Abstract
Cultured trabecular meshwork (TM) cells are a valuable model system to study the cellular mechanisms involved in the regulation of conventional outflow resistance and thus intraocular pressure; and their dysfunction resulting in ocular hypertension. In this review, we describe the standard procedures used for the isolation of TM cells from several animal species including humans, and the methods used to validate their identity. Having a set of standard practices for TM cells will increase the scientific rigor when used as a model, and enable other researchers to replicate and build upon previous findings.
Keywords: Aqueous humor dynamics, Conventional outflow, Glaucoma, Intraocular pressure, Ocular hypertension
1. Introduction
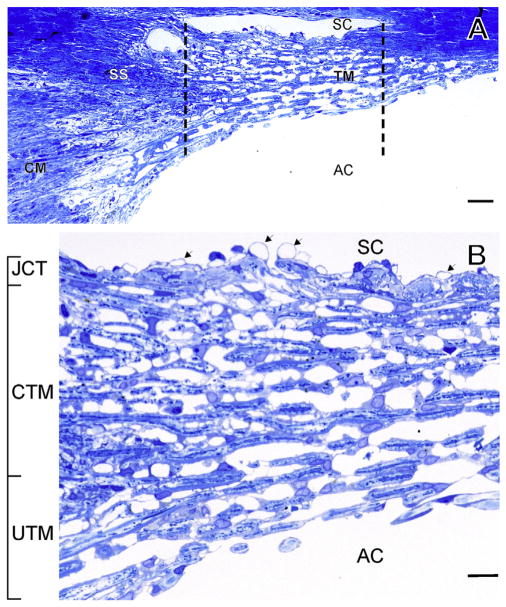
The conventional outflow pathway, primarily composed of the trabecular meshwork (TM) and Schlemm’s canal (SC) is the major outlet by which aqueous humor exits the anterior chamber of the eye (Fig. 1A). Anatomically it consists of several distinct cell layers (Fig. 1B) (Tamm, 2009). Starting from the anterior chamber, the first two layers of the TM are the uveal meshwork followed by the corneoscleral meshwork. The uveal meshwork consists of a loosely organized network of connective tissue beams that are covered by TM cells. The corneoscleral meshwork is composed of a series of lamellae (or sheets) of collagen and elastin, each of which are covered by a single layer of TM cells. In the deepest region of the tissue, the juxtacanalicular tissue (JCT) or cribriform region, the cells are less dense and embedded in connective tissue (Keller and Acott, 2013). In contrast to the uveal and corneoscleral beam cells, JCT cells do not form monolayers, but are connected to one another by cellular processes and make attachments to the elastin network extending from the anterior tendons of the ciliary muscle (Fuchshofer et al., 2006). It has proven difficult to isolate and culture the cells from these three regions; thus, TM cell cultures are generally a mixture of cells from all three regions. Whether the cells from these layers actually represent different cell types is a matter of debate (see “future directions” section below). A monolayer of endothelial cells that forms the inner wall of SC separates the TM from SC lumen and there is evidence of many cellular connections between JCT cells and SC cells in situ (Gong et al., 1996; Lutjen-Drecoll, 1999). In this paper we will focus on the methods to isolate, characterize and establish cultures of TM cells (Gong et al., 1996; Grierson et al., 1978; Lutjen-Drecoll, 1999). A recent review of TM cells can be accessed for more detail and perspective (Stamer and Clark, 2017).
Fig. 1.
Anatomy of the Trabecular Meshwork (TM). Light micrograph of meridional section through the human TM. Region between dotted lines indicate filtering portion of TM. Panel B is a magnification of A. Magnification bars: 20 μm (A), 5 μm (B); SC: Schlemm’s canal, SS: scleral spur, CM: ciliary muscle, UTM: Uveal TM, CTM: corneoscleral TM, JCT: juxtacanalicular tissue, AC: anterior chamber. Modified from Tamm (2009).
2. Tissue sources
Cultures of human TM cells are generated from donor eye tissue that is commonly received as either a whole globe or an anterior segment. A corneal rim discarded from a corneal transplant is also commonly used (Rhee et al., 2003). Tissue is generally stored on ice in a humidified vessel containing phosphate buffered saline or in Optisol, a specialized media that maintains corneal cell viability and enables storage of corneas that may be used in transplantation. Communication with the local eye bank, corneal surgeon or pathology department (autopsy tissue) is the most common ways to obtain human tissue. For tissue obtained from animal sources such as porcine, bovine, mouse, or monkeys, tissues are acquired from a vivarium or the local abattoir. Regardless of the source of the tissue, it is important to know how the tissue is stored and the length of time from death to culture since this can influence the establishment and viability of primary cell cultures.
It is also important that great care be taken when handling tissue obtained from human or animal donors. All tissues should be treated as though they may be contaminated or contain infectious agents. Human tissue for research is often not tested for virus (HIV, Hepatitis), sepsis, methicillin-resistant staphylococcus aureus and other infectious organisms. Monkey tissue can have a variety of viral contaminants including those for Marburg hemorrhagic fever/green monkey disease and porcine tissue can have viruses such as swine flu. Abattoir personnel have died from outbreaks, so extra care should be taken by the end user.
