Abstract
Parrot bornavirus (PaBV), the etiologic agent of proventricular dilatation disease (PDD), is a major cause of concern in the avian health community. Within an infected flock, some birds will develop PDD and succumb to disease, while others remain healthy. Until now, there has been no study describing the results of long-term infection in apparently healthy carriers. For the last 5 years, the Schubot Exotic Bird Health Center at Texas A&M University has monitored individual PaBV shedding data in a flock of 66 naturally infected cockatiels. Of these birds, 53 were detected shedding PaBV4 in their droppings by reverse transcriptase polymerase chain reaction on at least one occasion. However, the prevalence of shedding declined over time, with the last positive cloacal swab being in October 2013. To determine whether the decline and eventual lack of shedding was an indication of virus elimination, seven previously shedding birds were euthanized and necropsied in 2016. Neither any gross lesion of PDD was observed nor was there any evidence of PDD or bornaviral encephalitis detected by histopathology. All tissues tested were negative for the presence of PaBV by reverse transcriptase polymerase chain reaction and immunohistochemistry. Thus, there was no evidence of an ongoing, productive infection in these birds. There are two possible explanations for these results. One possibility is that the birds were previously infected and have subsequently eliminated the virus. Alternatively, there may have been as few as three truly infected birds in the flock and the transient detection of PaBV in the droppings of other birds may simply be a “pass-through” phenomenon.
Keywords: cockatiel, immunohistochemistry, parrot bornavirus, polymerase chain reaction, proventricular dilatation disease
Introduction
Parrot bornavirus (PaBV) is the etiologic agent of proventricular dilatation disease (PDD), an immune-mediated, neurologic disease of parrots.1 PaBV was identified as the causative agent of PDD in 2008.2,3 The disease is readily induced by appropriate challenge with PaBV.4–6 During natural outbreaks, the virus may spread rapidly through aviaries, although its mode of transmission remains unclear.7,8 PaBV causes a noncytopathic infection; also, PDD develops as a result of T cell-mediated neuronal damage (Hameed et al, unpublished data, 2017), a process similar to that seen in mammals.9,10 The development of disease in birds is associated with a lymphoplasmacytic ganglioneuritis that affects the central, peripheral, and autonomic nervous systems, especially the enteric nervous system. These lesions are the basis of histologic diagnosis. Grossly, PDD-affected birds develop a dilated proventriculus probably caused by a loss of enteric neurons and resulting in a failure in proventricular motility. Not all birds that are infected with PaBV will develop PDD.1 Many infected birds remain apparently healthy for many years and during that time, PaBV may be shed intermittently in the urofeces. As a result, repeated reverse transcriptase polymerase chain reaction (RT-PCR) testing of cloacal swabs has been found to be the most reliable, noninvasive method for diagnosis of infection.1
Counseling owners of PaBV-infected birds is often difficult in that a PaBV-positive cloacal swab confirms infection, but it cannot be predicted when, if, or why clinical disease will develop. Because of the intermittent nature of virus shedding, a negative RT-PCR result is not indicative of being uninfected. It is also possible that the presence of virus in droppings may simply reflect viruses “passing through” without actually infecting the bird.11,12 In addition, it is not uncommon for single pet birds housed without contact with other birds for many years to develop PDD. For these reasons, it is generally believed that parrots are unable to rid themselves of the virus and that PaBV infection persists for life.
We studied the PaBV status of an isolated flock of naturally infected cockatiels for over 5 years. We observed a progressive decline in the number of birds shedding PaBV over this time and no shedding birds have been detected for 2 years. We, therefore, investigated the presence of PaBV in the tissues of selected individuals from this flock. Although the tested birds had a prior history of shedding, they now appear to be free of the virus, as determined by immunohistochemistry (IHC) and RT-PCR of multiple organs. These results suggest that either some cockatiels may eliminate the virus or that the detection of PaBV in cloacal samples may simply reflect “pass-through” rather than true infection. This is a significant result since it materially affects the prognosis of birds shown to be shedding PaBV.
