Abstract
Background
The effect of a pre-exercise meal as countermeasure to exercise induced immunodepression is poorly known. Also, sedentary behavior is associated with increased cardiometabolic risk but studies on immune changes are lacking. Therefore, we aimed to assess: 1) the impact of a pre-exercise Mediterranean meal (MdM) compared with a fast-food type meal (FFM) on exercise-induced immunological changes and 2) the impact of an induced acute period of sedentary behavior on neuro-immune-endocrine status.
Methods
/Design: This is a two steps clinical trial including: (a) randomized crossover clinical trial, comparing the effect a high-fat/low-nutrient dense meal, FFM, with an isoenergetic similar high-nutrient dense meal, MdM, in the immune response to an exercise challenge (EC) and (b) a pilot trial assessing the neuro-immune-endocrine change induced by acute decreasing by half the usual physical activity level.
Results
A total of 46 participants (26 females), median aged 25 years were included. Of those 39-completed protocol, including overweight, physical active and inactive and participants with asthma. There were no differences in the EC between interventions. Dietary factors and physical activity were closely monitored during interventions and kept similar. During physical inactivity induction, 31% reached the target of 50% reduction in mean step number and 77% reached a 30% reduction.
Conclusion
The use of a pre-exercise meal to modulate immune response and the understanding of the immunological impact of physical inactivity might help to establish future recommendations on how to practice exercise in a safer way and to recognize the potential impact of inactivity.
Keywords: Allostatic load, Physical inactivity, Meal, Exercise challenge
1. Introduction
Exercise modulates the inflammatory and immune response. Very high and no or low training loads have been associated with higher risk of illness [1]. Lymphocyte proliferation is suppressed by acute and repeated bouts of strenuous exercise [2]. A reduction in peripheral blood Th1 cell number and production of interferon γ occurs in parallel with an increase in blood Th2 and regulatory T-cells with prolonged and exhaustive exercise [3]. Physical inactivity has been associated with an increased low grade inflammation [4] and longer periods to immunodepression [5]. Therefore, a J-shaped relationship has been proposed between absolute training load and illness, however this is not applicable to all populations [1].
Response to a stressor, like strenuous exercise [6], depends on several individual non-modifiable determinants such as genetic, gender, physiologic and psychological history and changeable ones related with individual fitness and nutritional status [[7], [8], [9], [10]]. The dynamic process of adjusting to perturbations of homeostasis is referred to as allostasis. Allostatic load is a complex clinical construct that takes in consideration the repeated stress and wear-and-tear on the body and brain [11,12]. Although much effort has been devoted to examining responses to physical activity changes, no holistic metric to measure sedentarism related immune dysfunction has been widely applied within exercise physiology.
Nutritional strategies have been implemented as potential countermeasures to exercise-induced immunodepression [7]. Dietary supplements, namely carbohydrate ingestion have been associated with a blunt of the inhibitory effects of exercise on T-cell proliferation and neutrophil phagocytosis/oxidative burst activity [9,13]. N-3 polyunsaturated fatty acids (PUFA) supplementation has shown conflicting results [14]. The intake of fruit and vegetables was correlated to an increase of oxidative capacity [15]. Antioxidant vitamins, have shown positive effects particularly in individuals that engage in repeated bouts of vigorous exercise [7]. However, high doses and over-supplementation might diminish body's natural antioxidant defenses [7]. Few studies have address the potential additional benefit of using whole food versus supplements in immune and health-related outcomes [16].
Mediterranean diet has antioxidant and anti-inflammatory properties [17]. Therefore, it could be used to modulate acute immune response to exercise in a favorable direction in comparison to a Western diet, which is characterized by high intake of saturated and trans fatty acids, high glycemic load foods, and has low quantities of n-3 PUFA. High glycemic and high fat meals have been correlated with postprandial lipemia and inflammation [18,19] and has been associated with acute airway neutrophilic inflammation [20]. However, these meals where never compared in their response to exercise response.
Accordingly, we hypothesized that (1) a pre-exercise Mediterranean diet meal may induce a blunted immune and inflammatory response to exercise, and (2) an induced sedentary behavior may increase the allostatic load. Therefore, we aimed to assess in a randomized cross-over trial the effects of two isoenergetic nutrient different meals (MdM vs FFM) in the acute neuro-endocrine immune response to an exercise challenge; followed by a pilot trial assessing the impact of an induced short-term period of sedentarism on the allostatic load and immune outcomes.
2. Methods
2.1. Study design
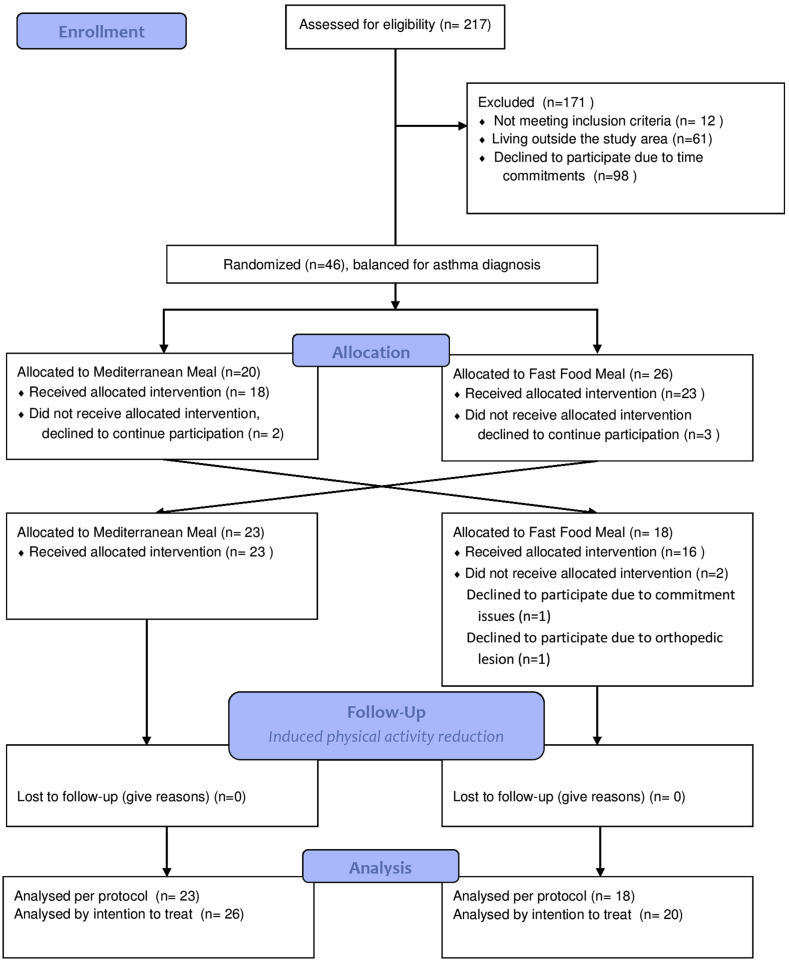
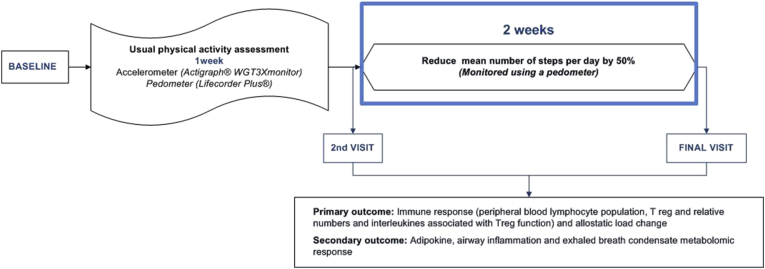
This is a two steps clinical trial including: (a) randomized crossover clinical trial, comparing the effect a high fat micronutrient poor meal, fast-food like meal (FFM) versus a isoenergetic similar, micronutrient different, Mediterranean like meal (MdM) in the inflammatory and immune response to an exercise challenge (Fig. 1) and (b) non-randomized pilot trial assessing the impact of an induced physical activity reduction, decreasing by half the mean step numbers per day, for two weeks (Fig. 2).
Fig. 1.
Study design of the interventional randomized cross over trial, participants allocation accordingly to intervention order and flow chart of trial participants.
Fig. 2.
Study design of physical activity reduction.
A wash-out period of 7 days was performed between exercise challenges, after which participants crossed over to the alternate diet sequence. Study period length was selected in order to reach complete normalization of post prandial inflammation and plasma lipid level markers and to avoid carry-over of the exercise immune response, accordingly to previous published studies [18,21]. Baseline physical activity and diet were assessed during the 7 days washing-out period. Outcomes were evaluated before and after each meal; after exercise challenge and after physical activity reduction.
This trial was registered in clinicaltrials.gov NCT02027675.
2.2. Participants
Subjects were invited to participate through trial posters bulletin boards, newspapers, internet advertisements and during hospital visits. All those with interest to participate completed a generic online questionnaire that was linked to the online recruitment platform http://meriitproject.weebly.com/, which included contact information details and questioned age, height and weight and previous asthma reported diagnosis. The flow chart of recruitment strategy is described in Supplementary Fig. 1.
2.2.1. Inclusion and exclusion criteria
Subjects with asthma diagnosis, with excess of weight or obese, with body mass index (BMI) ≥25kg/m2, or healthy with normal BMI (18.5–24.9kg/m2) between 18 and 35 years old were eligible for participation after providing informed consent.
Participants were excluded if they met any of the following criteria: suffered from a respiratory disease other than asthma, except for severe asthma according to GINA guidelines; had any major systemic diseases (diabetes, cardiac arrhythmia, angina, congestive heart failure, abnormal electrocardiogram, renal or hepatic failure, mal-absorption disease, intestinal inflammatory disease, chronic infectious diseases); women who were breastfeeding, pregnant or intending to be pregnant; subjects unable to comply with the study and follow-up procedures; had dietary restrictions (ex. food allergy, vegetarians) or were on a weight losing diet during the last 3 months before the study.
