Abstract
Incontinence is a common health problem among nursing home (NH) residents. Differences between black and white NH residents in incontinence prevalence have been reported. Although reducing health disparities is a principal objective of the national health care agenda, little is known about disparities in incidence of new incontinence in NHs. The purpose of this study was to assess whether there were racial/ethnic disparities in the time to development of incontinence in adults over age 65 who had been continent on NH admission. If no racial or ethnic disparities in time to incontinence were found, other predictors of time to incontinence would be explored. Three national databases were sources of data on 42,693 adults over 65 admitted to 446 for-profit NHs in a national chain. Multilevel predictors of time to any type of incontinence were analyzed, using Cox proportional hazards regression for white Non-Hispanic NH admissions and the Peters-Belson method for minority NH admissions: American Indians/Alaskan Natives, Asians/Pacific Islanders, Black non-Hispanics, and Hispanics. No racial/ethnic disparities in time to incontinence were found. Approximately 30% of all racial/ethnic groups had developed incontinence by 6 months. Those who developed incontinence sooner were older and had greater deficits in activities of daily living (ADL) and cognition. Results were consistent with past evidence and suggest that interventions to maintain continence from the time of admission should be applied across racial/ethnic groups.
Keywords: incontinence, disparities, epidemiology, nursing home, race and ethnicity, incidence, proportional hazards
Approximately half of residents in nursing homes (NHs) in the United States are incontinent (Bliss et al., 2013; Shamliyan, Wyman, Bliss, Kane, & Wilt, 2007), and Boyington et al. (2007) reported a significantly greater post-admission prevalence of urinary incontinence among Black versus White NH residents. Given the growing elderly and minority populations and increasing percentages of older minorities in NHs (Feng, Fennell, Tyler, Clark, & Mor, 2011; Lakdawalla et al., 2003), incontinence may add to racial or ethnic differences in clinical outcomes of NH residents. The goal of this study was to assess racial and ethnic disparities in the time to incontinence in a cohort of older individuals admitted to NHs.
Incontinence Prevalence
Most of what is known about incontinence or racial and ethnic disparities in incontinence in NHs is based on cross-sectional prevalence studies (Boyington et al., 2007; Shamliyan et al., 2007) that cannot be used to establish risk factors for incontinence development after admission or fully ascertain the impact of care practices during the NH stay. Incidence of incontinence in NHs has been determined mainly by changes in prevalence over time, and most studies have focused only on urinary incontinence. Among 293 individuals admitted to eight NHs in Maryland, at 2 months after admission, the prevalence of daytime urinary incontinence was 23%, and at 1 year it was 22% (Ouslander, Palmer, Rovner, & German, 1993). Saxer, Halfens, de Bie, and Dassen (2008) observed that a 51% prevalence of urinary incontinence among admissions to 42 Swiss NHs increased by 15% at 1 year and by 26% at 2 years after admission. In Wisconsin NH residents, there was an increase of 7.1% in the prevalence of any urinary incontinence (with or without fecal incontinence), from 53.9% to 61.0% over 2 years (Nelson, Furner, & Jesudason, 2001).
Disparities in Incontinence Rates in Nursing Home Residents
There have been no investigations of racial and ethnic disparities in the incidence (new cases) of incontinence in NHs. A health disparity is present when disadvantaged social groups who have systematically experienced disadvantage or discrimination experience worse health or greater health risks than more advantaged social groups, and those differences could be reshaped by healthcare policies and actions (Braveman, 2006). Reducing health disparities has been proposed as the most promising opportunity to improve health outcomes of disadvantaged, minority individuals (House, Lantz, & Herd, 2005) and is a priority of the national health care agenda (Centers for Medicare and Medicaid Services, 2013; US Department of Health and Human Services, 2011). Because incontinence is an important risk factor for pressure ulcers, and the pressure ulcer prevalence is greater in Blacks than Whites among high risk NH residents (Baumgarten et al., 2004; Li, Yin, Cai, Temkin-Greener, & Mukamel, 2011) reducing disparities in incontinence may reduce the disparity in pressure ulcers.
Individual-Level Correlates of Incontinence in Nursing Home Residents
In cross-sectional studies of NH residents, factors that have been associated with incontinence are at the individual level (i.e., resident characteristics) and from bivariate analyses in some cases (Nelson, Furner, & Jesudason, 1998; Nelson et al., 2001). Correlates vary across studies, and populations and methods differ. Fecal and urinary incontinence are not always clearly distinguished.
The most consistent factors associated with incontinence appear to be deficits in activities of daily living (ADLs) and cognition. Other factors include older age, male or female gender, mobility impairment, vision problems, depression, receiving medications for emotional and mental problems, restraint use, urinary tract infection (for urinary incontinence), diarrhea (for fecal incontinence), constipation or fecal impaction, weight, stroke, Parkinson's disease, and diabetes (Aggazzotti et al., 2000; Aslan, Beji, Erkan, Yalcin, & Gungor, 2009; Borrie & Davidson, 1992; Chen, Hwang, Chen, Chen, & Lan, 2009; Nelson et al., 1998; Nelson et al., 2001; Offermans, Du Moulin, Hamers, Dassen, & Halfens, 2009; Sgadari, Topinkova, Bjornson, & Bernabei, 1997; Shamliyan et al., 2007). No multivariate analyses were found of risk factors for the development of incontinence in residents who had been continent on admission.
Institutional and Community-Level Contributors to Disparities in Incontinence
An “individualistic fallacy” may occur when population-level patterns in outcomes are presumed to be due solely to individual-level characteristics (Diez-Roux, 1998; Kreiger et al., 2005). Disparities in institutions or communities may contribute to the etiology of incontinence or other conditions (Diez-Roux, 1998; Kreiger et al., 2005). For example, if treatment within nursing homes varies based on race or ethnicity, this may contribute to disparities in the time to development of incontinence. Disparities have been reported in treatment for some of the correlates of incontinence, such as Parkinson's disease (Lapane, Fernandez, & Friedman, 1999). Antidiabetic agents were administered at a lower rate for Black and Hispanic NH residents with diabetes than for Whites with diabetes (Allsworth, Toppa, Palin, & Lapane, 2005; Christian, Lapane, & Toppa, 2003). Racial/ethnic minority NH residents were less likely than White residents to receive secondary stroke prevention agents such as warfarin (Christian et al., 2003).
Greater deficiencies in the quality of nursing care have been reported in NHs with higher percentages of minorities, and minority residents have been segregated into NHs in poorer communities with fewer resources (Fennell, Feng, Clark, & Mor, 2010; Mor, Zinn, Angelelli, Teno, & Miller, 2004; Smith, Feng, Fennell, Zinn, & Mor, 2007). Disparate health outcomes have been reported based on the racial/ethnic composition of the NH, socioeconomic status or sociodemographic characteristics of the community around the NH, and the NH's geographic region (Diez-Roux, 1998; Miller, Papandonatos, Fennell, & Mor, 2006; Osypuk, 2010; Porell & Miltiades, 2002). As an example, residents in NHs with higher percentages of Hispanic residents were more likely to have pressure ulcers, which are associated with incontinence, after adjusting for resident characteristics (Gerardo, Teno, & Mor, 2009). Restraint use in 270 NHs in 10 states in different geographic areas varied after controlling for residents' physical and cognitive status (Phillips et al., 1996). Therefore, inclusion of multi-level predictors is critical in the analysis of racial and ethnic disparities in health outcomes (Diez-Roux, 1998; Krieger, Chen, Waterman, Rehkopf, & Subramanian, 2005).