Since a major concern with cell culture is bacterial or other contamination, it is important to clean, wash and prepare these tissues carefully. Human or animal eyes are generally not collected in a sterile environment and often have surface contaminating bacteria, mold or yeast. To reduce sources of contamination of cultures, sterilizing the exterior surface of the eye before dissection is recommended. Be certain that conjunctiva is dissected or scraped off the globe. In addition, eyes should be rinsed for short periods (1–2 min) in Betadine (iodine-based antiseptic) prior to handling. Some investigators also rinse globes in 70% ethanol. Following these sterilization procedures, eye tissue should be washed extensively in sterile PBS prior to dissection.
3. Donor age and tissue storage
3.1. Human
Tissue obtained from human donors < 60 years of age provides an adequate number of TM cells with appropriate growth characteristics that enhances successful culture development. Tissue > 60 years of age can also provide adequate primary TM cell cultures, although the number of cell isolates and cellular growth rates tend to drop significantly with donor age. This is most likely due to a reduction of TM cellularity that occurs with increasing age (Alvarado et al., 1981). Eyes obtained from very young donors (< 5 years old) can be used to establish TM cell cultures, but the TM is more difficult to dissect due to less distinct tissue margins and softer tissues, so contamination from neighboring cell types can be more problematic.
The time from death to culture can also influence the cellular yield of TM cultures. Use of fresh tissue is preferred as cells tend to be more viable and less stressed. As a guideline, whole globes or anterior segments from 5 to 40 year old individuals produce good cellular yields if obtained within 48 hours of death. For tissue obtained from older individuals, particularly that obtained from donors > 60 years of age, TM cell yields will be higher if the tissue can be obtained within 24 hours post mortem.
The age guidelines used for whole globe donors or anterior segments also apply to corneal rims stored in Optisol. The time from death to dissection is extended for corneal rims stored in Optisol (up to 7 days), since the Optisol also helps maintain TM cell viability required for the establishment of primary TM cell cultures. Yet, best results are obtained from rims stored less than 4 days.
Whenever possible, care should be taken to determine and record any history relevant to the donor. Eyes that are obtained from eye banks are assigned numbers and contain no identifying donor information. At present, the National Institutes of Health does not consider eye bank donor eyes as human subjects’ research. However, if any additional information is needed (e.g. clinical data), which could lead to the identification of the donor, the tissue/TM cells are subject to Institutional Review Board (IRB) requirements. It is good practice to have your IRB examine the consent used by the eye bank, as often it contains language that allows access to medical records. In all cases, the use of tissue from human cadavers should follow the tenets of the Declaration of Helsinki. Factors that should be considered when accepting tissue in addition to age are any history of ocular disease and prior treatments, such as recent use of ocular steroids or intraocular pressure lowering medications. Prior treatment with radiation and chemotherapy or if the donor was on a respirator for an extended period of time (> 48 hours) should also be noted as these eyes tend to have lower cell viability. It is also important to note the age and gender of the donor.
3.2. Animal
Porcine and most other animal tissues have the advantage that the animals are usually younger, and tissue can be obtained with shorter postmortem times; thus cellular yields and growth rates are better. Very old monkeys appear to have lower cellular yields as observed in humans, although this has not been studied in great detail. Antibiotic resistance is more common in abattoir-derived tissue since antibiotics are often included in animal feeds. Thus, the betadine wash prior to opening the eye is more important. Where possible, recording the age, gender and breed of the animal is important as future studies may identify genetic and/or sex differences.
4. Dissection techniques
4.1. Dissection of TM from human tissue
Once tissue has been obtained and undergone sterilization procedures, whole globes are bisected near the equator using scissors, a scalpel or single-edged razor blade. Using a binocular dissecting scope, the iris, lens, and ciliary processes are gently removed from the anterior segment before dissection of the TM begins. To achieve a flatter tissue surface and obtain some idea of the position of SC, it is often useful to cut the anterior segment into several radial wedges before dissection. By tipping the cut surface up and viewing through the dissecting scope, SC and the filtering portion of the TM can be visualized to help with orientation before the dissection. Several methods (described below) are used to isolate the TM and it is noteworthy that all of these dissection methods include varying amounts of the SC inner wall endothelium due to their attachments to JCT cells.
4.1.1. Sharp dissection
Vertical cuts are made with a scalpel down from the anterior chamber/TM side toward the sclera – along the anterior and posterior margins of the TM, i.e. just posterior to Schwalbe’s line on the corneal side and just anterior to the scleral spur, trying to clip the two edges of SC (see region between lines in Fig. 1A). Using fine-tipped forceps (e.g. 5 or 6 Dumont), the TM is lifted out and isolated for further preparation (Fig. 2). If making cuts, it is important to show that the scleral spur, cornea and sclera are not invaded. This can be done via histological examination (e.g. hematoxylin and eosin staining of sagittal paraffin sections through iridocorneal angle tissues) of the remaining eye tissue post-dissection.
