Abstract
Background
Three low molecular weight thiols are synthesized by Mycobacterium tuberculosis (M.tb), namely ergothioneine (ERG), mycothiol (MSH) and gamma-glutamylcysteine (GGC). They are able to counteract reactive oxygen species (ROS) and/or reactive nitrogen species (RNS). In addition, the production of ERG is elevated in the MSH-deficient M.tb mutant, while the production of MSH is elevated in the ERG-deficient mutants. Furthermore, the production of GGC is elevated in the MSH-deficient mutant and the ERG-deficient mutants. The propensity of one thiol to be elevated in the absence of the other prompted further investigations into their interplay in M.tb.
Methods
To achieve that, we generated two M.tb mutants that are unable to produce ERG nor MSH but are able to produce a moderate (ΔegtD-mshA) or significantly high (ΔegtB-mshA) amount of GGC relative to the wild-type strain. In addition, we generated an M.tb mutant that is unable to produce GGC nor MSH but is able to produce a significantly low level of ERG (ΔegtA-mshA) relative to the wild-type strain. The susceptibilities of these mutants to various in vitro and ex vivo stress conditions were investigated and compared.
Results
The ΔegtA-mshA mutant was the most susceptible to cellular stress relative to its parent single mutant strains (ΔegtA and ∆mshA) and the other double mutants. In addition, it displayed a growth-defect in vitro, in mouse and human macrophages suggesting; that the complete inhibition of ERG, MSH and GGC biosynthesis is deleterious for the growth of M.tb.
Conclusions
This study indicates that ERG, MSH and GGC are able to compensate for each other to maximize the protection and ensure the fitness of M.tb. This study therefore suggests that the most effective strategy to target thiol biosynthesis for anti-tuberculosis drug development would be the simultaneous inhibition of the biosynthesis of ERG, MSH and GGC.
Keywords: Thiols, Compensation, Tuberculosis, Therapeutic targets, ROS, RNS
Background
Upon host invasion, Mycobacterium tuberculosis (M.tb) encounters various cellular stresses generated by macrophages such as acidic, oxidative and nitrosative stress. However, M.tb is able to escape this, multiply, causing active tuberculosis (TB) or become dormant within necrotic macrophages (granuloma) [1, 2]. The host susceptibility to TB depends largely on its ability to fight invading mycobacteria by generating reactive oxygen species (ROS) and reactive nitrogen species (RNS) [3]. This became evident when the NADPH oxidase deficient (NOX2) mice and iNOS (inducible nitric oxide synthase) deficient mice were found to be more susceptible to TB infection than the wild-type mice [4, 5]. In addition, children with chronic granulomatous disease (a disorder characterized by phagocytic oxidative bursts resulting in recurrent pyogenic infections) were found to be highly susceptible to TB and to present complications during BCG vaccination [6]. Previous studies suggest a role of the phagocyte NADPH oxidase in the release of cytokines, implicating an interplay between the production of cytokines and the production of ROS, though the mechanism remains ambiguous. In addition, this interplay may also be implicated in the structural organization and formation of granuloma [7].
Several enzymes have been implicated in the detoxification of M.tb [8–10], however these enzymes required redox buffers during enzymatic reactions. Low molecular weight thiols such as MSH and ERG are efficient redox buffers [8, 11, 12]. Though it was shown that MSH and ERG are required for the survival of mycobacteria during adverse conditions [11, 13–15], the ability to generate mycobacteria mutants deficient in either MSH or ERG or both [11, 13, 16–18], suggests that they are compensated for by another thiol. Recently, another thiol, gamma-glutamylcysteine (GGC), was shown to provide mycobacteria protection against nitrosative and oxidative stress [18]. In addition, the production of ERG is elevated in the absence of MSH and vice versa [11, 15–19], while the production of GGC was found to be elevated in a MSH-deficient mutant and ERG-deficient mutants [18]. Elevation of one thiol following the loss of another as a compensation mechanism to maximize the protection of M.tb was therefore investigated in this study. Double mutants lacking MSH and ERG but producing a moderate amount of GGC (ΔegtD-mshA), another lacking both MSH and ERG but producing a high level of GGC (ΔegtB-mshA) and a last one lacking both MSH and GGC but producing a low level of ERG (ΔegtA-mshA) were generated. These were further characterized in vitro and ex vivo. This study demonstrates for the first time the significance of the compensatory roles of ERG, MSH and GGC in M.tb.
Methods
Generation and genotyping of M.tb double mutants
The gene mshA was deleted in the previously generated single M.tb CDC1551 mutants (egtA, egtB and egtD) as previously described [18, 20, 21]. To maximize the chances of obtaining the double mutants, plates were supplemented with OADC and ERG during the double cross over step. The deletion of mshA was investigated by PCR as previously described [18] and confirmed by southern blotting as follows.The genomic DNA was extracted from each strain as previously described [22]. Following extraction, it was digested (4–6 μg) with ClaI restriction enzyme overnight. Confirmation of complete digestion was performed by running a little amount of the digested DNA on an agarose gel (test gel). The digested genomic DNA along with the digoxigenin (DIG)-labelled molecular weight ladder (Roche) were separated on a 1% agarose gel and depurinated by incubating the gel in 250 mM HCl for 10 min (mins) at room temperature (RT). The gel was later rinsed with distilled H2O.
Southern transfer of DNA onto the membrane
This was performed as previously described [23] with few modifications. After depurination, the DNA was denatured by incubating the gel at room temperature (RT) for 30 mins in the denaturing buffer (0.5 M NaOH, 1.5 mM NaCl). It was subsequently neutralised in the neutralization buffer (0.5 M Tris/HCl pH 7.5, 1.5 M NaCl) for another 30 mins. Southern transfer was performed using the positively charged nylon membrane (Roche Diagnostic GmbH Mannheim, Germany) overnight in 20X SSC (3 M NaCl, 300 mM sodium citrate, pH 7). The membrane was washed in 2X SSC the following day and baked for 2 h (hrs) at 80 °C between 2 Wattman papers.
The DIG High Prime DNA labelling and detection kit II and the DIG wash and block Buffer set (Roche) were used at this stage. The principle is described briefly as follows. DIG binds to the T and A bases of the single stranded (denatured) probe, which would hybridize to the single stranded fragments from the genomic DNA digestion. Subsequently, an anti-DIG antibody (conjugated to alkaline phosphatase) would be added. The conjugated alkaline phosphatase would be able to dephosphorylate CSPD (Disodium3-(4-methoxyspiro{l,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl) phenyl phosphate) leading to photons emission at 477 nm.
