Abstract
Objectives
There has been debate regarding the safety of performing elective procedures in patients with vascular manifestations associated with Ehlers-Danlos syndrome (EDS). The purpose of this study was to review the surgical management and clinical outcomes of EDS patients undergoing vascular procedures at a tertiary medical center with multimodality expertise in connective tissue disorders.
Methods
All patients with EDS undergoing endovascular and open vascular procedures at a single-institution academic medical center from 1994 to 2009 were retrospectively reviewed. Clinical data were evaluated including patient demographics, length of stay (LOS), and mortality outcomes during hospital course and long-term follow-up.
Results
A total of 40 patients with EDS were identified, including individuals diagnosed with classic (n = 15), hypermobility (n = 16), and vascular (n = 9) types of EDS. These patients collectively underwent 45 endovascular and 18 open procedures for vascular disease during the time period, including embolization (n = 37), angioplasty (n = 8), arterial bypass (n = 5), and aortic aneurysm repair (n = 13). All cases were performed electively, except for one (2%) urgent endovascular and one (5%) emergent open procedure. Endovascular procedures were associated with a median LOS (interquartile range [IQR]) of 2 (1 to 3) days with no procedure-related mortality or in-hospital deaths among all EDS types, whereas open vascular procedures had median LOS (IQR) of 6 (5 to 8) days with one (6%) in-hospital death occurring in a vascular EDS patient. Survival free of any complication at 5 years was 85% and 54% following endovascular and open procedures, respectively.
Conclusions
The elective surgical management of vascular disorders in EDS patients using open and endovascular procedures has been associated with good outcomes. Our results suggest that vascular interventions in these EDS patients can be safely performed and should not be withheld until rupture or acute symptoms arise.
Ehlers-Danlos syndrome (EDS) is a group of clinically and genetically heterogeneous heritable connective tissue disorders resulting from mutations in genes involved in extracellular matrix formation and organization, leading to a predisposition for loss of structural integrity in tissues within multiple organ systems.1 While six different forms of EDS are currently recognized, including classical, hypermobile, kyphoscoliotic, arthrochalasic, and dermatosparactic types, the vascular type (formerly EDS IV) is the most severe form of the disorder.2 Disease-related symptoms vary based on each EDS type, but are generally characterized by joint hypermobility, skin hyperextensibility, and tissue fragility affecting skin, ligaments, joints, internal organs, and blood vessels.3
Vascular manifestations are among the most severe complications of EDS and involve a spectrum of arterial and venous anomalies, including progressive aneurysm formation or spontaneous vascular dissection and rupture. While vascular type EDS patients are generally recognized as having the most severe complications, vascular disease can present in many types of EDS patients. While it is likely that any given EDS patient will require a vascular procedure at some point during their lifetime, there is ongoing debate regarding the optimal surgical management of these patients.4–7 There is limited awareness about the distinctions among the different types of EDS and their cardinal manifestations in surgical patients. Since the historical experience detailing high complication rates and surgical failures in vascular type EDS patients is often taken as representative of the whole group,4–7 many elective vascular procedures are deferred or declined in EDS patients, thereby inviting significant and potentially unwarranted anxieties into the patient-doctor relationship. The objective of this study is to review the contemporary surgical management and clinical outcomes of EDS patients at a medical center with multimodality expertise in treating connective tissue disorders.
METHODS
Study patients
All patients diagnosed with EDS who were treated at the Johns Hopkins Hospital in Baltimore, Maryland between January 1994 and December 2008, were identified from an institutional database. Permission to review patient records was granted following Institutional Review Board approval. Cases were retrospectively identified by using the International Classification of Diseases, 9th Edition code for EDS (756.83) and the specific subtype diagnosis was confirmed by reviewing the medical history, clinical features of disease, and radiology imaging studies. Diagnostic criteria for EDS were defined using the revised nosology of Villefranche, and all patients were categorized into specific EDS subtypes based on this modern classification system.8 Confirmation of subtype diagnosis was made using results of biochemical testing of skin biopsy for collagen typing or genetic mutation analysis whenever applicable data were available.
Hospital and longitudinal data pertaining to all endovascular or open vascular surgical procedures undertaken at the Johns Hopkins Hospital in identified EDS patients were extracted for analysis. This included vascular procedures performed by vascular surgeons, cardiothoracic surgeons, and interventional radiologists. Therapeutic decision making for the EDS patients was guided by interactions among the treating surgeon or interventionalist in concert with medical geneticists with extant expertise in management of connective tissue disorders. In addition, the results of all preoperative and postoperative radiology imaging (CT, MRI, angiography) studies were reviewed and used to confirm patient diagnosis and procedure undertaken.
Study variables
Patient demographics and characteristics associated with EDS symptoms were collected from a careful review of paper and electronic patient records. Variables extracted included age at diagnosis, age at time of first operation, age at time of elective vascular procedure, gender, race, smoking history, and EDS subtype. In addition, records were reviewed to ascertain whether a family history of EDS, sudden death, or early onset of severe cardiovascular disease existed. The age and specific diagnosis of first-degree relatives with EDS symptoms were collected, as well as their reported outcomes.