Fig. 2.
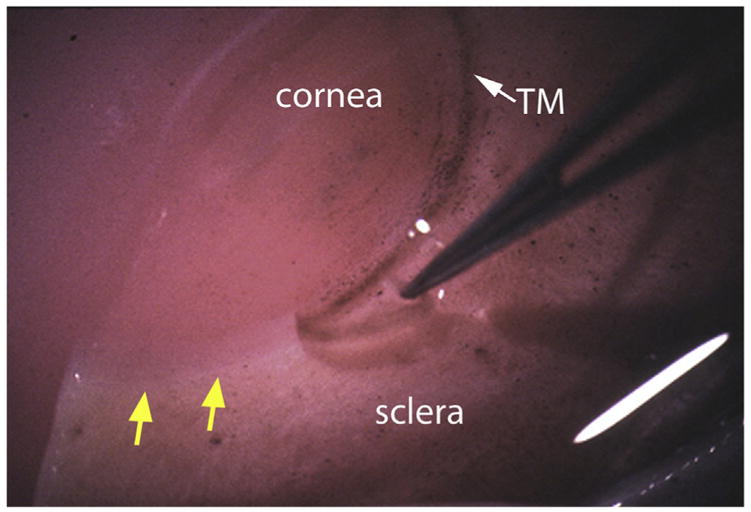
Micrograph showing removal of TM tissue from a human donor eye as a strip. Yellow arrows indicate region where TM has been removed. White arrow shows intact TM.
4.1.2. Blunt dissection
From the cut edge of the wedge, TM tissue is grabbed by placing one tip of fine-tipped forceps into the SC lumen and the other on top of the anterior TM. The TM tissue is pulled away, which separates it from the scleral spur, Schwalbe’s line, and SC to generate a continuous strip of TM. To assist in keeping an intact tissue strip (particularly in older eyes), TM tissue can be “scooped” out behind the TM strip using a 0.5 mm curret.
4.1.3. Kahook-dual blade
This device is designed to remove the TM and inner wall of SC. It contains a sharp tip to penetrate the TM and dual blades separated by a narrow well to cut the TM. Once the tip of the Kahook-dual blade is inserted through the TM into SC, it can be slowly moved around SC with the dual blades cutting out a strip of TM. This is very efficient at removing the TM but it is also more expensive.
4.2. Human TM dissection tips
TM is often pigmented, except in blue-eyed people, and it is more pigmented in older people. When looking down at the tissue, a raised ridge is visible with a sheen at the scleral spur and on the opposite side, the cornea is clearer with a little ridge (Schwalbe’s line). Contaminating corneal tissue is also very obvious if you look at the pieces of tissue under a microscope after dissection. Pieces of TM appear semi-transparent with a white/opaque hue and some pigment, while the cornea is transparent and reflective. Corneal endothelia can be scraped away from Schwalbe’s line toward the center of the cornea to remove potential cell contaminants before TM dissection. Finally, looking at the cut edge of the wedge, the TM and SC opening can often be seen. Another useful trick is to pipette India ink into SC or insert a colored suture into the canal to act as a guide. This is particularly useful for beginners learning the dissection technique. With the India ink or colored suture inside the canal, the semi-transparent TM tissue appears directly on top.
5. Culturing human TM cells
The dissected strips of human TM tissue (each 2–5 mm in length and cumulatively about 25–35 mm, the circumference of the human TM) are usually placed into one well (35 mm2) of a six-well culture plate and media is carefully added to minimize tissue disturbance. However, smaller 16 mm wells can also be used, and in this case only 4–6 strips are placed in each well. All cultures are maintained in a 37° humidified and 5% CO2-controlled culture incubator. TM tissue strips are then left undisturbed for 1–2 weeks, while the TM cells migrate out of the tissue and onto the bottom of the dish (Video of cells growing out of TM explant) (Polansky et al., 1979). Once cells have started to migrate out, the TM strips can be moved and re-plated into a new well. Throughout the initial stages of culture development, media should be carefully changed every 3–5 days, without disturbing the strips. TM cells derived from a single individual should be described as cell strains so as not to be confused with cell lines, which are immortalized.
A common problem with growing cells out from a TM explant is that the tissue tends to float up, especially when the media is changed. When this happens, the cells often die. Several approaches have been used to overcome this issue. Tissue culture plates are sometimes coated with collagen or gelatin. This not only helps adhere the tissue to the plate, but also helps encourage attachment and migration of TM cells out of an explant. Another approach is to place a coverslip on top of the tissue to weigh it down, keeping it in contact with the surface of the culture dish. This is particularly helpful when changing media. Once cells start to migrate out of the explant and attach to the plate, the coverslip and tissue strips can be removed. Cells may attach to the coverslip, so it is useful to remove the coverslip and place it in a separate well with media to enable these cells to expand. Another option is to place the TM strip(s) onto the bottom of a dry well for a few minutes to allow it to adhere to the surface, and to then carefully add a small amount of media (~1 ml) to cover the tissue. After one or two days, additional media can be added but care must be taken not to disturb the tissue. In all of these techniques, it is important to make sure that the tissue does not dry out, preventing cell death.