Probe labelling
An amount of 150–200 ng of the probe DNA was denatured at 100 °C for 10 mins, snapped cooled on ice for 5 mins, added in 1 X DIG High prime and incubated for 24–48 h at 37 °C for the labelling reaction to occur. The reaction was stopped by incubating the mixture at 65 °C for 10 mins.
Prehybridization and hybridization of the membrane
The membrane from the southern transfer, was pre-hybridized at 65 °C for 30–60 min in the pre-hybridization buffer using a shaking water bath (optimal hybridization temperature is equal to the melting temperature of the probes minus forty two). The denatured probe was added in the hybridization buffer and the pre-hybridization buffer was replaced with the hybridization buffer containing the probe. Using a heat sealer, the membrane was sealed without air bubbles in a plastic bag and incubated at 65 °C overnight. The following day, the membrane was washed twice for 5 mins at RT in a high stringency preheated (65 °C) buffer (2X SSC/0.1% SDS (sodium dodecyl sulphate)). It was subsequently washed twice in a preheated (65 °C) low stringency buffer (0.5X SSC/0.1% SDS) for 15 mins. After these washes, the membrane was incubated with shaking at RT for 2 mins in 1X maleate buffer. After discarding the maleate buffer, the membrane was incubated further in the blocking solution for 30 mins to 3 hrs. The blocking solution was discarded and replaced with another blocking solution containing the specific antibody and the membrane was incubated further for another 30 mins. The membrane was subsequently washed twice in 1X washing buffer at RT for 15 mins and equilibrated with the detection buffer for 3 mins.
Detection
The equilibrated membrane was placed in a hybridization bag and 1-3 ml CSPD (chemiluminescent substrate for alkaline phosphatase) was applied to it. After removal of air bubbles, the membrane was heat-sealed. Following, incubation at RT for 5 mins, excess fluid was removed, and the membrane was sealed again and incubated further at 37 °C for 10 mins. Bands detection and image acquisition were performed by the ChemiDoc™ MP System (BioRad; 2000 Alfred Nobel Drive, Hercules, California 94,547, USA).
Quantification of thiols
After culturing mycobacteria for ~ 2 weeks in 7H9 liquid media, 100 μl of each culture was serially diluted and plated for colony forming unit (CFU) counts. Five millilitres of each culture was pelleted by centrifugation. One millilitre of the supernatant was filtered twice (using a syringe filter) and lyophilized overnight. The pellets were washed twice in double distilled water and stored at − 80 °C. Lyophilized supernatants (extracellular fractions) and frozen pellets (intracellular fractions) from all biological replicates were re-suspended in the lysis buffer (50% warm acetonitrile + 20 mM hepes pH 8 + 2 mM monobromobimanne), sonicated in a water bath sonicator for ~ 30 mins at 60 °C and acidified with a final concentration of 1 mM acetic acid. Following centrifugation, they were filtered twice and stored for liquid chromatography tandem mass spectrometry (LC-MS) analyses [24].
Growth curve analysis of the mutants
This was performed as previously described [25]. The growth rate was evaluated by subtracting the OD600 at day 4 from the OD600 at day 10, which represents the linear exponential phase of growth [26].
Susceptibility testing of the mutants
Mycobacteria from frozen stocks were cultured on plates, 7H11 solid cultures supplemented with 1X OADC (oleic acid, albumin, dextrose and catalase). The lawn of mycobacteria formed on the plates were scrapped off, re-suspended in 7H9 supplemented with 1X ADS (albumin, dextrose, sodium chloride). After disrupting cell aggregates by trituration, cells suspensions were re-suspended to an OD600 of ~ 0.02. Then they were exposed in a 96-well plate to either 0.2 mM diamide (DIM), or 5 mM vitamin C (VC) for ~ 72 h, or ~ 4 mM diethylaminetriamine nitric oxide adduct (DETA-NO) for 14 days. Their susceptibilities to diamide (DIM) and vitamin C (VC) were estimated from the CFU counts of the treated cells relatively to the untreated cells. Their susceptibilities to DETA-NO were estimated from the fluorescence intensity of resazurin of the treated cells relative to the untreated cells. Similarly, their susceptibilities to antibiotics were investigated as follows. Logarithmic phase liquid cultures (in ADS) were diluted to an OD600 of ~ 0.007 and added to an equal volume of serially diluted drugs in black 96-well plates (optical bottom) and incubated for ~ 7 days at 37 °C. After adding resazurin and incubating the plates for 24–48 h at 37 °C, susceptibilities were estimated from the measured fluorescence intensity of resazurin (excitation at 544 nm and emission at 590 nm) of the treated cells relative to the untreated cells.
Infection of macrophages
Double mutants were inoculated 3–7 days before other strains, to ensure that they are at the same growth stage on the day of infection. On the day of infection, logarithmic phase mycobacteria were washed in phosphate buffer saline (PBS) or in the defined cell media supplemented with 10% fetal bovine serum (RPMI 1640 (Roswell Park Memorial Institute medium) for human primary macrophages or DMEM (Dulbecco’s modified Eagle’s medium) for RAW 264.7 murine cell lines). Re-suspensions were triturated by mixing with a syringe (25GA × 5/8in (0.5 x16mm)) (≥10X). The OD600 was adjusted to 0.1 with the cell culture media and the re-suspension was triturated. Previous optimizations revealed that the concentration of the double mutants, ΔegtA-mshA and ΔegtD-mshA was ~ 107/ml, of the ∆egtB and ∆egtB-mshA mutants was ~ 4 X 107 and of the other mutants and the wild-type was ~ 3.5 X 107/ml at OD600 of ~ 0.1. This enabled to calculate the volume required for each strain to obtain the targeted multiplicity of infection (MOI). One ml of each adjusted suspension was added in triplicate and accordingly to each well of a 24-well plate containing seeded macrophages (~ 5 X 105 per well). Few microliters of the mycobacterial suspensions added to the macrophages were serially diluted and plated for CFU counts to estimate the number of mycobacteria at time zero (before infection). Macrophages were gently washed (two-four times) after three hours of infection. Following the wash step, they were either lysed with 0.05% SDS in order to estimate the intracellular mycobacteria load at time ~ 4 h or incubated further for later time points. For prolong exposure, additional washes were performed on the third day. M0 macrophages differentiated from peripheral blood mononuclear cells (PBMC) isolated over the Histopaque (Sigma Aldrich) gradient method from the blood of a healthy donor were infected as described above with the only difference in the number of macrophages seeded per plate (~ 3 X 105 instead of ~ 5 X 105).
Statistical analyses
Statistical analyses were performed with Prism using a multiple t-test approach, assuming a uniform distribution with alpha set to 0.05 *(P < 0.05), **(P < 0.01), ***(P < 0.001), ****(P < 0.0001).
