progression, aggravating factors, present and past treatments, and family history were recorded. Extent of skin involvement and severity/grading of sclerosis was assessed by modified Rodnan skin score (mRSS).[15] It consists of evaluation of patient's skin thickness rated by clinical palpation on a 0–3 scale (0 = normal skin; 1 = mild thickness; 2 = moderate thickness; 3 = severe thickness with inability to pinch the skin into a fold) for each of 17 surface anatomic areas of the body, that is, face, anterior chest, abdomen, (right and left separately) fingers, forearms, upper arms, thighs, lower legs, dorsal hands, and feet. The sum total of individual values is total skin score.
Table 1.
Diagnosis and classification of systemic sclerosis and pulmonary hypertension
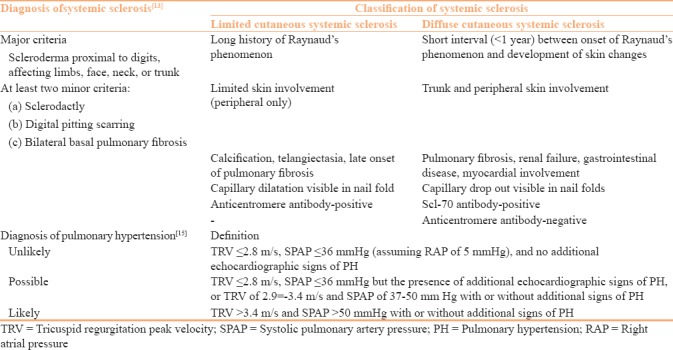
Laboratory workup included complete blood counts, erythrocyte sedimentation rate (ESR), CRP, blood sugar (fasting, postprandial), serum urea, creatinine, serum proteins/albumin/globulin levels, serum bilirubin, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), pulmonary function tests, chest radiograph, chest computed tomography (if indicated), antinuclear antibody (ANA), anticentromere antibody, anti-topoisomerase (Scl-70) antibody, echocardiography, and urinalysis. The diagnosis of pulmonary hypertension was by measuring tricuspid regurgitation peak velocity and systolic pulmonary artery pressure on echocardiography as per European Society of Cardiology guidelines [Table 1].[16] Vitamin D levels were measured by using 25(OH) Vitamin D ELISA kit (Calbiotech Life science Co, California) as per manufacturer's instructions. Vitamin D serum levels (normal 30–100 ng/ml) between 10 and 30 ng/ml were classified as vitamin D insufficiency, whereas levels <10 ng/ml were considered as vitamin D deficiency.[7]
Statistical analysis
Baseline characteristics of cases and controls were analyzed using descriptive statistics. Median ± IQR was calculated for serum vitamin D levels due to extreme values with wide and uneven distribution (outliers and non-normal data). Unpaired Student's t-test was used for numerical data and a P value <0.05 calculated at 5% level (95% confidence limit) was considered statistically significant. Pearson correlation coefficient (r) was used to find the correlation between mRSS and serum vitamin D levels.