Operative data were collected from a review of anesthesia and operative notes. Variables collected for analysis included the specific type of procedure, whether the procedure was converted from percutaneous to open, and estimated blood loss. Moreover, the total number of units of blood products received during patients’ hospital stay was extracted from a review of blood bank records and categorized by the number of units of packed red blood cells (PRBC), fresh frozen plasma (FFP), or platelets transfused.
Several outcome variables were collected to assess the morbidity and mortality related to elective vascular surgery in EDS patients. The main in-hospital outcome measures were operative mortality, in-hospital mortality, major complications, and median length of hospital stay following elective endovascular and open vascular surgery procedures. Late follow-up data and outcomes related to death or need for secondary vascular procedures were obtained from medical records, office visits, and the national death index.
Statistical analysis
Differences between EDS patients and study outcomes were compared using one-way analysis of variance (ANOVA) or the Wilcoxon rank-sum test for continuous variables and the Kruskal-Wallis test or Fisher’s exact test for categorical data. Survival curves were calculated using the Kaplan-Meier method. P values less than .05 were considered to be statistically significant for all tests and models. All statistical analyses were perfomed using Stata statistical software, version 9.1 (StataCorp, College Station, Tex).
RESULTS
A total of 183 patients with EDS were identified that underwent treatment for a broad range of medical conditions at the Johns Hopkins Hospital between 1994 and 2009. Among these EDS patients, 40 (22%) were admitted and underwent at least one elective endovascular or open vascular procedure during this defined time period. Patient characteristics and demographic data for these 40 individuals are shown in Table I. Briefly, this cohort primarily consisted of female patients (82%) of Caucasian descent (95%), with a median age of 34 years at the time of their elective procedure. Fifteen patients (37%) met diagnostic criteria for classical EDS, 16 (40%) met diagnostic criteria for hypermobility EDS, and nine patients (23%) met diagnostic criteria for vascular EDS. Only 12 (31%) patients within this cohort were found to have a known family history of EDS or early cardiovascular disease, and six (16%) additional patients had a family history of sudden death of unknown etiology.
Table I.
Characteristics and demographics of EDS patients (n = 40) undergoing elective vascular procedures
| Variable | Value |
|---|---|
| Age – median (IQR) | |
| Age at EDS diagnosis | 25 (16–39) |
| Age at time of first vascular procedure* | 34 (22–40) |
| Gender – No. (%) | |
| Male | 7 (17) |
| Female | 33 (82) |
| Race – No. (%) | |
| Caucasian | 38 (95) |
| African American | 2 (5) |
| EDS subtype†– No. (%) | |
| Classical | 15 (37) |
| Hypermobile | 16 (40) |
| Vascular | 9 (23) |
| Other EDS subtypes | 0 (0) |
| Family history – No. (%) | |
| History of EDS | 12 (31) |
| History of sudden death | 6 (16) |
| History of early CV disease | 12 (31) |
CV, Cardiovascular; EDS, Ehlers-Danlos syndrome; IQR, interquartile range; No., number.
Median age at which patients underwent their first endovascular or open vascular procedure.
EDS classification defined using the revised nosology of Villefranche.
Endovascular procedures
Forty-five endovascular procedures were undertaken in EDS patients, including 15 (33%) in patients with classic EDS, 27 (60%) in patients with hypermobility EDS, and three (7%) in patients with vascular EDS (Table II). All endovascular procedures were performed electively, except for one (2%) urgent procedure in a patient with vascular EDS. Arterial and venous embolizations were the most common type of endovascular procedure performed among patients in all three EDS diagnostic subgroups (N = 37 embolization procedures). Embolization materials included scleroembolization by gelfoam and sodium morrhuate in 34 of the procedures (92%) or coils in 24 procedures (65%). Twenty-one procedures (57%) were performed using both coils and scleroembolization material, and only three procedures (8%) used coils alone (which was in arterial locations only). Angioplasty (± stenting) was undertaken for arterial stenosis in five procedures in patients with classic EDS (two primary subclavian angioplasty, one secondary subclavian angioplasty, and two primary renal artery angioplasty procedures). Three procedures were performed in patients with hypermobility EDS for vein stenosis associated with DVT. None of these percutaneous procedures were performed in patients with vascular EDS. While patients with vascular EDS were significantly more likely (P < .05) to require general anesthesia during endovascular procedures due to surgeon or interventionalist preference, nevertheless, there were no conversions to open procedures or need to transfuse blood products in EDS patients within any diagnostic subgroups (Table II). Open femoral access was performed in all cases of patients with vascular type EDS with repair and reinforcement of access site punctures using suture repair and felt pledget and/or buttressing.
Table II.