Results
The M.tb mutants deficient in more than one thiol have a growth defect in vitro
The gene mshA was deleted in the previously generated ΔegtA, ΔegtB, ΔegtD single thiol-deficient mutants as previously described [18] (Fig. 1). This resulted in the loss of MSH production in the generated double mutants (Table 1). The ΔegtA-mshA mutant could not produce GGC, neither MSH, but did produce a low level of ERG, the ΔegtB-mshA could not produce ERG neither MSH but produced a significantly high level of GGC, and the ΔegtD-mshA mutant was also unable to produce ERG, neither MSH but produced a high level of GGC (Table 1). Complementation of the ΔegtA-mshA by inserting a copy of egtA at the attP site of the genome as previously described [18], restored the production of ERG and GGC in this strain (ΔegtAc-mshA in Table 1).
Fig. 1.
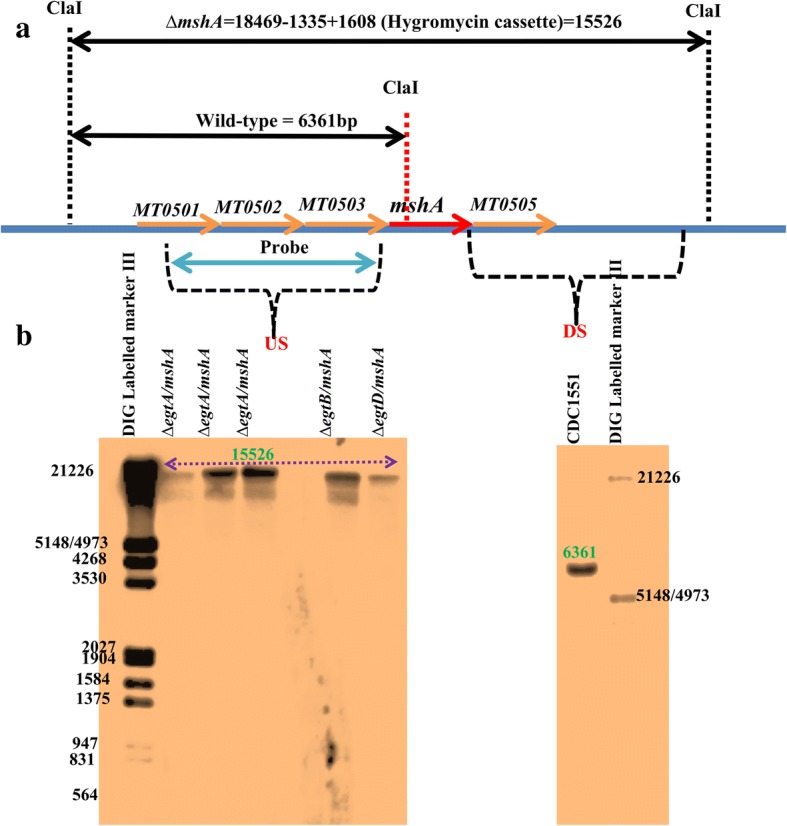
Southern blotting analysis of the double mutants. a Southern blotting design of mshA deletion. The gene mshA (1335 bp) was replaced by a hygromycin cassette (1608 bp) in the mutant. The restriction enzyme ClaI cuts within mshA but not within the hygromycin gene. It also cuts outside the cloned upstream (US) and downstream (DS) regions. Therefore, ClaI digestion of the wild-type genomic DNA and the mutant genomic DNA, yield distinct fragment sizes at the deleted region. These fragments are detected using a digoxigenin labelled DNA fragment (probe) that is able to hybridize to both fragments b Southern blotting results of the wild-type and mutants. Since the gene mshA is still intact in the wild-type strain, the 6361 bp fragment is detected. However, since mshA is replaced by the hygromycin cassette in the mutant, a bigger fragment is detected (15,526 bp)
Table 1.
Level of thiols in the generated double mutants pg/105CFUs
| Wild-type | ΔegtA-mshA | ΔegtB-mshA | ΔegtD-mshA | ΔegtAc-mshA | |
|---|---|---|---|---|---|
| IE1 | 111 | 10 | < 5 | < 5 | 486 |
| EE1 | 33 | 15 | < 5 | < 5 | 303 |
| IE2 | 296 | 48 | < 5 | < 5 | 184 |
| EE2 | 93 | 44 | < 5 | < 5 | 118 |
| IM1 | 41 | < 5 | < 5 | < 5 | < 5 |
| IM2 | 12 | < 5 | < 5 | < 5 | < 5 |
| IG1 | 1.7 | < 5 | 112 | 24 | 25 |
| IG2 | 0.6 | < 5 | 91 | 23 | 21 |
IE intracellular ERG, EE extracellular ERG, IM intracellular MSH, IG intracellular gamma-glutamyclcysteine
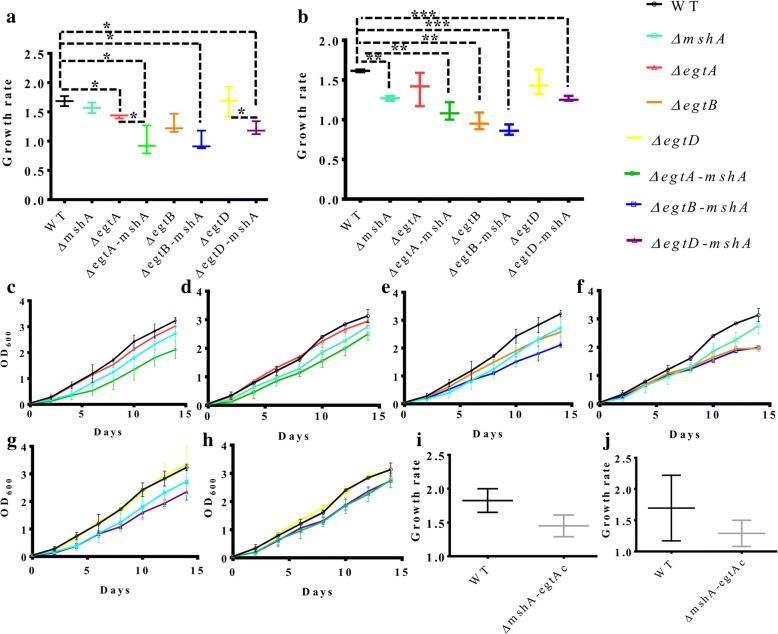
The growth profiles of the generated double mutants were evaluated in liquid cultures supplemented with either ADS or OADC. Growth fitness of the strains were evaluated by investigating their growth rate as previously described [26]. All double mutant had a growth defect in media supplemented with ADS or OADC relative to the wild-type strain (Fig. 2a and b). The growth defect of the ΔegtA-mshA mutant relative to its parent strains is more pronounced in the media supplemented with ADS (Fig. 2c) relative to media supplemented with OADC (Fig. 2d). This was not the case with the ΔegtB-mshA mutant (Fig. 2e and f), but was with the ΔegtD-mshA mutant (Fig.2g and h). The production of GGC and ERG is restored (Table 1) in the complemented strain of the ΔegtA-mshA mutant and analysis of the growth rate of this complemented strain relative to the wild-type reveals that it is not significantly (P > 0.05) different (Fig. 2i and j).