The purpose of this study was to assess racial and ethnic disparities in the time to incontinence in a cohort of older individuals admitted to NHs, using predictors at the individual, NH, and community levels. If no racial or ethnic disparity in time to incontinence was found, a model would be created of a wider range of factors present at NH admission that predicted time to incontinence among all residents.
Methods
Data Sources
Data from the following sources were linked and analyzed: (1) the Minimum Data Set (MDS) version 2.0, containing demographic and comprehensive health assessment data of individual residents of a national for-profit chain of NHs from years 2000–2002; (2) the Online Survey, Certification, and Reporting (OSCAR), containing measures of NH staffing, the care environment, and deficiencies in quality of care, also from years 2000–2002; and (3) the 2000 US Census, containing socioeconomic and sociodemographic measures of the census tracts where the NHs were located. Data were received in coded and de-identified form, and the study was determined by a university institutional review board to be exempt from review.
Sample Selection
The cohort for analysis of time to incontinence consisted of older adults (over 65 years) admitted to a NH who were continent or usually continent of urine and/or feces (defined by a score of ≤ 1 for MDS items H1a and H1b) on their first full/admission MDS record. Residents with indwelling urinary catheters or ostomies were excluded because the MDS v. 2.0 classified residents with indwelling urinary catheters as continent, so it was not possible to distinguish between residents who had indwelling urinary catheters because of incontinence or for another reason (e.g., urinary retention); similarly, continence status could not be determined in those with ostomies.
Measures
Race and Ethnicity
Race and ethnicity were defined per the MDS, as American Indian or Alaskan Native (AIAN), Asian or Pacific Islander (API), Black non-Hispanic (Black), White non-Hispanic (White), and Hispanic. The racial and ethnic profile of the cohort was very comparable to older residents admitted to all Medicare/Medicaid-certified NHs in the United States during the same time period (Bliss et al., 2013).
Predictors of Incontinence
Relevant predictors were identified using the literature and expertise of the investigators and three clinical consultants and selected based on available data. Potential individual-level predictors were defined using single items in the data-sets when appropriate and established scales with good psychometric properties when possible. When no scale existed or single items were insufficient, composite measures were developed. Previously established statistical procedures for developing composite measures using the MDS (Savik, Fan, Bliss, & Harms, 2005) were followed. Scales and composite variables used in these models were coded so that a higher score indicated worse status. Single and composite predictors at the individual resident level are shown in Table 1.
Table 1.
Demographic, Functional, and Physical Characteristics of a Cohort of Older Nursing Home Admissionsby Race and Ethnicity1 Followed for Developing Incontinence
| Variable/Scale | MDS Item | AIANs2 | APIs3 | Blacks | Hispanics | Whites |
|---|---|---|---|---|---|---|
| # of Admissions | n = 229 | n = 530 | n = 2,615 | n = 618 | n = 38,698 | |
| n (%) unless otherwise indicated |
||||||
| Demographics | ||||||
| Age at Admission4 | AA3, AB1 | 77.92 (8.18) | 81.58 (7.25) | 79.14 (8.18) | 78.78 (8.10) | 81.33 (7.52) |
| Female Gender | AA2 | 131 (57.2) | 348 (65.7) | 1,615 (61.8) | 364 (58.9) | 26,810 (69.3) |
| ≥ High School Education | AB7 | 91 (39.7) | 298 (56.2) | 947 (36.2) | 206 (33.3) | 24,143 (62.4) |
| Functional and Physical Characteristics | ||||||
| Activities of Daily Living Deficit Scale4 (Morris et al., 1999) | G1aA, G1bA, G1eA, G1gA, G1hA, G1iA, G1jA, Range 0–28 | 8.32 (6.45) | 12.88 (5.38) | 9.68 (6.63) | 10.58 (6.60) | 10.07 (6.30) |
| Body Mass Index4 | K2a-b | 25.64 (5.28) | 23.02 (3.86) | 25.95 (5.48) | 26.40 (5.11) | 25.63 (5.19) |
| Bowel Problems | I2b, H2b-d | 36 (15.7) | 147 (27.7) | 281 (10.7) | 86 (13.9) | 7,206 (18.6) |
| Comorbidity Index Charlson Index4 (Charlson et al., 1987) | I3a-e, and/or I1, Range 0–30 | 1.91 (1.48) | 1.85 (1.65) | 2.12 (1.61) | 1.98 (1.66) | 1.61 (1.50) |
| Mortality risk CHESS Scale4 (Hirdes et al., 2003) | J1c, J1g, J1l, J1o, K3a,K4c, J5c, B6, G9, Range 0–5 | 1.28 (1.14) | 1.86 (0.94) | 1.31 (1.04) | 1.31 (0.98) | 1.63 (1.05) |
| Medications – Number in last week4 | O4a-e, Range 0–35 | 4.61 (5.10) | 3.03 (4.42) | 5.28 (5.54) | 4.99 (5.74) | 6.30 (5.97) |
| Oxygenation Problems Number of Indicators | J1b, J1k-l, P1ag, P1ai-j, P1al, P1bdA | |||||
| 1 | 18 (7.9) | 50 (9.4) | 217 (8.3) | 68 (11.0) | 4,462 (11.5) | |
| 2 | 18 (7.9) | 17 (3.2) | 136 (5.2) | 35 (5.7) | 3,074 (7.9) | |
| ≥3 | 8 (3.5) | 24 (4.5) | 92 (3.5) | 24 (3.9) | 2,261 (5.8) | |
| Perfusion Problems Number of Indicators | J1a, J1c-d, J1g | |||||
| 1 | 41 (17.9) | 133 (25.1) | 629 (24.1) | 119 (19.3) | 10,692 (27.6) | |
| ≥2 | 1 (0.4) | 18 (3.4) | 31 (1.2) | 17 (2.8) | 969 (2.5) | |
| Poor Nutrition Number of Indicators | K3a, K4c | |||||
| 1 | 70 (30.6) | 224 (42.3) | 838 (32.0) | 186 (30.1) | 14,407 (37.2) | |
| 2 | 15 (6.6) | 55 (10.4) | 102 (3.9) | 33 (5.3) | 2,520 (6.5) | |
| Highest Stage Pressure Ulcer = Stages 2, 3, and 4 | M2a | 23 (10.0) | 49 (9.2) | 217 (8.3) | 38 (6.1) | 2,293 (5.9) |
| Restraint use - Any | P4c-e | 3 (1.3) | 11 (2.1) | 29 (1.1) | 7 (1.1) | 319 (0.8) |
| Tube Feeding | K5b | 3 (1.3) | 17 (3.2) | 34 (1.3) | 11 (1.8) | 477 (1.2) |
