Abstract
Pediatric reading disorder (RD) is associated with an increased risk of anxiety symptoms, yet understudied are the neurobiological factors that might underlie anxiety in children with RD. Given the role of the amygdala in anxiety, we assessed resting state functional connectivity of amygdalar subregions in children with RD to identify functional correlates of anxiety and reading impairment. We collected resting state functional MRI data from 22 children with RD and 21 typically developing (TD) children, ages 7 to 13 years. We assessed group differences in resting state functional connectivity (RSFC) from amygdalar subregions. Associations of amygdalar RSFC and volume with reading impairment, reading fluency scores, and anxiety symptoms were explored. Relative to TD children, those with RD showed increased RSFC from amygdalar nuclei to medial prefrontal cortex. Across all subjects, RSFC from right centromedial amygdala to left medial prefrontal cortex positively predicted both reading impairment and self-reported anxiety, and anxiety mediated the relationship between RSFC and reading impairment. These findings are consistent with amygdalar functional abnormalities in pediatric anxiety disorders, suggesting a common neurobiological mechanism underlying anxiety and reading impairment in children. Thus, aberrant patterns of RSFC from amygdalar subregions may serve as potential targets for the treatment of anxiety symptoms that typically co-occur with RD. Our dimensional approach to studying anxiety in RD revealed how amygdalar connectivity underlies anxiety and reading impairment across a continuum from normal to abnormal.
1 | INTRODUCTION
Reading disorder (RD) is characterized by persistent difficulties in reading that are unexpected given a child’s developmental level (American Psychiatric Association, 2013). RD is the most common learning disorder, affecting 5–17% of children, and children with RD experience academic difficulties throughout their lives (Lyon, 1996). Children with RD are at increased risk for anxiety symptoms (Greenham, 1999) and epidemiological findings suggest high rates of comorbid RD and anxiety disorders (AD) in children (Margari et al., 2013). Increased anxiety symptoms predict deficits in oral reading fluency (Grills-Taquechel, Fletcher, Vaughn, & Stuebing, 2012), as well as future academic problems in children with RD (Raskind, Goldberg, Higgins, & Herman, 1999). The co-occurrence of reading impairment and anxiety symptoms may arise from the negative influence of anxiety on achievement (Everson, Smodlaka, & Tobias, 1994), or from the influence of reading problems on anxiety (Carroll, Maughan, Goodman, & Meltzer, 2005). Alternatively, a bidirectional relationship might explain the co-occurrence wherein reading difficulties lead to anxiety symptoms, which in turn lead to continued difficulty reading (Grills-Taquechel et al., 2012). Both reading impairment and anxiety symptoms may arise from a common neurobiological mechanism such as functional connectivity from amygdala to prefrontal cortices since prefrontal cortices modulate amygdala responses to exert control over emotions, thereby implicating these circuits in anxiety and emotion regulation (Diamond, 2013; Ochsner & Gross, 2005; Taylor & Whalen, 2015). FMRI findings suggest that age-related decreases in connectivity from amygdala to medial and dorsolateral prefrontal cortices from childhood to adolescence coincides with the development of emotion regulation (Gee et al., 2013; Prater, Hosanagar, Klumpp, Angstadt, & Phan, 2013), and that connectivity from amygdala to ventral and medial prefrontal regions is altered in children with anxiety disorders (Hamm et al., 2014; Prater et al., 2013; Roy et al., 2013; Sylvester et al., 2013; Toazza et al., 2016). Amygdala to prefrontal connectivity is also altered in association with self-reported math anxiety (Supekar, Iuculano, Chen, & Menon, 2015; Wu, Willcutt, Escovar, & Menon, 2014; Young, Wu, & Menon, 2012), but the neural correlates of anxiety related to reading have not yet been investigated. Thus, we used fMRI in conjunction with neuropsychological assessments of anxiety and reading ability to assess resting state functional connectivity (RSFC) within amygdala-prefrontal circuits in children with reading disorder and to explore the relationship between RSFC, anxiety, and reading impairment.
Resting state fMRI studies of RD have focused exclusively on connectivity within the reading network (Koyama et al., 2011, 2013). Findings from these studies suggest that reading competence is associated with positive connectivity between regions within a left hemisphere reading network, including Broca’s and Wernicke’s areas, and relative to their typically developing peers, children with RD show weaker connectivity from left intraparietal sulcus to left fusiform gyrus. In contrast to the sparse findings from RD samples, RSFC has been used extensively to study the neural underpinnings of anxiety, with focus on the amygdala-prefrontal circuits involved in emotion regulation (Brown et al., 2014; Etkin, 2010; Gee et al., 2013; Hahn et al., 2011; Hamm et al., 2014; S.H. Kim & Hamann, 2007; Liao et al., 2010; Prater et al., 2013; Qin et al., 2014; Roy et al., 2009, ****2013; Sripada et al., 2012; Sylvester et al., 2013; Toazza et al., 2016).
