Abstract
Palbociclib has been shown to be a highly effective therapy in hormone receptor positive metastatic breast cancer when used in combination with letrozole or fulvestrant. Grade 3/4 neutropenia is a common side effect although febrile neutropenia is relatively uncommon. Insufficient data exist to describe the hematological safety of palbociclib in African American women (AAW) known to have a high incidence of benign ethnic neutropenia (BEN). PALOMA 1, 2 and 3, the initial phase II/III studies that led to the U.S. Food and Drug Administration (FDA) approval of palbociclib in metastatic breast cancer, only included participants with baseline absolute neutrophil count (ANC) of 1500/mm3 or higher. African American women (AAW) were underrepresented in the PALOMA trials and this may be partially explained by strict requirements for minimal ANC ≥1500/mm3. The ANC of 1500/mm3 for initiation of treatment in those with BEN has been previously challenged. In this study, we propose to lower the ANC cutoff for enrollment to 1000/mm3. PALINA (NCT02692755) is a phase II, single arm, multicenter clinical trial that will enroll 35 patients. The primary endpoint is to assess the proportion of patients who complete therapy without the development of febrile neutropenia or treatment discontinuation due to neutropenia. The secondary endpoints include number of patients who required dose delays or dose reductions in palbociclib attributed to neutropenia, rate of grade 3/4 neutropenia, clinical benefit rate at 24 weeks, the association between metabolite and exosomal signature with disease response and the association between baseline ANC prior to cancer diagnosis and the Duffy Null polymorphism (SNP rs2814778) with hematological safety. PALINA will provide important information about the hematologic safety of palbociclib in AAW with advanced breast cancer.
Keywords: Palbociclib, Letrozole, Fulvestrant, African-American
1. Introduction
Approximately one-third of patients with hormone receptor (HR) positive human epidermal growth factor receptor 2 (HER2) negative tumors experience disease recurrence despite being initially diagnosed with early stage disease [1]. Palbociclib is a highly selective oral reversible inhibitor of cyclin-dependent kinases (CDK) 4 and 6. Palbociclib has been approved for women with advanced or metastatic HR-positive HER2-negative breast cancer in combination with either letrozole or fulvestrant [2,3]. Grade 3/4 neutropenia was the most commonly noted toxicity in the clinical trials that led to the approval of palbociclib (in up to 65% of patients), however, febrile neutropenia was relatively uncommon [[4], [5], [6]]. In these trials, an absolute neutrophil count (ANC) of at least 1500/mm3 was required for enrollment [[4], [5], [6]]. This cutoff could unnecessarily exclude patients with benign ethnic neutropenia (BEN) which affects 25–50% of individuals of African descent and some ethnic groups in the Middle East [7,8]. Due to a variety of reasons that include general lack of awareness of trials, mistrust in the medical system, communication gaps and structural barriers such as transportation, childcare and access to health care [9,10], African American women (AAW) continue to be underrepresented in clinical trials. Without representation in trials, the lack of appreciation of genetic factors particular to African-Americans and other minority communities will continue to increase [11]. The diversity gap that results from low minority accrual in clinical trials can lead to sub-optimal development of new medicines, compromise the generalization of clinical trial results and further exacerbate minority health disparities.
1.1. Study rationale
In this study, we propose to evaluate the hematological safety of palbociclib in combination with letrozole or fulvestrant in AAW with HR-positive, HER2-negative advanced breast cancer. Currently, there is insufficient data to describe the hematological safety of palbociclib in this population and the strict requirement of ANC of 1500/mm3 for initiation of treatment could exclude patients with BEN, which is common in this population [12,13]. Therefore we propose to lower the ANC cutoff for the enrollment on this trial to 1000/mm3 or higher. Presence of Duffy Null Polymorphism as a predictive marker for neutrophil count will be assessed at baseline.
2. PALINA study design
The PALINA study (NCT02692755) is a phase II, single arm, multicenter clinical trial designed to assess the proportion of patients who complete therapy without the development of febrile neutropenia or treatment discontinuation due to neutropenia (primary endpoint). We will also assess the following as secondary endpoints: number of patients who required dose delays or dose reductions in palbociclib attributed to neutropenia, rate of grade 3/4 neutropenia, clinical benefit rate at 24 weeks, the association between metabolite and exosomal signature with disease response and the association between baseline ANC prior to cancer diagnosis and the Duffy Null polymorphism (SNP rs2814778) with hematological safety. The Institutional Review Board at Georgetown University Medical Center has approved the study.