To encourage TM cells to migrate out from the tissue more easily, TM strips can be incubated with bacterial collagenase (10 mg/ml) and/or dispase (10 mg/ml) for up to 30 min (37 °C with rotation) prior to placement in culture. This additional step disrupts contacts between TM cells and the extracellular matrix of the TM explant presumably enabling enhanced opportunity for cells to detach and move onto the culture dish. When performing this step, it is important to use high quality collagenase (e.g: Fisher, Cat. #17-104-019 or Worthington Cat. #4188) that has low tryptic and caseinase activity. After digestion, the TM tissue strips should be pelleted by centrifugation at low speed (1500g) and the supernatant should be removed. The pelleted tissue is carefully resuspended into fresh media and plated into a single well of a 6-well culture dish as described above.
TM cells derived from animal tissues are generally cultured as described for humans. For porcine TM cells, TMs are dissected from 10 to 20 eyes and are combined into a single T25 cm2 tissue culture flask producing TM cells of a mixed genetic background. This reduces the chance that experimental data are due to biological variation. However, as with humans, pig TM cells can be grown from a single source animal if genetic and gender variations are important factors that need to be considered.
5.1. Culture media
The standard media used for TM cell culture is a mixture of Dulbecco’s Modified Eagle’s Medium (DMEM) containing low glucose (1 mg/L), 2 mM L-glutamine, antibiotics [0.5% gentamicin or penicillin (100 units/ml) and streptomycin (100 μg/ml)] and 10% fetal bovine serum (FBS). Since not all sera are identical, it is important to “lot test” several to find one that is best at promoting growth of your TM cell cultures. If TM cells are isolated from older donor eyes (> 60 years old), 20% FBS supplementation to culture media may help stimulate and maintain growth. An antifungal agent like 1% amphotericin B can also be used, and is recommended for TM cells derived from animal eyes.
Other agents have also been used in TM cell culture to enhance culture development. Some laboratories use DMEM containing a 1:1 mix of low glucose and high glucose (Anderssohn et al., 2011). Addition of fibroblast growth factors (bFGF/FGF-2, 250 ng/ml) can enhance cell growth and is especially helpful if the donor tissue is from an older individual (Polansky et al., 1979), and helps TM cells outgrow contaminating cells. Once the cultures reach confluence, the FGF can be removed and the serum concentration can be reduced to as low as 1%. TM cells can be maintained in low or no serum for up to 4 weeks, but serum is needed to expand cell numbers in subconfluent cultures.
One of the potential drawbacks of current TM cell culture methods is that media containing FBS is rich in growth factors and cytokines. In vivo, TM cells are bathed and nourished in aqueous humor, a sparsely proteinated fluid which contains about 1% serum proteins derived from leakage out of the iris root (Freddo, 2013). Thus it has been suggested that use of aqueous humor would provide an environment more similar to in vivo conditions, enabling in vitro TM gene expression and cell function to more closely mimic those of TM cells in situ. While it would be ideal to culture TM cells in aqueous humor that contains soluble factors important for TM homeostasis (Resch et al., 2010), aqueous humor alone does not provide sufficient growth factors to stimulate cell proliferation and expansion for cells in culture. If using aqueous humor, TM cells should be grown and expanded in DMEM containing 10% FBS until the culture has expanded appropriately for use in experiments. At this time, the media can be switched to a media containing aqueous humor (Fautsch et al., 2005). However, this methodology is limited by the ability to obtain sufficient amounts of aqueous humor and the lack of an artificial aqueous humor supplement.
5.2. Passaging of TM cells
To passage TM cells, two methods are commonly used. The first involves the traditional method of trypsinization (e.g.: 0.05% trypsin/0.5 mM EDTA) to dissociate cell-cell and cell-matrix adhesions and lift the cells off the plates. When passaging, fibroblasts detach rapidly, but TM cells require a much longer incubation (3–5 min). Varying trypsin incubation times can be used to enrich TM cells from contaminating fibroblasts since the fibroblasts can be simply washed away following light trypsinization (30 s–1 min). Once trypsinized, TM cells at 70–90% confluence are generally split 1:3 to maintain appropriate cell densities. TM cells appear to require paracrine factors from surrounding cells for growth, so splitting cells at lower cell densities may result in slow or no growth. Once initial isolates are trypsinized (passage 1), cells are considered “secondary” cultures and should be referred to as “normal” TM cells, not “primary” TM cells.