| Vision Impairment Number of Indicators | D1, D2a, D2b | |||||
| 1 | 43 (18.8) | 106 (20.0) | 690 (26.4) | 123 (19.9) | 7,631 (19.7) | |
| 2 | 15 (6.6) | 29 (5.5) | 202 (7.7) | 46 (7.4) | 2,350 (6.1) | |
| ≥3 | 12 (5.2) | 5 (0.9) | 107 (4.1) | 23 (3.7) | 1,443 (3.7) | |
| Cognitive and Emotional Characteristics | ||||||
| Cognitive deficits MDS-COGS4 (Hartmaier et al., 1994) | B2a, B2b, B3b, B3d, B3e, B4, C4, G1gA, Range 0–10 | 1.72 (2.07) | 1.73 (1.92) | 2.28 (2.33) | 1.77 (2.29) | 1.72 (2.16) |
| Communication Difficulties4 adapted from (Hopper, Bayles, Harris, & Holland, 2001) | C1, C5, C6, C3b-f, Range 0–9 | 0.89 (1.3) | 1.16 (1.42) | 0.70 (1.04) | 0.85 (1.20) | 0.74 (1.11) |
| Delirium MDS-CAM (Dosa, Intrator, McNicoll, Cang, & Teno, 2007) | B5a-f, B6, E5 | |||||
| Subsyndromal Delirium | ||||||
| Level 1 | 41 (17.9) | 79 (14.9) | 227 (8.7) | 63 (10.2) | 4,577 (11.8) | |
| Level 2 | 15 (6.6) | 32 (6.0) | 141 (5.4) | 23 (3.7) | 2,598 (6.7) | |
| Full delirium | 0 (0) | 2 (0.4) | 10 (0.4) | 0 (0) | 183 (0.5) | |
| Depression – Any | E1a, E1d, E1f, E1h, E1i, E1l, E1m | 70 (30.6) | 81 (15.3) | 497 (19.0) | 127 (20.6) | 10,308 (26.6) |
| Discomfort Behavior Scale4 (Stevenson, Brown, Dahl, Ward, & Brown, 2006) | E1c, E1k, E1l-p, E4a-eA, E4a-eB, Range 0–102 | 4.68 (8.82) | 2.06 (5.20) | 3.00 (7.43) | 3.03 (6.70) | 3.07 (7.05) |
Race/ethnicity data were missing for three admissions that are not included in this table or the analysis of disparity;
AIAN=American Indian, Alaskan Native;
API=Asian, Pacific Islander;
mean (standard deviation)
NH composite variables were developed in four areas: (1) resident conditions (e.g., activities of daily living (ADLs), mental status, etc.), (2) facility deficiencies (summarizing the scope and severity levels of relevant deficiencies as well as calculating the total number of deficiencies), (3) percentages of NH admissions by gender, race, and ethnicity, and (4) staffing by the various types of clinical NH personnel.
Census-level variables included some variables in their original form, such as census tract divisions and indicators of census tract socioeconomic status (e.g., percent-age identified as working-class and median household income), and some composite variables, such as tract sociodemographic data (e.g., proportion of males <65 years of age). Key predictors that were evaluated for inclusion in the models are presented in Table 2. A more detailed description of variables at these levels and the formation of composite scores has been previously published (Bliss et al., 2015).
Table 2.
Characteristics of Nursing Homes and Their Surroundings
| Licensed Nurses (FTE1/ resident) |
Licensed Nurses (hours/ resident/d) |
CNA2 (FTE/ resident) |
CNA (hours/ resident /d) |
Total number of deficiencies3 |
Index of deficiency in quality of nursing home care4 |
% Residents on Medicaid | ||
|---|---|---|---|---|---|---|---|---|
| Mean (sd) | 0.22 (0.10) | 1.09 (0.48) | 0.44 (0.43) | 2.20 (2.13) | 3.78 (2.35) | 7.59 (6.30) | 73.79 (15.89) | |
|
| ||||||||
| Characteristics of Census Tract Community around Nursing Homes | ||||||||
|
| ||||||||
| Levels of Census Tract Community Characteristic | % American Indians/Asians/Pacific Islanders5 | % Non-Hispanic Blacks | % Hispanics | % Non-Hispanic Whites | % Below Poverty | % Working class | Proportion in an Urban Area | Proportion in a Rural Area |
|
| ||||||||
| n (%) of Nursing Home | ||||||||
|
| ||||||||
| < 25% | 434 (97.3)6 | 388 (87.0) | 406 (91.0) | 17 (3.8) | 406 (91.0) | 2 (0.4) | 210 (47.1) | 342 (76.7) |
| 25 to < 50% | 10 (2.2) | 34 (7.6) | 28 (6.3) | 41 (9.2) | 38 (8.5) | 46 (10.3) | 1 (0.2) | 36 (8.1) |
| 50 to < 75% | 0 (0.0) | 17 (3.8) | 9 (2.0) | 74 (16.6) | 2 (0.4) | 332 (74.4) | 13 (2.9) | 9 (2.0) |
| ≥ 75% | 2 (0.4) | 7 (1.6) | 3 (0.7) | 314 (70.4) | 0 (0.0) | 66 (14.8) | 222 (49.8) | 59 (13.2) |
Full-time equivalents/resident were calculated as total licensed nurse (registered and licensed practical nurses) and certified nursing assistant staffing reported for a two-week period (including full-time, part-time, and contract positions) divided by the total number of residents in a NH;.
CNA=Certified Nursing Assistant;
selected deficiencies relevant to the outcome;
scope/severity of selected quality of care deficiencies relevant to the outcome;
racial and ethnic categories are according to US Census;
as an example, 97.3% of NHs (n=434) in our sample were located in Census tract communities with <25% American Indian/Asian/Pacific Islander population
As a broad array of predictors was available, and evidence directing their selection was limited, all relevant predictors were initially considered for inclusion in the models. Those with a bivariate association with the outcome of time to incontinence at p < .05 were considered candidates. Bivariate associations between variables also were examined for potential collinearity. If an individual-level and NH/community-level variable were highly correlated, the individual-level variable was included in the model, as it was more specific.
Based on these analyses, predictors included in the model of disparities in time to incontinence were age, gender, ADL deficits (Morris, Fries, & Morris, 1999), comorbidities (Charlson index; Charlson, Pompei, Ales, & MacKenzie, 1987), indicators of vision problems, cognitive deficits (MDS COGS; Hartmaier, Sloane, Guess, & Koch, 1994), index of deficiencies in NH quality of care, percentage of NH residents receiving Medicaid, proportion of NH admissions that was White, proportion of the census tract community around the NH that was below poverty level or in an urban area, and the census divisions in which NHs were located.