2.3. Randomization, allocation and blinding
Participants were randomly assigned to the intervention order in a double-blinded fashion. A research assistant, generated the random allocation of the meals sequence and assigned the subjects to the intervention order, stratified by asthma diagnosis. This researcher was not involved in the evaluation or intervention procedures. The research nutritionist who prepared the meals was informed which meals to prepare for that day, but was unaware of participant's allocation and was not involved in the recruitment procedures or in the outcomes evaluations. The investigator that performed exercise challenge was also blinded for the intervention. The investigators that assessed the outcomes were blinded to the participant's allocation order. Participants were informed about the two different isoenergetic meals, but were unaware until the study visit of the content of each the meals or the order of the meals.
2.4. Study procedures
All participants performed a baseline visit. Then they would be submitted to (a) randomized cross over trial that included two different meals (FFM vs MdM) followed by an exercise challenges, each meal separated by a wash out period of seven days and then (b) two weeks of induced physical activity reduction. During the follow-up period they were asked to maintain their dietary habits.
On the days of both interventions participants were requested to have the same breakfast, at the same time, so that residuals from breakfast could be minimized, avoiding coffee or caffeine containing products; abstain smoking habits 12 h before the meal and during intervention and alcohol ingestion during the preceding seven days.
The evaluations for outcomes and confounding factors were performed before and after each meal and after each of the exercise challenges, during wash-out period and at the end of the physical activity reduction (Fig. 1, Table 1).
Table 1.
Primary and secondary outcomes and moment of evaluation accordingly to study design.
| Type of Variables | Baseline | Before each meal | After each meal | After exercise challenge | Wash out-period | After induced physical activity reduction |
|---|---|---|---|---|---|---|
| Primary outcome and immune response outcomes | ||||||
| IL-6 | IL-6 | |||||
| Peripheral blood lymphocyte subpopulations, including NK, B, T and T reg relative numbers | Peripheral blood lymphocyte subpopulations, including NK, B, T and T reg relative numbers | Peripheral blood lymphocyte subpopulations, including NK, B, T and T reg relative numbers | ||||
| TNF-α; IL8; IL2; IL10; TGF β; IL1RA |
TNF-α; IL8; IL2; IL10; TGF β; IL1RA |
|||||
| Secondary outcomes | ||||||
| Anthropometry: weight, body mass index, percentage of body fat and fat free mass | Anthropometry: weight, body mass index, percentage of body fat and fat free mass | |||||
| Lung function, airway inflammation | Lung function, airway inflammation | |||||
| Adipokine | Adipokine | |||||
| Allostatic load | Allostatic load | |||||
| Pupillometry and substance P | Pupillometry and substance P | |||||
| Metabolomic markers, pH, Na, K+ and conductivity in EBC | Metabolomic markers, pH, Na, K+ and conductivity in EBC | |||||
| Primary and oxidative DNA damage |
Primary and oxidative DNA damage |
|||||
| Potential confounding factors | Age, gender, smoking habits, physical activity, dietary intake, atopy, asthma diagnosis and control, education and occupation, pupillometry, Anxiety and depression questionnaire | Asthma control questionnaire, Anthropometry: weight, height, BMI, waist circumference, percentage of body fat and fat free mass |
Meal taste score | Physical fitness: cardiorespiratory fitness | Accelerometer & Pedometer 3 day Food consumption diary |
Anxiety, depression and insomnia questionnaires |
2.5. Interventions
2.5.1. Meal and exercise challenge
2.5.1.1. Fast food like and mediterranean like meal preparation and analysis
Meals were chosen to be energetically similar and respecting the Dietary Reference Intakes (DRI) for macronutrient distribution [22]. Two different meal were performed one Fast Food (FFM) and the other Mediterranean like meal (MdM). After each meal, participants were not allowed to eat or drink, and could not perform any physical activity.
Fast food meal was acquired in a typical restaurant, contents and nutritional composition were provided from the food manufacturer database. For the development of MdM the main characteristics of a Mediterranean dietary pattern were considered, and typical ingredients were chosen, namely vegetables, bread and other whole grain cereals (pasta), nuts, olive oil, cheese (as a dairy product), fish and fruits. MdM was created to be energetically similar to the FFM and respecting DRI for macronutrient distribution: carbohydrate 45–65% of total energy value (TEV) (added sugars less than 25% of TEV), protein 10–35% of TEV and fat 20–35% of TEV (polyunsaturated fatty acids 5–10%, monounsaturated fatty acids 0.6–1.2%, cholesterol and saturated fatty acids “as low as possible”) [23]. The MdM meal's nutritional composition was calculated according to information provided by Portuguese Food Composition Table (National Health Institute Doutor Ricardo Jorge, IP [24]). N-3 PUFA were estimated according to McCance & Widdowson's Composition of Foods Integrated datase [25]. MdM was prepared in an experimental kitchen by one independent investigator and accordingly to a recipe (supplementary file 2). All ingredients were rigorously weighted as confection cuisine; preparation methods were always the same. MdM contained a spinach and chickpea soup, whole bread with fresh tomatoes, garlic and extra-virgin olive oil, whole pasta mixed with olives, sardines and tomatoes with some fresh green salad and as desert fresh fruit salad (apple, orange, and dry figs), and walnuts; and water (40cl) as beverage. In Table 2 both meals composition are specified. The Food Processor Plus program version SQL (ESHA Research, Salem, OR, USA) was used to compare both meals composition, in terms of energy and nutrient intakes and there were no significant differences between meal nutritional composition and the calculated version.
Table 2.
Characterization of meals nutritional composition for both Mediterranean Meal (MdM) and Fast Food Meal (FFM).
| MdM | FFM | |
|---|---|---|
| Energy (kcal) | 1070 | 1015 |
| Protein (g) | 45.0 | 31.0 |
| Protein (% TEV) | 16.8 | 12.2 |
| Carbohydrates (g) | 137.1 | 126.0 |
| Carbohydrates (% TEV) | 51.3 | 49.7 |
| Sugars (g) | 31.3 | 52.0 |
| Sugars (% TEV) | 11.7 | 20.5 |
| Fiber (g) | 23.0 | 7.0 |
| Fiber (g% TEV) | 4.3 | 1.4 |
| Fat (g) | 37.9 | 43.0 |
| Fat (% TEV) | 31.9 | 38.1 |
| saturated (g) | 7.4 | 12.0 |
| saturated (% TEV) | 6.2 | 9.3 |
| MUFA (g) | 12.2 | NA |
| MUFA (% TEV) | 10.3 | NA |
| PUFA (g) | 8.4 | NA |
| n-3 (g) | 4.0 | NA |
| n-3 (% TEV) | 3.4 | NA |
| n-6 (g) | 2.3 | NA |
| n-6 (% TEV) | 1.9 | NA |
| Trans (g) | 0.2 | NA |
| Trans (%TEV) | 0.17 | NA |
| NaCl (g) | 3.0 | 2.9 |
| Vitamin A (retinol) (mcg REA) | 386.5 | NA |
| Vitamin C (mg) | 50.7 | NA |
| Vitamin E (mg) | 5.3 | NA |
NA-not available; REA- Retinol equivalent activity; TEV-total energy value.
2.5.1.2. Exercise challenge
Three hours after each meal an exercise challenge was performed and blood sample was obtained immediately after the end of the challenge.
Progressive multistage exercise test, accordingly to Bruce Protocol was performed in a treadmill until exhaustion [26]. During the challenge, heart rate was monitored. Participants were fitted with a tightly sealing breathing mask connected to an airflow sensor. Ventilation and respiratory gas analysis was measured via dynamic breath-by-breath measurement using the Oxycon Pro Metabolic Cart, Jaeger™, Hochberg, Germany, including breathing frequency (BF), oxygen consumption (VO2) carbon dioxide production (VCO2), respiratory exchange ratio (RER) and minute ventilation (VÈ) was performed.
2.5.2. Physical activity reduction
Participants were requested to reduce their mean steps per day by half for two weeks. Number of daily steps were assessed using a pedometer (Lifecorder Plus®) during the wash-out period, which was considered the run-in period for this intervention. Subjects were instructed to wear the pedometer in the waistline above the knee, as previously published [27], being removed only for sleep or other activity that could damage the device, namely bath. Mean steps number per day were calculated using the mean of number of steps per day performed during the run-in period. Reduction was independent of the mean number of steps performed per day.
During the two weeks of physical activity reduction participants were advised to: take the elevators instead of the stairs and riding car instead of walking or bicycling, reduce or stop daily exercise training. They were also requested to maintain their usual dietary habits. Mean number of steps reduction target was calculated using the performed mean number of steps per day during the run-in period. Participants could monitor their daily steps to access if they were following the target step number. Accelerometer was used to evaluate physical activity changes between the run-in period versus the reduced two weeks of physical activity.
2.6. Study variables
2.6.1. Primary outcome and immune response outcomes
IL-6 is a pleiotropic cytokine [28,29], exercise-induced IL-6 is modulated by nutritional interventions, therefore is the primary outcome to evaluate the immune response of meal-exercise challenge and physical activity reduction. It is further modulated by leukocyte and lymphocyte subset response and other cytokines, which were also measured and evaluated as immune response outcomes.
Peripheral blood lymphocyte subpopulations, including NK, B, T and Treg relative numbers are determined to evaluate if different meals modulate the response to exercise challenge and to address the acute immune impact of induced short term physical activity reduction. For exercise related inflammatory response [8,9], Treg related cytokine response [30] and evaluation of immediate inflammatory response to the different meals the following cytokines are evaluated: IL-6; TNF-α; IL-8; IL-2; IL-10; TGF-β and IL1-RA.
Blood is obtained by venipuncture from an antecubital vein and collected into two vacutainer tube containing EDTA, 7ml and two Terumo Venosafe® serum-gel tubes 10 ml.