Operative data for EDS patients (n = 40) undergoing elective endovascular and open vascular procedures
| Endovascular procedures (n = 45) | ||||
|---|---|---|---|---|
|
| ||||
| Variable | Classic EDS (n = 15) |
Hypermobile EDS (n = 27) |
Vascular EDS (n = 3) |
P value* |
| Procedure type – No. (%) | .52 | |||
| Embolization | 10 (67) | 24 (89) | 3 (100) | |
| Angioplasty | 5 (33) | 3 (11) | 0 (0) | |
| Anesthesia type – No. (%) | <.05 | |||
| General | 0 (0) | 2 (7) | 2 (67) | |
| CS/local | 15 (100) | 25 (93) | 1 (33) | |
| Conversion to open – No. (%) | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| Blood products§– median (IQR) | ||||
| PRBC (units) | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| FFP (units) | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| Platelets (6 packs) | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| Open vascular procedures (n = 18) | ||||
|---|---|---|---|---|
|
| ||||
| Variable | Classic EDS (n = 7) |
Hypermobile EDS (n = 2) |
Vascular EDS (n = 9) |
P value* |
| Procedure type – No. (%) | .05 | |||
| Arterial bypass† | 3 (42) | 2 (100) | 0 (0) | |
| TAAA repair (Crawford I–III) | 2 (29) | 0 (0) | 5 (55) | |
| AAA repair (Crawford IV) | 2 (29) | 0 (0) | 4 (45) | |
| Anesthesia type – No. (%) | 1.0 | |||
| General | 7 (100) | 2 (100) | 9 (100) | |
| CS/local | 0 (0) | 0 (0) | 0 (0) | |
| EBL (L) – median (IQR) | 1 (0.3–4.0) | 0.4 (0.2–0.6) | 9 (3–21) | <.05 |
| Blood products§ – median (IQR) | ||||
| PRBC (units) | 2 (0–4) | 1 (0–2) | 8 (4–26) | .07 |
| FFP (units) | 0 (0–4) | 0 (0–0) | 6 (4–19) | <.05 |
| Platelets (6 packs) | 0 (0–0) | 0 (0–0) | 2 (1–4) | <.05 |
AAA, Abdominal aortic aneurysm; CS/local, combined conscious sedation and local anesthesia; EBL, estimated blood loss; EDS, Ehlers-Danlos syndrome; FFP, fresh frozen plasma; IQR, interquartile range; No., number; PRBC, packed red blood cells; TAAA, thoracoabdominal aortic aneurysm.
P value calculated using the Kruskal-Wallis test for categorical data and one-way analysis of variance for continuous variables.
Includes peripheral and reno visceral arterial bypass procedures.
Number of units of blood products transfused during entire course of hospitalization.
In-hospital outcomes for EDS patients undergoing elective endovascular and open vascular procedures are shown in Table III. There were no operative or in-hospital deaths following endovascular procedures among EDS patients belonging to the three diagnostic subtypes. Moreover, no significant differences were found in median LOS or rate of major postoperative complications following endovascular procedures between EDS patients belonging to any of the three diagnostic subgroups. There were no perioperative complications (including bleeding) associated with endovascular procedures, except for a transient bradycardic episode experienced by a single patient undergoing embolization with hypermobile EDS that responded with pharmacologic agents.
Table III.
In-hospital outcomes for elective endovascular and open vascular procedures in EDS patients (n = 40)
| Endovascular procedures (n = 45) | ||||
|---|---|---|---|---|
|
| ||||
| Outcome | Classic EDS (n = 15) |
Hypermobile EDS (n = 27) |
Vascular EDS (n = 3) |
P value* |
| Operative death – No. (%) | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| In-hospital death – No. (%) | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| LOS – median (IQR) | 1 (1–2) | 2 (1–2) | 3 (1–6) | .51 |
| Any complication†– No. (%) | 0 (0) | 1 (4) | 0 (0) | .37 |
| Open vascular procedures (n = 18) | ||||
|---|---|---|---|---|
|
| ||||
| Outcome | Classic EDS (n = 7) |
Hypermobile EDS (n = 2) |
Vascular EDS (n = 9) |
P value* |
| Operative death – No. (%) | 0 (0) | 0 (0) | 1 (11) | .61 |
| In-hospital death – No. (%) | 0 (0) | 0 (0) | 1 (13) | .56 |
| LOS – median (IQR) | 7 (5–8) | 5 (2–8) | 7 (6–8) | .86 |
| Any complication† – No. (%) | 3 (29) | 0 (0) | 3 (38) | .58 |
EDS, Ehlers-Danlos syndrome; FFP, fresh frozen plasma; IQR, interquartile range; LOS, length of stay; No., number.
P value calculated using the Kruskal-Wallis test.
Any major complications occurring during the perioperative period and the duration of hospital stay.