Outcome Measurement
The outcome was incident incontinence of any type, defined as the report of urinary incontinence, i.e., “inadequate control” (Centers for Medicare and Medicaid Services, 2008), fecal incontinence, or dual (i.e., both urinary and fecal) incontinence (indicated by MDS item >1 for MDS items H1a and H1b, signifying incontinent, frequently incontinent, or occasionally incontinent). Data in MDS records were searched forward after admission until a record of incident incontinence was identified or a resident's data were censored due to the end of the available records or the time period under study. Days to incontinence were computed as (incident incontinence date - admission date) for incident cases and (date of last record—admission date) for censored cases.
Data Analysis
The Peters-Belson Method The Peters-Belson method was used to assess racial and ethnic disparities in the time to incontinence. In this method, two statistical models are developed. First, a model is built of of factors that predict an outcome for a presumed advantaged group (in this case, White non-Hispanic NH admissions), and then the regression coefficients from the advantaged group model are applied to the disadvantaged group's data (Eberly et al., 2015). The outcome of the second model is what would be expected if a particular group of minority NH residents were members of the group of White residents. The overall disparity (difference between the observed outcome of Whites and each observed minority group) has two components: (1) a disparity that can be explained by predictors in the model, estimated by the difference between the observed outcome in the White group and the expected outcome in the minority group, and (2) an unexplained disparity, which is the difference between the minority group's observed outcome and its expected outcome. This unexplained disparity, if significant, represents the race- and ethnicity-based disparity between the white and minority groups (Graubard, Sowmya Rao, & Gastwirth, 2005; Rao, Graubard, Breen, & Gastwirth, 2004).
In implementing the Peters-Belson method, each racial and ethnic minority group was analyzed separately. Because members of the cohort were clustered within NHs, minority residents were included only if they were in NHs that also housed White residents whose coefficients would be applied in modeling, hence referred to as mixed-race NHs.
To identify predictors of the outcome of time to incontinence, first, data for White residents were analyzed using proportional hazards regression, first including only individual-level predictors, and then both individual- and NH/community-level factors. The estimated regression coefficients (beta weights) of this model were then applied to each minority group separately as described above. Predicted and actual (Kaplan–Meier) time-without-incontinence curves were plotted together for visual comparison, and these two survival curves were compared to assess disparity using a one-sample two-sided log-rank test (Eberly et al., 2015). When the log-rank statistic was significant (p < .05), this was defined as a disparity based on race or ethnicity.
Multivariate Analysis of Time to Incontinence in All Nursing Home Admissions
If no racial or ethnic disparity were found for time to incontinence, the follow-up analysis would include all residents of all racial and ethnic groups in one model, to examine other predictors of time to incontinence. To account for potential within-NH correlation and unobserved heterogeneity, a Cox proportional hazards regression model with a random effect for NH would be fit. The screening of variables to include in the model was done independently and followed the same procedures used for the screening of potential covariates for the analysis of racial and ethnic disparities. Covariates included were age, gender, deficits in ADLs and cognition, indicators of vision problems, percentage of NH residents receiving Medicaid, index of deficits in NH quality of care, and proportion of the community in the census tract of the NH that was in an urban area.
Data management and descriptive statistics were conducted using SPSS v. 21 (Chicago, IL) or SAS v. 9.2 (SAS Institute Inc., Cary, NC). All steps of the Peters-Belson analysis were performed using R 2.14. The follow-up analysis for time to incontinence for all residents together would be done in SAS v. 9.3 (SAS Institute Inc., Cary, NC). Results were considered statistically significant if p < .05.
Results
Characteristics of the Cohort
The cohort for analysis was 42,693 older adults admitted to 446 NHs located in 27 states in all 9 census divisions. The characteristics at NH admission of each racial and ethnic group that was followed for developing any incontinence are presented in Table 1. The average age at admission was >75 years, and more than half was female. In comparison to other racial or ethnic groups, a larger percentage of White and API residents had more than a high school education.
Health status and types of health problems varied across the racial and ethnic groups. All groups had a moderate level of deficits in ADLs; API residents had the most deficits and AIANs had the fewest. The comorbidity and mortality risk among all groups was low to moderate. Black residents had more comorbidities in relation to other groups, and API residents had the highest mortality risk as measured by the MDS-CHESS scale (Hirdes, Frijters, & Teare, 2003). A higher percentage of White residents had oxygenation and perfusion problems overall. White residents, followed by Black residents, received the most medications. Indicators of poor nutrition were present in at least one-third of all groups on average. API residents had the most indicators of poor nutrition and the lowest BMIs, and more received tube feeding than the other groups. Tube feeding, however, was low (approximately 1–3%) across all groups, as was restraint use. Indicators of vision problems were common among all groups, and more AIAN residents than others had ::3 indicators. Cognitive deficits were relatively low among all groups, with Black residents having the highest scores on average (2.3 of 10). However, delirium was less common in Black residents than in other groups. API residents had the most communication difficulties. Compared to other groups, more AIAN residents had signs of depression and more discomfort behaviors.
Characteristics of the NHs and Surrounding Communities
Licensed nurse staffing averaged 1.1 hours/resident/day, and CNA staffing was approximately twice that. The total number of deficiencies per NH on average was less than 4 (Table 2). Over 70% of the residents in the NHs received Medicaid. The NHs were located in census tract communities with diverse racial and ethnic populations, although the percentages of minorities in most of the communities was small. Approximately three-quarters of the NHs were in census tract communities where the percentage of working class was at least half of the population, and approximately half were in census tract communities where a majority (>75%) of the population lived in urban areas.
Absence of Disparities in Time to Incontinence
The overall incidence of new incontinence of any type among individuals who were continent on admission to NHs was 20.2%. Approximately half of residents in all racial and ethnic groups had developed incontinence at 1 year after NH admission (Table 3). There was no significant difference between the observed and expected incidence of incontinence for any of the minority groups, indicating that there was no racial or ethnic disparity. Table 3 and Figure 1 show the similarity (closeness) of the proportions of minorities observed and expected to develop incontinence at various time points. For example, at 6 months after admission, 37% of Black residents were observed to develop incontinence, while 35% were expected to do so had they had been in the White group. The proportion of Black residents expected to develop incontinence was similar to the proportion (34%) of White residents observed to do so.
Table 3.
Observed and Expected Proportions of Minority Groups and Observed Proportion of Whites Developing Any Incontinence at Selected Times Points after Nursing Home Admission
| Racial or Ethnic Group |
Time after Nursing Home Admission |
Observed Percentage of Racial/Ethnic Group Developing Incontinence |
Expected Percentage of Racial/Ethnic Group Developing Incontinence |
Observed Percentage of Whites Developing Incontinenc |
|---|---|---|---|---|
| AIAN1 | 3 months | 14.17 | 17.35 | 19.36 |
| 6 months | 27.62 | 29.93 | 33.34 | |
| 12 months | 48.02 | 45.13 | 50.22 | |
| 24 months | 57.20 | 64.44 | 70.73 | |
| API2 | 3 months | 19.67 | 20.60 | 18.35 |
| 6 months | 36.70 | 38.10 | 34.61 | |
| 12 months | 58.08 | 54.66 | 51.69 | |
| 24 months | 88.07 | 72.80 | 73.77 | |
| Black | 3 months | 21.39 | 19.64 | 18.83 |
| 6 months | 37.36 | 35.07 | 34.14 | |
| 12 months | 52.41 | 51.30 | 50.85 | |
| 24 months | 73.21 | 71.50 | 75.52 | |
| Hispanic | 3 months | 16.21 | 19.34 | 18.52 |
| 6 months | 32.87 | 35.63 | 34.74 | |
| 12 months | 46.75 | 51.91 | 51.83 | |
| 24 months | 70.92 | 70.86 | 73.88 |
AIAN=American Indian, Alaskan Native;
API=Asian, Pacific Islander
FIGURE 1.