Hematological parameters, total and differential leukocyte counts are performed in an automated cell-counter. Two of the samples with serum and plasma are centrifuged for 10 min at 400 × g and then stored −80 °C. Lymphocyte subpopulations (including Treg cells) are measured by flow cytometry. Venous blood samples are stained with CD3-FITC, CD3-APC CD56-PE, CD45-PerCP-Cy5.5,CD19-APC,CD4-FITC,CD8-PE (BD Biosciences®, San Jose, CA, USA) and CD16-PE. Human Regulatory T Cell Cocktail and FoxP3-PE (BD Pharmingen®) used for T regs cells evaluation. Samples are acquired on BD FACS Calibur flow cytometer and analyzed using BD Infinicyt ™ software version 1.7.
Immune response measured by cytokines is assessed by a bioassay multiplex technology, based on Luminex xMAP®. This system uses color-code microspheres with two fluorescent dyes. After an analyte from a test sample is captured by the bead, a biotinylated detection antibody is introduced. The reaction mixture is the incubated with Streptavidin-PE conjugate, the reporter molecule, to complete the reaction on the surface of each microsphere. The procedure performed in Luminex 200™. Each individual microsphere is identified and the result of its bioassay is quantified based on fluorescent reporter signals. IL-6, TNF-α, IL-2 and IL 10 will be evaluated using Magnetic Luminex® Assay, with a human premixed multi-analyte kit (LXSAHM-05, R&D systems®). TGF β1 will be analyzed through ELISA by DRG®.
2.6.2. Secondary outcomes
Lung function was evaluated with spirometry measured by Spirolab® spirometer (MIR, Italy), with the WINSPIROPRO® software. Lung function was performed at baseline, before and after each meal and 5, 10 and 15 min after exercise challenge and after the period of physical activity reduction. The procedure was carried out in the sitting position, with participants wearing a nose clip. Lung function values included forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), forced expiratory flow between 25% and 75% of FVC (FEF25–75%) and peak expiratory flow (PEF). Participants were asked to inhale completely after three to four cycles of tidal breathing, and then exhale with maximum force until no more air was expelled while maintaining an upright posture. Throughout the procedure, loud verbal encouragement was given to obtain the expiratory and inspiratory manoeuvres completely with maximal effort. The procedure was monitored for compliance by a researcher, and all tests were performed in accordance with the acceptability and repeatability criteria according to the American Thoracic Society criteria [31]. For lung function evaluation, the following parameters were used as outcomes: forced expiratory volume in 1sec (FEV1) in L, FEV1% predicted, forced vital capacity (FVC) in L, FVC% predicted, Maximum mid-expiratory flow 25–75% (MMEF25/75) in L and MMEF2575% predicted and FEV1/FVC%. For comparing subgroups only % of predicted values of lung function outcomes were used.
Airway inflammation was assessed by determining fraction of exhaled nitric oxide (FeNO) measured via electrochemical sensor (NOBreath®, Bedfont Scientific, Kent, England). The test required that participants exhale during 10 s at 50 ml/s, with a minimum of 6 s plateau achieved in the final of the manoeuvre. The mean of 3 measurements were used to calculate final exhaled NO in parts per billion (ppb), according to ATS/ERS recommendations [32,33].
Anthropometric measures Weight, height and waist circumference were evaluated. Participants were lightly clothed and bare-footed. Height was measured using a portable stadiometer (SECA® model 214). Weight and body composition were measured using a digital scale Tanita BC-418® (bioimpedance scale analysis). The percentage of body fat and the fat-free mass were estimated with a four-electrode Tanita®. Readings were performed before each of the meal-exercise challenges, at the same time and after physical activity reduction and they were obtained under controlled temperature and humidity conditions, with the participant being shoeless.
Body mass index (BMI) was calculated as body weight divided by square height (w/h2) and participants were classified according to World Health Organization recommendations [34] in: underweight (<18.5Kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) and obesity (≥30kg/m2). Waist circumference was measured using a tape measure Seca model, accordingly to International Society for the Advancement of Kinanthropometry (ISAK) [35] to the nearest 0.5 cm.
2.6.2.1. Allostatic load
Measurement of allostatic load is quantified by the allostatic load score (ALS), which is used to measure the physiologic response to stress [36]. The biomarkers included in allostatic load fall in three categories [37,38]: (1) primary mediators include cortisol, sympathetic and parasympathetic activity and pro and anti-inflammatory cytokines and neuromodulators; (2) secondary mediators, reflect the cumulative actions of primary mediators in tissue/organ specific and represent abnormal metabolism and cardiovascular risk, such as waist-hip ratio, blood pressure, glycosylated hemoglobin, cholesterol, HDL; (3) tertiary outcomes are actual diseases or disorders.
Due to the complexity and interdisciplinary index of personal and public health, there is an issue of which biomarker panel best operationalizes AL. Therefore recently, computational modeling has been use to optimize the ALS [39]. Biomarkers are mainly organized in five physiological system, as previously reviewed by Juster et al. [40], biomarkers from neuroendocrine system, cardiovascular and respiratory; metabolic system, immune system and anthropometric status (see Table 3).
Table 3.
Allostatic load biomarkers accordingly to the five-physiological system classification.
| Biomarkers |
|---|
| Neuro-endocrine |
| Cortisol |
| Dehydroepiandrosterone (DHEA-S) |
| Epinephrine |
| Norepinephrine |
| Immune |
| High sensitivity C-reactive protein [mg/dl] |
| Metabolic |
| High density lipoprotein [mg/dl] |
| Low density lipoprotein[mg/dl] |
| Total cholesterol [mg/dl] |
| Triglycerides[mg/dl] |
| Glucose |
| Insulin |
| Cardiovascular and respiratory |
| Systolic blood pressure[mmhg] |
| Diastolic blood pressure[mmhg] |
| FEV1 |
| Heart rate/pulse [bit/min] |
| Anthropometric |
| Body mass index |
| Fat-free Mass (kg) |
| Total body water (kg) |
There is a wide variability of biomarkers included and classifications [41]. Regarding the allostatic load scores, they will be divided into sex-specific quartiles with high-risk (levels ≥75th percentile) defined by the highest quartile for each variable except for HDL, which used the lowest quartile (≤25 percentile) [[41], [42], [43]]. For each variable, a dichotomous indicator will be created, reflecting those with “high-risk” values (assigned a score of “1”) and “lower risk” values (assigned a score of “0”). A total score of 18 will be used, a higher ALS indicates worse stress associated physiologic dysfunction. An allostatic load over 3 is considered a high risk [44].
High density lipoprotein (HDL), total cholesterol, triglycerides levels, insulin, glycose, dehydroepiandroterone sulfate (DHEA-S) and high sensitivity PCR are analyzed by ELISA technique. Low-density lipoprotein (LDL) cholesterol estimated indirectly using Friedewald et al. [45] formula.
2.6.2.2. Adipokine measurement
Adipocytes secrete adipokines which functions include appetite and energy balance, insulin sensitivity and lipid metabolism. These changes might modulate the response to an exercise challenge accordingly to a specific meal content. Therefore, the following evaluations are performed using a bioassay multiplex technology, based on Luminex xMAP® with a human adipokine panel that included Adiponectin, Resistin, PAI-1 (Total), Lipocalin-2/NGAL and Adipsin, as previously described [46] using a human adipokine panel (HADK1MAG-61 K, EMD Miillipore®). All samples are analyzed in duplicates and measurements performed in serum.
2.6.2.3. Pupillometry
Autonomic nervous function is assessed by pupillary activity parameters and rest heart rate, which has been previously compared with heart rate variability outcomes with promising results [47]. Pupillary measurements are performed with a portable infrared PLR-200™ Pupillometer (NeurOptics Inc, CA, USA), as previously published [48]. The subjects are asked to spend at least 15 min in a room to allow their eyes to adjust to the low lighting levels. Two pairs of infrared-emitting diodes of an 880-nm wavelength illuminate each eye and the measure was obtained 3 s after. At the end of the measurement cycle, a graph of the pupil diameters as a function of time was obtained for each eye and pupil light response curves is recorded. The following parameters are obtained: the initial pupil diameter (Init Dia) and at constriction peak (Min Dia), in millimeters; the percent of the constriction; the time of the onset of the constriction (latency), in seconds; the average and the maximum constriction velocities (ACV and MCV respectively), and the dilation velocity (ADV), all given in millimeters/second; and time at which pupil has re-dilated 75% of the reflex amplitude (TM75%), in seconds. Pupil diameters, latency, ACV, MCV, and the constriction amplitude are related to parasympathetic activity, while ADV and T75 are measures of sympathetic activity.
2.6.2.4. Primary and oxidative DNA damage
For oxidative stress analysis, blood is cooled to approximately 4 °C with freeze packs. Since the samples are not immediately analyzed after being processed to cryopreserve the cells, an equal amount of a 20:80 (V/V) mixture of dimethyl sulfoxide (DMSO) and Roswell Park Memorial Institute Medium 1640 cell culture (RPMI) is added, and after that sample aliquots are progressively frozen to −80 °C. The alkaline comet assay is performed as described by Singh [49] with minor modifications to detect basal levels of DNA damage (strand breaks, alkaline labile sites, and transient repair sites). The assay is carried out with an additional step of incubation with the restriction enzyme formamidopyrimidine DNA-glycosylase (FPG) that can detect oxidized purines, including 8-oxoGua, FaPyGua and 4,6-diamino-5-formamidopyrimidine (FaPyAde) and other ring-opened purines.