2.1. Treatment
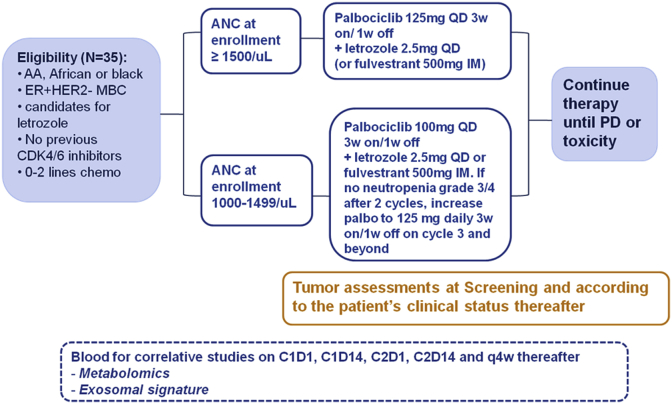
Patients enrolled with baseline ANC of 1500/mm3 or greater will receive palbociclib 125 mg daily for 21 days followed by 7 days off in addition to letrozole 2.5 mg daily or fulvestrant 500 mg on days 1, 15, and 29, and as maintenance dose of 500 mg every 28 days. If baseline ANC 1000–1499/mm3, initial dose of palbociclib will be 100 mg daily 21 days on, 7 days off and increase to 125 mg after cycle 2 if well tolerated (Fig. 1). Palbociclib recommended dose modifications for hematological related toxicities requiring treatment interruption/delay follow the FDA label and are described in Table 1.
Fig. 1.
Study design.
Table 1.
Dose modification and management for hematologic toxicities.
| CTCAE grade | Dose Modifications |
|---|---|
| Grade 1 or 2 | No dose adjustment is required. |
| Grade 3 neutropenia (ANC<1000/mm3), thrombocytopenia (PLC < 50,000/mm3) or anemia (Hgb < 8 g/dL) |
No dose adjustment is required. Consider repeating CBC monitoring one week later. Withhold initiation of next cycle until recovery to Grade ≤2. |
| Grade 3 neutropenia (<1000 to 500/mm3) + fever ≥38.5 °C and/or infection | Withhold palbociclib and initiation of next cycle until recovery to Grade ≤2 (≥1000/mm3). Resume at next lower dose. |
| Grade 4 neutropenia (ANC<500/mm3), thrombocytopenia (PLC < 25,000/mm3) or anemia (life threatening consequences) | Withhold palbociclib and initiation of next cycle until recovery to Grade ≤2. Resume at next lower dose. |
2.2. Correlative studies
Presence of Duffy Null Polymorphism as a predictive marker for neutrophil count will be assessed at baseline. Metabolite and exosomal signature (proteins and RNA) of drug resistance will be evaluated at different time points (every 14 days for first two cycles and then every 28 days while on study). Untargeted metabolomics analysis of serum may allow identification of metabolite markers of endocrine resistance by comparing metabolite profile of responders to that of non-responders. In addition, the characterization of exosomes has previously been shown to be a potential predictive biomarker of drug resistance in the clinic [14,15]. Metabolomics analysis of the serum will be performed using ultra-performance liquid chromatography mass spectrometer (UPLC MS) and gas chromatography-mass spectrometry (GC MS). Analysis will provide a global distribution of specific metabolites in patients' serum at the beginning and end of the study. To perform an exosomal signature of drug resistance, exosomes will be isolated from serum followed by purification of RNA and proteins for next generation sequencing (NGS) and mass spectrometry analysis, respectively. RNA-seq and proteomics profile from serum samples of patients will be compared with patient's tumor assessments to determine biochemical changes associated with response to palbociclib.
2.3. Key inclusion criteria
Self-identified Black, African or African American women of ≥18 years of age with HR-positive HER2-negative advanced adenocarcinoma of the breast. Patients must have an Eastern Cooperative Oncology Group performance status of 0–2. Adequate bone marrow function defined as ANC ≥1000/mm3, Platelets ≥100,000/mm3 and hemoglobin ≥9 g/dL (90 g/L).
2.4. Key exclusion criteria
Patients with previous use CDK4/6 inhibitor or those currently on potent inhibitors or inducers of CYP3A4 are excluded. Also, patients with active uncontrolled or symptomatic brain metastases are excluded.