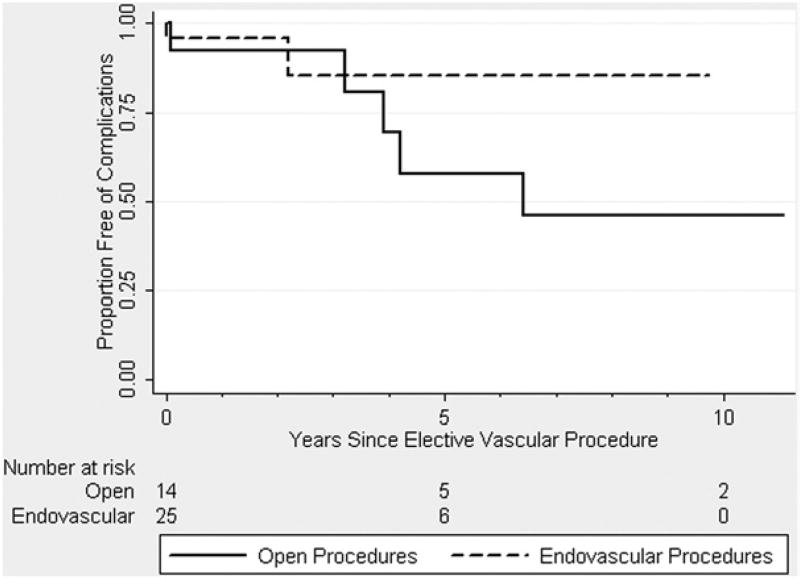
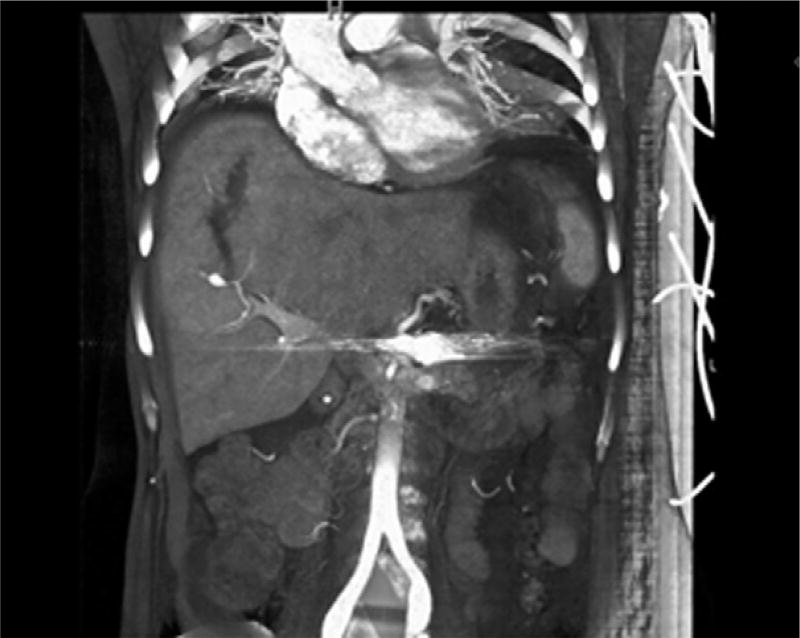
Long-term outcomes in patients undergoing elective endovascular procedures were obtained in all but three individuals who were lost to follow-up. The median follow-up of all EDS patients undergoing endovascular procedures was 1.6 (range, 0.5–9) years, with no reported deaths. Cumulative survival free of any complication was 85% at 5 years (see Fig 1); only one vascular complication occurred in a patient who presented with a ruptured right hepatic artery aneurysm and intraparencymal liver hematoma 2.5 years after her index operation, a splenic artery embolization (see Fig 2). This complication was successfully treated by selective coil embolization of the right hepatic artery branch, and the patient recovered without any further vascular events to present. Notably, the appreciated hepatic aneurysms developed within the 2.5-year time frame, as high-quality axial and 3D imaging demonstrated normal hepatic vasculature earlier.
Fig 1.
Kaplan-Meier estimates of cumulative survival free of any vascular complication among 40 patients with Ehlers-Danlos syndrome (EDS) who underwent 45 endovascular (dashed line) and 17 open vascular procedures (solid line). SEM > 10% after 4 years for open procedures and after 3 years for endovascular procedures.
Fig 2.
Reconstruction of abdominal CT angiography scan showing a ruptured right anterior hepatic arterial aneurysm and associated hemoperitoneum in a patient with vascular Ehlers-Danlos syndrome (EDS) who presented with 2.4 years following successful elective coil embolization of a splenic artery aneurysm.
Open vascular procedures
Eighteen open vascular procedures were performed in EDS patients, including seven (39%) in patients with classical EDS, two (11%) in patients with hypermobility EDS, and nine (50%) in patients with vascular EDS (Table II). All cases were elective, with the exception of one (6%) emergent TAAA repair in a patient with vascular EDS. Peripheral or visceral arterial bypass procedures were more frequently performed in classical and hypermobility EDS patients within our cohort, whereas vascular EDS patients were more likely to undergo thoracoabdominal aortic aneurysm (TAAA) repair and abdominal aortic aneurysm (AAA) repair procedures (P = .05). Correspondingly, estimated blood loss during open cases was significantly higher (P < .05) for vascular EDS patients, and they received a higher number of blood product transfusions during the entire course of their hospital stay (Table II), but this was certainly reflective of the greater extent of arterial replacement. All arterial reconstruction procedures in vascular EDS patients were undertaken using Teflon or felt reinforcement at the anastamoses.