2.6.2.5. Exhaled breath condensate breathprint, pH, sodium, potassium and conductivity analysis
Exhaled breath condensate (EBC) is obtained by cooling exhaled breath through contact with a cold surface or condenser, accordingly to previous published guidelines [[50], [51], [52]]. This is a non-invasive technique that requires tidal breathing, via a system of exhaled air cooling. Participants breath through a special mouthpiece with salivary trap and a single-way valve, a Disposable Collection Circuit DECSS was used associated with a Peltier-type electric cooling system, ECoScreen® Turbo, Carefusion®, wearing a nose clip. Participants breath quietly, at tidal volume, for 15–20 min. The volume of condensate collected from exhaled volume was registered. Immediately after collection, samples were stored in an inert material at −80 °C.
pH measurement is performed before and after deaeration of the samples for 10 min using a handheld high resolution and accuracy device pHenomenal 1100H, VWR®, as previously published [51,52].
Sodium, potassium and conductivity in exhaled breath condensate. For sodium and potassium, a battery-operated analyzer composed of a control unit (meter) and a detachable sensor, which integrate the ion selective electrode unit are used: B-722 LAQUAtwin® Horiba for sodium and B-731 for potassium. These devices include a flat sensor and applies the same test principle as standard laboratory electrodes. It has been previously used in biological samples, sweat and urine, with an excellent relative and absolute reliability [53,54]. Evaluation of electrolyte in exhaled breath condensate has been previously performed in various respiratory diseases, using other methodologies [55]. Conductivity is evaluated using B-771 LAQUAtwin® Horiba, where it was measured by the 2-AC bipolar method. In patients with sarcoidosis, conductivity in exhaled breath condensate was significantly higher than healthy controls [56].
Exhaled breath condensate breathprint analyzes (a) differences before and after each meal and (b) before and after reducing physical activity. For both experiments the Cyranose 320 (C-320, Sensigent) is used, which is a hand-geld device capable of detecting smell prints through the analysis of mixtures of VOCS. It is equipped with 32 chemical sensors that respond differently to each mixture of VOCs through the change in resistance of each chemical sensor. For the measurement with the electronic nose 200 μl of the collected EBC is heated up to 37 °C. Ambient air is used as reference air while baselining for 10 s. The snout of the C-320 is hold above the surface drawing a sample for 10 s. Each measurement with C-320 includes three steps: baseline, sensor is exposed to reference air, sampling sensors are exposed to sample air and purging the sensors are refreshed by exposing them to ambient air.
2.6.3. Potential confounding factors
Participants were evaluated at baseline with sociodemographic, lifestyle habits questionnaire, including smoking and drinking habits, as well as previous medical history. Furthermore, the following confounding factors were evaluated during the trial.
2.6.3.1. Dietary reports
Usual dietary intake was assessed by: (1) Food Frequency questionnaire, validated for a Portuguese population [57], to assess dietary intake in the previous 12 months; (2) dietary record method, consisting on a record about all food and drinks consumed over three consecutive days [58].
FFQ is a semiquantitative questionnaire, comprising 86 food items or beverage categories and a frequency section with nine possible responses ranging from never to six or more times per day, and a seasonal alternative. Blank lines were included to add any food that were not listed. The portion size in grams was multiplied by the multiple/fraction of daily frequency intake and by a seasonality variation factor for each option selected, and dietary intake was estimated. The Food Processor Plus program version SQL (ESHA Research, Salem, OR, USA), supplemented with the Portuguese food-composition databases, was used to convert food to energy and nutrient intakes.
Food consumption during the wash-out period was assessed using a three-consecutive day food diary. Food portion sizes and beverages consumed were estimated using household measures (cups, glasses, spoons, slices, food wrappers, containers, etc.) as an aid in determining serving sizes. A description of each food and beverage consumed was recorded, including the method of preparation, the time it was eaten, amounts (weight or portion sizes) and, if appropriate, the brand name of the product. Nutrient analysis was performed using Food Processor, complemented with Portuguese Food Composition Table [24].
2.6.3.2. Physical activity
Baseline physical activity was measured by Baecke questionnaire validated for the Portuguese population [59,60]. It has 16 items with Likert-type response (from 1 to 5), designed to assess different categories of the broad concept of physical activity (work/school, sport and leisure).
To evaluate changes in physical activity throughout the study a short seven days self-administered version of International Physical Activity Questionnaire (IPAQ-7) was also used and applied before the intervention visits and after physical activity reduction intervention [61]. IPAQ reports separately vigorous-intensity, moderate-intensity and walking in terms of frequency and duration of each specific type of activity, in the past 7 days and time spent sitting in an ordinary weekday. IPAQ was expressed classified in three categories, low PA level, moderate PA level and high PA, according to the guidelines for data processing and analysis in as MET-min/week (metabolic equivalent) [62].
Physical activity was also evaluated using a pedometer and accelerometer during the 7 days wash out-period and during the physical activity reduction intervention. The accelerometer (Actigraph®WGT3Xmonitor) was used on the right mid-axillary line of the hip on an elastic belt or clip and wear during the study period, it was only removed for sleep or any activity that could cause harm to either the monitor or another person. Physical activity data was collected by accelerometer in 60 s' epochs; a minimum of 8 h on at least 4 days were considered valid; 60 min of consecutive zeros were considered invalid. Troiano 2008 [63] physical activity cut points were used. Data was presented as average time spent in moderate and vigorous, light and sedentary physical activity per day.
2.6.3.3. Atopy
Skin prick tests with common aeroallergens (Dermatophagoides pteronyssinus, grass pollen and weed pollen mixture, cat and dog epithelium, Alternaria) with histamine and saline as controls (Hal-allergy®) were performed. Atopy was defined by the presence of positive skin prick tests with aeroallergen (≥3mm), with adequate controls reaction accordingly to published guidelines [64].
2.6.3.4. Asthma and rhinitis
Clinical asthma diagnosis was determined at baseline, as well as asthma pattern, accordingly to Global Initiative for Asthma recommendations [65]. Asthma stability was defined as no exacerbation, respiratory tract infection, or oral corticosteroid in the past 4 weeks. Those individuals under regular asthma treatment were requested to maintain treatment as usual.
Lung function and airway responsiveness were evaluated with spirometry (Spirobank, Winspiro pro software, MIR®) and carried out according to the American Thoracic Society criteria reversibility was evaluated 15 min after 400 μg of salbutamol in aerochamber was administered. Clinical significant reversibility was defined as a ≥12% and 200 ml increase in FEV1 after inhalation [66]. Presence of bronchodilation response supported asthma diagnosis.
Rhinitis was diagnosed at baseline accordingly to the Allergic Rhinitis and its impact on asthma guidelines [67]. Asthma and Rhinitis control were evaluated by using Control of Allergic Rhinitis and Asthma Test [68].
2.6.3.5. Cortisol, adrenaline and noradrenaline
Cortisol is performed in serum sample using a solid phase enzyme-linked immunosorvent assay (ELISA), based on the principle of competitive binding with the DRG Cortisol ELISA Kit in the automatic ELISA Triturus analyzer. All samples are analyzed in duplicates. Acute response to major physiological systems to a single bout of exercise are usually proportional to the exercise intensity. In endurance acute exercise response, the most common and important change occur in adrenaline, cortisol and insulin. As these changes might cause different immune response they will be measured and addressed. Namely, insulin using multiplex analysis technique and cortisol which will be evaluated by an automated ELISA analyzer Triturus®. Levels of adrenaline and noradrenaline are determined from plasma by ELISA. For these measurements, blood samples are obtained in plasma-EDTA, separated and frozen within 60 min after the blood sample was taken.
2.7. Statistical analysis
All statistical analyses were performed using the SPSS 24.0 software (Chicago, Ill) or STATA 15, accordingly to the statistical tests used. Excel (Microsoft Office) and SPSS was used for data assembly and graphical analysis. Normality of data distribution was assessed by the Kolmogorov-Smirnov test, histogram and QQ plot distribution. Results are expressed as median and inter-quartile range or mean and standard deviation, as appropriate, and in discrete variables as count and percentage. Count and percentage were analyzed with Pearson-Chi Square or Fisher exact test.
For comparison between groups, if a normal distribution was described t-test-student would then be used in continuous variables, for non-normal data, non-parametric tests, Mann-Whitney was used to compare groups. Paired samples Wilcoxon signed rank test or paired sample t-test were used as appropriate, particularly to compare changes before and after physical activity reduction, meals and exercise challenge. The minimum threshold for statistical significance used was p ≤ 0.05. In data with skewed distributions, logarithmic transformation was done and if appropriate (normal distribution is achieved) comparisons will be made with this change.
For the evaluation of the effect of the meals and exercise challenge using the randomized cross-over an analysis by intention to treat will be performed. Primary and secondary endpoints will be analyzed for the presence of a carry-over effect [69]. For missing data, if missing completely at random (MCAR) was verified, a complete case analysis would be done. Analysis for the presence of a carryover effect between interventions was performed using a two-way mixed ANOVA, after normalization of the study variables.
Mixed effect linear models, linear mixed model (LMM) or generalized linear mixed model (GLMM) will be used to evaluate the differences between interventions for primary and secondary outcomes, adjusted for confounders.
2.7.1. Sample size calculation
For the 2-intervention, 2-period cross over design part of the study, estimates of between-subject variability have been derived from previous studies evaluating meal high carbohydrate ingestion [70]. Standard-deviation was estimated for interleukin-6, considering 5.3(1.9) pg/ml in the intervention group vs 6.6(3.0)pg/ml in the placebo group, the mean difference between groups was 1.30 with a 1.34 standard deviation(sd). Another previous study evaluated a high fat challenge in asthma and obese patients and showed an increase of IL-6 2 h after the meal with a mean difference of 0.42 pg/ml with a standard deviation of 0.4 [20].
Estimates for sample size calculation were derived from cross-over trial from Schoenfeld D. software [72], aiming to evaluate the sample size for a cross-over study where the outcome is a measurement. Assuming a mean difference of 0.8 pg/ml and a standard deviation of 0.8 for IL-6, in order to assess both exercise and meal challenge, a number of 20 participants in each sequence would allow a power above 90% at a 0.05 two tailed level of significance.