Materials and methods
Birds
The 66 cockatiels used in this study were acquired by the Schubot Exotic Bird Health Center at Texas A&M University from a private aviary in Rhode Island on 18 July 2011. Their donation was prompted by the death of two flock members 6 weeks prior to donation. One had died and one was euthanized upon manifestation of clinical signs of disease. Both birds showed clinical signs as well as gross PDD lesions on a necropsy performed at and reported by Ocean State Veterinary Specialists in East Greenwich, Rhode Island in April and May of 2011. After arrival at our aviary, the birds were housed in an isolation building in groups of five to six in suspended cages and were fed a commercial cockatiel pellet seed diet (Zupreem Inc., Shawnee, KS, USA). These studies were conducted with the approval of the Texas A&M University Laboratory Animal Care Committee under the Animal Use Protocol, 2011–024 entitled Studies on the Treatment and Control of Avian Bornavirus-induced Disease.
Routine screening for PaBV
Cloacal swabs obtained approximately monthly, from individual birds, were tested for the presence of PaBV by RT-PCR as previously described.13 After 14 months, the flock was tested less frequently. Since the beginning of 2014, the flock was tested for PaBV shedding by cloacal swabbing four times yearly.
Assessing infection after 5 years
Six birds were selected for further investigation after 5 years. All were apparently healthy, but had been documented as having shed the virus on at least three occasions during the initial monthly cloacal swab testing. An additional bird from the flock was euthanized due to the development of multiple lipomas. This seventh bird was otherwise healthy and had shed the virus on a single occasion. None were shedding virus at the time of necropsy.
Fifteen days before euthanasia, each bird was swabbed cloacally. In addition, urofeces was pooled from the floor beneath all cages and used to screen the entire flock for PaBV. The selected birds were bled, euthanized, and immediately necropsied. Additional cloacal and choanal swabs were taken at the time of death.
Necropsies were performed and multiple tissue samples taken from each bird. Whole blood and brain samples were extracted separately and tested using conventional RT-PCR, using an M primer set, as described previously.13 Brain, liver, and proventriculus were also tested using a real-time RT-PCR and a P primer set.13 Tissues collected for histopathology were collected and fixed in buffered formalin. IHC using a rabbit anti-N serum was performed as described previously.14
Results
Flock health and cloacal shedding
On arrival at the aviary in July 2011, all birds were swabbed and four were determined to be shedding PaBV4 (Supplementary material). All birds in the flock were tested monthly thereafter and by 12 months, 53/66 had shed at least once. Seven birds died between July 2011 and March 2013. These birds were necropsied and submitted to an independent laboratory for histologic diagnosis. All except one were reported to have died from causes unrelated to PaBV infection. The lesions reported included megabacteriosis, nephrosis, salpingitis, hepatitis, atherosclerosis, gout, cardiomyopathy, bronchopneumonia, splenosis, euthanasia, and trauma. While none of these seven deaths were attributed to PDD, RT-PCR analysis of the organs of three of these birds (two at 9 months and one at 20 months after arrival) detected the presence of PaBV4 in multiple organs (Table 1), confirming that at least some members of the flock were indeed infected with bornavirus. One 4-year-old male bird died during a blood draw 18 months after arrival at the aviary. There were no gross lesions in the proventriculus or brain, but the pathologist reported on the presence of a mild multifocal ganglioneuritis in the heart consistent with PDD. This bird had detectable PaBV in its droppings once, 10 months after arrival, but its tissues were PaBV negative.
Table 1.