2.5. Statistical considerations
A total of 35 patients will be recruited across MedStar Cancer Network in the greater Washington, DC area, the University of Chicago and Thomas Jefferson University in Philadelphia. The first patient was enrolled in November 2016 and we anticipate the enrollment to be completed within 24 months. Simon's two-stage design with a maximum of 35 patients is used. The null hypothesis that the true completion rate is 60% will be tested against a one-sided alternative. This design yields a type I error rate of 0.05 and power of 80% when the true completion rate is 80%. Early stopping rules will be incorporated for safety based on febrile neutropenia. Safety monitoring plan consists of a Data Safety Monitoring Committee (DSMC).
3. Conclusions
Women of African descent are often underrepresented in clinical trials and this was also verified in the clinical trials that led to the approval of palbociclib for the treatment of HR positive advanced breast cancer. The design of a trial such as the PALINA study that mimics real-world conditions will hopefully address barriers to enrollment and help answer the question of the hematological safety of palbociclib in African American patients [12]. The results of this novel trial will provide important contribution to close the gap in cancer health disparities and further advance towards the promise of personalized medicine.
Disclosures
This research is funded by an ASPIRE Breast Cancer Research Award from Pfizer (WI208808).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2018.05.012.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (London, England) May 14-20 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Walker A.J., Wedam S., Amiri-Kordestani L. FDA approval of palbociclib in combination with fulvestrant for the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer. Clin. Canc. Res. Official J. Amer. Assoc. Canc. Res. Oct 15 2016;22(20):4968–4972. doi: 10.1158/1078-0432.CCR-16-0493. [DOI] [PubMed] [Google Scholar]
- 3.Beaver J.A., Amiri-Kordestani L., Charlab R. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin. Canc. Res. Official J. Amer. Assoc. Canc. Res. Nov 01 2015;21(21):4760–4766. doi: 10.1158/1078-0432.CCR-15-1185. [DOI] [PubMed] [Google Scholar]
- 4.Finn R.S., Crown J.P., Lang I. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. Jan 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 5.A study of Palbociclib (PD-0332991) + letrozole vs. letrozole for first line treatment of postmenopausal women with ER/HER2- advanced breast cancer (PALOMA-2) https://clinicaltrials.gov/ct2/show/NCT01740427 Available at:
- 6.Verma S., Bartlett C.H., Schnell P. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3) Oncol. Oct 2016;21(10):1165–1175. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J. Lab. Clin. Med. 133(1):15–22. [DOI] [PubMed]
- 8.Reich D., Nalls M.A., Kao W.H. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. Jan 2009;5(1) doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris Y., Gorelick P.B., Samuels P. Why African Americans may not be participating in clinical trials. J. Natl. Med. Assoc. Oct 1996;88(10):630–634. [PMC free article] [PubMed] [Google Scholar]
- 10.Rivers D., August E.M., Sehovic I., Lee Green B., Quinn G.P. A systematic review of the factors influencing African Americans' participation in cancer clinical trials. Contemp. Clin. Trials. 2013 Jul;35(2):13–32. doi: 10.1016/j.cct.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Chen M.S., Jr., Lara P.N., Dang J.H. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. Apr 01 2014;120(Suppl 7):1091–1096. doi: 10.1002/cncr.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh M.M., Tisdale J.F., Rodgers G.P. Neutrophil count in African Americans: lowering the target cutoff to initiate or resume chemotherapy? J. Clin. Oncol. Official J. Amer. Soc. Clin. Oncol. Apr 01 2010;28(10):1633–1637. doi: 10.1200/JCO.2009.24.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly D.L., Kreyenbuhl J., Dixon L. Clozapine underutilization and discontinuation in African Americans due to leucopenia. Schizophr. Bull. Sep 2007;33(5):1221–1224. doi: 10.1093/schbul/sbl068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu D.D., Wu Y., Zhang X.H. Exosomes from adriamycin-resistant breast cancer cells transmit drug resistance partly by delivering miR-222. Tumour Biology J. Int. Soc. Oncodevelopmental Biol. Med. Mar 2016;37(3):3227–3235. doi: 10.1007/s13277-015-4161-0. [DOI] [PubMed] [Google Scholar]
- 15.Lv M.M., Zhu X.Y., Chen W.X. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour biology J. Int. Soc. Oncodevelopmental Biol. Med. Nov 2014;35(11):10773–10779. doi: 10.1007/s13277-014-2377-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.