Results
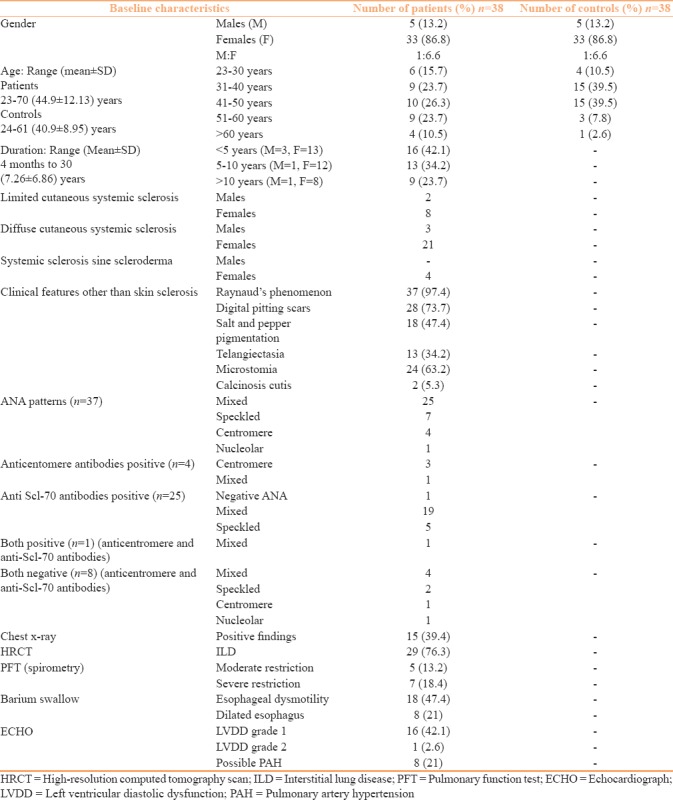
Baseline and clinical and investigative characteristics of patients and controls are tabulated [Table 2]. All female patients and controls were homemakers and involved in farming activities as well, whereas all men were farmers or farm laborers staying in sun during working hours. Majority of the patients, 28 (73.7%) were aged between 31 and 60 years. Ten (26.3%) patients had limited and 24 (63.1%) had diffuse cutaneous SSc but 4 (10.5%) patients did not have thickened hide bound skin (SSc sine scleroderma). History of Raynaud's phenomenon was present in 37 (97.4%) patients and was corroborated clinically from digital pitting scar in 28 (73.7%) patients. Salt and pepper skin dyspigmentation was observed in 18 (47.4%) patients, mat-like telangiectasia in 13 (34.2%), microstomia in 24 (63.2%), and calcinosis cutis in 2 (5.3%) patients,. ANA was positive in 37 (97.3%) patients with mixed pattern in majority. Five patients had anticentromere antibody positivity and 26 patients had positive anti-topoisomerase (Scl-70) antibody including one patient who was seropositive for both. Eight patients were seronegative for both anticentromere and anti-topoisomerase antibody. Chest x-ray changes seen in 15 (39.4%) patients included interstitial thickening, prominent reticular marking, radio opaque, and reticulonodular shadows were indicative of interstitial lung disease (ILD) and were corroborated on high resolution computed tomography (HRCT) scan. Other 14 patients with normal chest x-rays also had early ILD changes on HRCT scans. Esophageal dysmotility was found in 18 (47.4%), and 8 (21%) patients also showed dilated esophagus on barium meal studies. Echocardiography showed left ventricular diastolic dysfunction in 17 (44.7%), and possible pulmonary artery hypertension in 8 (21%) patients each.
Table 2.
Baseline characteristics of patients and controls
The serum Vitamin D levels in 38 patients varied between 0 and 100 (median ± IQR = 19.5 ± 77.8) ng/ml and 16.5–100 (median ± IQR = 100 ± 31.3) ng/ml in controls [Table 3]. Vitamin D deficiency was recorded in 13 (34.2%) patients and 10 (26.3%) patients had insufficient levels. Only two healthy controls had vitamin D insufficiency. Statistically, the difference between serum vitamin D levels of patients and controls was significant [Table 3]. However, serum vitamin D levels when sorted by clinical and serological parameters showed no significant correlation statistically [Table 4] but had an inverse correlation (r = −.267) with mRSS [Figure 1].
Table 3.
Serum vitamin D levels in patients and controls
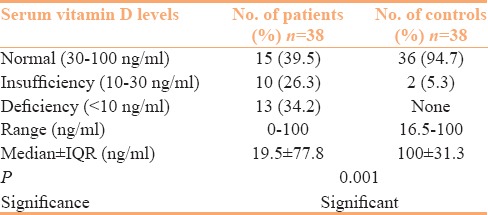
Table 4.