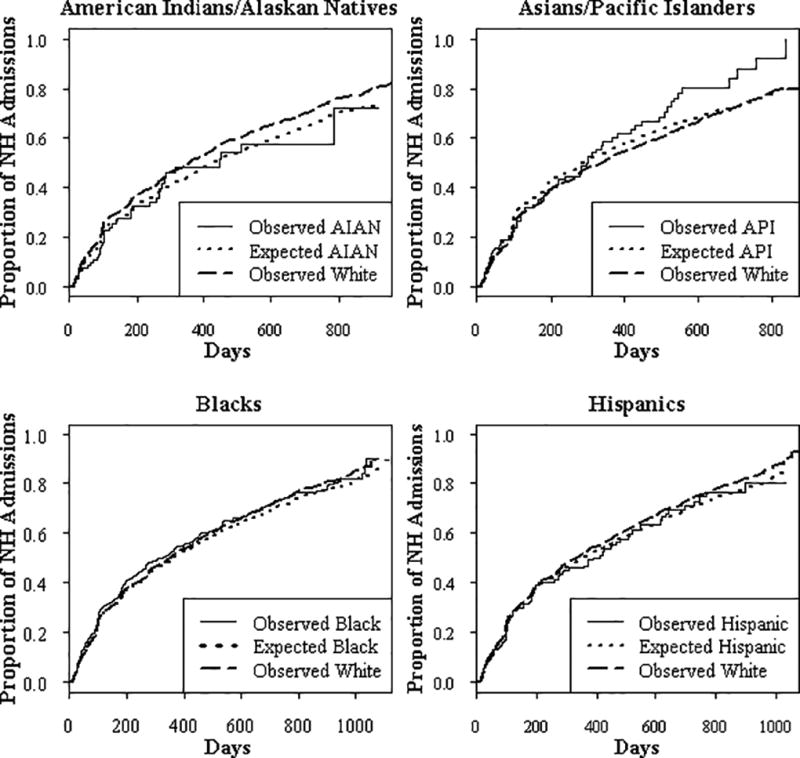
Graphs show no racial or ethnic disparities in time to incontinence. Results are presented as observed and predicted proportions of admissions that developed incontinence over time. Compared to the proportion of each minority group of admissions that was expected to develop incontinence (signified by dotted line), the proportion of each minority group (solid line) observed to develop incontinence was not significantly different (p > .05 for each group). The proportion of White residents in the mixed race nursing homes observed to develop incontinence is also shown (dashed dotted line). Analyses adjusted for individual and nursing home/community predictors. Potential predictors included in the model of disparities in time to incontinence were age, gender, limitations in ADLs (Morris et al., 1999), cognitive deficits (MDS COGS) (Hartmaier et al., 1994), comorbidities (Charlson index; Charlson et al., 1987), indicators of vision problems, percentage of NH residents receiving Medicaid, index of deficiencies in NH quality of care, proportion of admissions that were White, proportion of the Census tract of the NH that was in an urban area or below poverty level, and the Census divisions in which NHs were located.
Risk Factors for Development of Incontinence
Older age, more limitations in ADLs, and greater cognitive deficits at NH admission were significant predictors of developing incontinence after admission to NH and developing it sooner. In Table 4, the results of the follow-up analysis of risk factors for development of incontinence among all NH residents are shown.
Table 4.
Predictors of Any Incontinence in Older Nursing Home Admissions
| Predictor | HR (95% CI) |
|---|---|
| Individual Level | |
| Age at Admission | 1.009 (1.006, 1.012) |
| Female gender | 1.04 (0.99, 1.09) |
| Activities of daily living deficit scale (Morris et al., 1999) | 1.06 (1.05, 1.06) |
| Vision impairment | 1.01 (0.98, 1.03) |
| Cognitive deficits MDS-COG scale (Hartmaier et al., 1994) | 1.15 (1.13, 1.16) |
| Nursing Home/Community Level | |
| Quality of care deficiency index | 1.000 (0.996, 1.004) |
| % Nursing Home residents on Medicaid | 1.000 (0.999, 1.001) |
| % Census tract in an urban area | 1.03 (0.98, 1.08) |
Discussion
This is the first investigation to our knowledge of racial or ethnic disparities in the time to incontinence after admission to NHs. In contrast to differences suggested in the few previous cross-sectional reports, no disparity was found. Given the dearth of studies about the epidemiology of incident incontinence and lack of evidence regarding the association of a large number of potential multi-level predictors, strengths of the present study were the cohort design and inclusion of NH- and community-level data along with detailed individual-level data, making these conclusions stronger theoretically and scientifically than previous designs.
Both the methods and results of this study make contributions to the field. Most previous research on health disparities and factors associated with incontinence has been focused only on individual-level factors. Both theory and a growing literature support the value of multi-level analyses of multi-factorial problems (Diez-Roux, 1998; Krieger et al., 2005). The multi-level analysis was strengthened by use of data at all three levels of analysis (from the MDS, OSCAR and census) from the same years. In addition, the Peters-Belson method has notable advantages for analyzing racial and ethnic disparities (Eberly et al., 2015).
The current analysis was the first to our knowledge in which the time to incontinence in individuals who were continent at NH admission was described and the risk factors that predicted development of new incontinence were identified. Nearly 20% of individuals from all racial and ethnic groups were observed to develop incontinence within 3 months after NH admission, and that percentage steadily increased over time. With a prevalence of 46–78% (Gorina, Schappert, Bercovitz, Elgaddal, & Kramarow, 2014) in NHs overall, incontinence continues to be a leading health problem among NH residents. Robinson (2000) reported that some NH residents considered the development of incontinence as an inevitable norm of aging but responded with emotional and behavioral distress. The fairly rapid onset of incontinence revealed in this study and the deleterious emotional impact of incontinence in NH residents reported by others (DuBeau, Simon, & Morris, 2006; Robinson, 2000) emphasize the importance of initiating interventions at the time of admission to maintain continence.