Elective open vascular procedures were successfully undertaken in all three EDS diagnostic subgroups, with only one operative and in-hospital death occurring in a vascular EDS patient undergoing a juxtarenal AAA repair (Table III). Likewise, there was no significant difference in median LOS or the in-hospital complication rate between EDS patients in the three diagnostic subgroups. Postoperative complications experienced in classical EDS patients included an incisional hernia and pneumothorax in one patient and an episode of atrial fibrillation in a second patient. Complications following open procedures in vascular EDS included a sternal hematoma requiring washout and draining in one patient and a thoracic duct injury with chylous leak during proximal descending thoracic aortic repair that was managed conservatively with parenteral nutrition for 2 weeks and gradual introduction of oral intake.
Long-term follow-up was obtained in all EDS patients undergoing open vascular procedures. The median follow-up in this group of patients was 3.2 (range, 1–11) years. One death occurred in a patient 4.2 years after a suprarenal AAA repair, although the exact cause of mortality could not be determined. While there were no late graft-related complications or anastomotic aneurysms found on follow-up imaging, three patients developed aneurysms in noncontiguous aortic segments that required further elective operative repair. The overall survival free of any complication was 54% at 5 years and 42% at 10 years, as vascular events predominated the later complications (89%) over other gastrointestinal or orthopedic complications (11%) referable to the disorders.
DISCUSSION
EDS is a heterogeneous disorder with an estimated prevalence of 1:5000 to 1:25,000 births.2,3 The relative frequencies of different EDS types are not known precisely, but classical and hypermobile types account for >90%. In comparison, vascular EDS likely accounts for less than 5% of EDS patients.2 The classification of EDS is first made on clinical grounds using the major and minor criteria defined by the Villefranche nosology8 and substantiated by biochemical and molecular analysis when possible. The classical, hypermobile, and vascular types of EDS are autosomal dominant disorders, and affected individuals have a 50% risk of passing the disorder to offspring. Nonetheless, many features of EDS may not be recognized until adulthood. Confounding issues include the high incidence of joint laxity in young children in the normal population9 and frequent bruising in active youngsters. In addition, many affected persons do not develop widened scars until they have sustained an injury that results in skin laceration. Delays in recognition of the disorder until the occurrence of an arterial catastrophic event are common. Indeed, only 31% of our patients had an antecedent family history and prior reports of vascular type patients presenting with arterial complications revealed the patient was aware of their EDS in only 4% to 26% of cases.4,7 Therefore, clinical awareness of the treating physician to consider the EDS diagnosis in patients with findings suspicious for connective tissue disorder is of utmost importance.
Genotype-phenotype correlation remains elusive, and location of the genetic mutation within the collagen genes (Classical: COL5A1 and COL5A2; Hypermobile: COL5A1 and Tenascin X; Vascular: COL3A1) has not been informative to predict tissue integrity. In molecular analysis of COL3A1 genes from 135 vascular type patients, Pepin et al10 revealed point mutations in most of the subjects that led to substitution of some other amino acid for glycine through the triple-helical collagen domain, and no single mutation correlated with the type or frequency of vascular complications. Biochemically, since the procollagen molecule is a homopolymer consisting of three identical chains, only 1/8 of the assembled molecules would be normal if the pool of procollagen chains contained equal numbers of normal and mutant chains, as one would expect in heterozygous mutation that left only one normal allele. The 7/8 abnormal collagen molecules might be retained or degraded in a process called “protein suicide.”11 This theoretical biochemical explanation has proven operant in many of the EDS spectrum. Indeed the amount of collagen deposited in the skin and vessels of vascular type and classical type patients may be as low as 10% to 15% of normal.2,12 Moreover, there is evidence that mutation in one type of collagen gene may interfere with the organization and correct assembly of other types of collagens, making it difficult to predict the impact of a specific mutation in tissue integrity.13 Tensile strength of the skin is similarly unable to be correlated with genotype but may correlate with severity of disease.14 There are no available studies to correlate tensile strength of the skin with a patient’s capacity to tolerate surgery or to model a perioperative risk analysis.