Fig. 2.
Growth curves of double mutants. a Growth rate of mutants in media supplemented with ADS, all double mutant display a growth defect relative to their parent single mutants and the wild-type (WT) strain. b Growth rate of mutants in media supplemented with OADC, all double mutant display a growth defect relative to the wild-type (WT) strain c Growth curves of the ΔegtA-mshA double mutant relative to its parent strains in media supplemented with ADS. d Growth curves of the ΔegtA-mshA double mutant relative to its parent strains in media supplemented with OADC e Growth curves of the ΔegtB-mshA double mutant relative to its parent strains in media supplemented with ADS f Growth curves of the ΔegtB-mshA double mutant relative to its parent strains in media supplemented with OADC. g Growth curves of the ΔegtD-mshA double mutant relative to its parent strains in media supplemented with ADS. h Growth curves of the ΔegtD-mshA double mutant relative to its parent strains in media supplemented with OADC. i Growth rate of the complemented strain of ΔegtA-mshA relative to the wild-type in media supplemented with ADS. j Growth rate of the complemented strain of ΔegtA-mshA relative to the wild-type in media supplemented with OADC. Data represent 2–3 experiments. Statistical analyses were performed with Prism using a multiple t-test approach, assuming a uniform distribution with alpha set to 0.05 *(P < 0.05), **(P < 0.01), ***(P < 0.001)
The M.tb mutant, deficient in all three thiols, is the most sensitive to oxidative and nitrosative stress
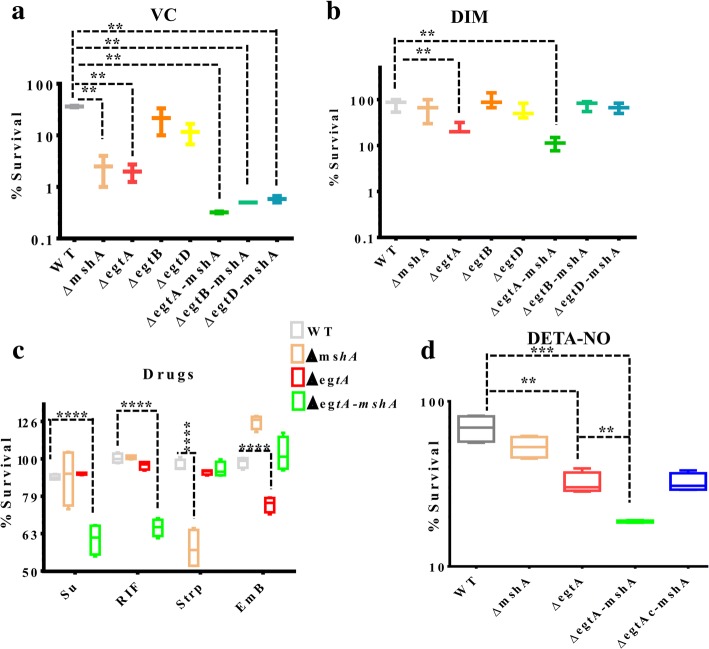
Ferric ions (Fe3+) can be reduced by VC to ferrous ions (Fe2+) (Fe3+ + VC = Fe2+), which in turn can react with oxygen to yield superoxide (Fe2+ + O2 = O°2− + Fe3+). The generated superoxide can be converted to hydrogen peroxide by dismutation (O°2− + 2H+ = H2O2 + O2). Hydrogen peroxide can also react with ferrous ions to yield hydroxyl radicals (H2O2 + Fe2+ = Fe3+ + OH° + OH−) [27, 28]. As such, VC is able to generate oxidative stress. Previous studies indicated that the MSH-deficient ΔmshA mutant is sensitive to VC [29]. Therefore, in order to determine if the loss of more than one thiol would aggravate the sensitivity of M.tb, the double mutants with their respective parent single mutants were exposed to VC. While the ΔegtA-mshA mutant was the most sensitive strain (Fig. 3a), all three double mutants were more sensitive than their respective parent strains (Fig. 3a). Compounds such as DIM generate oxidative stress by oxidizing thiols [30, 31]. The mutant ΔegtA-mshA was the most sensitive double mutant to DIM (Fig. 3b). The susceptibility of this mutant to antibiotics known to generate oxidative stress such as rifampicin [32] (RIF) and sulfaguanidine [25] (Su) was investigated. Preliminary investigations at lethal concentrations revealed no significant difference between ΔegtA-mshA and its parent single mutant strains (∆egtA and ∆mshA) since they were already highly sensitive to these antibiotics as previously shown [11, 25]. However, sub-lethal concentrations of these drugs significantly inhibited the growth of the ΔegtA-mshA mutant (Fig. 3c). This was not observed with other tested antibiotics namely, streptomycin (Strp) and ethambutol (EmB) (Fig. 3c). It was previously indicated that the high level of MSH in the ∆egtA mutant was able to protect it against a short exposure to a low concentration of nitric oxide. In addition, the high level of GGC in the ∆mshA mutant was able to protect it against an extended exposure to a high concentration of nitric oxide [18]. In this study, the ΔegtA-mshA mutant (deficient in both MSH and GGC, Table 1) was more sensitive to nitric oxide stress generated by DETA-NO than the wild-type, the GGC-deficient ΔegtA and MSH-deficient ΔmshA single mutants (Fig. 3d).
Fig. 3.