The risk factors for incontinence identified in this study, older age and greater functional and cognitive deficits on admission, are consistent with a clinical status of greater frailty. While the hazard ratios may appear small and of little consequence clinically, the scale of impact of functional and cognitive deficits was such that an increase of one point in the ADL score (possible score range ¼ 0–28), increased the hazard of developing incontinence by 6% (hazard ratio ¼ 1.06), while controlling for other covariates such as age. These findings echo those of a systematic review of studies of incontinence prevalence in NHs (Shamliyan et al., 2007), in which incontinence in NH residents appeared to be associated with functional dependency. The ADL scores of the racial or ethnic groups varied on average by 2–4 points, corresponding to 12–26% differences across groups in the ADL-associated hazards of developing incontinence. As ADL function is a modifiable risk factor, the findings of this study provide evidence in support of resident-centered plans of care to maintain or improve functional abilities to prevent incontinence. Existing urinary incontinence of NH residents was reduced by a combined exercise and prompted toileting intervention, but an intervention that combined prompted toileting, physical activity, and food and fluid intake did not reduce the frequency of fecal incontinence (Schnelle et al., 2002; Schnelle et al., 2010). Further studies are needed to determine whether these types of interventions are effective for maintaining continence of urine and feces and to assess their burden and acceptability to NH residents. Although timely responsiveness to toileting needs is a fundamental goal of nursing care of individuals with functional dependency, a less staff-intensive approach to toileting assistance may improve incontinence prevention efforts. In addition to ADL impairment, cognitive impairment was a risk factor for incontinence in the present analysis. Evidence-based interventions for this group are sorely needed.
Regulations promoting individualized care planning for incontinence management in NHs came into effect after data in the present study were collected (Department of Health and Human Services Centers for Medicare and Medicaid, 2005). However, incontinence prevention was not specified in these directives. More recently, a NIH consensus conference identified prevention of incontinence in NHs as a priority for research and clinical practice (Landefeld et al., 2008). The risk factors identified in this study can guide NHs in their prevention efforts and resource planning. The finding of no racial ethnic disparities in time to incontinence suggest that older residents of all backgrounds may benefit from such interventions. This approach to care, however, seems to be underutilized. In a systematic review of care practices in NHs related to urinary incontinence, Roe et al. (2011) found no reports of interventions aimed at maintaining continence of residents.
Limitations of this study included use of data of a single national chain of for-profit NHs, reducing the ability to generalize results to non-profit NHs. For-profit NHs comprise 69% of US NHs (Centers for Medicare & Medicaid Services, 2013). The characteristics of our admission cohort were comparable to those of all US NHs at the time (Bliss et al., 2013) but may not be representative of residents in all NHs. Our models included incontinence of any type as an outcome, and they excluded residents with indwelling urinary catheters or ostomies. Findings may differ if type and definition of incontinence are defined differently. Not all relevant predictors of time to incontinence or that could increase the explained disparity were known, available in our datasets, identifiable by screening procedures, or possible to include in our models. For example, other factors include the knowledge of NH nursing staff about incontinence, its risk factors, and management (Bostroom, Slaughter, Chojecki, & Estabrooks, 2012; Campbell, Knight, Benson, & Colling, 1991; Resnick et al., 2006), organizational/administrative leadership and nursing staff valuing continence efforts (Campbell et al., 1991; Palmer, 2008; Resnick et al., 2006), a system of positive feedback or reward for appropriate incontinence-related practice (Burgio et al., 1990; Resnick et al., 2006), and a NH environment with better technology (e.g., lifts, bedside commodes, and a bladder scanner for assessing urinary retention; Resnick et al., 2006). Changing practice requires comprehensive, multi-level strategies at the NH and community levels included in this study (Flynn, Liang, Dickson, & Aiken, 2010; Mueller, 2004). Risk factors for incontinence confirmed in this study (older age, more limitations in ADLs, and greater cognitive deficits) can be used in the education of NH staff to improve practice. The time to develop incontinence identified in this analysis can serve as a benchmark against which to measure success in promoting continence. The lack of racial and ethnic disparity in time to incontinence can be communicated to staff in reinforcing good practice across all patient groups.
Conclusion
Development of incontinence among NH residents is a multifactorial problem. In this study we examined whether racial and ethnic disparities contributed to its incidence. Our findings suggested no such disparities in the incidence of incontinence in NH residents. Results suggest a need for initiating a continence maintenance plan for all residents from the time of admission, as the ability to remain continent appears to decline fairly quickly. Strategies focused on maintaining continence after NH admission can include maintaining or improving functional/ADL status, particularly for individuals with cognitive impairment. The burden on residents should be taken into consideration in developing continence interventions (Robinson, 2000).
Acknowledgments
This study was funded by National Institute of Nursing Research, NIH, 1R01NR010731 and the Minnesota Supercomputing Institute, University of Minnesota, Minneapolis, MN.
References
- Aggazzotti G, Pesce F, Grassi D, Fantuzzi G, Righi E, DeVita, Artibani W. Prevalence of urinary incontinence among institutionalized patients: A cross-sectional epidemiologic study in a midsized city in northern Italy. Urology. 2000;56:245–249. doi: 10.1016/s0090-4295(00)00643-9. [DOI] [PubMed] [Google Scholar]
- Allsworth JE, Toppa R, Palin NC, Lapane KL. Racial and ethnic disparities in the pharmacologic management of diabetes mellitus among long-term care facility residents. Ethnicity & Disease. 2005;15:205–212. [PubMed] [Google Scholar]
- Angel JL, Angel RJ, Aranda MP, Miles TP. Can the family still cope? Social support and health as determinants of nursing home use in the older Mexican-origin population. Journal of Aging and Health. 2004;16:338–354. doi: 10.1177/0898264304264203. [DOI] [PubMed] [Google Scholar]
- Aslan E, Beji NK, Erkan HA, Yalcin O, Gungor F. The prevalence of and the related factors for urinary and fecal incontinence among older residing in nursing homes. Journal of Clinical Nursing. 2009;18:3290–3298. doi: 10.1111/j.1365-2702.2009.02936.x. [DOI] [PubMed] [Google Scholar]
- Baumgarten M, Margolis D, Van Doorn C, Gruber-Baldini AL, Hebel JR, Zimmerman S, Magaziner J. Black/white differences in press ure ulcer incidence in nursing homeresidents. Journal of the American Geriatrics Society. 2004;52:1293–1298. doi: 10.1111/j.1532-5415.2004.52358.x. [DOI] [PubMed] [Google Scholar]
- Bliss DZ, Gurvich O, Savik K, Eberly LE, Harms S, Mueller C, Virnig B. Are there racial-ethnic disparities in time to pressure ulcer development and pressure ulcer treatment in older adults after nursing home admission? Journal of Aging and Health. 2015;27:571–593. doi: 10.1177/0898264314553895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss DZ, Harms S, Garrard JM, Savik K, Gurvich O, Wyman JF, Cunanan K. Prevalence of incontinence by race and ethnicity of older people admitted to nursing homes. Journal of the American Medical Directors Association. 2013;14(451):e1–e7. doi: 10.1016/j.jamda.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrie MJ, Davidson HA. Incontinence in institutions: Costs and contributing factors. CMAJ: Canadian Medical Association Journal. 1992;147:322–328. [PMC free article] [PubMed] [Google Scholar]
- Bostroom A, Slaughter SE, Chojecki D, Estabrooks CA. What do we know about knowledge translation in the care of older adults? A scoping review. Journal of the American Medical Directors Association. 2012;13:210–219. doi: 10.1016/j.jamda.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Boyington JE, Howard DL, Carter-Edwards L, Gooden KM, Erdem N, Jallah Y, Busby-Whitehead J. Differences in resident characteristics and prevalence of urinary incontinence in nursing homes in the southeastern United States. Nursing Research. 2007;56:97–107. doi: 10.1097/01.NNR.0000263969.08878.51. [DOI] [PubMed] [Google Scholar]
- Braveman P. Health disparities and health equity: Concepts and measurement. Annual Review of Public Health. 2006;27:167–194. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- Burgio LD, Engel BT, Hawkins A, McCormick K, Scheve A, Jones LT. A staff management system for maintaining improvements in continence with elderly nursing home residents. Journal of Applied Behavior Analysis. 1990;23:111–118. doi: 10.1901/jaba.1990.23-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EB, Knight M, Benson M, Colling J. Effect of an incontinence training program on nursing home staff’s knowledge, attitudes, and behavior. The Gerontologist. 1991;31:788–794. doi: 10.1093/geront/31.6.788. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. Chapter 3: Item-by-item guide to the MDS. 2008 Retrieved from https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/downloads/MDS20rai1202ch3.pdf.