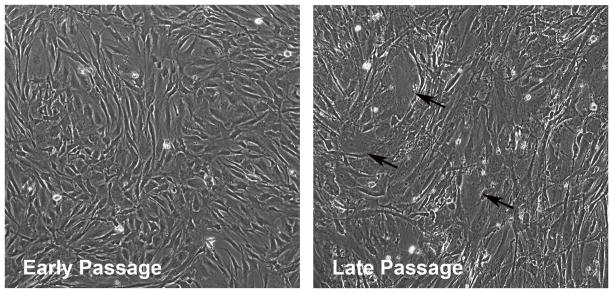
TM cells have a finite number of doublings and begin to change in appearance after 6–8 passages (Fig. 3). By passage 9 or 10, they can no longer be described as TM cells since they start to develop senescence features such as vacuoles, increased size and reduced doubling time. Major changes in gene expression patterns are also observed (Hernandez et al., 1987). For this reason, it is generally not recommended to use TM cells from human eyes at greater than 7 passages. For TM cells derived from animal sources, the recommended passage number is often decreased. For instance, porcine TM cells are typically used within 5 passages. Since duration of passaging of individual cell strains depends upon initial number of cell isolates, it is critical to visually monitor all cultures for senescence features no matter the passage number.
Fig. 3.
Phase contrast images of early and late passage (p2 vs p6) confluent TM cell cultures from the same human donor (11 month old). Arrows point to senescent cells.
5.3. Isolating/passaging TM cells with cytodex beads
A second method of isolating and passaging TM cells utilizes cytodex beads (C-3275; Sigma, St. Louis, MO) (Morgan et al., 2014). Five millimeter segments of dissected TM tissue are incubated with 0.2% collagen-coated cytodex beads (Sigma-Aldrich) in DMEM/Nutrient Mixture F-12 (50:50; DMEM/F12) supplemented with 10% FBS, and 1% penicillin/streptomycin/fungizone. The beads ‘latch’ on to tissue segments and the cells ‘crawl’ from the tissues to the beads. Once the beads have sufficient cells, they get heavier and settle down to the bottom of the dish. TM cells then grow from the beads onto the tissue culture plastic dishes, from which the beads can be dislodged by “tapping” the plate prior to expansion of the cultures. Cells that migrate out of these beads are maintained in supplemented DMEM/F12 and used before passage 7.
Passaging with Cytodex beads is executed using a 2% suspension of beads in sterile PBS, which is added to confluent TM monolayers (Steely et al., 1992). After about a week, gentle washing of the beads with a stream of media from a Pasteur pipette dislodges the cell-covered beads from the confluent cultures. Beads with cells are then aspirated and transferred to new plates. The cells on the original plate are left to re-grow as before. Replicative senescence (increased cell and nuclear size) is also observed at higher passage numbers using this method. Positive senescence-associated β-galactosidase staining verifies senescence regardless of the method used to passage cells (Morgan et al., 2015).
5.4. TM cell storage and transport
5.4.1. Freezing
TM cells are sensitive to extreme changes in temperature so cells need to be frozen down through a temperature gradient process. TM cells are trypsinized, spun down at 1500 g, and resuspended in freezing medium containing 0.5–1 million cells/tube. Freezing medium contains DMEM, 10% sterile DMSO and at least 50% (up to 90%) of FBS. Antibiotics and antimycotics are not added. Cell cryotubes (1.5 or 2 ml size) are frozen slowly by either suspending in the vapor phase of the liquid nitrogen tank or by placing the cryotube in an isopropanol bath at -80 °C. After freezing overnight, the tubes are directly transferred into liquid nitrogen for long term storage.
5.4.2. Thawing
TM cells in cryotubes are thawed in a 37 °C water bath with gentle shaking. DMSO is toxic to cells so as soon as a frozen ball of cells in center of the tube is observed, the cells are immediately transferred to a culture plate containing pre-warmed DMEM to dilute out DMSO.
5.4.3. Shipping
TM cells are generally shipped in T25 or T75 flasks filled to the rim with media containing 10% FBS at room temperature with the lid sealed with parafilm. TM cells can survive up to 4 days of transport under these conditions, which is an advantage if shipping internationally. Additionally, shipping flasks of cells allows TM morphology to be verified before shipping and after arrival. Alternatively, TM cells can be shipped frozen on dry ice, although potential fluctuations in temperature during transport can have a detrimental impact on some cultures, and shipping is more expensive.
6. Culturing glaucomatous TM cells
The culture efficiency of TM cells from glaucomatous donors is lower than that of cells from age-matched normal donors because: (A) TM cell numbers are reduced with glaucoma (Alvarado et al., 1984) and (B) eyes are usually treated for many years with glaucoma medications with potential side effects such as reducing aqueous humor flow, which reduces nutrient supply to the TM. However, some researchers have successfully cultured glaucoma TM cells and they appear to retain many of their glaucomatous characteristics after prolonged culture (Clark et al., 1995; Grant et al., 2013; Stamer et al., 2000). As a caution, signs of cell senescence often appear earlier in glaucomatous TM cells than in normal TM cells (due to fewer initial isolates and senescence in situ), so the number of passages should be limited. Unfortunately for researchers, glaucoma eyes are infrequently available, since disease incidence is about 4% in elderly populations and often donor families do not consent for donation because they think that diseased eyes are not useful for research.