Susceptibility of the double mutants to oxidative and nitrosative stress. a Susceptibility of the mutants to vitamin C (VC) (5 mM for ~ 72 h). Every double mutant was more sensitive than its parent single mutant strains; however, ΔegtA-mshA appeared to be the most sensitive. Data are representative of two experiments. b Susceptibility of the mutants to diamide (DIM) (0.2 mM for ~ 72 h), ΔegtA-mshA is the most sensitive mutant. Data are representative of three independent experiments c Susceptibility of the mutants to sulfaguanidine (Su, 3.8 μg/ml), rifampicin (RIF, 0.0007 μg/ml), streptomycin (Strp, 0.16 μg/ml) and ethambutol (Emb, 0.6 μg/ml). ΔegtA-mshA is more sensitive than its parent strains to RIF and Su, while ΔmshA is sensitive to Strep and ΔegtA is sensitive to EmB. Data are representative of two experiments and two technical replicates for each experiment. d Susceptibility of the mutants to nitric oxide generated by diethylaminetriamine nitric oxide adduct (DETA-NO) (4 mM for 14 days). Even though, the single mutants display a sensitivity trend, ΔegtA-mshA is more sensitive than its parent strains. Data are representative of two experiments and two technical replicates for each experiment. Statistical analyses were performed with Prism using a multiple t-test approach, assuming a uniform distribution with alpha set to 0.05 *(P < 0.05), **(P < 0.01), ***(P < 0.001), ****(P < 0.0001)
The M.tb mutant, deficient in all three thiols, has the most severe growth defect in macrophages
During TB infection, human macrophages are able to generate oxidative and nitrosative stress to destroy invading mycobacteria. M. tuberculosis is able to escape this defence mechanism to some extent [1]. It was previously shown that the thiol-deficient single mutants survived within the first few days and became sensitive after three days of infection in mouse macrophages at multiplicity of infection (MOI) ≥ 5:1 (mycobacteria: macrophages) [11, 33]. The ability of the double thiol-deficient mutants to survive within the first few days of infection of mouse macrophages was therefore investigated. The ΔegtA-mshA mutant was the most sensitive double mutant at MOI 1:1 (Fig. 4a). Investigation at a higher multiplicity of infection MOI 10:1 further confirms that the ΔegtA-mshA mutant was more sensitive than its parent single mutant strains during the first few days of infection (Fig. 4b). However, to ensure that these results could be related to humans treated with potential compounds inhibiting the biosynthesis of all three thiols, human blood monocyte-derived macrophages (HBMM) isolated from a healthy donor were infected with these mutants. As opposed to the observation in mouse macrophages (Fig. 4a and b), the ΔegtA-mshA mutant became more sensitive than its parent strains only after 3 days of infection at MOI 2.5:1 (Fig. 4c), a trend that was observed as well at a higher MOI (MOI 5:1, data not shown).
Fig. 4.
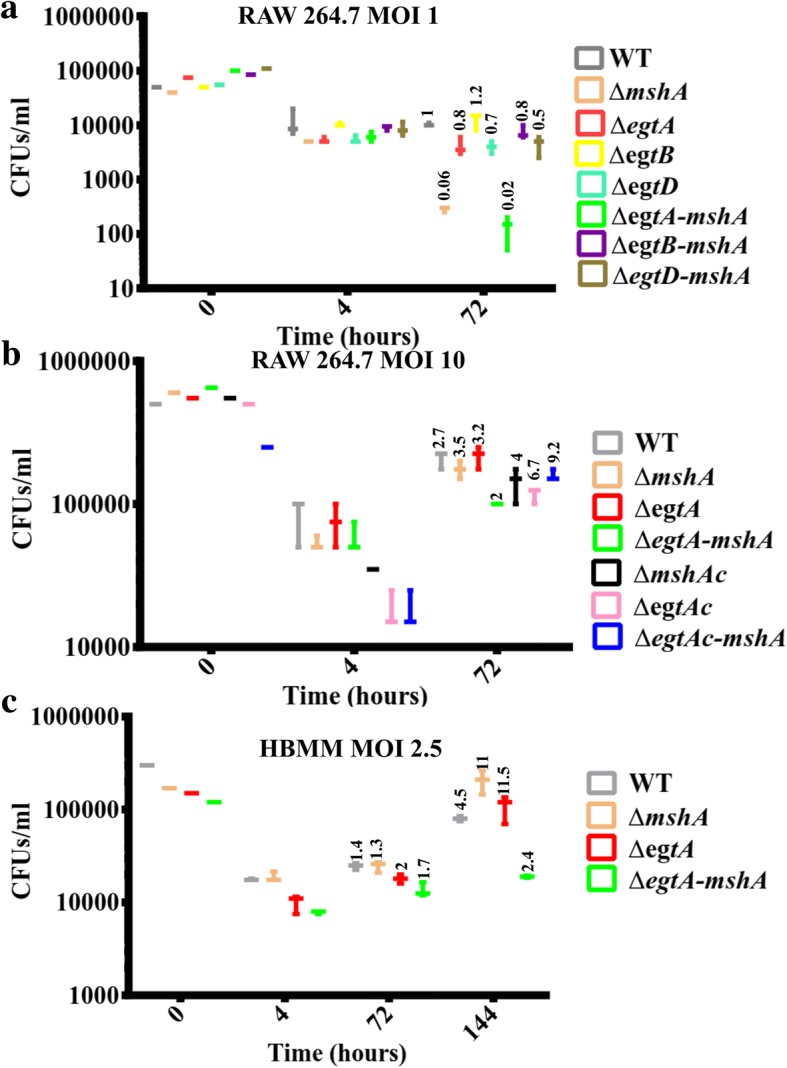
Survival of thiols-deficient mutants in macrophages. a Investigation of the survival of all double mutant relative to their parent strains in murine cell lines RAW264.7 at an MOI of~ 1:1. The ΔegtA-mshA double mutant was the most sensitive. b Further, investigation of its survival relative to its parent and complemented strains at an MOI of~ 10:1. The ΔegtA-mshA mutant was still the most sensitive. c This was also observed when human blood monocyte-derived macrophages (HBMM) were infected at an MOI of ~ 2.5:1 and 5:1 (data not shown), however at a later time point. The 0-h time-point represents the amount of mycobacteria added to the macrophages and the 4-h time point represents the amount of mycobacteria taken up by macrophages. For accuracy, the growth index (GI) was determined based on the amount of each ingested mycobacterial strain (4-h time point). The average GI indicated at the top of each whisker, was determined by dividing the CFUs obtained at the defined time point by the CFUs obtained at the 4-h time point (that indicates the uptake amount of each stain). Data are representative of three replicates
Discussion
The ability of thiols to protect mycobacteria against oxidative and nitrosative stress is well documented [11, 17, 18, 33–35]. However, it was unclear if the fitness of M.tb would be more affected in case all thiols were depleted simultaneously. In this study, by generating a series of double mutants, it was shown that the loss of more than one thiol affects the growth rate of M.tb. In addition, the growth defect of the double mutants was more severe relative to their parent strains in the absence of catalase (media supplemented with ADS) (Fig. 2a, b, c, d, g, h). This suggests that catalase may facilitate the growth of these mutants by reducing hydrogen peroxide or peroxynitrite [36]. The growth defect phenotype was partially reversed in the complemented strain of the ΔegtA-mshA mutant (ΔegtAc-mshA, Fig. 2i and j). Since the production of ERG and GGC is restored in the complemented strain (Table 1), this suggests that all three thiols are required to ensure the optimal growth of M.tb.