. To allow for dropouts the study was designed to recruit 25 participants in each sequence. Regarding the evaluation of physical activity reduction effect on other immune response, the absence of studies evaluating the immunological outcomes precluded the power analysis. Within subject's ANOVA will be used to adjust results for possible confounders, namely the effect of age, gender, asthma and BMI.
2.7.2. Ethical consideration
This study was conducted in full conformity with the principles set in the Declaration of Helsinki. The Ethics Committee of the University of Porto approved the study, that is registered in clinicalTrials.gov ID NCT02027675.
3. Results
3.1. Baseline characteristics
A total of 46 subjects, median aged 25 years (26 females), were included. During the trial, 5 participants withdraw before any of the interventions, mainly due to unavailability to attend the visits. Two participants dropped out before the second meal, a fast food meal, one due to an adverse event, an ankle sprain not related with the study intervention, nevertheless the participant was unable to attend and complete the exercise challenge; the other participant due to commitment issues. Accordingly, to the intervention order assignment, the participants differed in gender, height and smoking habits, as described in Table 4. Of those that consumed alcohol, there were no differences between the allocation order (MdM then FFM 2.6[1.0; 5.2] vs FFM then MDM 1.9[1.3; 3.2], p = 0.647).
Table 4.
Baseline characteristics and comparison accordingly to allocation for intervention order, Mediterranean and then Fast Food (MdM-FFM) or Fast food followed by Mediterranean meal (FFM-MdM).
| Total (n = 46) | MdM-FFM (n = 20) | FFM-MdM (n = 26) | p | |
|---|---|---|---|---|
| Age years, median [IQR] | 25 [22,30] | 25 [21,30] | 25 [22,30] | 0.911‡ |
| Female | 26(57) | 17(85) | 9(35) | 0.001§ |
| Caucasian | 43(94) | 19(95) | 24(92) | 0.599§ |
| Height in cm, median [IQR] | 168[162; 178] | 165[158; 171] | 171[165; 180] | 0.011‡ |
| Weight in kg, median [IQR] | 66.8 [58,77] | 62,3 [5,8,54,71] | 70.5 [2,7,60,82] | 0.054‡ |
| Waist circumference in cm, median [IQR] | 77 [69,84] | 74 [67,79] | 81[71; 86] | 0.085‡ |
| Body mass index (BMI) | 0.732§ | |||
| Normal weight (18.5–24.9) | 24(52) | 10(50) | 14(54) | |
| Overweight (25–29.9) | 17(37) | 7(35) | 10(39) | |
| Obese (≥30) | 5(11) | 3(15) | 2(8) | |
| Smoking habits, active smokers | 7(15) | 6(30) | 1(4) | 0.014§ |
| Alcohol consumption, n(%) | 31(67) | 14(70) | 17(65) | 0.553§ |
| Atopy | 32(70) | 14(70) | 18(69) | 0.955§ |
| Rhinitis diagnosis | 19(41) | 10(50) | 9(35) | 0.633§ |
| Asthma diagnosis | 13(28) | 7(35) | 6(23) | 0.511§ |
| FVC %prev, median [IQR] | 103[96; 112] | 103[94; 112] | 103[98; 113] | 0.657‡ |
| FEV1 %prev, median [IQR] | 103[93; 112] | 101[93; 112] | 103[93; 112] | 1.000‡ |
| FEV1/FVC %, median [IQR] | 85.6[82.1; 90.1] | 85.7[83.2; 90.8] | 85.6[80.3; 88.6] | 0.451‡ |
| MMEF2575%prev, median [IQR] | 94.0[72.5; 103.0] | 94.0[77; 97.3] | 95.0[68.8; 106] | 0.748‡ |
| FeNO,ppb, median [IQR] | 29 [16,54] | 28 [11,45] | 30 [18,70] | 0.276‡ |
| IPAQ activity levels classification | 0.521§ | |||
| Low | 23(50) | 11(55) | 12(46) | |
| Moderate | 5(11) | 1(5) | 4(15) | |
| High | 18(39) | 8(40) | 10(39) | |
| Dietary Intake FFQ, median [IQR] | ||||
| Energy kcal, median [IQR] | 2352.5[1886.7; 2838.0] | 2260.1[1494.2; 3177.1] | 2364.1[2020.4; 2790.5] | 0.909‡ |
| Carbohydrates, % TEV, median[IQR] | 46.8[42.4; 51.8] | 44.9[41.9:47.9] | 48.7[43.2; 53.2] | 0.103‡ |
| Protein, g, % TEV, median[IQR] | 19.1[17.4; 21.5] | 19.8[18.3; 21.8] | 18.2[16.7; 21.2] | 0.113‡ |
| Lipids, g, % TEV, median[IQR] | 34.4[31.7; 37.6] | 35.4[32.7; 37.8] | 33.3[31.5; 36.3] | 0.135‡ |
| Cholesterol, %TEV, median[IQR] | 0.13[0.11; 0.17] | 0.14[0.12; 0.17] | 0.13[0.11; 0.15] | 0.154‡ |
| Saturated, % TEV, median[IQR] | 9.8[8.8; 11.5] | 10.0[8.2; 11.8] | 9.7[8.9; 11.0] | 0.713‡ |
| Polyunsaturated, % TEV, median[IQR] | 5.8[5.2; 6.8] | 6.0[5.6; 6.8] | 5.6[4.8:6.6] | 0.141‡ |
| Monounsaturated, % TEV, median[IQR] | 15.0[13.5; 17.3] | 15.0[13.5; 17.9] | 14.9[13.4; 17.0] | 0.629‡ |
| Fiber, % TEV, median[IQR] | 2.3[1.7; 2.7] | 2.3[1.6; 2.9] | 2.2[1.9; 2.6] | 0.765‡ |
Data is presented as n(%), except if otherwise specified; IQR-interquartile range; TEV-total energetic value * A score ≥ 24 indicates good disease control; ‡Independent samples Mann-Whitney U test; § Qui-square test; ¤ Independent samples t-test.
For those participants with asthma and allergic rhinitis, median value of CARAT score was 25 [23,29], and 58% had not well controlled asthma (CARAT score ≤24).
3.2. Meal-exercise challenge
Exercise challenge lasted in median 15:22 min with an interquartile range of 13:46 to 16:43, the maximal oxygen uptake (VO2max) was 43.91 ml/min/kg [38.3; 49.6] and anaerobic threshold median value was 63.0% [56.0; 72.0], with no differences after FFM or MdM (Table 5).
Table 5.
Exercise challenge evaluated outcomes after each of the meals Mediterranean Meal (MDM) and Fast Food Meal (FFM).
| MdM (n = 41) | FFM (n = 39) | pa | |
|---|---|---|---|
| Duration, min | 15:16[13:25; 16:57] | 15:25[13:52; 16:40] | 0.775 |
| Time reached VO2 max, min | 14:05[13:08; 16:44] | 14:35[13:20; 16:15] | 0.472 |
| Speed at VO2 max, km/h | 6.8[6.7; 8.0] | 6.8[6.7; 8.0] | 0.778 |
| Elevation at VO2 max | 16.3[16.0; 18.0] | 16.3[16.0; 18.0] | 0.757 |
| VCO2, ml/min | 3301[2786; 4614] | 3485[3009; 4510] | 0.219 |
| VO2, ml/min | 2706[2364; 3945] | 2967[2453; 3502] | 0.558 |
| VO2/kg, ml/min/kg | 43.9[38.4; 49.8] | 43.9[38.2; 49.6] | 0.485 |
| RER | 1.2[1.2:1.3] | 1.2[1.2:1.3] | 0.155 |
| Heart rate, bpm | 188[182; 194] | 190[185; 199] | 0.001 |
| Breathing frequency, per min | 51 [47,53] | 51 [46,53] | 0.911 |
| Anaerobic treshold | 64.5[57.3; 71] | 63.0[53.3; 74] | 0.426 |
| VE | 107[96; 142] | 110[99:128] | 0.889 |
Wilcoxon signed rank.
Values of VO2max obtained in both challenges, accordingly to previous published reference values [73], corresponded to non-athletes in 47% (n = 21) of the participants (40.1 ml/min/kg [36.4; 45.7]), 35% had levels above non-athletes reference values (48.5[45.0; 55.0]) and 10% had lower than normal values (33.0[32.2; 37.2]). Those with levels above normal in maximal oxygen uptake had reported previous high level of physical activity in the IPAQ score and, conversely, those with under normal values had a low level of physical activity.
Using the Food processor, the FFM had slight more energy than the MdM, nevertheless percentage of protein, carbohydrates and fat were similar to what was previously calculated to build the meals (Table 2).
3.3. Changes in diet and physical activity during the wash-out period
Regarding dietary intake during the wash out period, those individuals which started with MdM food had a higher consumption of saturated and monounsaturated fat in comparison with those that started with FFM (Table 4). Levels of physical activity were assessed during wash out both with accelerometer - 46.4min/day 95%CI [40.0; 52.8] of moderate vigorous physical activity - and using mean number of steps per day: 9648 95% CI [8628; 106668]. IPAQ was used to evaluate differences of behavior during wash-out and there was no change between the two intervention visits, (2057 METS/min/day IQR [920; 3832] first visit vs 2079 [750; 4031], p = 0.285.
There were no differences between FeNO before each meal (24 [16,47]) vs 27 [15,57], p = 0.472), even when patients were stratified for asthma diagnosis.
3.4. Physical activity reduction
Sedentary behavior was induced for all the participants, using a target level of mean steps per day that corresponded to half of the number of steps per day performed during the wash-out week. Evaluating both pedometer mean number of steps per day and moderate vigorous physical activity there was a significant decrease in all parameters (Table 6).
Table 6.