Necropsy and organ RT-PCR results of four birds from the flock that died within a year of arrival in the aviary
| Bird band number | L green 36L 0425 Dante | L Purple 28 Bobo | L 23P 08 01 Camelot | No band white 23 |
|---|---|---|---|---|
| Date of death | 12 February 2012 | 6 March 2012 | 19 March 2013 | 7 February 2012 |
| Necropsy results | Moderately dilated proventriculus | Enlarged black liver | Pale heart, distended pericardium. Mildly distended proventriculus | Mildly enlarged proventriculus containing fluid. Pale mottled kidneys |
| Histopathology | Mild hepatitis, myocardial degeneration Mild proventriculitis |
Hepatitis, myocarditis, nephritis. No evidence of PDD | Myocardial degeneration Nephritis Mild encephalitis |
Mild nephrosis and enteritis |
| Shedding detected | 8× | 8× | 12× | 1× |
| PCR results | ||||
| Crop | Pos | Pos | Pos | Neg |
| Proventriculus | Pos | Pos | Pos | Neg |
| Ventriculus | Pos | Pos | Pos | Neg |
| Intestine | Pos | Pos | Pos | Neg |
| Cloaca | Pos | Pos | Pos | Neg |
| Adrenals | Pos | – | Pos | Neg |
| Liver | Pos | Pos | Pos | Neg |
| Spleen | Pos | – | Pos | Neg |
| Kidney | Pos | Pos | Pos | Neg |
| Heart | Pos | Pos | Pos | Neg |
| Lung | Pos | – | Pos | Neg |
| Sciatic nerve | Pos | Pos | Pos | Neg |
| Brachial plexus | Pos | Pos | Pos | Neg |
| Pancreas | Pos | – | Pos | Neg |
| Brain | Pos | Pos | Pos | Neg |
| Spinal cord | Pos | Pos | Pos | Neg |
| Optic nerve | Pos | – | Pos | Neg |
| Eye | Pos | – | Pos | – |
| Gonads | – | Pos | Pos | – |
Notes: Three other birds that died were also necropsied. They had neither gross nor microscopic lesions of PDD, although their tissues were not tested by RT-PCR.
Abbreviations: Neg, negative; PDD, proventricular dilatation disease; Pos, positive; RT-PCR, reverse transcriptase polymerase chain reaction.
Detection of positive droppings varied from month to month with two peaks, one in August 2011 and the other one in January 2012. However, the number of birds shedding virus then dropped progressively (Figure 1). The last two individual positive cloacal swabs were detected in October 2013, 27 months after arrival at the aviary and approximately 29 months after the last clinical case of PDD. This was 7 months after the last necropsy confirmed the bird died. Testing of the flock in March 2014; January, June, September and November 2015; and January 2016 failed to detect any positive birds.
Figure 1.
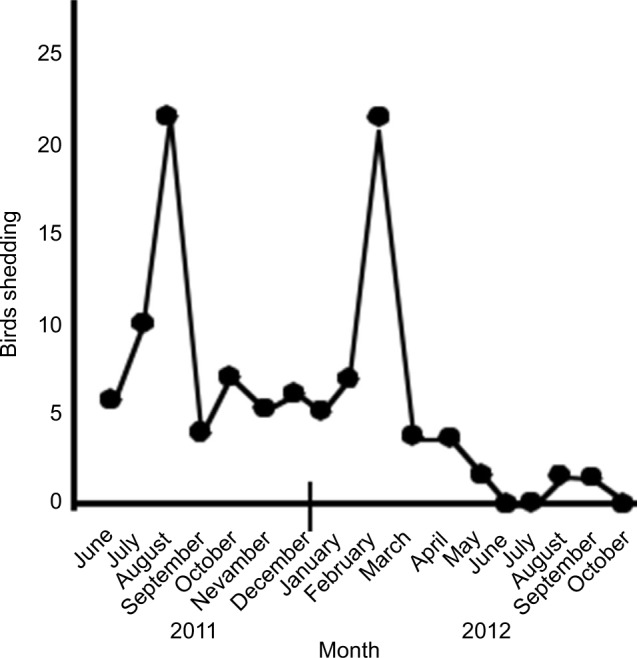
The number of birds shedding detectable PaBV in their urofeces each month beginning on arrival (June 2011) until November 2012.
Note: No shedding was detected after November 2012.