Clinico-investigative parameters and serum vitamin D levels
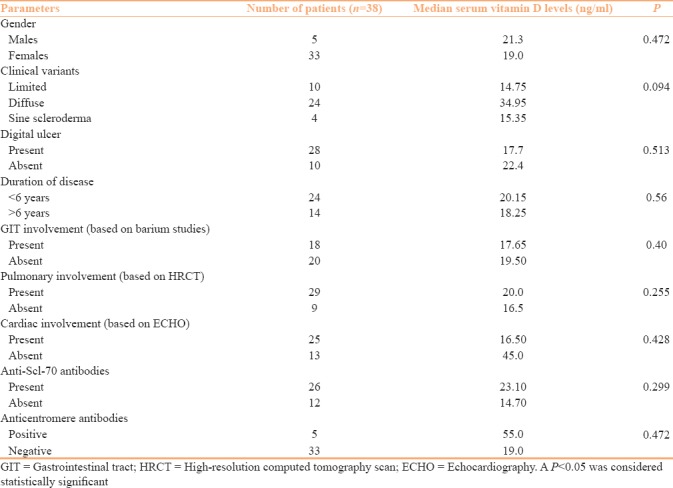
Figure 1.
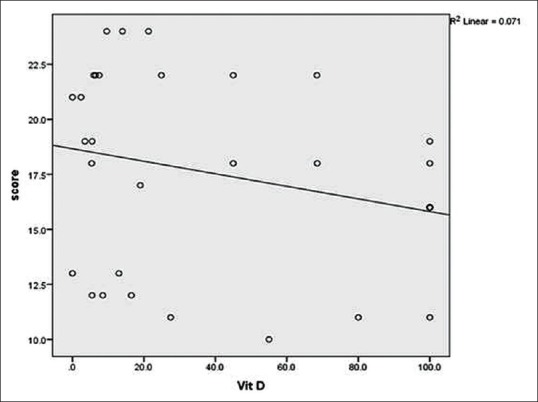
Correlation between serum vitamin D levels and modified Rodnan skin score (mRSS): The sign of correlation coefficient (r) indicates the direction whereas the magnitude indicates the strength o association. Small circles denote serum vitamin D levels of patients and line shows inverse correlation (r = −.267) between them meaning serum vitamin D levels decreases with increasing mRSS
Discussion
The clinico-investigative and autoantibody profile of patients in this study is more or less similar to its characteristics established in literature.[3,17] Although the exact etiopathogenesis of SSc remains obscure despite extensive knowledge gained over the years, the role of vitamin D due to its anti-inflammatory, immunomodulatory, and anti-proliferative effects has been highlighted in recent years in causation of autoimmune diseases including SSc in view of several studies across geographical locations observing low serum levels.[3,5,6,7,8,9,10,11,12,18,19,20] Arnson et al.[3] compared serum vitamin D levels in SSc patient and healthy controls and observed significant low levels of vitamin D in patients than controls. Caramaschi et al.[7] in a study of 65 patients observed 19 (29.2%) patients with vitamin D deficiency and 43 (66.2%) patients having insufficient levels. Similarly, vitamin D deficiency was seen in 13 (34.2%) patients and insufficient levels in 10 (26.3%) patients as compared to insufficiency noted in only two controls in our study, and the difference was statistically significant (P =0.001). However, low serum vitamin D levels in our patients were independent of gender, occupation, duration, presence of digital ulcers or systemic involvement, or variant of SSc, and type of auto-antibodies corroborating an earlier study.[21] Contrarily, vitamin D levels have been also observed previously correlating with severity of skin involvement, longer disease duration, severe pulmonary and cardiac involvement and low diffusion capacity, higher prevalence of pulmonary arterial hypertension, acroosteolysis, and calcinosis in 80% of SSc patients across studies.[3,5,7,8,9,17,22,23,24] MRSS varied between 10 and 24 (mean ± SD = 17.44 ± 4.29) and showed an inverse correlation (r = −.267) with serum vitamin D levels in our patients, which suggests that progression of fibrosis might be influenced by vitamin D deficiency.