When culturing TM cells from potential “glaucomatous” donor eyes, it is critical to provide as much information as possible about the donor. Examples of useful information include: documentation of glaucoma medications, IOP history and surgical/laser intervention to lower IOP and/or documentation of optic nerve head cupping. It is also possible to perform an outflow facility measurement of whole eyes before beginning the TM cell isolation procedure. Anterior chambers of enucleated whole eyes from putative glaucomatous donors can be cannulated and outflow facility estimated over a short time (e.g.: 2 hr) at a constant pressure (e.g.: 10 mmHg) while measuring change in reservoir volume, or by using more sophisticated methods (Sherwood et al., 2016).
If myelinated portions of the optic nerve can be secured from the donor eye (which is only possible if the optic nerve has been cut not too shortly upon enucleation), a reliable method to confirm glaucoma is to process for histology, cutting cross sections and staining for myelin. In glaucomatous optic nerves, degenerated axon bundles can be identified. If the material is embedded in plastic, semithin sections can be processed that allow quantification to estimate the extent of glaucomatous damage (i.e.: number of remaining axons). Alternatively, retinas from donor eyes can be examined in whole mounts, enabling labeling and quantification of remaining neurofilaments or retinal ganglion cell bodies.
7. Immortalized human TM cells
Immortalized TM cell lines can be generated using SV40 origin defective vector and can be useful for a variety of purposes (Filla et al., 2002; Pang et al., 1994; Tamm et al., 1996). However, during immortalization some “TM like” properties can be lost, and this findings must be replicated with normal, non-immortalized TM cells. For example, some immortalized TM cells no longer express myocilin or express lower levels (Jacobson et al., 2001).
8. Fetal TM tissue dissection and culture
Due to limitations in using adult eye tissues, cultivation of the cells that line the anterior chamber angle of the developing fetus is an alternative way to obtain cells with better growth potential. These primitive cells of the fetal angle are known to be the future site of the TM. Histology studies have shown an identifiable TM at gestational age 20–25 weeks (Hosaka et al., 2013) and the successful isolation and culture of TM cells from 24-week-old fetuses has been carried out previously (Lin et al., 2007). The anterior portion of the angle, which is approximately 1–2 mm posterior to limbus in the fetal eye, can be identified under the operating microscope. The dissection should be performed under high-power magnification to easily identify the light-gray line of tissue adjacent to the cornea where the TM resides. The iris and ciliary body, which are the posterior structures closest to the angle, are then carefully removed to avoid damage to the angle area. The light-gray line of tissue is delicately removed (predominantly uveal and corneoscleral meshwork, with JCT and inner wall left intact) and then placed into 6-well plates coated with extracellular matrix according to previously described techniques with standard culture media (Gospodarowicz and Ill, 1980).
Fetal TM cells demonstrate faster and more consistent doubling times (~14 hours) when compared with adult TM cells (~48 hours). The cultured fetal TM cells have a typical monolayer appearance (cobblestone-like pattern) and cell size that are comparable to cultured adult TM cells. At passages 7 to 8, fetal TM cells start to show morphology closer to fibroblasts, which are longer, spindle-shaped cells and arrange in multilayer fashion. When this occurs cells should be discarded. Fetal TM cells show similar expression patterns for actin, vimentin, fibronectin, laminin, aquaporin-1, CD-44, and myocilin as adult TM cells. Western blot analysis for dexamethasone-induced myocilin expression is also similar between fetal and adult TM cells.
9. Dissection and culture of TM cells from animal eyes
9.1. Non-human primate
The first TM cells to be cultured were from a vervet eye (Rohen et al., 1975). Monkey TM is very similar to human, although the tissue is a little softer. The dissection itself is essentially identical to humans and yields are normally good except with very old monkeys. Since monkeys can develop glaucoma at an advanced age (although rare), it is also conceptually possible to obtain glaucomatous cells. Another key advantage of monkey tissues is the freshness; however, a limited number of primate centers restricts access to this valuable resource.
9.2. Mouse
As in humans and non-human primates, neural crest-derived mesenchymal cells cover the inner endothelial lining of mouse SC to form the TM outflow pathways (Smith et al., 2001; Tamm and Kellenberger, 2008; Tamm et al., 1999a). The TM outflow pathways consist of an inner part with one or two connective tissue strands or lamellae, each of which are covered by flat cells. The lamellae are fixed posteriorly at the sclera behind SC and anteriorly at the periphery of the cornea. Accordingly, the inner part resembles the corneoscleral TM in the human eye. The outer part does not form organized lamellae, but resembles a loose connective tissue similar to the JCT in the human eye.
Despite the similarities with regards to SC, JCT and corneoscleral TM, it is important to keep in mind that there are several distinct and quite pronounced structural differences in the architecture of the out-flow pathways between the mouse eye and that of humans. The most obvious difference relates to the position of the ciliary body. Unlike the posterior location in humans, the root of the ciliary body in the mouse eye is located much further anteriorly, overlapping the inner TM (Tamm and Kellenberger, 2008). Similar to other non-primate mammals, the ciliary body is attached to the cornea by pectinate ligaments that originate near the junction between the iris and ciliary body, and attach to the periphery of the cornea anterior to the TM. Aqueous humor passes through the pectinate ligaments into a meshwork of uveal connective tissue strands that are covered by flat cells.