Findings of altered amygdala-prefrontal RSFC in children with anxiety disorders are inconsistent, with some reporting increased (Roy et al., 2013; Toazza et al., 2016) and others reporting decreased (Hamm et al., 2014; Sylvester et al., 2013) connectivity from amygdala to prefrontal cortices compared to healthy children. These discrepancies likely arise from differences across the ages and specific anxiety disorders included in the samples and methodological differences, including the regions or subregions used as seeds in the RSFC analyses. Nevertheless, positive amygdala-prefrontal RSFC in children with anxiety disorders contrasts with negative RSFC in healthy children (Gee et al., 2013; Qin et al., 2014), suggesting that positive amygdala-frontal connectivity may be a mechanism underlying anxiety in children.
Studies of pediatric anxiety have increasingly focused on RSFC from amygdalar subregions, as specific amygdala nuclei have been identified and implicated in distinct processes involved in fear learning and extinction (Roy et al., 2009). For example, the basolateral amygdala (BLA) is typically associated with associative learning and fear conditioning (LeDoux, 2003; Roy et al., 2009), and the centromedial amygdala (CMA) with control of responses to fearful stimuli (LeDoux, 2003; Qin, Young, Supekar, Uddin, & Menon, 2012; Roy et al., 2009). Pediatric RSFC findings suggest that sub-clinical anxiety symptoms are associated with increased functional connectivity from BLA and CMA to multiple brain regions involved in emotion perception and regulation, including ventromedial prefrontal cortex, with BLA-based connectivity emerging as a stronger predictor of subclinical anxiety than CMA-based connectivity (Qin et al., 2014).
Herein, we used resting state fMRI to assess whether aberrant RSFC from amygdalar nuclei underlies the anxiety symptoms that frequently accompany RD (Carroll et al., 2005). Given previous findings from children with subclinical anxiety (Qin et al., 2014), we hypothesized that functional connectivity from BLA to prefrontal cortices would be increased in children with RD compared to their healthy counterparts. We further hypothesized that such increased connectivity would predict reading impairment and anxiety symptoms in school-aged children, and that anxiety would mediate the relationship between amygdala-prefrontal RSFC and reading impairment. Finally, consistent with prior findings that anxiety associates with poor oral reading fluency, we explored the relationship between RSFC and reading fluency in children with and without RD. This study represents an initial effort to investigate functional correlates of anxiety associated with RD and to identify predictive biomarkers to identify children who are at risk for learning disorders and co-occurring anxiety symptoms.
2 | METHODS
2.1 | Participants
Twenty-four children diagnosed with RD and 21 typically developing (TD) children were recruited from private schools and clinics in the New York City metropolitan area. All children were between the ages of 7 and 12 years and were native, monolingual English speakers. Children were excluded if they had taken any psychotropic medication or had any lifetime diagnosis of neurological or neurodevelopmental disorder other than Specific Learning Disorder, such as ADHD, or any current/past Axis 1 disorder, as determined by clinical interview and administration of the Kiddie-SADS by a trained research assistant and confirmed by a licensed psychologist. Children were included in the RD group if an RD diagnosis was indicated by clinical history and by their poor performance (at or below 25th percentile) on at least three measures of reading (described below). Two independent licensed psychologists with expertise in the assessment of RD reviewed case information to confirm diagnosis and group membership.
All participants were provided a $120 Toys R Us gift card, pictures of their brain from the MRI scan, and an abbreviated neuropsychological report in exchange for participation. The Institutional Review Board of the New York State Psychiatric Institute approved this study, including the informed consent and assent procedures for all participants.
2.2 | Neuropsychological, reading, and psychosocial measures
A comprehensive neuropsychological test battery was administered by a trained research assistant (see Supplemental Information). Children completed measures of intellectual functioning (Wechsler Abbreviated Scale of Intelligence [WASI]), including verbal ability (Vocabulary and Similarities subtests) and non-verbal ability (Block Design and Matrix Reasoning subtests), as well as verbal memory (Children’s Memory Scale [CMS] Story Memory, Word Pairs, and Numbers) and motor functioning (Wechsler Intelligence Scale for Children [WISC-IV] Coding). Children also completed measures of cognitive skills underlying reading including phonological processing (Test of Word Reading Efficiency [TOWRE-2] Phonemic Decoding Efficiency, Woodcock Johnson Achievement Tests [WJ-III] Word Attack) and rapid naming (Comprehensive Test of Phonological Processing [CTOPP-2] Rapid Digit Naming, Rapid Letter Naming). In addition, children completed measures of reading achievement, including decoding/encoding (WJ-III Letter-Word Identification, WJ-III Spelling, TOWRE-2 Sight Word Efficiency, Gates Oral Reading Test [GORT-5] Accuracy), reading fluency (GORT-5 Rate, WJ-III Reading Fluency), and reading comprehension (Gates-MacGinitie Reading Comprehension, GORT-5 Comprehension). Finally, children completed psychosocial measures of anxiety (Revised Children’s Manifest Anxiety Scale [RCMAS]) and depression (Children’s Depression Rating Scale [CDRS-R]). Parents completed the DuPaul AD/HD rating scale – fourth edition (DRS) and the Hollingshead Four-Factor Index of Socioeconomic Status (SES). See Supplemental Information for detailed test descriptions.