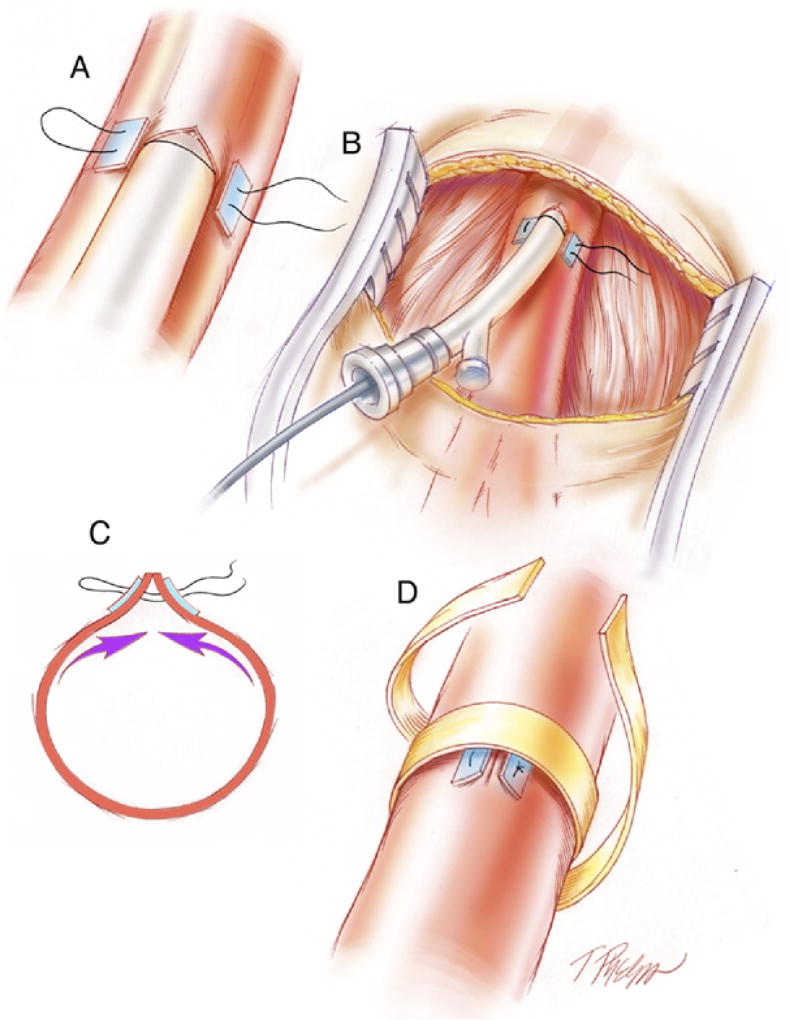
Endovascular approaches to coil embolize aortic branch vessels and other medium sized-arteries have been successful in EDS patients in our contemporary experience and that of others.7,15–17 Historical estimates of mortality related to arteriography in vascular type EDS ranged up to 17%, with 67% of patients experiencing major complications.4,18 We believe the excessive rate of arterial complication was related to large-diameter sheaths and devices used in prior decades. Modern endovascular technology has evolved to lower-profile systems with less traumatic catheters and wires. Nonetheless, arterial access can precipitate femoral rupture and pseudoaneurysm formation, especially when large devices are necessary. Consideration should be given to open repair of any access puncture, especially when a larger French size is introduced (see Fig 3). Indeed, an endovascular suite with hybrid abilities to perform both open and endovascular procedures is strongly preferred for most patients with arterial aneurysm interventions, and we have used this setting in the past 5 years for all patients with classical and vascular type EDS arterial aneurysm embolizations.
Fig 3.
Technique for management of the access vessel in fragile vasculature of Ehlers-Danlos syndrome (EDS) patient. (A) A “U” stitch monofilament suture is placed with a pledget buttress, and needle entry is made within the area. (B) Sheath access is obtained and manipulated or exchanged minimally to avoid femoral tears. (C) The pledgetted “U” stitch suture is tied down as sheath is removed. (D) Circumferential felt reinforcement is approximated to reduce systolic pulse wave stretch on sutures and to prevent late pseudoaneurysm formation.
Stent-graft therapy for abdominal or thoracic aortic aneurysm in EDS has not been reported in significant samples and with no long-term follow-up.19 Clearly, the long-term durability and threat to the fixation zones in the setting of chronic outward radial force of the device against an abnormal host vessel may increase secondary interventions. Emerging reports suggest stent-graft therapy in connective tissue disorders may be wrought with complications with perforation and erosion at fixation zones and very high rates of secondary reintervention.18,19 As such, we are in agreement with recent consensus documents that stentgraft therapy in EDS patients (and those with other connective tissue disorders) should be avoided.20 Recent litigation suggests physician reliance on consensus statements to determine medically reasonable levels of care in patients with cardiovascular abnormalities is very appropriate.21
Open surgical therapy of EDS is associated with elevated rates of intraoperative and postoperative complications.7,18 Prior reports have estimated open mortality in vascular reconstruction in vascular type patients to be between 20% to 65%.4–7 Most importantly, regardless of EDS subtype, we found the vessels display marked thinness and abnormal handling characteristics, being prone to develop adventitial hematoma and dissection. Again, this suggests the type of EDS disorder and its mutation (genotype-phenotype relationship) was less relevant than the clinical history of fragile tissues. Our technique of repair in elective cases included induced hypotension (usually systolic pressure 70 mm Hg to 90 mm Hg) during all clamping sequences, wide exposure to re-clamp for anastomotic bleeding across a new, more proximal segment of vessel thus avoiding repeated clamp application on the same segment, and circumferential felt reinforcement where possible. For central cardioaortic operations, hypothermic circulatory arrest was employed in all cases. Anesthesia preparation includes large-bore venous access placed under ultrasound guidance to reduce inadvertent arterial punctures. Our immediate surgical results compare very favorably with the extant surgical literature,4–7 with less operative mortalities (N = 1, 8%) and postoperative hemorrhages (N = 1, 8%). Our appreciably improved surgical results demonstrate our approach to address the EDS patients electively, and only two of 15 patients (13%) underwent procedures for urgent or emergent indication. There were also three patients who underwent secondary open vascular procedures on vessels noncontiguous with the original operation in an elective fashion. This contrasts strongly with the experience of Oderich et al7 wherein 70% of the patients had an open operation performed on an emergent or urgent basis, with 47% performed for rupture with active bleeding. For each EDS patient presenting acutely (N = 2, both vascular type: patient 1, liver rupture from hepatic aneurysm, and patient 2, aortic arch rupture with hypotension), patient 1 had a prolonged hospital stay, and patient 2 experienced a postoperative hematoma requiring exploration.