Vitamin D deficiency in SSc patients has been attributed to reduced drawing of provitamin D3 synthesized from dehydrocholesterol by UVB radiation in the epidermis due to dermal fibrosis with capillary damage, skin hyperpigmentation and low sun exposure, and insufficient intake or malabsorption of dietary vitamin D from gastrointestinal involvement.[3,7,10,23] Recently, subclinical hepatic fibrosis in SSc patients too has been implicated for impaired vitamin D metabolism and its deficiency.[25] Although topical vitamin D analog was found useful for localized scleroderma (morphoea), administration of cholecalciferol and/alfacalcidiol (2 mcg/d) has shown no effect or determined improvement in SSc symptoms.[26,27,28] It is possible that vitamin D deficiency perhaps plays some role in the subsequent development of a well-defined variant from undifferentiated connective tissue disease.
Conclusions
It can be inferred that geographic origin or clinical presentation of SSc patients perhaps does not influence vitamin D levels in them as compared to healthy controls as the levels do not correlate well with age, gender, disease duration, or its variants, type of autoantibodies, or presence of digital ulceration. An inverse correlation observed between mRSS and serum vitamin D levels suggests that progression of sclerosis/fibrosis might be influenced by it. However, it remains conjectural at the moment and better-designed studies will perhaps resolve issues related to potential benefits of vitamin D supplementation on disease progression or associated complications, or the regimen that suffices to modulate immunological homeostasis, and possibly reduce disease activity or severity. The clinical implications of this study also remain limited by small number of patients, its cross-sectional nature, no assessment for dietary vitamin D intake or exact amount of sun exposure, and lack of treatment outcome measures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Eason RJ, Tan PL, Gow PJ. Progressive systemic sclerosis in Auckland: A 10-year review with emphasis on prognostic features. Aust NZ J Med. 1981;11:657–62. doi: 10.1111/j.1445-5994.1981.tb03542.x. [DOI] [PubMed] [Google Scholar]
- 2.Medsger TA Jr, Masi AT. Epidemiology of systemic sclerosis (scleroderma) Ann Intern Med. 1971;74:714–21. doi: 10.7326/0003-4819-74-5-714. [DOI] [PubMed] [Google Scholar]
- 3.Arnson Y, Amital H, Agmon-Levin N, Alon D, Sánchez-Castañón M, López-Hoyos M, et al. Serum 25-OH Vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: A retrospective cohort study and review of the literature. Autoimmun Rev. 2011;10:490–4. doi: 10.1016/j.autrev.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev. 2011;10:276–81. doi: 10.1016/j.autrev.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Zerr P, Vollath S, Palumbo-Zerr K, Tomcik M, Huang J, Distler A, et al. Vitamin D receptor regulates TGF-β signalling in systemic scleros is. Ann Rheum Dis. 2015;74:e20. doi: 10.1136/annrheumdis-2013-204378. [DOI] [PubMed] [Google Scholar]
- 6.Alyami A, Soares MJ, Sherriff JL, Mamo JC. Vitamin D and endothelial function. Indian J Med Res. 2014;140:483–90. [PMC free article] [PubMed] [Google Scholar]
- 7.Caramaschi P, Dalla GA, Ruzzenente O, Volpe A, Ravagnani V, Tinazzi I, et al. Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol. 2010;12:1419–25. doi: 10.1007/s10067-010-1478-3. [DOI] [PubMed] [Google Scholar]
- 8.Vacca A, Cormier C, Piras M, Mathieu A, Kahan A, Allanore Y. Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. J Rheumatol. 2009;36:1924–9. doi: 10.3899/jrheum.081287. [DOI] [PubMed] [Google Scholar]
- 9.Braun-Moscovici Y, Furst DE, Markovits D, Rozin A, Clements PJ, Nahir AM, et al. Vitamin D, Parathyroid hormone, and acroosteolysis in systemic sclerosis. J Rheumatol. 2008;35:2201–5. doi: 10.3899/jrheum.071171. [DOI] [PubMed] [Google Scholar]