2.3 | Reading impairment score
A reading impairment score was created by assigning one point for each of the following: (1) each reading measure on which participants scored at or below the 25th percentile, (2) a history of academic difficulty (teacher report), (3) a history of having received reading intervention, (4) a prior diagnosis of a reading disorder, and (5) placement in a special education school. A higher reading impairment score indicates worse overall reading proficiency with high concurrent and convergent validity (ps < .001, see Supplemental Information).
2.4 | Imaging data acquisition
Participants were scanned using a General Electric Signa 3-Tesla LX scanner (Milwaukee, WI). A high resolution T1-weighted fast field echo (FFE) structural scan was acquired, followed by two 5-minute resting state axial echo-planar imaging (EPI) scans (TR = 2200 ms, TE = 30 ms, 90 degree flip angle, slice thickness = 3.5 mm, 140 slices, 24 × 24 cm field of view and 64 × 64 matrix, providing a resolution of 3.75 × 3.75 × 3.5 mm and whole-brain coverage), using a standard quadrature 32-channel head coil. For resting state image acquisition, participants were instructed to rest quietly and let their minds wander while focusing their eyes on a white fixation cross without falling asleep for the duration of two 5-minute scans. An eye-tracking camera allowed the examiner to ensure that participants kept their eyes open during these scans.
2.5 | Resting state functional connectivity analysis
Preprocessing was carried out using Statistical Parametric Mapping 12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and the CONN-fMRI Functional Connectivity Toolbox v 16.b (http://www.nitric.org/projects/conn/), with MATLAB version R2015a. Preprocessing of functional images included slice timing correction, motion correction using a six-parameter rigid body transformation, band-pass filtering (0.01 Hz<f<0.1 Hz), spatial coregistration to subject-specific structural scans, spatial normalization to MNI space, and spatial smoothing using a 6-mm full-width-at-half-maximum Gaussian kernel. T1 structural scans were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using Voxel-Based Morphometry 8 toolbox (http://www.neuro.uni-jena.de/vbm/) and then normalized to the Montreal Neurological Institute (MNI) template. Normalization parameters were applied to functional images to bring them into MNI space. Preprocessing procedures also included outlier detection using Artifact Detection Tools implemented in CONN, and within each subject, runs requiring artifact repair at more than 15% total time points were discarded.
2.6 | Seed-to-voxel connectivity analysis
Blood-oxygen level dependent (BOLD) signal was correlated voxel-by-voxel for each participant across the resting state time series. Fisher z transformation was applied. Seed-to-voxel connectivity maps were then generated in CONN using bilateral BLA and CMA seeds, derived from the Juelich Histological Atlas (Eickhoff et al., 2005). Head motion realignment parameters were used as nuisance regressors, and aCompcor was used to correct for physiological noise by regressing out signal from white matter and CSF regions that was unlikely to be related to neural activity. This approach limits the influence of confounds such as head motion, peripheral physiology, and other imaging artifacts (Behzadi, Restom, Liau, & Liu, 2007). The resulting residual time series was band-pass filtered (0.01–0.1 Hz) to reduce the effect of very low frequency and high frequency physiological noise. Additional denoising included frame censoring, applied using the image scrubbing ART method in the CONN toolbox with a frame-wise displacement threshold of 0.5, as well as linear de-trending. A recent systematic evaluation of confound regression methods (Ciric, 2017) suggests that global signal regression (GSR) with 36 parameters and aCompCor are similarly effective in the mitigation of motion. Both GSR and aCompCor regress signal from white matter and cerebral spinal fluid, but GSR additionally regresses signal from gray matter, thus moving the distribution of the correlation coefficients closer to 0 and affecting the interpretability of negative correlation coefficients (Murphy, Birn, Handwerker, Jones, & Bandettini, 2009). Since negative correlations from amygdala nuclei are characteristic of healthy children included in studies of pediatric anxiety disorders (Gee et al., 2013; Qin et al., 2014), we elected to use aCompCor. However, given controversy in the field regarding selection of confound regression methods, supplemental analyses were conducted after denoising with GSR and 36 parameters (see Supplemental Information).
Group differences in RSFC from amygdalar subregions were assessed via independent two-sample t tests, and we present findings that reached significance at p < .05, corrected using family-wise error correction for multiple comparisons implemented in SPM12, with a voxel level significance of p < .001. We also present findings corrected for multiple comparisons with a more lenient primary threshold (p < .01), which should be interpreted with caution and need to be replicated.