On the other hand, though the ΔegtB-mshA and ΔegtD-mshA mutants were more sensitive than their parent single mutant strains to oxidative stress (Fig. 3a), the ΔegtA-mshA mutant was the most sensitive strain to oxidative and nitrosative stress (Fig. 3). In addition, it was sensitive to sub-lethal concentrations of RIF and Su, but not Strp neither EmB. Since the mode of action of Strp and EmB is not directly associated with the generation of oxidative stress [37], as is of the case for Su [25] and RIF [32], these results suggest that compounds targeting the biosynthesis of all three thiols would reduce the therapeutic dose of drugs that generate oxidative stress such as RIF and Su.
Though it was indicated that ERG is able to scavenge peroxyntrite [38], and decompose S-nitrosoglutathione [39], it is unclear if it is able to protect eukaryotic or prokaryotic cells against nitrosative stress. Preliminary investigations of the susceptibility of all three double mutants to nitrosative stress generated by DETA-NO revealed that the ∆egtA-mshA mutant, which still produced low levels of ERG (Table 1), was more sensitive than the ΔegtB-mshA and ΔegtD-mshA mutants which can’t produce ERG (Table 1) (data not shown). In addition, it was shown that chemical complementation of the sensitivity phenotype of the ∆egtA mutant to nitrosative stress generated by DETA-NO could be achieved with GGC but not ERG [18]. Therefore, it is less likely that the high sensitivity of the ∆egtA-mshA mutant to nitrosative stress relative to its parent strains (Fig. 3d) is associated with the depletion of ERG in this strain (Table 1). Nevertheless, according to results in Table 1, dealing with the anti-nitrosative roles of MSH [40] and GGC [18, 41], the sensitivity of the double mutant lacking both thiols (∆egtA-mshA) is more likely associated with the loss of both thiols (Table 1) in this strain.
Further investigations of the survival of these mutants revealed that the ΔegtA-mshA mutant had the most severe growth defect within macrophages (Fig. 4), indicating that the elevated GGC in the other double mutants (Table 1) may have protected them during the first three days of infection. The growth defect of the ΔegtA-mshA mutant within the mouse macrophages was observed during early infections (~ 3 days, Fig. 4a and b). On the other hand, the ERG-deficient single mutant strains were shown to display a growth defect within mouse macrophages after 4 days of infection [11, 33]. This suggests that the elevated levels of MSH and GGC in the ERG-deficient single mutants [18, 25] are not enough to protect them during a prolong exposure to ROS and RNS generated by macrophages (after 4 days) but are enough to protect them during the first few days of infection. Further explaining why, the ΔegtA-mshA mutant, deficient in all thiols was the most sensitive during the first few days of infection (Fig. 4a and b). However, this was not the case with human blood monocyte-derived macrophages (HBMM), as the ΔegtA-mshA mutant displayed the most severe sensitivity only after 3 days (Fig. 4c). This suggests agreement with previous studies that showed that mycobacteria are better adapted to survive and grow exponentially in the human primary macrophages than in mouse macrophages [42]. Probably, because mouse macrophages display increased iNOS (inducible nitric oxide synthase) activity and consequently increased production of RNS during early infections [43]. The sensitivity phenotype of the ∆egtA-mshA double mutant observed during the early time point in mouse macrophages (Fig. 4a and b) may therefore be due to this initial burst, which is not the case in HBMM where the growth defect was only observed at a later time point (6-day) (Fig. 4c). Therefore, thiols interplay to ensure an optimal protection of M.tb against nitrosative and oxidative stress. It is also worth noting that the ΔegtA-mshA mutant still produced a low level of ERG (Table 1), yet, it displayed the most severe sensitivity (Figs. 3 and 4) suggesting that the absolute simultaneous inhibition of all three thiols could be lethal to M.tb. However, this requires further investigations.
Conclusions
In conclusion, thiols are able to protect M.tb against various cellular stresses and ensuring their fitness in vitro and within macrophages. However, to ensure the potential complete eradication of invading M.tb during infection, this study suggests that targeting the production of all three thiols simultaneously would be more efficient.
Acknowledgements
Authors would also like to thank Abhilasha Madhvi Mishra for collecting and isolating the PBMC, Jomien Mouton and Danicke Willemse for kindly donating the RAW 264.7 cells lines, Andile Ngwane for his intellectual input during the design of the drug testing experiments, Malcolm Taylor and the mass spectrometry unit of the Central Analytical Facilities of Stellenbosch University for performing the LC-MS.
Funding
Research reported in this publication was supported by the South African Medical Research Council (SAMRC) and the DST-NRF Centre of Excellence for Biomedical Tuberculosis Research.
Availability of data and materials
The first author (karallia@sun.ac.za) on reasonable request can provide raw data of results.
Abbreviations
- ADS
Albumin, dextrose, sodium chloride
- CFU
Colony forming unit
- CSPD
Disodium3-(4-methoxyspiro{l,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl) phenyl phosphate
- DETA-NO
Diethylaminetriamine nitric oxide adduct
- DIG
digoxigenin
- DIM
Diamide
- DMEM
Dulbecco’s modified Eagle’s medium
- EmB
Ethambutol
- ERG
Ergothioneine
- GGC
Gamma-glutamylcysteine
- HBMM
Human blood monocyte-derived macrophages
- Hr
hour
- iNOS
Inducible nitric oxide synthase
- M.tb
Mycobacterium tuberculosis
- Min
minute
- MOI
Multiplicity of infection
- MSH
Mycothiol
- OADC
Oleic acid, albumin, dextrose and catalase
- PBMC
Peripheral blood mononuclear cells
- PBS
Phosphate buffer saline
- RIF
Rifampicin
- RNS
Reactive nitrogen species.
- ROS
Reactive oxygen species
- RPMI 1640
Roswell Park Memorial Institute medium
- RT
Room temperature
- SDS
Sodium dodecyl sulphate
- Strp
Streptomycin
- Su
Sulfaguanidine.