Effect of the intervention in physical activity outcomes, comparison between usual and reduced physical activity (PA) period (n = 39).
| Usual PA | Reduced PA | p* | |
|---|---|---|---|
| Pedometer mean number of steps per day [95%CI] | 9648[8628; 10668] | 5870[5315; 6426] | <0.001 |
| Physical activity (min/day MVPA), mean [95%CI] | 46.4[40.0; 52.8] | 24.7[20.0; 29.3] | <0.001 |
| Sedentary activity min/day, mean [95%CI] | 520.5[495.4; 545.6] | 459.3[419.9; 498.7] | 0.001 |
| Light activity min/day, mean [95%CI] | 272.7[253.1; 292.4] | 217.9[199.2; 236.6] | <0.001 |
| Daily average of sedentary bouts, median [IQR] | 149.0 [103.0; 276.2] | 130.0[101.5; 238.7] | <0.001# |
IQR-interquartile range; MVPA minutes per day in moderate to vigorous physical activity; Paired sample t-test, except if otherwise specified # Wilcoxon Rank test.
The global sedentary activity and daily average of sedentary bouts also decreased, although this reduction was milder than the moderate and vigorous physical activity. Twelve participants (31%) reached the target level of mean steps reduction reducing by half or more than half and 30 (77%) reached levels equal or higher than a 30% step reduction. Two participants included in the trial were not able to reduce the mean number of steps per day. Accordingly, to Tudor-Locke [74], pedometer-determined physical activity cutoff points for healthy adults 10(22%) had were “low active”, 13(28%) “somewhat active”, 11(24%) “active” and 5(11%) “highly active”. Those that reduced lower than 30% the number of steps per day were from the group that were “low active” and “somewhat active”.
4. Discussion and conclusion
This clinical trial approaches the effects of two different meals, that differ in micronutrients content, in the acute immune response to exercise challenge. This intervention was controlled not only for baseline confounding factors, namely physical fitness and biological response to stress, but also during the intervention with close monitoring of physical activity and dietary factors. Furthermore, an exploratory study was designed to assess the immune and allostatic response to induced sedentary behavior.
Our study has some potential limitations based on baseline participant's characteristics that could have an impact on the interpretation of the main outcomes and generalizability of the results. At first, considering the main inclusion criteria, both interventions cannot be generalized to other specific populations, namely those that are immune depressed, elderly or children [75] our highly trained individuals. A population bias related to the recruitment strategy may also occur, as about a third of the included participants had values of maximal oxygen uptake within the athlete levels during the exercise challenge. The effectiveness of recruitment strategies for adult participants to physical activity interventions has been previously reported to be difficult, in order to reach unhealthy or inactive participants, and when representative samples are obtained, lower participation rate was found in previous studies [76]. The number of participants that completed protocol did not reach the target of 20 participants in each arm, this might limit the possibility to identify a difference in the main outcomes. Nevertheless, our population sample size of 39 participants is one of the largest reported to address nutritional intervention and IL-6 response to exercise [28]. Despite including individuals that were overweight or inactive, it is not possible to exclude that the participants had an increase interest in a healthy life style than the general population. The impact of this behavior was minimized by the exclusion of those participants that were in the last 3 months on weight losing diet and/or exercise programs and by doing a strict follow-up during the intervention of physical activity, using objective measures like accelerometry and dietary registries. Finally, though we have recruited a gender balanced population, there was an imbalance in gender allocation to the intervention order. This explains the difference both in weight and height and could be avoided by gender stratification in allocation. However using a cross-over design, where the same patient performed both interventions, and the stability of this characteristic, it is unlikely that this imbalance would affect the main outcomes evaluation. A high retention rate of 85% for the meal-exercise challenge intervention was reached, when the long term physical activity reduction intervention was performed, only 31% could complete the targeted 50% reduction of mean steps number per day and 77% were able to reach at least a 30% reduction.
The exploratory trial of induced sedentary behavior is limited by the pre-post design. Nevertheless, this is, to our knowledge, the largest trial evaluating the reduction of physical activity on immunological, inflammatory and allostatic load. Recently a trial protocol was published that proposes to evaluate the usefulness of specific exercise strategies during a step-reduction, but in an older population and using as a primary outcome the muscle function and size [77]. The high attrition rate was probably correlated to the included population, particularly individuals which were “low active”. Previous studies have mainly focused on population that performed more than 10 000 steps per day and in our sample, most of those that performed more than 10 000 steps reached reductions higher than 50% in mean step number.
Regarding the outcomes used, they are mainly an in-vitro evaluation. Nevertheless, in-vivo immediate impact of acute response to exercise is difficult to access and most of the studies are focused in long-term impact of exercise [78]. In a recent study, a correlation with specific lymphocyte subset profile, namely low naïve CD8+ T cell and high T reg percentage with a reduced risk of respiratory viral infection was observed [79]. Therefore, the evaluation of this surrogate outcomes, as is included in our study protocol, might be indicative of a potential immune susceptibility.
Our study has also important strengths. For the first time the effect of a complete meal in the exercise induced immune response is studied. This new line of investigation will have an important impact in the population that will start or usually practices regular exercise training and particularly for those in high level competition, who are particularly prone to upper respiratory infection due to the described open window theory [80]. The use of a balanced meal that potentially will boost the immune system in a safe manner, without high doses of vitamin supplements, will have an important impact on their health. In a recent review, it was hypothesized that the use of a sufficient caloric meal to meet energy requirements before and during exercise might reduce both muscle-derived IL-6 immediately after exercise, mediated by carbohydrate effects, and also the recovery levels of IL-6 serum response through the action of ω-3 fatty acids and antioxidants [28]. A meal that includes both interventions, as we are evaluating in our intervention, might be of a potential additional benefit.
There are previous studies comparing the nutrigenomic effect of a tocopherol-enriched Mediterranean versus a western high-fat meal, which showed that oxidized-LDL measured 3 h after meal was significantly higher with high fat meal versus the tocopherol enriched meal (52.20 ± 9.50 versus 37.13 ± 5.68) and also an immediate up-regulation of genes related to human inflammasome and oxidative pathways were found [81]. Both meals were similar in calories content, so it was hypothesized that the synergism of different nutrients, would modulate the immune system and the Mediterranean meal exert a protective action reducing LDL cholesterol oxidation through the induction of antioxidant enzymes. Detrimental cardiovascular effects of high fat or saturated meals have been shown promoting an increase of metabolic syndrome associated outcomes [82], namely in endothelial function [83] and post prandial lipemia [29]. In a recent study, airway inflammation increased after a high fat meal when exercise was performed in the postprandial period, both in exhaled NO and sputum neutrophils, independently of the baseline physical activity level [84], this supports an interaction between these two interventions and the need to evaluate different meals response.
Previous studies regarding induced prolonged bed rest have proven the concept that inactivity modulates immune response [5]. The effects of induced sedentary behavior in endocrine outcomes were also reported [85]. Changes in physical activity and acute physical inactivity, frequently secondary to a lesion, will also potentially change the immune response, particularly if participants have another inflammatory disease like asthma. In summary, this study may provide an insight on the neuro-endocrine, inflammatory and immune response changes after acute exercise and inactivity, and help establish future recommendations on how to practice exercise in a safer way, reducing the risk of immunodepression.
Funding
Authors gratefully acknowledge the funding of Project NORTE-01-0145-FEDER-000010 – Health, Comfort and Energy in the Built Environment (HEBE), cofinanced by Programa Operacional Regional do Norte (NORTE2020), through Fundo Europeu de Desenvolvimento Regional (FEDER).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2018.05.010.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Schwellnus M., Soligard T., Alonso J.M., Bahr R., Clarsen B., Dijkstra H.P., Gabbett T.J., Gleeson M., Hagglund M., Hutchinson M.R., Janse Van Rensburg C., Meeusen R., Orchard J.W., Pluim B.M., Raftery M., Budgett R., Engebretsen L. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016;50(17):1043–1052. doi: 10.1136/bjsports-2016-096572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siedlik J.A., Benedict S.H., Landes E.J., Weir J.P., Vardiman J.P., Gallagher P.M. Acute bouts of exercise induce a suppressive effect on lymphocyte proliferation in human subjects: a meta-analysis. Brain Behav. Immun. 2016;56:343–351. doi: 10.1016/j.bbi.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Shaw D.M., Merien F., Braakhuis A., Dulson D. T-cells and their cytokine production: the anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine. 2018;104:136–142. doi: 10.1016/j.cyto.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Jurdana M., Jenko-Praznikar Z., Mohorko N., Petelin A., Jakus T., Simunic B., Pisot R. Impact of 14-day bed rest on serum adipokines and low-grade inflammation in younger and older adults. Age (Dordr) 2015;37(6):116. doi: 10.1007/s11357-015-9848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoff P., Belavy D.L., Huscher D., Lang A., Hahne M., Kuhlmey A.K., Maschmeyer P., Armbrecht G., Fitzner R., Perschel F.H., Gaber T., Burmester G.R., Straub R.H., Felsenberg D., Buttgereit F. Effects of 60-day bed rest with and without exercise on cellular and humoral immunological parameters. Cell. Mol. Immunol. 2015;12(4):483–492. doi: 10.1038/cmi.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackney A.C. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expet Rev. Endocrinol. Metabol. 2006;1(6):783–792. doi: 10.1586/17446651.1.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gleeson M. Immunological aspects of sport nutrition. Immunol. Cell Biol. 2016;94(2):117–123. doi: 10.1038/icb.2015.109. [DOI] [PubMed] [Google Scholar]
- 8.Walsh N.P., Gleeson M., Shephard R.J., Woods J.A., Bishop N.C., Fleshner M., Green C., Pedersen B.K., Hoffman-Goetz L., Rogers C.J., Northoff H., Abbasi A., Simon P. Position statement. Part one: immune function and exercise. Exerc. Immunol. Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 9.Simpson R.J., Kunz H., Agha N., Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Mills P.J., Hong S., Redwine L., Carter S.M., Chiu A., Ziegler M.G., Dimsdale J.E., Maisel A.S. Physical fitness attenuates leukocyte-endothelial adhesion in response to acute exercise. J. Appl. Physiol. 2006;101(3):785–788. doi: 10.1152/japplphysiol.00135.2006. [DOI] [PubMed] [Google Scholar]
- 11.McEwen B.S. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 12.McEwen B.S., Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 13.Ihalainen J.K., Vuorimaa T., Puurtinen R., Hamalainen I., Mero A.A. Effects of carbohydrate ingestion on acute leukocyte, cortisol, and interleukin-6 response in high-intensity long-distance running. J. Strength Condit Res. 2014;28(10):2786–2792. doi: 10.1519/JSC.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 14.Da Boit M., Hunter A.M., Gray S.R. Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism. 2017;66:45–54. doi: 10.1016/j.metabol.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieman D.C., Mitmesser S.H. Potential impact of nutrition on immune system recovery from heavy exertion: a metabolomics perspective. Nutrients. 2017;9(5) doi: 10.3390/nu9050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton-Freeman B., Sesso H.D. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr. 2014;5(5):457–485. doi: 10.3945/an.114.005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casas R., Sacanella E., Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune Disord. - Drug Targets. 2014;14(4):245–254. doi: 10.2174/1871530314666140922153350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herieka M., Erridge C. High-fat meal induced postprandial inflammation. Mol. Nutr. Food Res. 2014;58(1):136–146. doi: 10.1002/mnfr.201300104. [DOI] [PubMed] [Google Scholar]
- 19.Dickinson S., Hancock D.P., Petocz P., Ceriello A., Brand-Miller J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. Am. J. Clin. Nutr. 2008;87(5):1188–1193. doi: 10.1093/ajcn/87.5.1188. [DOI] [PubMed] [Google Scholar]
- 20.Wood L.G., Garg M.L., Gibson P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011;127(5):1133–1140. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Baila-Rueda L., Mateo-Gallego R., Perez-Calahorra S., Lamiquiz-Moneo I., de Castro-Oros I., Cenarro A., Civeira F. Effect of different fat-enriched meats on non-cholesterol sterols and oxysterols as markers of cholesterol metabolism: results of a randomized and cross-over clinical trial. Nutr. Metabol. Cardiovasc. Dis. 2015;25(9):853–859. doi: 10.1016/j.numecd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 22.I.o. Medicine . The National Academies Press; Washington, DC: 2005. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. [Google Scholar]
- 23.I.o.M. Food and Nutrition Board, National Academies, Dietary Reference Intakes (DRIs): Estimated Average Requirements, Institute of Medicine, National Academies, Institute of Medicine, National Academies.