2.7 | Neuropsychological correlates and mediating effects with reading impairment and anxiety
Beta weights corresponding to average connectivity strengths in significant clusters were extracted from CONN. These values were then used as predictors in multiple regression analyses (SPSS; SPSS Inc., Chicago, IL) to assess the significance of RSFC–neuropsychological associations across the entire sample, as all subjects (i.e., with and without RD) presented with at least some symptoms of both anxiety and reading impairment. Neuropsychological measures included as dependent variables were RCMAS total scores, reading impairment scores, and, given our specific interest in oral reading fluency, scores on the GORT-5 (i.e., oral reading fluency, accuracy, and rate). Age and sex were included as covariates. To test our a priori hypothesis that anxiety mediates the relationship between amygdalar RSFC and reading impairment, connectivity values were entered as independent variables in a mediation model with reading impairment scores as dependent variables, RCMAS scores as mediators, and age and sex as covariates. The mediation model used a bootstrapping approach (5000 simulations; Hayes, 2013), which makes fewer assumptions about the shape of the sampling distribution of the indirect effect than do other commonly used techniques such as the Sobel test, and is thus more valid than a simple regression approach. Models using connectivity values as either mediators or dependent variables were not tested given prior data suggesting that aberrant amygdala-prefrontal connectivity causes anxiety, rather than the reverse (Gee et al., 2013; Liao et al., 2010; Rigoux & Daunizeau, 2015).
3 | RESULTS
3.1 | Subject characteristics
Following quality control, resting state data were available from 22 RD and 21 TD children. Complete sets of resting state data were excluded from two RD children and one run of data was excluded from 10 participants (5 RD and 5 TD) due to excessive motion. The groups did not differ in age (RD mean age 122.50 months, SD = 22.17; TD mean age 117.19 months, SD = 14.85), gender, handedness, race/ethnicity, SES, or ADHD symptomatology (Table 1). The groups differed in Full Scale IQ scores (p = .009), but all participants had scores at or above the top of the average range.
TABLE 1.
Group characteristics
| Characteristic | Participants | Analysis | |||
|---|---|---|---|---|---|
|
| |||||
| RD (n = 22) | TD (n = 21) | ||||
|
|
|
||||
| Mean | SD | Mean | SD | p | |
| Age, months | 122.50 | 22.17 | 117.19 | 14.85 | .36 |
|
| |||||
| Sex | .46 | ||||
|
| |||||
| Ethnicity | .13 | ||||
|
| |||||
| SES | 54.65 | 4.51 | 56.43 | 4.45 | .21 |
|
| |||||
| Handedness | .74 | ||||
|
| |||||
| DuPaul ADHD Rating Scale | 11.05 | 7.52 | 8.71 | 8.98 | .367 |
|
| |||||
| Reading impairment | 10.96 | 3.98 | 1.29 | 1.06 | <.01 |
|
| |||||
| WASI | |||||
|
| |||||
| Full-4 IQ | 113.91 | 15.43 | 126.71 | 13.51 | .01 |
|
| |||||
| Verbal IQ | 111.96 | 16.18 | 126.29 | 12.85 | <.01 |
|
| |||||
| Performance IQ | 112.82 | 15.61 | 120.62 | 13.06 | .08 |
|
| |||||
| GORT-5 | |||||
|
| |||||
| Rate | 7.00 | 3.98 | 12.14 | 2.54 | <.01 |
|
| |||||
| Accuracy | 7.18 | 1.68 | 11.57 | 2.66 | <.01 |
|
| |||||
| Fluency | 7.18 | 1.89 | 11.91 | 2.53 | <.01 |
|
| |||||
| RCMAS Total Score | 11.14 | 5.68 | 6.19 | 5.72 | .01 |
Note. FSIQ, full scale IQ; GORT, Gray Oral Reading Test; RCMAS, Revised Children’s Manifest Anxiety Scale; RD, Reading Disorder; SES, socioeconomic status; TD, Typically Developing.
3.2 | Neuropsychological data
Independent samples t tests were used to compare RD and TD groups on neuropsychological measures (Table 1). As expected, compared to TD children, those with RD had significantly more reading impairment (p < .001) and, specifically, worse oral reading fluency (GORT-5 rate, accuracy, fluency; all ps < .001). Although all subjects had RCMAS scores within the normal range, compared to TD children, those with RD had significantly more anxiety symptoms (p = .008). Across the entire sample, anxiety was positively associated with reading impairment (p = .006) and inversely associated with reading fluency (p = .024). We further assessed associations with specific skills that comprise reading fluency (i.e., reading rate and accuracy), and reading rate more strongly predicted symptoms of anxiety than reading accuracy (rate p = .005, accuracy p = .026); thus, reading rate was used in all subsequent analyses.