- Centers for Medicare and Medicaid Services. CMS Quality Strategy 2013-Beyond. 2013a Retrieved from http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/CMS-Quality-Strategy.pdf.
- Centers for Medicare and Medicaid Services. Nursing home data compendium 2013 edition. 2013b Retrieved from http://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/downloads/nursinghomedatacompendium_508.pdf.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hwang S, Chen L, Chen D, Lan C. Urinary incontinence among institutionalized oldest old Chinese men in Taiwan. Neurourology and Urodynamics. 2009;28:335–338. doi: 10.1002/nau.20628. [DOI] [PubMed] [Google Scholar]
- Christian JB, Lapane KL, Toppa RS. Racial disparities in receipt of secondary stroke prevention agents among US nursing home residents. Stroke. 2003;34:2693–2697. doi: 10.1161/01.STR.0000096993.90248.27. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services Centers for Medicare and Medicaid. CMS manual system Pub 100-07: State operations provider certification. 2005 Retrieved from https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R8SOM.pdf.
- Diez-Roux AV. Bringing context back into epidemiology: Variables and fallacies in multilevel analysis. American Journal of Public Health. 1998;88:216–222. doi: 10.2105/ajph.88.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosa D, Intrator O, McNicoll L, Cang Y, Teno J. Preliminary derivation of a nursing home confusion assessment method based on data from the Minimum Data Set. Journal of the American Geriatrics Society. 2007;55:1099–1105. doi: 10.1111/j.1532-5415.2007.01239.x. [DOI] [PubMed] [Google Scholar]
- DuBeau CE, Simon SE, Morris JN. The effect of urinary incontinence on quality of life in older nursing home residents. Journal of the American Geriatrics Society. 2006;54:1325–1333. doi: 10.1111/j.1532-5415.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- Eberly LE, Cunanan K, Gurvich O, Savik K, Bliss DZ, Wyman JF. Statistical approaches to assessing health and healthcare disparities. Research in Nursing & Health. 2015 doi: 10.1002/nur.21679. (advance online publication) [DOI] [PubMed] [Google Scholar]
- Feng Z, Fennell ML, Tyler DA, Clark M, Mor V. The care span: Growth of racial and ethnic minorities in US nursing homes driven by demographics and possible disparities in options. Health Affairs. 2011;30:1358–1365. doi: 10.1377/hlthaff.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell ML, Feng Z, Clark MA, Mor V. Elderly Hispanics more likely to reside in poor-quality nursing homes. Health Affairs. 2010;29:65–73. doi: 10.1377/hlthaff.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn L, Liang Y, Dickson GL, Aiken LH. Effects of nursing practice environments on quality outcomes in nursing homes. Journal of the American Geriatrics Society. 2010;58:2401–2406. doi: 10.1111/j.1532-5415.2010.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardo MP, Teno JM, Mor V. Not so black and white: Nursing home concentration of Hispanics associated with prevalence of pressure ulcers. Journal of the American Medical Directors Association. 2009;10:127–132. doi: 10.1016/j.jamda.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina Y, Schappert S, Bercovitz A, Elgaddal N, Kramarow Prevalence of incontinence among older Americans. Vital & Health Statistics Series 3, Analytical and Epidemiological Studies. 2014;36:1–33. [PubMed] [Google Scholar]
- Graubard BI, Sowmya Rao R, Gastwirth JL. Using the Peters-Belson method to measure health care disparities from complex survey data. Statistics in Medicine. 2005;24:2659–2668. doi: 10.1002/sim.2135. [DOI] [PubMed] [Google Scholar]
- Hartmaier SL, Sloane PD, Guess HA, Koch GG. The MDS Cognition Scale: A valid instrument for identifying and staging nursing home residents with dementia using the Minimum Data Set. Journal of the American Geriatrics Society. 1994;42:1173–1179. doi: 10.1111/j.1532-5415.1994.tb06984.x. [DOI] [PubMed] [Google Scholar]
- Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS scale: A new measure to predict mortality in institutionalized older people. Journal of the American Geriatrics Society. 2003;51:96–100. doi: 10.1034/j.1601-5215.2002.51017.x. [DOI] [PubMed] [Google Scholar]
- House JS, Lantz PM, Herd P. Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans’ Changing Lives Study) The Journals of Gerontology, Series B, Psychological Sciences and Social Sciences. 2005;60(Spec No 2):15–26. doi: 10.1093/geronb/60.special_issue_2.s15. [DOI] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: The Public Health Disparities Geocoding Project. American Journal of Public Health. 2005;95:312–323. doi: 10.2105/AJPH2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakdawalla D, Goldman DP, Bhattacharya J, Hurd MD, Joyce GF, Panis CW. Forecasting the nursing home population. Medical Care. 2003;41:8–20. doi: 10.1097/00005650-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Landefeld CS, Bowers BJ, Feld AD, Hartmann KE, Hoffmann E, Ingber MJ, Trock BJ. National Institutes of Health State-of-the-Science Conference statement: Prevention of fecal and urinary incontinence in adults. Annals of Internal Medicine. 2008;148:449–458. doi: 10.7326/0003-4819-148-6-200803180-00210. [DOI] [PubMed] [Google Scholar]
- Lapane KL, Fernandez HH, Friedman J HSAGE Study Group. Prevalence, clinical characteristics, and pharmacologic treatment of Parkinson’s disease in residents in long-term care facilities. Pharmacotherapy. 1999;19:1321–1327. doi: 10.1592/phco.19.16.1321.30877. [DOI] [PubMed] [Google Scholar]
- Li Y, Yin J, Cai X, Temkin-Greener J, Mukamel DB. Association of race and sites of care with pressure ulcers in high-risk nursing home residents. JAMA. 2011;306:179–186. doi: 10.1001/jama.2011.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SC, Papandonatos G, Fennell M, Mor V. Facility and county effects on racial differences in nursing home quality indicators. Social Science & Medicine. 2006;63:3046–3059. doi: 10.1016/j.socscimed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Mor V, Zinn J, Angelelli J, Teno JM, Miller SC. Driven to tiers: Socioeconomic and racial disparities in the quality of nursing home care. The Milbank Quarterly. 2004;82:227–256. doi: 10.1111/j.0887-378X2004.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1999;54:M546–M553. doi: 10.1093/gerona/54.11.M546. [DOI] [PubMed] [Google Scholar]