- TB
Tuberculosis
- VC
Vitamin C
Authors’ contributions
CSE, MJW, IJW and BB conceived the experiments, read and revised the manuscript. CSE wrote the manuscript, designed and performed the experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The ethics committee of the faculty of Medicine and Health Sciences of Stellenbosch University (Cape Town, South Africa) approved the study PBMC isolation protocol (S17/10/211). The blood donor signed a written consent form.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
C. Sao Emani, Email: karallia@sun.ac.za
M. J. Williams, Email: moniquejw@sun.ac.za
I. J. Wiid, Email: bukslucky@gmail.com
B. Baker, Email: brubaker@sun.ac.za
References
- 1.Stallings CL, Glickman MS. Is Mycobacterium tuberculosis stressed out? A critical assessment of the genetic evidence. Microbes Infect [Internet]. NIH Public Access; 2010 [cited 2018 Feb 27];12:1091–101. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20691805. [DOI] [PMC free article] [PubMed]
- 2.Xu G, Wang J, Gao GF, Liu CH. Insights into battles between Mycobacterium tuberculosis and macrophages. Protein Cell [Internet]. Springer; 2014 [cited 2018 Feb 27];5:728–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24938416. [DOI] [PMC free article] [PubMed]
- 3.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun [Internet]. 2008;76:2333–40. Available from: http://view.ncbi.nlm.nih.gov/pubmed/18347040. [DOI] [PMC free article] [PubMed]
- 4.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A [Internet]. 1997;94:5243–8. Available from: http://view.ncbi.nlm.nih.gov/pubmed/9144222. [DOI] [PMC free article] [PubMed]
- 5.Adams LB, Dinauer MC, Morgenstern DE, Krahenbuhl JL. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis Off J Int Union Against Tuberc Lung Dis. 1997;78:237–246. doi: 10.1016/S0962-8479(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee PPW, Chan K-W, Jiang L, Chen T, Li C, Lee T-L, et al. Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr Infect Dis J. 2008;27:224–230. doi: 10.1097/INF.0b013e31815b494c. [DOI] [PubMed] [Google Scholar]
- 7.Deffert C, Cachat J, Krause K-H. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell Microbiol [Internet]. 2014 [cited 2018 Jun 6];16:1168–78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24916152. [DOI] [PubMed]
- 8.Nambi S, Long JE, Mishra BB, Baker R, Murphy KC, Olive AJ, et al. The oxidative stress network of Mycobacterium tuberculosis reveals coordination between radical detoxification systems. Cell Host Microbe. 2015;17:829–837. doi: 10.1016/j.chom.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryk R, Lima CD, Erdjument-Bromage H, Tempst P, Nathan C. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Sci (80- ) [Internet]. 2002;295:1073–7. Available from: http://view.ncbi.nlm.nih.gov/pubmed/11799204. [DOI] [PubMed]
- 10.Lee SP, Hwang YS, Kim YJ, Kwon KS, Kim HJ, Kim K, et al. Cyclophilin A Binds to Peroxiredoxins and Activates Its Peroxidase Activity. J Biol Chem. 2001;276:29826–32. [DOI] [PubMed]
- 11.Saini V, Cumming BM, Guidry L, Lamprecht DA, Adamson JH, Reddy VP, et al. Ergothioneine Maintains Redox and Bioenergetic Homeostasis Essential for Drug Susceptibility and Virulence of Mycobacterium tuberculosis. Cell Rep. 2016;14(3):572–585. doi: 10.1016/j.celrep.2015.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ordóñez E, Van Belle K, Roos G, De Galan S, Letek M, Gil JA, et al. Arsenate Reductase, Mycothiol, and Mycoredoxin Concert Thiol/Disulfide Exchange. J Biol Chem [Internet]. 2009 [cited 2018 Feb 27];284:15107–15116. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19286650 [DOI] [PMC free article] [PubMed]
- 13.Xu X, Vilchèze C, Av-Gay Y, Gómez-Velasco A, Jacobs WRJ. Precise null deletion mutations of the mycothiol synthesis genes reveal their role in isoniazid and ethionamide resistance in Mycobacterium smegmatis. Antimicrob Agents Chemother [Internet] 2011;55:3133–3139. doi: 10.1128/AAC.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ung KSE, Av-Gay Y. Mycothiol-dependent mycobacterial response to oxidative stress. FEBS Lett. 2006;580:2712–2716. doi: 10.1016/j.febslet.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Ta P, Buchmeier N, Newton GL, Rawat M, Fahey RC. Organic Hydroperoxide resistance protein and Ergothioneine compensate for loss of Mycothiol in Mycobacterium smegmatis mutants. J Bacteriol [Internet] 2011;193:1981–1990. doi: 10.1128/JB.01402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vilchèze C, Av-Gay Y, Attarian R, Liu Z, Hazbón MH, Colangeli R, et al. Mycothiol biosynthesis is essential for ethionamide susceptibility in Mycobacterium tuberculosis. Mol Microbiol [Internet]. 2008;69:1316–1329. Available from: http://view.ncbi.nlm.nih.gov/pubmed/18651841. [DOI] [PMC free article] [PubMed]
- 17.Sao Emani C, Williams MJ, Wiid IJ, Hiten NF, Viljoen AJ, Pietersen R-DD, et al. Ergothioneine is a secreted antioxidant in Mycobacterium smegmatis. Antimicrob Agents Chemother [Internet]. 2013;57:3202–7. Available from: http://view.ncbi.nlm.nih.gov/pubmed/23629716. [DOI] [PMC free article] [PubMed]
- 18.Sao Emani C, Williams MJ, Van Helden PD, Taylor MJC, Wiid IJ, Baker B. Gamma-glutamylcysteine protects ergothioneine-deficient Mycobacterium tuberculosis mutants against oxidative and nitrosative stress. Biochem Biophys Res Commun. 2018;495(1):174–178. doi: 10.1016/j.bbrc.2017.10.163. [DOI] [PubMed] [Google Scholar]
- 19.Shea RJ, Mulks MH. ohr, Encoding an organic hydroperoxide reductase, is an in vivo-induced gene in Actinobacillus pleuropneumoniae. Infect Immun [Internet]. 2002;70:794–802. Available from: http://view.ncbi.nlm.nih.gov/pubmed/11796613. [DOI] [PMC free article] [PubMed]
- 20.Parish T, Stoker NG. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology [Internet]. 2000 [cited 2018 May 9];146(Pt 8):1969–75. Available from: http://view.ncbi.nlm.nih.gov/pubmed/10931901. [DOI] [PubMed]
- 21.Muttucumaru DGN, Parish T. The molecular biology of recombination in mycobacteria: what do we know and how can we use it? Curr Issues Mol Biol [Internet] 2004;6:145–157. [PubMed] [Google Scholar]
- 22.Warren R, de Kock M, Engelke E, Myburgh R, Gey van Pittius N, Victor T, et al. Safe Mycobacterium tuberculosis DNA extraction method that does not compromise integrity. J Clin Microbiol [Internet] American Society for Microbiology (ASM); 2006 [cited 2018 Jun 8];44:254–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16390984. [DOI] [PMC free article] [PubMed]
- 23.Warren R, Richardson M, Sampson S, Hauman JH, Beyers N, Donald PR, et al. Genotyping of Mycobacterium tuberculosis with additional markers enhances accuracy in epidemiological studies J Clin Microbiol [Internet]. American Society for Microbiology (ASM); 1996 [cited 2018 Jun 8];34:2219–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8862588. [DOI] [PMC free article] [PubMed]
- 24.Capone DL, Ristic R, Pardon KH, Jeffery DW. Simple Quantitative Determination of Potent Thiols at Ultratrace Levels in Wine by Derivatization and High-Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC-MS/MS) Analysis. Anal Chem [Internet]. 2015 [cited 2018 Jun 8];87:1226–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25562625. [DOI] [PubMed]
- 25.Sao Emani C, Williams MJ, Wiid I, Baker B, Carolis C. Compounds with potential activity against Mycobacterium tuberculosis. Antimicrob Agents Chemother [Internet]. American Society for Microbiology; 2018 [cited 2018 Feb 15];AAC.02236-17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29437626. [DOI] [PMC free article] [PubMed]
- 26.Sandegren L, Lindqvist A, Kahlmeter G, Andersson DI. Nitrofurantoin resistance mechanism and fitness cost in Escherichia coli. J Antimicrob Chemother [internet]. Oxford University Press; 2008 [cited 2018 Mar 9];62:495–503. Available from: https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dkn222 [DOI] [PubMed]
- 27.Haber F, Weiss J. The Catalytic Decomposition of Hydrogen Peroxide by Iron Salts. Proc R Soc A Math Phys Eng Sci [Internet]. The Royal Society; 1934 [cited 2018 Jun 7];147:332–51. Available from: http://rspa.royalsocietypublishing.org/cgi/doi/10.1098/rspa.1934.0221.