3.3 | Seed-to-voxel connectivity analysis
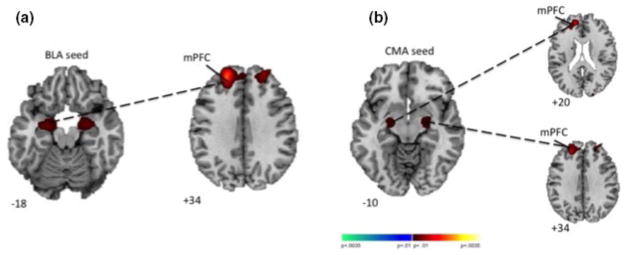
Group differences in RSFC were detected from left BLA to a medial prefrontal cluster comprising left superior medial and middle frontal gyri (peak: −20, 46, 34; FWE-corrected with voxel-level p < .001; Figure 1, Figure S1, Table 2), deriving from positive connectivity in the RD group in contrast to negative connectivity in the TD group. Group differences were also detected from bilateral CMA to medial prefrontal clusters comprising left superior medial and middle frontal gyri (left CMA peak: −22, 42, 34; right CMA peak: −14, 50, 20; FWE-corrected with voxel-level p < .01; Figure 1, Figure S1, Table 2), deriving from positive connectivity in the RD group in contrast to negative connectivity in the TD group. A supplemental analysis after denoising with GSR and 36 parameters produced results that were nearly identical to, but more right lateralized, than those using aCompCor (see Supplemental Information).
FIGURE 1.
Group differences in RSFC from amygdalar nuclei. In the RD compared to TD groups, increased RSFC was detected from (A) left BLA to left mPFC and from (B) bilateral CMA to left mPFC. Increases in RSFC in the RD compared to TD group are shown in red. All statistical maps were generated in SPM, thresholded at p < .01, uncorrected, for display purposes. Abbreviations: BLA, basolateral amygdala; CMA, centromedial amygdala; mPFC, medial prefrontal cortex; RD, reading disorder; RSFC, resting state functional connectivity; TD, typically developing
TABLE 2.
Between-group connectivity differences (RD>TD)
| Seed | Target | Cluster size | MNI coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left BLA | Left mPFC | 549* | −20 | 46 | 34 |
| Left CMA | Left mPFC | 950** | −22 | 42 | 34 |
| Right CMA | Left mPFC | 983** | −14 | 50 | 20 |
Note. BLA, basolateral amygdala; CMA, centromedial amygdala; mPFC, medial prefrontal cortex; RD, Reading Disorder; TD, Typically Developing;
cluster sizes reported at height threshold p < .001, FDR-corrected cluster threshold p < .05,
cluster sizes reported at height threshold p < .01, FDR-corrected cluster threshold p < .05.
3.4 | Neuropsychological correlates and mediating effects on reading impairment and anxiety
Across all subjects, RSFC from left BLA and bilateral CMA to medial prefrontal cortex (mPFC) positively predicted reading impairment and negatively predicted reading fluency, including both reading rate and reading accuracy (ps < .01). RSFC from right CMA to left mPFC positively predicted anxiety symptoms (p < .013). In the RD group only, this pattern of connectivity from right CMA also increased with more anxiety symptoms, but this correlation was not statistically significant (p > .05; Figure S2). Anxiety symptoms partially mediated the relationship between this RSFC and reading impairment, explaining 32.2% of the association between RSFC and reading impairment (Figure 2, Direct Effect = 15.0773, CI: 0.6180, 29.5365; Indirect Effect = 7.1568, CI: 0.7992, 19.9076; p = .0035).
FIGURE 2.
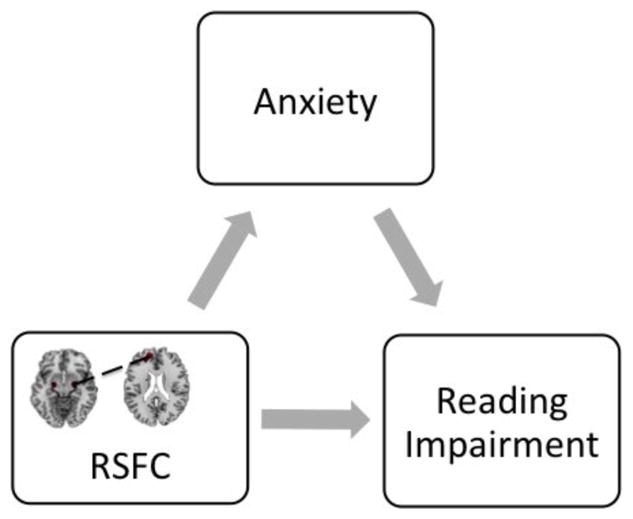
Mediating effects of anxiety symptoms on RSFC from right CMA to left mPFC and Reading impairment. Anxiety symptoms mediated the relationship between RSFC from right CMA to left mPFC and reading impairment. Direct Effect = 15.0773, CI: 0.6180, 29.5365; Indirect Effect = 7.1568, CI: 0.7992, 19.9076; p = .0035. CMA, centromedial amygdala; mPFC, medial prefrontal cortex; LLCI, lower level confidence interval; RSFC, resting state functional connectivity; ULCI, upper level confidence interval
4 | DISCUSSION
To our knowledge, this study is the first to demonstrate that abnormalities in the RSFC of amygdalar subregions might underlie the co-occurrence of reading impairment and anxiety symptoms in school-aged children. Compared to TD children, children with RD had significantly more anxiety than TD children. Across the entire sample, those with more anxiety symptoms had more reading impairment and, specifically, worse oral reading fluency. Consistent with our hypotheses, children with RD showed increased RSFC from amygdalar nuclei to mPFC. Across the entire sample, RSFC from CMA to mPFC correlated positively with both anxiety and reading impairment, and anxiety mediated the relationship between RSFC and reading impairment, thereby pointing to a potential mechanism underlying the co-occurrence of reading impairment and anxiety symptoms in children with RD.