Hereditable connective tissue disorders such as EDS are associated with an increased lifelong risk of developing progressive vascular complications and sudden death. As shown in Fig 1, approximately 50% of EDS patients in our cohort who underwent open vascular procedures had died or presented with a secondary vascular complication after their elective index case. None of these complications was related the index surgical anastomosis, and noncontiguous vessels were the site of event. We continue to follow all EDS patients closely with regular annual or semiannual radiology studies, typically using either computed tomography angiography or magnetic resonance angiography whole-body imaging, to detect asymptomatic vascular pathology. It is important to recognize vascular disease in these patients early in order to allow preparation for elective surgery and be able to intervene when patients have the best risk profile. Indeed, our center’s experience is that each EDS patient’s operative risk is individualized and may even be predicted by reviewing their performance with prior surgical procedures. On the other hand, as EDS patients age, we also recognize their tissue integrity and ability to tolerate surgical procedures may wane.
The limitations of the study include the demographic of a tertiary referral population that may bias the population to improve our results-patients die before referral or are not referred at all given severe clinical history. Typical of any genetic disease, alternatively, referral bias may also overestimate treatment risk – less-affected patients are not referred or even clinically diagnosed before undergoing vascular procedures in their own locale. In general, the rarity of EDS in the community, as well as the rarity of needing repair (less than three per year in our tertiary center on average), should invoke consideration of referral to specialized centers with surgeons, interventionalists, and medical geneticists with expertise in evaluation of connective tissue disorder patients.
We rely strongly on clinical criteria for diagnosis, yet biochemical and genetic testing for collagen abnormalities is more accurate in most cases. It is possible that some of our study patients were misclassified as having EDS but may have another disorder. Particularly for the vascular type patients, three out of nine did not have biochemical or molecular confirmation. We have reviewed imaging on these patients and do not appreciate the carotid and vertebral tortuosity typical of Loeys-Dietz syndrome or suggestive skeletal anthropometrics. In Loeys-Dietz syndrome,22 peripheral aneurysms and aggressive clinical behaviors are common, yet surgical handling is known to be very favorable versus EDS patients.
Our study included many EDS subjects from all three of the common subtypes. In the previous literature, arterial complications have been underreported in nonvascular type EDS. We contend that genotype-phenotype correlations in EDS are not relevant in the surgical results, as tissue characteristics are noticeably abnormal in our experience with the many subtypes. Nonetheless, longer follow-up will be required to ascertain if our vascular reconstructions, neighboring portions of the arterial tree, and survival are distinguishable among the EDS subtypes who suffer vascular complications.
There remains no effective medical therapy for EDS. Our data suggest that EDS patients can safely undergo elective operations, including both endovascular and open vascular repair procedures. Our approach includes a multidisciplinary approach to evaluate the patients by a skilled medical geneticist, proper anesthetic preparation, and liberal use of adjunctive techniques to reduce operative trauma in the open and endovascular setting. For patients presenting with vascular complications amenable to coil embolization, endovascular approaches demonstrate an excellent safety profile. We remain committed to open surgical reconstruction for EDS patients when the nature of the vascular event requires, and refrain from stent-graft therapy given the serious nature of the associated fixation zone complications and high rates of reintervention appreciated by others.
CONCLUSION
Our contemporary results suggest that the majority of EDS patients with vascular disease can be managed electively with minimal morbidity and mortality. Prior recommendations to defer vascular interventions in EDS patients with known vascular abnormalities until urgent or emergent presentation may not be warranted. Further research to determine tissue integrity and suitability for surgical handling is likely to aid accurate risk stratification for treatment planning. Referral of EDS patients with vascular manifestations to centers with experience in the diagnosis and treatment of patients with connective tissue disorders should be encouraged.
Acknowledgments
This study was supported, in part, by funds originating at the Intramural Program, National Institutes on Aging, National Institutes of Health.