To harvest murine trabecular cells for culture, an approach similar to human TM cell culture has been used which involves the isolation of both corneoscleral TM and the JCT covering the SC and placing them as an explant in one well of a 6-well culture dish. Outgrowing cells have an endothelial-like phenotype and form a cobblestone-like layer at confluence (Begley et al., 1991; Tamm et al., 1999a). The cells synthesize collagens type I, III, IV and VI, laminin, fibronectin, αB-crystallin, neural cell adhesion molecule, aquaporin 1 and receptors for acetylated low-density lipoprotein. Moreover, cultured mouse TM cells are induced to express myocilin upon treatment with dexamethasone and to synthesize α-smooth muscle actin upon treatment with transforming growth factor-β1 (Mao et al., 2013a) (Tamm et al., 1999b).
Since the dissection procedure needed to isolate TM from the small mouse eye is not trivial, an alternative approach has been developed (Mao et al., 2013b). Magnetic microbeads are injected intracamerally into mouse eyes, which leads to their uptake into cells of the chamber angle within a week. To harvest cells for cultures, anterior eye segments from multiple eyes are digested with collagenase A. Cells containing microbeads are isolated by using a magnetic field and by repeated washing. A putative caveat of the magnetic bead method is that “professional immune cells” such as macrophages, which have a much higher phagocytic activity than TM cells, are likely to be preferentially isolated and brought to culture. Checking for macrophage cell surface markers to exclude their presence in bead-derived mouse TM cultures is recommended. Since the number of cells in an individual mouse eye is much smaller than a human eye, pooled TM explants from numerous mouse eyes are recommended. With proliferation, beads are eventually diluted out of the culture. Remaining cells containing beads can be removed from the culture during passaging to avoid confounding effects of beads on proposed experiments.
9.3. Canine/feline
A slightly different approach, which also relies on TM cell phagocytic properties, can be used to isolate and purify TM cells from dog or cat eyes. Unlike rodents and primates, the bovine, feline, canine and porcine conventional outflow pathway have an aqueous plexus (looped collection vessels) rather than a continuous SC (McMenamin and Steptoe, 1991). The iridocorneal angle structures of dog and cat eyes make it very difficult to precisely dissect TM tissues. Thus, the iridocorneal tissue containing mainly TM, but small amounts of other surrounding tissues, is also present. Cells are dissociated from the tissue by digestion with collagenase-A (as described above). The tissue digest is centrifuged at 500×g for 5 min at 4 °C to remove debris and undigested tissue. The dissociated cells are extensively washed with ice cold PBS and sequential centrifugation to remove pigment and debris. The remaining cells are plated and cultured overnight in DMEM containing 10% FBS, L-glutamine, and penicillin/streptomycin. The cells attach to the coated plate and the remaining debris and pigment are removed by successive washing. [Note: It is essential to remove free pigment because pigment is toxic to the attached cells]. The cells are cultured for an additional 1–2 days, and then magnetic microbeads are added to the culture medium and the plate is gently rocked. After 2–3 days in culture, the adherent cells are trypsinized, and TM cells that have phagocytized the magnetic microbeads are isolated by sequential magnet purification and washing steps. (Hernandez, McDowell et al., unpublished data). Isolated cells become senescent and consistently stop growing at passages 5–6, despite high glucose and/or high serum (> 10%) conditions; thus, using cells at passage 4 or less is recommended.
9.4. Porcine
There is a layer of grey soft reticular material over the TM of pig eyes, which needs to be gently removed or pushed out of the way from the corneal side onto the scleral spur before dissection of the TM. Although there are very few cells in the reticular material, it does obstruct the TM and can be a source of cellular contamination if it is not removed. The sharp dissection method described for human TM is less effective than just teasing-out (blunt dissection) the TM with forceps, since pigs have an aqueous plexus but not a standard SC. Instead of attempting to insert one point of the forceps into SC, the TM is just grabbed from above and pulled out in small strips. Porcine TM strips are then placed into plates and cultured as for human TM cells (Bradley et al., 2003).
9.5. Bovine
TM cells are easily isolated from cow eyes. Freshly enucleated cow eyes are divided to isolate the anterior segment by cutting along the pars plana (approximately 8 mm from the limbus). Using a dissecting microscope, the zonules are carefully cut to release the lens enclosed in the lens capsule, and the vitreous attached to the posterior lens capsule will be removed with the lens. Using two pairs of forceps (one to grab the iris and the other grabbing the posterior ciliary body opposite the iris forceps), the iris is gently pulled to separate and remove it from the eye. Using the same two forceps, one grabbing the sclera and the other grabbing the ciliary body, the ciliary body is gently teased away to expose the TM tissue. [Note: If you see that the TM is also “tearing away” you will need to use a small pair of scissors to cut along the ciliary body and TM margin]. Once uncovered, the exposed TM tissue can be removed with scissors. The TM tissue is cut into small pieces and placed as explants into wells of a 6-well plate and growth medium is added (DMEM containing 10% FBS, L-glutamine, and penicillin/streptomycin).