- Mueller CA. Quality improvement and incontinence in long-term care. Clinics in Geriatric Medicine. 2004;20:539–551. doi: 10.1016/j.cger.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Nelson R, Furner S, Jesudason V. Fecal incontinence in Wisconsin nursing homes: Prevalence and associations. Diseases of the Colon and Rectum. 1998;41:1226–1229. doi: 10.1007/BF02258218. [DOI] [PubMed] [Google Scholar]
- Nelson R, Furner S, Jesudason V. Urinary incontinence in Wisconsin skilled nursing facilities: Prevalence and associations in common with fecal incontinence. Journal of Aging and Health. 2001;13:539–547. doi: 10.1177/089826430101300406. [DOI] [PubMed] [Google Scholar]
- Offermans MP, Du Moulin MF, Hamers JP, Dassen T, Halfens RJ. Prevalence of urinary incontinence and associated risk factors in nursing home residents: A systematic review. Neurourology and Urodynamics. 2009;28:288–294. doi: 10.1002/nau.20668. [DOI] [PubMed] [Google Scholar]
- Osypuk TL. Beyond individual neighborhoods: A geography of opportunity perspective for understanding racial/ethnic health disparities. Health Place. 2010;16:1113–1123. doi: 10.1016/j.healthplace.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouslander JG, Palmer MH, Rovner BW, German PS. Urinary incontinence in nursing homes: Incidence, remission and associated factors. Journal of the American Geriatrics Society. 1993;41:1083–1089. doi: 10.1111/j.1532-5415.1993.tb06456.x. [DOI] [PubMed] [Google Scholar]
- Palmer MH. Nurses’ knowledge and beliefs about continence interventions in long-term care. Journal of Advanced Nursing. 2008;21:1065–1072. doi: 10.1046/j.1365-2648.1995.21061065.x. [DOI] [PubMed] [Google Scholar]
- Phillips CD, Hawes C, Mor V, Fries BE, Morris JN, Nennstiel ME. Facility and area variation affecting the use of physical restraints in nursing homes. Medical Care. 1996;34:1149–1162. doi: 10.1097/00005650-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Porell FW, Miltiades HB. Regional differences in functional status among the aged. Social Science & Medicine. 2002;54:1181–1198. doi: 10.1016/S0277-9536(01)00088-0. [DOI] [PubMed] [Google Scholar]
- Rao RS, Graubard BI, Breen N, Gastwirth JL. Understanding the factors underlying disparities in cancer screening rates using the Peters-Belson approach: Results from the 1998 National Health Interview Survey. Medical Care. 2004;42:789–800. doi: 10.1097/01.mlr.0000132838.29236.7e. [DOI] [PubMed] [Google Scholar]
- Resnick B, Keilman LJ, Calabrese B, Parmelee P, Lawhorne L, Pailet J, Ouslander J. Nursing staff beliefs and expectations about continence care in nursing homes. Journal of Wound Ostomy & Continence Nursing. 2006;33:610–618. doi: 10.1097/00152192-200611000-00004. [DOI] [PubMed] [Google Scholar]
- Robinson JP. Managing urinary incontinence in the nursing home: Residents’ perspectives. Journal of Advanced Nursing. 2000;31:68–77. doi: 10.1046/j.1365-2648.2000.01258.x. [DOI] [PubMed] [Google Scholar]
- Roe B, Flanagan L, Jack B, Barrett J, Chung A, Shaw C, Williams K. Systematic review of the management of incontinence and promotion of continence in older people in care homes: Descriptive studies with urinary incontinence as primary focus. Journal of Advanced Nursing. 2011;67:228–250. doi: 10.1111/j.1365-2648.2010.05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savik K, Fan Q, Bliss D, Harms S. Preparing a large data set for analysis: Using the Minimum Data Set to study perineal dermatitis. Journal of Advanced Nursing. 2005;52:399–409. doi: 10.1111/j.1365-2648.2005.03604.x. [DOI] [PubMed] [Google Scholar]
- Saxer S, Halfens RJ, de Bie RA, Dassen T. Prevalence and incidence of urinary incontinence of Swiss nursing home residents at admission and after six, 12 and 24 months. Journal of Clinical Nursing. 2008;17:2490–2496. doi: 10.1111/j.1365-2702.2007.02055.x. [DOI] [PubMed] [Google Scholar]
- Schnelle JF, Alessi CA, Simmons SF, Al-Samarrai NR, Beck JC, Ouslander JG. Translating clinical research into practice: A randomized controlled trial of exercise and incontinence care with nursing home residents. Journal of the American Geriatrics Society. 2002;50:1476–1483. doi: 10.1046/j.1532-5415.2002.50401.x. [DOI] [PubMed] [Google Scholar]
- Schnelle JF, Leung FW, Rao SS, Beuscher L, Keeler E, Clift JW, Simmons S. A controlled trial of an intervention to improve urinary and fecal incontinence and constipation. Journal of the American Geriatrics Society. 2010;58:1504–1511. doi: 10.1111/j.1532-5415.2010.02978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgadari A, Topinkova E, Bjornson J, Bernabei R. Urinary incontinence in nursing home residents: A cross-national comparison. Age & Ageing. 1997;26(Suppl 2):49–54. doi: 10.1093/ageing/26.suppl_2.49. [DOI] [PubMed] [Google Scholar]
- Shamliyan T, Wyman J, Bliss DZ, Kane RL, Wilt TJ. Preventionofurinaryandfecalincontinenceinadults. Evidence Report/Technology Assessment. 2007;161:1–379. [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Feng Z, Fennell ML, Zinn JS, Mor V. Separate and unequal: Racial segregation and disparities in quality across U.S. nursing homes. Health Affairs. 2007;26:1448–1458. doi: 10.1377/hlthaff.26.5.1448. [DOI] [PubMed] [Google Scholar]
- Stevenson KM, Brown RL, Dahl JL, Ward SE, Brown MS. The Discomfort Behavior Scale: A measure of discomfort in the cognitively impaired based on the Minimum Data Set 2.0. Research in Nursing & Health. 2006;29:576–587. doi: 10.1002/nur.20168. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. HHS action plan to reduce racial and ethnic health disparities: A nation free of disparities in health and health care. 2011 Retrieved from http://minorityhealth.hhs.gov/npa/files/Plans/HHS/HHS_Plan_complete.pdf.