Abbreviation: PaBV, parrot bornavirus.
Of the original 66 birds, 13 were never detected shedding, 32 shed once, 15 shed twice, three shed three times, two shed eight times, and one shed 12 times (Figure 2). Thus, while most birds shed on only one occasion, some birds shed many times and three birds had been detected shedding on 8–12 occasions.
Figure 2.
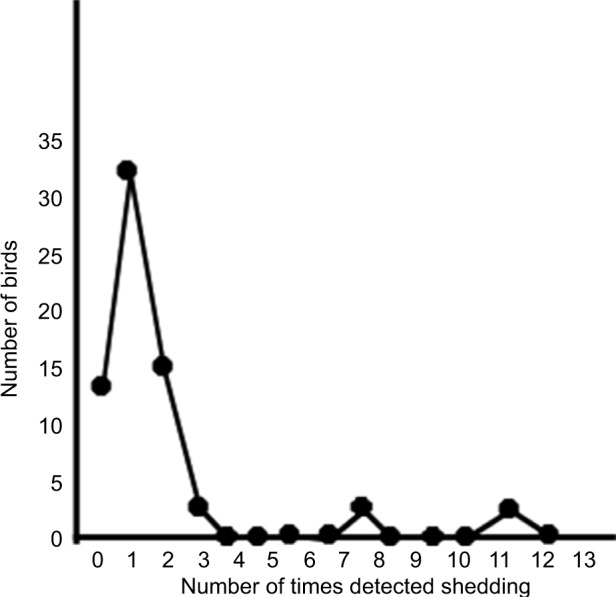
The number of times each bird was detected shedding parrot bornavirus in their urofeces when sampled monthly for 18 months.
Overall, flock health remained good and some birds were used for nonterminal projects. Most recently, all members of the flock were used in a PaBV vaccine safety trial. (The birds were no longer shedding at that time and the experimental vaccine is known to have no effect on viral shedding [Hameed et al, unpublished observations, 2017]).
Necropsy
Approximately 5 years after first entering the aviary, and almost 3 years after the last detectable shedding, seven birds, six with a history of shedding at least three times, and one with a single shedding event, were euthanized and their organs tested for the presence of PaBV. Necropsy failed to show lesions typical of PDD. All birds were in good body condition (3/5–5/5 keel scores). Most were overweight or obese. Four of the seven birds appeared to have abnormal livers, ranging from mottled in color (two birds), to moderate (one bird), to severe (one bird). One bird (R2617) had yolk coelomitis. The additional euthanized bird (R2618) had developed multiple lipomas.
RT-PCR and histopathology
All cloacal and choanal swabs taken on the day of necropsy were negative by both conventional and real-time RT-PCR (Table 2). In addition, histopathology showed no evidence of PDD lesions in any organ (data not shown). IHC for the N-protein of PaBV was also uniformly negative (data not shown). Four birds had a severe hepatopathy consistent with the gross necropsy lesions. One bird had severe degeneration, vacuolation, and fibrosis of smooth muscle cells in the ventriculus.
Table 2.
Necropsy and organ RT-PCR results from seven birds euthanized 5 years after arrivala
| Bird ID | R2612 | R2613 | R2614 | R2615 | R2616 | R2617 | R2618 |
|---|---|---|---|---|---|---|---|
| Shedding detected | 3× | 3× | 3× | 3× | 3× | 3× | 1× |
| Blood | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Feather calami | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Cloacal swab | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Choanal swab | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Feathered skin between scapulae | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Feathered skin on wing | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Feathered skin behind neck | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Heart | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Thyroid/parathyroid | – | Neg | Neg | Neg | Neg | Neg | Neg |
| Brachial plexus | Neg | – | – | – | – | – | Neg |
| Crop | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Spleen | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Liverb | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Proventriculusb | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Ventriculus | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Small intestine | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Large intestine | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Kidney | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Adrenal glands | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Gonads | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Brainb,c | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Spinal cord | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
Notes:
All had a prior history of shedding PaBV.