These findings of amygdalar hyperconnectivity to mPFC may reflect abnormal connectivity within the emotion regulation system in children with RD (Qin et al., 2014). Theoretical frameworks of cognition– emotion correspondence identify two basic means of emotion regulation: extinction and reappraisal. Neuroimaging findings suggest that overlapping neural mechanisms underlie both strategies, suggesting a common emotion regulation circuit; specifically, increased prefrontal activity and concurrent decreased amygdala activity suggests that prefrontal cortex exerts control over amygdala during successful emotion regulation (M.J. Kim et al., 2011). In healthy individuals, negative coupling between amygdala and BA 9 suggests successful control of anxiety, and CMA is the amygdalar subregion primarily responsible for controlling responses to threat (LeDoux, 2003). In individuals with sub-clinical anxiety and anxiety disorders, increased amygdala-mPFC connectivity likely reflects ineffective inhibition of amygdala by medial prefrontal frontal regions or compensatory activation of medial prefrontal regions in the service of engaging regulatory control over excessive anxiety (Etkin, Prater, Schatzberg, Menon, & Greicius, 2009; Qin et al., 2012). Thus, positive coupling of CMA and mPFC in our sample may reflect similar circuit-level dysfunction across reading and anxiety disorders, thereby contributing to poor emotional control in children with RD.
Our finding that anxiety mediated the relationship between RSFC from right CMA to left mPFC and reading impairment suggests that reading deficits and anxiety symptoms may co-occur, in part, due to a shared neurobiological mechanism. This finding is consistent with prior findings that right CMA is implicated in fast, automatic processing of fearful visual stimuli (Baas, Aleman, & Kahn, 2004; Qin et al., 2012), and left mPFC is implicated in language and reading (Binder et al., 1997; Gabrieli, Poldrack, & Desmond, 1998; Hagoort & Indefrey, 2014; Smallwood et al., 2013), suggesting that visual detection of text may be automatically accompanied by heightened emotional reactivity in children with RD, making the visual sensory experience of reading emotionally provocative. Although anxiety and reading impairment may derive from aberrations in the same emotion regulation network, our mediation findings further suggest that anxiety may contribute to or exacerbate reading impairment, pointing to the need for more refined measures and treatment of anxiety in children with RD. Future task-based fMRI studies should assess whether RSFC from right amygdala to left mPFC is further increased during reading and reading-related tasks to better assess causal pathways linking RSFC, reading impairment, and anxiety. Moreover, future longitudinal studies should attempt to elucidate the development of this hyperconnectivity and its association with anxiety in children at risk for RD by examining changes in RSFC before children are exposed to print and after they learn to read. Findings from such a study would shed light on whether amygdala-mPFC hyperconnectivity is an epiphenomenon or, rather, contributes directly to reading problems and concurrent anxiety.
Our finding that RSFC associated with both anxiety and oral reading rate extends prior findings that children with anxiety have slow oral reading rate (Grills-Taquechel et al., 2012). In our sample, those with the most anxiety had the slowest oral reading rate, and both anxiety and slow reading rate associated with RSFC from CMA to mPFC. These data suggest that abnormal amygdalar connectivity may contribute to both anxiety and reading dysfluency. Such an interpretation is consistent with the role of these amygdalar circuits in the manifestation of emotion dysregulation (LeDoux & Pine, 2016; Qin et al., 2014). Thus, abnormal amygdalar connectivity may contribute to making the act of reading a slow, effortful, and anxiety-provoking experience for some children with RD. These findings suggest a potential opportunity for the development of novel cognitive-behavioral treatments for RD that could improve reading ability by targeting anxiety symptoms underlying slow and dysfluent reading.
These findings highlight the dimensional nature of pediatric learning disorders and associated anxiety symptoms. Reading proficiency and emotion regulation change across development, and behaviors that are considered normal at one age may be considered abnormal at another (Hudziak, Achenbach, Althoff, & Pine, 2007). Since children tend to manifest multiple kinds of problems, both within and among diagnostic categories (Hudziak et al., 2007), we quantified the degree to which children manifest both anxiety and reading problems across a continuum ranging from normal to abnormal, exploring how amygdalar connectivity associates with the entire range of those symptoms. Our RSFC data also complement categorical models of nosology, distinguishing children with and without RD by amygdalar connectivity. Thus, both dimensional and categorical models should be applied to future studies of neurodevelopmental disorders including reading disorder.