- 24.INSA PortFir. 2017. http://portfir.insa.pt/# 2014.
- 25.PHE, McCance, Widdowson’s . In: The Composition of Foods Integrated Dataset 2015. England P.H., editor. 2015. [Google Scholar]
- 26.Bruce R.A., Blackmon J.R., Jones J.W., Strait G. Exercising testing in adult normal subjects and cardiac patients. 1963. Ann. Noninvasive Electrocardiol. 2004;9(3):291–303. doi: 10.1111/j.1542-474X.2004.93003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knudsen S.H., Hansen L.S., Pedersen M., Dejgaard T., Hansen J., Hall G.V., Thomsen C., Solomon T.P., Pedersen B.K., Krogh-Madsen R. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J. Appl. Physiol. 2012;113(1):7–15. doi: 10.1152/japplphysiol.00189.2011. [DOI] [PubMed] [Google Scholar]
- 28.Hennigar S.R., McClung J.P., Pasiakos S.M. Nutritional interventions and the IL-6 response to exercise. Faseb. J. 2017;31(9):3719–3728. doi: 10.1096/fj.201700080R. [DOI] [PubMed] [Google Scholar]
- 29.Teeman C.S., Kurti S.P., Cull B.J., Emerson S.R., Haub M.D., Rosenkranz S.K. The effect of moderate intensity exercise in the postprandial period on the inflammatory response to a high-fat meal: an experimental study. Nutr. J. 2016;15:24. doi: 10.1186/s12937-016-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoeppli R.E., Wu D., Cook L., Levings M.K. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front. Immunol. 2015;6:61. doi: 10.3389/fimmu.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., Crapo R., Enright P., van der Grinten C.P., Gustafsson P., Jensen R., Johnson D.C., MacIntyre N., McKay R., Navajas D., Pedersen O.F., Pellegrino R., Viegi G., Wanger J., Force A.E.T. Standardisation of spirometry. Eur. Respir. J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 32.American Thoracic S., European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 33.N.I.f.H.a.C . NICE diagnostics guidance National Institute for Health and Care Excellence; 2014. Excellence, Measuring Fractional Exhaled Nitric Oxide Concentration in Asthma: NIOX MINO, NIOX VERO and NObreath. [Google Scholar]
- 34.WHO Global database on body mass index: BMI classification. 2015. http://apps.who.int/bmi/index.jsp
- 35.ISAK International standards for anthropometric assessment. 2001. http://xa.yimg.com/kq/groups/83631355/1318405609/name/6692536-ISAK-BOOK.pdf
- 36.McEwen B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 37.McEwen B.S., Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 38.McEwen B.S. Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism. 2015;64(3 Suppl 1):S2–S10. doi: 10.1016/j.metabol.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Buckwalter J.G., Castellani B., McEwen B., Karlamangla A.S., Rizzo A.A., John B., O'Donnell K., Seeman T. Allostatic load as a complex clinical construct: a case-based computational modeling approach. Complexity. 2016;21(Suppl 1):291–306. doi: 10.1002/cplx.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juster R.P., McEwen B.S., Lupien S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Duong M.T., Bingham B.A., Aldana P.C., Chung S.T., Sumner A.E. Variation in the calculation of allostatic load score: 21 examples from NHANES. J Racial Ethn Health Disparities. 2017;4(3):455–461. doi: 10.1007/s40615-016-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauss D., Li J., Schmidt B., Angerer P., Jarczok M.N. Measuring allostatic load in the workforce: a systematic review. Ind. Health. 2015;53(1):5–20. doi: 10.2486/indhealth.2014-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bingham B.A., Duong M.T., Ricks M., Mabundo L.S., Baker R.L., Jr., Utumatwishima J.N., Udahogora M., Berrigan D., Sumner A.E. The association between stress measured by allostatic load score and physiologic dysregulation in african immigrants: the africans in America study. Front Public Health. 2016;4:265. doi: 10.3389/fpubh.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X., Redline S., Shields A.E., Williams D.R., Williams M.A. Associations of allostatic load with sleep apnea, insomnia, short sleep duration, and other sleep disturbances: findings from the National Health and Nutrition Examination Survey 2005 to 2008. Ann. Epidemiol. 2014;24(8):612–619. doi: 10.1016/j.annepidem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 46.Martos-Moreno G.A., Burgos-Ramos E., Canelles S., Argente J., Barrios V. Evaluation of a multiplex assay for adipokine concentrations in obese children. Clin. Chem. Lab. Med. 2010;48(10):1439–1446. doi: 10.1515/CCLM.2010.276. [DOI] [PubMed] [Google Scholar]
- 47.Stang J., Couto M., Stensrud T., Mowinckel P., Moreira A., Carlsen K.H. Assessment of parasympathetic activity in athletes: comparing two different methods. Med. Sci. Sports Exerc. 2016;48(2):316–322. doi: 10.1249/MSS.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 48.Couto M., Silva D., Santos P., Queiros S., Delgado L., Moreira A. Exploratory study comparing dysautonomia between asthmatic and non-asthmatic elite swimmers. Rev. Port. Pneumol. 2006;21(1):22–29. doi: 10.1016/j.rppnen.2014.05.004. 2015. [DOI] [PubMed] [Google Scholar]
- 49.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 50.Konstantinidi E.M., Lappas A.S., Tzortzi A.S., Behrakis P.K. Exhaled breath condensate: technical and diagnostic aspects. Sci. World J. 2015;2015:435160. doi: 10.1155/2015/435160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horvath I., Barnes P.J., Loukides S., Sterk P.J., Hogman M., Olin A.C., Amann A., Antus B., Baraldi E., Bikov A., Boots A.W., Bos L.D., Brinkman P., Bucca C., Carpagnano G.E., Corradi M., Cristescu S., de Jongste J.C., Dinh-Xuan A.T., Dompeling E., Fens N., Fowler S., Hohlfeld J.M., Holz O., Jobsis Q., Van De Kant K., Knobel H.H., Kostikas K., Lehtimaki L., Lundberg J., Montuschi P., Van Muylem A., Pennazza G., Reinhold P., Ricciardolo F.L.M., Rosias P., Santonico M., van der Schee M.P., van Schooten F.J., Spanevello A., Tonia T., Vink T.J. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur. Respir. J. 2017;49(4) doi: 10.1183/13993003.00965-2016. [DOI] [PubMed] [Google Scholar]
- 52.Horvath I., Hunt J., Barnes P.J., Alving K., Antczak A., Baraldi E., Becher G., van Beurden W.J., Corradi M., Dekhuijzen R., Dweik R.A., Dwyer T., Effros R., Erzurum S., Gaston B., Gessner C., Greening A., Ho L.P., Hohlfeld J., Jobsis Q., Laskowski D., Loukides S., Marlin D., Montuschi P., Olin A.C., Redington A.E., Reinhold P., van Rensen E.L., Rubinstein I., Silkoff P., Toren K., Vass G., Vogelberg C., Wirtz H. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur. Respir. J. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 53.Goulet E.D.B., Asselin A., Gosselin J., Baker L.B. Measurement of sodium concentration in sweat samples: comparison of 5 analytical techniques. Appl. Physiol. Nutr. Metabol. 2017:1–8. doi: 10.1139/apnm-2017-0059. [DOI] [PubMed] [Google Scholar]
- 54.Goulet E.D., Asselin A. Reliability and validity of a low cost, pocket-sized and battery operated sodium analyzer in measuring urinary sodium concentration. Technol. Health Care. 2015;23(6):881–891. doi: 10.3233/THC-151028. [DOI] [PubMed] [Google Scholar]
- 55.Gregus M., Foret F., Kindlova D., Pokojova E., Plutinsky M., Doubkova M., Merta Z., Binkova I., Skrickova J., Kuban P. Monitoring the ionic content of exhaled breath condensate in various respiratory diseases by capillary electrophoresis with contactless conductivity detection. J. Breath Res. 2015;9(2):027107. doi: 10.1088/1752-7155/9/2/027107. [DOI] [PubMed] [Google Scholar]
- 56.Mohan N., Akter R., Bryant K., Herbert C., Chow S., Thomas P.S. Exhaled breath markers of alveolar macrophage activity in sarcoidosis. Inflamm. Res. 2016;65(6):471–478. doi: 10.1007/s00011-016-0929-y. [DOI] [PubMed] [Google Scholar]
- 57.Lopes C., Aro A., Azevedo A., Ramos E., Barros H. Intake and adipose tissue composition of fatty acids and risk of myocardial infarction in a male Portuguese community sample. J. Am. Diet Assoc. 2007;107(2):276–286. doi: 10.1016/j.jada.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Authority E.F.S. General principles for the collection of national food consumption data in the view of a pan-European dietary survey. EFSA Journal. 2009;7(12):51. 1435. [Google Scholar]