Samples tested additionally by real-time RT-PCR.
Samples re-extracted and tested again by traditional RT-PCR.
Abbreviations: Neg, negative; PaBV, parrot bornavirus; RT-PCR, reverse transcriptase polymerase chain reaction.
In addition to cloacal/choanal swabs, 18–19 tissue samples from each bird were processed for conventional RT-PCR, all with negative results (Table 1). Finally, three samples from each bird (liver, proventriculus, and brain) were also tested using real-time PCR and again, the results were negative.
Discussion
In this paper, we describe the health and viral shedding history of a flock of PaBV-exposed/infected cockatiels housed at the Schubot Exotic Bird Center at Texas A&M University. Despite two confirmed deaths due to PDD prior to their arrival in Texas, no additional birds died of PDD. However, most birds in the flock had detectable PaBV in their urofeces on at least one occasion. Three birds were detected shedding virus on 8–12 occasions, and thus contributed significantly to the viral load within the flock. Organs from four birds that died of causes other than PDD were tested for the presence of bornavirus and viral RNA was detected in multiple organs of three of them.
However, these “megashedders” eventually died and the shedding frequency in the other birds decreased so that, by just over 2 years, all RT-PCR tests of cloacal swabs and feces were negative. In order to follow-up on this observation, we selected seven birds for necropsy and examined their organs by histopathology, IHC, and RT-PCR. Six of these selected birds had had at least three virus-positive cloacal swabs. No organs from any of these birds were virus positive by IHC or polymerase chain reaction (PCR), and we noted no lesions typical of PDD. RT-PCR was performed on 18–19 samples per bird using primers to detect the M protein gene. We use these primers for routine screening of cloacal swabs and feces and they detect multiple species of avian bornavirus.13 We determined that conventional PCR using our M primer set can detect down to three copies of viral genome (Guo, unpublished material, 2017). Following the first set of negative results, an additional brain sample from each bird was processed and retested, and the results were again negative. Finally, three organs from each bird were reprocessed and tested by real-time PCR using a primer set for the P protein gene, again with negative results.
It is highly unlikely that the flock in question was uninfected given that two birds were clinically diagnosed with PDD (died before we obtained the remainder of the flock), the majority of individuals had at least one RT-PCR–positive cloacal swab, and three birds that died had multiple RT-PCR–positive organs.
There are two possible explanations for the observed results. One is that the observed decrease in shedding was due to control and eventual elimination of the virus by infected individuals. We cannot state that the seven recently tested birds were completely free of bornavirus; however, had they not had a clear history of viral shedding, their necropsy and testing results would support a finding of bornavirus-free status.
An alternative explanation is that, while some birds in the flock were truly infected as confirmed by necropsy, most of the other positive results were a consequence of contamination or pass-through rather than true infection. The distribution pattern of positive results would support this explanation. Three birds were “megashedders” that had positive cloacal swabs on all occasions tested. The vast majority of the flock shed on very few occasions, and a significant fraction were never detected shedding. Transiently positive birds may have ingested contaminated feed or water or simply by inhalation of contaminated dust or dander. We have previously detected bornaviral RNA in the dust filtered from the air of this aviary.1 This hypothesis is also supported by the decline in positive cloacal samples after the death of the last megashedder. Antibody levels were never measured in these birds.
These results support several conclusions. First, some birds are naturally resistant to bornavirus infection, despite prolonged exposure to infected birds. Second, oral transmission of bornavirus is very inefficient. Third, PaBV infection of cockatiels may not be lifelong, as had been assumed previously. Alternatively, it may also indicate that PCR-positive feces may be a result of pass-through rather than established infection. More importantly, these results show that while there may initially be PDD cases in an infected flock, it is likely that many birds will remain healthy.