The current study has some limitations. First, our cross-sectional design precludes delineation of causal relationships between reading impairment, anxiety symptoms, and amygdalar connectivity. Although our primary mediation model was theory-driven and revealed that anxiety partially mediated the relationship between RSFC and reading impairment, longitudinal studies are indeed required to uncover the temporal order between RSFC, reading impairment, and anxiety as predictors, mediators and outcomes (de los Reyes, 2017). Second, our sample size was small and perhaps underpowered to detect significant brain–behavior correlations within the RD group. However, positive, albeit non-significant, relationships between RSFC and anxiety were detected within the RD group, suggesting that future studies including larger samples of children are necessary to verify these neural correlates of anxiety symptoms in RD. Third, our sample was quite homogeneous, consisting of participants who were recruited from private schools and clinics, thereby limiting the generalizability of our findings. Future studies with geographically, socioeconomically, and ethnically diverse populations are thus necessary. Fourth, our image resolution (64 × 64 matrix) may not be ideal for visualizing small amygdala subregions, but consistent with previous studies of amygdala subregions at 3T, we did not select a larger matrix in order to avoid image distortion (Li, Qin, Jiang, Zhang, & Yu, 2012). Future MRI research at ultra high field should continue to investigate functional connectivity of amygdala nuclei and clarify associations with anxiety symptoms in individuals with and without reading disorders. Finally, we reported some RSFC findings corrected at a voxel-level threshold that may be viewed as lenient. However, BOLD signal time series are significantly noisier in children than adults, making findings from studies of young children less likely to survive FDR-and FWE-corrected thresholds (Thomason, Burrows, Gabrieli, & Glover, 2005). Future studies should continue to attempt to overcome the technical issues that contribute to the increased noise in data from young children (i.e., developmental increases in brain electrical activity, respiration rates, heart rates, and vascular pulsivity, as well as in non-correlated head motion in the scanner; Thomason et al., 2005), and attempt to replicate these findings at more stringent thresholds. Group differences in patterns of connectivity from amygdala to mPFC remained when different confound regression methods were employed (i.e., aCompCor and GSR with 36 parameters), pointing to the similar effectiveness of these denoising procedures in mitigating motion in resting state data from child participants.
In conclusion, relative to TD peers, children with RD demonstrate increased symptoms of anxiety, as well as abnormal RSFC patterns from amygdala subregions. These amygdalar RSFC abnormalities are similar to those seen in children with anxiety disorder, suggesting that the symptoms of anxiety in pediatric RD are biologically based and, while oftentimes subclinical, interfere with functioning and warrant targeted assessment and treatment. Also, aberrant RSFC patterns may serve as potential biomarkers for anxiety symptoms that co-occur with RD. Consistent with the Research Domain Criteria (Insel et al., 2010), our examination of the neural correlates of anxiety symptoms across children with and without RD is a step towards conceptualizing RD and co-occurring anxiety within a new dimensional framework of developmental psychopathology.
Supplementary Material
RESEARCH HIGHLIGHTS.
Relative to typically developing children, those with reading disorder showed increased resting state functional connectivity from amygdalar nuclei to medial prefrontal cortex.
Connectivity from right centromedial amygdala to left medial prefrontal cortex predicted reading impairment and anxiety, and anxiety mediated the relationship between connectivity and reading impairment.
These findings are consistent with amygdalar functional abnormalities in pediatric anxiety disorders, suggesting a common neurobiological mechanism underlying anxiety and reading impairment in children.
Our dimensional approach to studying anxiety in reading disorder revealed how amygdalar connectivity underlies anxiety and reading impairment across a continuum from normal to abnormal.
Acknowledgments
Funding information
Promise Project at Columbia
This work was supported by Promise Project at Columbia. National Institute of Environmental health Sciences (NIEHS) K23ES026239
Footnotes
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: A systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Beall SK, Van Voorhees E, … Morey RA. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JM, Maughan B, Goodman R, Meltzer H. Literacy difficulties and psychiatric disorders: Evidence for comorbidity. Journal of Child Psychology and Psychiatry. 2005;46:524–532. doi: 10.1111/j.1469-7610.2004.00366.x. [DOI] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Satterthwaite TD. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage. 2017;154:174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Reyes A. Inaugural editorial: Making the Journal of Clinical Child & Adolescent Psychology your “home journal”. Journal of Clinical Child and Adolescent Psychology. 2017;46:1–10. doi: 10.1080/15374416.2016.1266649. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: A neural circuit perspective. Current Topics in Behavioral Neurosciences. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Everson HT, Smodlaka I, Tobias S. Exploring the relationship of test anxiety and metacognition on reading test performance: A cognitive analysis. Anxiety, Stress, and Coping. 1994;7:85–96. [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences, USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. Journal of Neuroscience. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenham SL. Learning disabilities and psychosocial adjustment: A critical review. Child Neuropsychology. 1999;5:171–196. [Google Scholar]
- Grills-Taquechel AE, Fletcher JM, Vaughn SR, Stuebing KK. Anxiety and reading difficulties in early elementary school: Evidence for unidirectional- or bi-directional relations? Child Psychiatry and Human Development. 2012;43:35–47. doi: 10.1007/s10578-011-0246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P. The neurobiology of language beyond single words. Annual Review of Neuroscience. 2014;37:347–362. doi: 10.1146/annurev-neuro-071013-013847. [DOI] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, … Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Hamm LL, Jacobs RH, Johnson MW, Fitzgerald DA, Fitzgerald KD, Langenecker SA, … Phan KL. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biology of Mood and Anxiety Disorders. 2014;4:15. doi: 10.1186/s13587-014-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. 2013 Retrieved 24 August 2016 from: http://www.guilford.com/books/Introduction-to-Mediation-Moderation-and-Conditional-Process-Analysis/Andrew-Hayes/9781609182304.
- Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International Journal of Methods in Psychiatric Research. 2007;16:S16–S23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, Schwartz SJ, … Milham MP. Cortical signatures of dyslexia and remediation: An intrinsic functional connectivity approach. PLoS ONE. 2013;8:e55454. doi: 10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, … Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. Journal of Neuroscience. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: A two-system framework. American Journal of Psychiatry. 2016;173:1083–1093. doi: 10.1176/appi.ajp.2016.16030353. [DOI] [PubMed] [Google Scholar]
- Li Y, Qin W, Jiang T, Zhang Y, Yu C. Sex-dependent correlations between the personality dimension of harm avoidance and the resting-state functional connectivity of amygdala subregions. PLoS ONE. 2012;7:e35925. doi: 10.1371/journal.pone.0035925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, … Chen H. Altered effective connectivity network of the amygdala in social anxiety disorder: A resting-state fMRI study. PLoS ONE. 2010;5:e15238. doi: 10.1371/journal.pone.0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GR. Learning disabilities. The Future of Children. 1996;6:54–76. [PubMed] [Google Scholar]
- Margari L, Buttiglione M, Craig F, Cristella A, de Giambattista C, Matera E, … Simone M. Neuropsychopathological comorbidities in learning disorders. BMC Neurology. 2013;13:198. doi: 10.1186/1471-2377-13-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depression and Anxiety. 2013;30:234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V. Amgydala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biological Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Supekar K, Uddin LQ, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences, USA. 2012;109:7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MH, Goldberg RJ, Higgins EL, Herman KL. Patterns of change and predictors of success in individuals with learning disabilities: Results from a twenty-year longitudinal study. Learning Disabilities Research & Practice. 1999;14:35–49. [Google Scholar]
- Rigoux L, Daunizeau J. Dynamic causal modelling of brain–behaviour relationships. NeuroImage. 2015;117:202–221. doi: 10.1016/j.neuroimage.2015.05.041. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JSA, Daniele T, Carlisi C, … Ernst M. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:290–299 e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, … Milham MP. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Gorgolewski KJ, Golchert J, Ruby FJM, Engen H, Baird B, … Margulies DS. The default modes of reading: Modulation of posterior cingulate and medial prefrontal cortex connectivity associated with comprehension and task focus while reading. Frontiers in Human Neuroscience. 2013;7:734. doi: 10.3389/fnhum.2013.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry and Neuroscience: JPN. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Iuculano T, Chen L, Menon V. Remediation of childhood math anxiety and associated neural circuits through cognitive tutoring. Journal of Neuroscience. 2015;35:12574–12583. doi: 10.1523/JNEUROSCI.0786-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Barch DM, Corbetta M, Power JD, Schlaggar BL, Luby JL. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:1326–1336. e5. doi: 10.1016/j.jaac.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Whalen PJ. Neuroimaging and anxiety: The neural substrates of pathological and non-pathological anxiety. Current Psychiatry Reports. 2015;17:49. doi: 10.1007/s11920-015-0586-9. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Burrows BE, Gabrieli JDE, Glover GH. Breath holding reveals differences in fMRI BOLD signal in children and adults. NeuroImage. 2005;25:824–837. doi: 10.1016/j.neuroimage.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Toazza R, Franco AR, Buchweitz A, Molle RD, Rodrigues DM, Reis RS, … Manfro GG. Amygdala-based intrinsic functional connectivity and anxiety disorders in adolescents and young adults. Psychiatry Research: Neuroimaging. 2016;257:11–16. doi: 10.1016/j.pscychresns.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Wu SS, Willcutt EG, Escovar E, Menon V. Mathematics achievement and anxiety and their relation to internalizing and externalizing behaviors. Journal of Learning Disabilities. 2014;47:503–514. doi: 10.1177/0022219412473154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CB, Wu SS, Menon V. The neurodevelopmental basis of math anxiety. Psychological Science. 2012;23:492–501. doi: 10.1177/0956797611429134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.