- 10.Calzolari G, Data V, Carignola R, Angeli A. Hypovitaminosis D in systemic sclerosis. J Rheumatol. 2009;36:2844. doi: 10.3899/jrheum.090439. [DOI] [PubMed] [Google Scholar]
- 11.Rios Fernández R, Fernández Roldán C, Callejas Rubio JL. Vitamin D deficiency in a cohort of patients with systemic scleroderma from the south of Spain. J Rheumatol. 2010;37:1355. doi: 10.3899/jrheum.091143. [DOI] [PubMed] [Google Scholar]
- 12.Gambichler T, Chrobok IS, Höxtermann S, Kreuter A. Significantly decreased serum 25-hydroxy vitamin D levels in a large German systemic sclerosis cohort. J Rheumatol. 2011;38:2492–3. doi: 10.3899/jrheum.110695. [DOI] [PubMed] [Google Scholar]
- 13.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 14.LeRoy EC, Black C, Fleischmajer R. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 15.Kaldas M, Khanna PP, Furst DE, Clements PJ, Wong WK, Seibold JR, et al. on behalf of the investigators of the human recombinant relaxin and oral bovine collagen clinical trials. Sensitivity to change of the modified Rodnan skin score in diffuse systemic sclerosis-assessment of individual body sites in two large randomized controlled trials. Rheumatology. 2009;48:1143–6. doi: 10.1093/rheumatology/kep202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 17.Viswanath V, Phiske MM, Gopalani VV. Systemic sclerosis: Current concepts in pathogenesis and therapeutic aspects of dermatological manifestations. Indian J Dermatol. 2013;58:255–68. doi: 10.4103/0019-5154.113930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand) 2003;49:277–300. [PubMed] [Google Scholar]
- 19.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxy vitamin D3: Basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Giuggioli D, Colaci M, Cassone G, Fallahi P, Lumetti F, Spinella A, et al. Serum 25-OH vitamin D levels in systemic sclerosis: Analysis of 140 patients and review of the literature. Clin Rheumatol. 2017;36:583–90. doi: 10.1007/s10067-016-3535-z. [DOI] [PubMed] [Google Scholar]
- 21.Belloli L, Ughi N, Marasini B. Vitamin D in systemic sclerosis. Clin Rheumatol. 2011;30:145–6. doi: 10.1007/s10067-010-1564-6. [DOI] [PubMed] [Google Scholar]
- 22.Zold E, Szodoray P, Gaal J, Kappelmayer J, Csathy L, Gyimesi E, et al. Vitamin D deficiency in undifferentiated connective tissue disease. Arthritis Res Ther. 2008;10:R123. doi: 10.1186/ar2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atteritano M, Sorbara S, Bagnato G, Miceli G, Sangari D, Morgante S, et al. Bone mineral density, bone turnover markers and fractures in patients with systemic sclerosis: A case control study. PLoS One. 2013;8:1–6. doi: 10.1371/journal.pone.0066991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groseanu L, Bojinca V, Gudu T, Predeteanu D, Balanescu A, Berghea F, et al. Low vitamin D status in systemic sclerosis and the impact on disease phenotype. Eur J Rheumatol. 2016;3:50–5. doi: 10.5152/eurjrheum.2015.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ursini F, D’Angelo S, Padula A, Leccese P, Abignano G, Mennillo GA, et al. Vitamin D deficiency in systemic sclerosis: A possible role of subclinical liver fibrosis? Retrospective analysis from an Italian cohort. Clin Rheumatol. 2017;36:2871–2. doi: 10.1007/s10067-017-3709-3. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham BB, Landells ID, Langman C, Sailer DE, Paller AS. Topical calcipotriene for morphea/linear scleroderma. J Am Acad Dermatol. 1998;39(2Pt 1):211–5. doi: 10.1016/s0190-9622(98)70077-5. [DOI] [PubMed] [Google Scholar]
- 27.Terao M, Yang L, Matsumura S, Yutani M, Murota H, Katayama I. A vitamin D analog inhibits Th2 cytokine- and TGFβ -induced periostium production in fibroblasts: A potential role for vitamin D in skin sclerosis. Dermatoendocrinol. 2015;7:e1010983. doi: 10.1080/19381980.2015.1010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:12736. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]