Footnotes
Presented at the Plenary Session of the Society for Vascular Surgery Annual Meeting, Denver, Colo, June 12, 2009.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
AUTHOR CONTRIBUTIONS
Conception and design: BB, JB
Analysis and interpretation: BB, JB
Data collection: BB, GA
Writing the article: JB, BB
Critical revision of the article: NM, JB
Final approval of the article: BB, JB
Statistical analysis: BB, GA
Obtained funding: JB, NM
Overall responsibility: JB
References
- 1.Barabas AP. Heterogeneity of the Ehlers-Danlos syndrome: description of three clinical types and a hypothesis to explain the basic defects. Br Med J. 1967;2:612–3. doi: 10.1136/bmj.2.5552.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman B, Royce PM. Superti-Furga: Ehlers-Danlos Syndrome. In: Royce P, Steinmann B, editors. Connective tissue and its heritable disorders. 5. New York: Wiley-Liss; 2002. pp. 431–523. [Google Scholar]
- 3.Byers PH. Ehlers-Danlos Syndrome. In: Rimoin DL, Connor JM, Pyereitz RE, editors. Emery and Rimoin’s principles and practice of medical genetics. 3. London: Churchill Livingstone; 1997. pp. 1067–81. [Google Scholar]
- 4.Cikrit DF, Miles JH, Silver D. Spotaneous arterial perforation: the Ehlers-Danlos specter. J Vasc Surg. 1987;5:248–55. [PubMed] [Google Scholar]
- 5.Bergqvist D. Ehlers-Danlos type IV syndrome: a review from a vascular surgical point of view. Eur J Surg. 1996;162:163–70. [PubMed] [Google Scholar]
- 6.Germain DP. Clinical and genetic features of vascular Ehlers-Danlos Syndrome. Ann Vasc Surg. 2002;16:391–7. doi: 10.1007/s10016-001-0229-y. [DOI] [PubMed] [Google Scholar]
- 7.Oderich GS, Panneton JM, Bower TC, Lindor NM, Cherry KJ, Noel AA, et al. The spectrum, management, and clinical outcome of Ehlers-Danlos Syndrome type IV: a 30-year experience. J Vasc Surg. 2005;42:98–106. doi: 10.1016/j.jvs.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 8.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos Syndromes: Revised Nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Am J Med Genet. 1998;77:31–7. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos Type IV, the vascular type. N Engl J Med. 2000;342:673–80. doi: 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- 10.Prockop DJ. Osteogenesis imperfecta: phenotypic heterogeneity, protein suicide, short and long collagen. Am J Hum Genet. 1984;36:499–505. [PMC free article] [PubMed] [Google Scholar]
- 11.Holbrook KA, Byers PH. Ultrastructural characteristics of the skin in a form of the Ehlers-Danlos syndrome type IV. Lab Invest. 1981;44:342–50. [PubMed] [Google Scholar]
- 12.Hauser I, Anton-Lamprecht I. Differential ultrastructural aberrations of collagen fibrils in Ehlers-Danlos Syndrome types I–IV as a means of diagnostics and classification. Hum Genet. 1994;93:394–407. doi: 10.1007/BF00201664. [DOI] [PubMed] [Google Scholar]
- 13.Hollands JK, Santarius T, Kirkpatrick PJ, Higgins JN. Treatment of a direct carotid-cavernous fistula in a patient with type IV Ehlers-Danlos syndrome: a novel approach. Neuroradiology. 2006;48:491–4. doi: 10.1007/s00234-006-0084-1. [DOI] [PubMed] [Google Scholar]
- 14.Matsushima K, Takara H. Endovascular treatment for a spontaneous rupture of the posterior tibial artery in a patient with Ehlers-Danlos syndrome type IV: Report of a case. Surg Today. 2009;39:523–6. doi: 10.1007/s00595-008-3881-9. [DOI] [PubMed] [Google Scholar]
- 15.Calvo P, Lanciego C, Krasniqi G, Cereceda C, Mórlan MA, Vega A, et al. Successful endovascular treatment of a splenic artery aneurysm in a patient with Ehlers-Danlos syndrome. J Vasc Inter Radiol. 2009;20:274–5. doi: 10.1016/j.jvir.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Lauwers G, Nevelsteen A, Daenen G, Lacroix H, Suy R, Frijns JP. Ehlers-Danlos syndrome type IV: a heterogeneous disease. Ann Vasc Surg. 1997;11:178–82. doi: 10.1007/s100169900031. [DOI] [PubMed] [Google Scholar]
- 17.Bade MA, Queral LA, Mukherjee D, Kong LS. Endovascular abdominal aortic aneurysm repair in a patient with Ehlers-Danlos syndrome. J Vasc Surg. 2007;46:360–2. doi: 10.1016/j.jvs.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Geisbüsch P, Kotelis D, von Tengg-Kobligk H, Hyhlik-Dürr A, Allenberg JR, Böckler D. Thoracic aortic endografting in patients with connective tissue diseases. J Endovasc Ther. 2008;15:144–9. doi: 10.1583/07-2286.1. [DOI] [PubMed] [Google Scholar]
- 19.van Keulen JW, Moll FL, Jahrome AK, van Herwaarden JA. Proximal aortic perforation after endovascular repair of a Type B dissection in a patient with Marfan Syndrome. J Vasc Surg. 2009;50:190–2. doi: 10.1016/j.jvs.2009.01.045. Epub 2009 May 15. [DOI] [PubMed] [Google Scholar]
- 20.Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA Society of Thoracic Surgeons Endovascular Surgery Task Force. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular grafts. Ann Thor Surg. 2008;85:S1–41. doi: 10.1016/j.athoracsur.2007.10.099. [DOI] [PubMed] [Google Scholar]
- 21.Maron BJ, Mitten MJ, Quandt EF, Zipes DP. Competitive athletes with cardiovascular disease: the case of Nicholas Knapp. N Engl J Med. 1998;339:1632–5. doi: 10.1056/NEJM199811263392211. [DOI] [PubMed] [Google Scholar]
- 22.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med. 2006;355:788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]