- 59.Baecke J.A., Burema J., Frijters J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 60.Freitas D., Beunen G., Maia J., Claessens A., Thomis M., Marques A., Gouveia E., Lefevre J. Tracking of fatness during childhood, adolescence and young adulthood: a 7-year follow-up study in Madeira Island. Portugal, Ann Hum Biol. 2012;39(1):59–67. doi: 10.3109/03014460.2011.638322. [DOI] [PubMed] [Google Scholar]
- 61.Craig C.L., Marshall A.L., Sjostrom M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., Oja P. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 62.IPAQ Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ) 2005. https://sites.google.com/site/theipaq/scoring-protocol Accessed 2014 2014. [PubMed]
- 63.Troiano R.P., Berrigan D., Dodd K.W., Masse L.C., Tilert T., McDowell M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 64.Heinzerling L., Mari A., Bergmann K.C., Bresciani M., Burbach G., Darsow U., Durham S., Fokkens W., Gjomarkaj M., Haahtela T., Bom A.T., Wohrl S., Maibach H., Lockey R. The skin prick test - european standards. Clin. Transl. Allergy. 2013;3(1):3. doi: 10.1186/2045-7022-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.G.E.a.S. Committees . GINA Executive and Science Committees; 2015. From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2015. [Google Scholar]
- 66.Pellegrino R., Viegi G., Brusasco V., Crapo R.O., Burgos F., Casaburi R., Coates A., van der Grinten C.P., Gustafsson P., Hankinson J., Jensen R., Johnson D.C., MacIntyre N., McKay R., Miller M.R., Navajas D., Pedersen O.F., Wanger J. Interpretative strategies for lung function tests. Eur. Respir. J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 67.Bousquet J., Khaltaev N., Cruz A.A., Denburg J., Fokkens W.J., Togias A., Zuberbier T., Baena-Cagnani C.E., Canonica G.W., van Weel C., Agache I., Ait-Khaled N., Bachert C., Blaiss M.S., Bonini S., Boulet L.P., Bousquet P.J., Camargos P., Carlsen K.H., Chen Y., Custovic A., Dahl R., Demoly P., Douagui H., Durham S.R., van Wijk R.G., Kalayci O., Kaliner M.A., Kim Y.Y., Kowalski M.L., Kuna P., Le L.T., Lemiere C., Li J., Lockey R.F., Mavale-Manuel S., Meltzer E.O., Mohammad Y., Mullol J., Naclerio R., O'Hehir R.E., Ohta K., Ouedraogo S., Palkonen S., Papadopoulos N., Passalacqua G., Pawankar R., Popov T.A., Rabe K.F., Rosado-Pinto J., Scadding G.K., Simons F.E., Toskala E., Valovirta E., van Cauwenberge P., Wang D.Y., Wickman M., Yawn B.P., Yorgancioglu A., Yusuf O.M., Zar H., Annesi-Maesano I., Bateman E.D., Ben Kheder A., Boakye D.A., Bouchard J., Burney P., Busse W.W., Chan-Yeung M., Chavannes N.H., Chuchalin A., Dolen W.K., Emuzyte R., Grouse L., Humbert M., Jackson C., Johnston S.L., Keith P.K., Kemp J.P., Klossek J.M., Larenas-Linnemann D., Lipworth B., Malo J.L., Marshall G.D., Naspitz C., Nekam K., Niggemann B., Nizankowska-Mogilnicka E., Okamoto Y., Orru M.P., Potter P., Price D., Stoloff S.W., Vandenplas O., Viegi G., Williams D., World Health O. Galen, AllerGen, allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World health organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 68.Fonseca J.A., Nogueira-Silva L., Morais-Almeida M., Azevedo L., Sa-Sousa A., Branco-Ferreira M., Fernandes L., Bousquet J. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy. 2010;65(8):1042–1048. doi: 10.1111/j.1398-9995.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- 69.Hills M., Armitage P. The two-period cross-over clinical trial. Br. J. Clin. Pharmacol. 1979;8(1):7–20. doi: 10.1111/j.1365-2125.1979.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robson-Ansley P., Barwood M., Eglin C., Ansley L. The effect of carbohydrate ingestion on the interleukin-6 response to a 90-minute run time trial. Int. J. Sports Physiol. Perform. 2009;4(2):186–194. doi: 10.1123/ijspp.4.2.186. [DOI] [PubMed] [Google Scholar]
- 71.Lenth R.V., Java Applets for Power and Sample Size [Computer software] 2006. Java Applets for Power and Sample Size [Computer Software]http://www.stat.uiowa.edu/∼rlenth/Power [Google Scholar]
- 72.Schoenfeld D. Java Applets for Statistical considerations for a cross-over study where the outcome is a measurement. 2010. http://hedwig.mgh.harvard.edu/sample_size/js/js_crossover_quant.html
- 73.Kenney L., Wilmore J., Costill D. Human Kinetics; United States: 2012. Physiology of Sport and Exercise. [Google Scholar]
- 74.Tudor-Locke C., Bassett D.R., Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 75.Cao Dinh H., Beyer I., Mets T., Onyema O.O., Njemini R., Renmans W., De Waele M., Jochmans K., Vander Meeren S., Bautmans I. Effects of physical exercise on markers of cellular immunosenescence: a systematic review. Calcif. Tissue Int. 2017;100(2):193–215. doi: 10.1007/s00223-016-0212-9. [DOI] [PubMed] [Google Scholar]
- 76.Cooke R., Jones A. Recruiting adult participants to physical activity intervention studies using sport: a systematic review. BMJ Open Sport Exerc Med. 2017;3(1) doi: 10.1136/bmjsem-2017-000231. e000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perkin O.J., Travers R.L., Gonzalez J.T., Turner J.E., Gillison F., Wilson C., McGuigan P.M., Thompson D., Stokes K.A. Exercise strategies to protect against the impact of short-term reduced physical activity on muscle function and markers of health in older men: study protocol for a randomised controlled trial. Trials. 2016;17:381. doi: 10.1186/s13063-016-1440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pape K., Ryttergaard L., Rotevatn T.A., Nielsen B.J., Torp-Pedersen C., Overgaard C., Boggild H. Leisure-time physical activity and the risk of suspected bacterial infections. Med. Sci. Sports Exerc. 2016;48(9):1737–1744. doi: 10.1249/MSS.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 79.Johnstone J., Parsons R., Botelho F., Millar J., McNeil S., Fulop T., McElhaney J., Andrew M.K., Walter S.D., Devereaux P.J., Malekesmaeili M., Brinkman R.R., Mahony J., Bramson J., Loeb M. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108481. e108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kakanis M.W., Peake J., Brenu E.W., Simmonds M., Gray B., Hooper S.L., Marshall-Gradisnik S.M. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc. Immunol. Rev. 2010;16:119–137. [PubMed] [Google Scholar]
- 81.De Lorenzo A., Bernardini S., Gualtieri P., Cabibbo A., Perrone M.A., Giambini I., Di Renzo L. Mediterranean meal versus Western meal effects on postprandial ox-LDL, oxidative and inflammatory gene expression in healthy subjects: a randomized controlled trial for nutrigenomic approach in cardiometabolic risk. Acta Diabetol. 2017;54(2):141–149. doi: 10.1007/s00592-016-0917-2. [DOI] [PubMed] [Google Scholar]
- 82.Monfort-Pires M., Ferreira S.R. Inflammatory and metabolic responses to dietary intervention differ among individuals at distinct cardiometabolic risk levels. Nutrition. 2017;33:331–337. doi: 10.1016/j.nut.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 83.Lacroix S., Des Rosiers C., Gayda M., Nozza A., Thorin E., Tardif J.C., Nigam A. A single Mediterranean meal does not impair postprandial flow-mediated dilatation in healthy men with subclinical metabolic dysregulations. Appl. Physiol. Nutr. Metabol. 2016;41(8):888–894. doi: 10.1139/apnm-2015-0490. [DOI] [PubMed] [Google Scholar]
- 84.Kurti S.P., Rosenkranz S.K., Chapes S.K., Teeman C.S., Cull B.J., Emerson S.R., Levitt M.H., Smith J.R., Harms C.A. Does chronic physical activity level modify the airway inflammatory response to an acute bout of exercise in the postprandial period? Appl. Physiol. Nutr. Metabol. 2017;42(2):173–180. doi: 10.1139/apnm-2016-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krogh-Madsen R., Thyfault J.P., Broholm C., Mortensen O.H., Olsen R.H., Mounier R., Plomgaard P., van Hall G., Booth F.W., Pedersen B.K. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J. Appl. Physiol. 2010;108(5):1034–1040. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.