- 28.Burkitt MJ, Gilbert BC. Model studies of the iron-catalysed Haber-Weiss cycle and the ascorbate-driven Fenton reaction. Free Radic Res Commun [Internet]. 1990 [cited 2018 Jun 7];10:265–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1963164. [DOI] [PubMed]
- 29.Vilchèze C, Hartman T, Weinrick B, Jacobs WRJ. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun [Internet]. 2013;4:1881. Available from: http://view.ncbi.nlm.nih.gov/pubmed/23695675. [DOI] [PMC free article] [PubMed]
- 30.Kosower NS, Kosower EM. Diamide: an oxidant probe for thiols. Methods Enzymol [Internet]. 1995 [cited 2018 Jun 6];251:123–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7651192. [DOI] [PubMed]
- 31.Rudyk O, Eaton P. Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biol [Internet]. Elsevier; 2014 [cited 2018 Feb 27];2:803–813. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25009782. [DOI] [PMC free article] [PubMed]
- 32.Piccaro G, Pietraforte D, Giannoni F, Mustazzolu A, Fattorini L. Rifampin Induces Hydroxyl Radical Formation in Mycobacterium tuberculosis. Antimicrob Agents Chemother [Internet]. 2014 [cited 2018 Feb 27];58:7527–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25288092. [DOI] [PMC free article] [PubMed]
- 33.Richard-Greenblatt M, Bach H, Adamson J, Pena-Diaz S, Wu L, Steyn AJC, et al. Regulation of Ergothioneine Biosynthesis and its effect on Mycobacterium tuberculosis Growth and Infectivity. J Biol Chem [Internet]. 2015; Available from: http://view.ncbi.nlm.nih.gov/pubmed/26229105. [DOI] [PMC free article] [PubMed]
- 34.Newton GL, Buchmeier N, Fahey RC. Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. Microbiol Mol Biol Rev [Internet] 2008;72:471–494. doi: 10.1128/MMBR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saikolappan S, Das K, Dhandayuthapani S. Inactivation of the organic hydroperoxide stress resistance regulator OhrR enhances resistance to oxidative stress and isoniazid in Mycobacterium smegmatis. J Bacteriol [Internet]. 2015;197:51–62. doi: 10.1128/JB.02252-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glorieux C, Calderon PB. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem [Internet]. 2017 [cited 2018 Mar 23];398:1095–108. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28384098. [DOI] [PubMed]
- 37.Almeida Da Silva PE, Palomino JC. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother [Internet]. 2011 [cited 2018 Feb 27];66:1417–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21558086. [DOI] [PubMed]
- 38.Aruoma OI, Whiteman M, England TG, Halliwell B. Antioxidant Action of Ergothioneine: Assessment of Its Ability to Scavenge Peroxynitrite. Biochem Biophys Res Commun [Internet]. Academic Press; 1997 [cited 2018 Mar 6];231:389–91. Available from: https://www.sciencedirect.com/science/article/pii/S0006291X9796109X. [DOI] [PubMed]
- 39.Misiti F, Castagnola M, Zuppi C, Giardina B, Messana I. Role of ergothioneine on S-nitrosoglutathione catabolism. Biochem J [Internet]. Portland Press Limited; 2001 [cited 2018 Mar 6];356:799–804. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11389687. [DOI] [PMC free article] [PubMed]
- 40.Miller CC, Rawat M, Johnson T, Av-Gay Y. Innate protection of Mycobacterium smegmatis against the antimicrobial activity of nitric oxide is provided by mycothiol. Antimicrob Agents Chemother [Internet]. 2007;51:3364–3366. Available from: http://view.ncbi.nlm.nih.gov/pubmed/17638697. [DOI] [PMC free article] [PubMed]
- 41.Alqahtani S, Mahmoud AM. Gamma-Glutamylcysteine ethyl Ester protects against cyclophosphamide-induced liver injury and hematologic alterations via upregulation of PPARγ and attenuation of oxidative stress, inflammation, and apoptosis. Oxidative Med Cell Longev. 2016;2016:4016209. doi: 10.1155/2016/4016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordao L, Bleck CKE, Mayorga L, Griffiths G, Anes E. On the killing of mycobacteria by macrophages. Cell Microbiol [Internet]. Wiley/Blackwell (10.1111); 2008 [cited 2018 Jun 7];10:529–548. Available from: http://doi.wiley.com/10.1111/j.1462-5822.2007.01067.x. [DOI] [PubMed]
- 43.Jordao L, Bleck CKE, Mayorga L, Griffiths G, Anes E. On the killing of mycobacteria by macrophages. Cell Microbiol [Internet]. 2007 [cited 2018 Mar 8];0:071106215315001–??? Available from: http://www.ncbi.nlm.nih.gov/pubmed/17986264. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The first author (karallia@sun.ac.za) on reasonable request can provide raw data of results.