There are well-recognized species differences in sensitivity to PaBV infection and the development of PDD in psittacines.1 Cockatiels appear quite resistant to natural infection by PaBV and natural cases of PDD are infrequent (although PDD is readily induced by experimental inoculation of PaBV).9 In fact, we have encountered several cockatiel aviaries where the owners deny having losses from PDD despite a high prevalence of PaBV in urofeces. For these reasons, it is possible that these observations may apply only to cockatiels
Acknowledgments
The authors would like to recognize the anonymous referee who made a convincing case for the possibility of viral pass-through rather than infection in many of these birds.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hoppes SM, Tizard I, Shivaprasad HL. Avian bornavirus and proventricular dilatation disease: diagnostics, pathology, prevalence, and control. Vet Clin North Am Exot Anim Pract. 2013;16(2):339–355. doi: 10.1016/j.cvex.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Kistler AL, Gancz A, Clubb S, et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J. 2008;5:88. doi: 10.1186/1743-422X-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honkavuori KS, Shivaprasad HL, Williams BL, et al. Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg Infect Dis. 2008;14(12):1883–1886. doi: 10.3201/eid1412.080984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne S, Shivaprasad HL, Mirhosseini N, et al. Unusual and severe lesions of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) acting as healthy carriers of avian bornavirus (ABV) and subsequently infected with a virulent strain of ABV. Avian Pathol. 2011;40(1):15–22. doi: 10.1080/03079457.2010.536978. [DOI] [PubMed] [Google Scholar]
- 5.Mirhosseini N, Gray PL, Hoppes S, Tizard I, Shivaprasad HL, Payne S. Proventricular dilatation disease in cockatiels (Nymphicus hollandicus) after infection with a genotype 2 avian bornavirus. J Avian Med Surg. 2011;25(3):199–204. doi: 10.1647/2010-030.1. [DOI] [PubMed] [Google Scholar]
- 6.Piepenbring AK, Enderlein D, Herzog S, et al. Pathogenesis of avian bornavirus in experimentally infected cockatiels. Emerg Infect Dis. 2012;18(2):234–241. doi: 10.3201/eid1802.111525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubbenstroth D, Brosinski K, Rinder M, et al. No contact transmission of avian bornavirus in experimentally infected cockatiels (Nymphicus hollandicus) and domestic canaries (Serinus canaria forma domestica) Vet Microbiol. 2014;172(1–2):146–156. doi: 10.1016/j.vetmic.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Kistler AL, Smith JM, Greninger AL, Derisi JL, Ganem D. Analysis of naturally occurring avian bornavirus infection and transmission during an outbreak of proventricular dilatation disease among captive psittacine birds. J Virol. 2010;84(4):2176–2179. doi: 10.1128/JVI.02191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983;148(2):305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- 10.Carbone KM, Duchala CS, Narayan O. Borna disease. An immunopathologic response to viral infection in the CNS. Ann N Y Acad Sci. 1988;540:661–662. doi: 10.1111/j.1749-6632.1988.tb27204.x. [DOI] [PubMed] [Google Scholar]
- 11.Piepenbring AK, Enderlein D, Herzog S, et al. Parrot Bornavirus (PaBV)-2 isolate causes different disease patterns in cockatiels than PaBV-4. Avian Pathol. 2016;45(2):156–168. doi: 10.1080/03079457.2015.1137867. [DOI] [PubMed] [Google Scholar]
- 12.Runge S, Olbert M, Herden C, et al. Viral vector vaccines protect cockatiels from inflammatory lesions after heterologous parrot bornavirus 2 challenge infection. Vaccine. 2017;35(4):557–563. doi: 10.1016/j.vaccine.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Payne S, Zhang S, Turner D, Tizard I, Suchodolski P. Avian bornaviruses: diagnosis, isolation, and genotyping. Curr Prot Microbiol. 2014;34:15i.1.1–33. doi: 10.1002/9780471729259.mc15i01s34. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Shivaprasad HL, Rech RR, Heatley JJ, Tizard I, Payne S. Characterization of a new genotype of avian bornavirus from wild ducks. Virol J. 2014;11:197. doi: 10.1186/s12985-014-0197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