10. TM cell characterization
10.1. Basic morphology
Once confluent, TM cell cultures demonstrate a cobblestone-like pattern with some overlapping processes and can be considered differentiated (i.e. have entered Go) similar to the situation in vivo. When compared to scleral spur cells, keratocytes, scleral fibroblasts or SC cells, TM cells appear wider, flatter and have more numerous cell extensions and processes (Polansky et al., 1979, 1981, 1984; Stamer et al., 1995, 2000; Tripathi and Tripathi, 1982). A primary contaminant of TM cultures is SC cells, which are longer, spindle-shaped and are contact inhibited (Stamer et al., 1998). Healthy and normal cultured TM cells are also contact inhibited and low passage number cells have a doubling time of two days or less (Polansky et al., 1979). Confluent cultures can maintain this stable morphology for weeks, which is not true for scleral fibroblasts, keratocytes and corneal endothelium. Pig TM cells grow faster in culture than human TM cells and look similar, but are slightly more elongated.
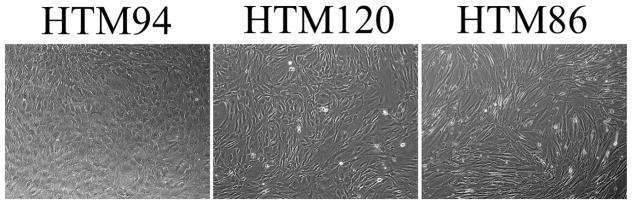
Essentially all TM cell cultures will contain a mixture of TM “beam” cells from the uveal and corneoscleral regions, with smaller proportions of JCT or cribriform TM cells and SC inner wall endothelium. Stem cells may also be a minor component (see “future directions” below). Cultures can have a spectrum of morphologies at confluence, from a near uniform “cobblestone-like” appearance to a mixture of cobblestone-like and elongated cells (Fig. 4). Contamination by a few Schwalbe’s line cells, corneal endothelium, corneal keratocytes/fibroblasts, scleral spur myofibroblasts, sclera fibroblasts, ciliary muscle cells, SC outer wall endothelium, corneal or scleral vascular endothelium or collector channel endothelium and macrophages are all possible. Clearly, analyzing morphology alone is not sufficient to identify TM cell cultures and additional characterization is required. Since no single feature is unique to a TM cell, it is highly recommended that several additional methodologies and criteria be used for identification when establishing TM cells from any species.
Fig. 4.
Phase-contrast images of three different human TM cell strains in culture, showing a spectrum of morphologies. Modified from Stamer and Clark (2017).
10.2. Additional criteria
To date, specific TM cell markers have not been identified and instead a panel of markers is used to differentiate TM cells from contaminating cells that reside in the vicinity of the TM and could reside in co-culture. For example, chitinase-3 like-1, aquaporin-1 protein and MGP are expressed by cultured TM cells and not by many of the potential contaminating cells (Bradley et al., 2003; Du et al., 2012; Wirtz et al., 2002; Xue et al., 2007). Upon stimulation by mechanical stretch, TNFα and/or IL-1, MGP and alpha-B-crystallin are strongly induced by TM cells and less so by potential contaminating cells (Bradley et al., 2003). For a more detailed description of markers, see (Stamer and Clark, 2017). For simplicity, we will only discuss the most commonly accepted criteria for characterizing TM cell cultures.
10.3. Myocilin
Myocilin induction in response to dexamethasone is a reliable marker for TM cells, since neighboring cells do not respond as robustly (Polansky et al., 2000). Typically, 100–500 nM dexamethasone added fresh every 2–3 days to the media for up to 7 days provokes robust myocilin production, which can be assessed by Western immunoblot or PCR. Further, immunohistochemical comparisons allow identification of responding and non-responding individual cells. On Western immunoblots, a doublet at 55–57 kDa is expected. A broad band often identified at around 65 kDa is likely albumin and signifies a “dirty” antibody. Since several anti-myocilin antibodies are not specific, a Western immunoblot is highly recommended as well as immunocytochemistry of a field of cells in culture to determine proportion of cells that are induced by dexamethasone (Fautsch et al., 2000; Jacobson et al., 2001; Stamer et al., 1998). A recommended threshold for degree of induction is greater than 50% of cells in the field of view are “responsive” of at least 100 cells. Antibodies used successfully to detect myocilin include those from commercial (R&D Systems MAB3446; Millipore MABN866; Abcam Ab41552) and academic (Fautsch et al., 2000; Stamer et al., 1998) sources. As a note, there are mixed reports of dexamethasone-induction of myocilin in bovine TM cells, possibly due to breed (Bermudez et al., 2017; Danias et al., 2011; Taniguchi et al., 2000).
Markers not expressed by TM cells, but by potentially contaminating cells, are also useful in characterizing TM cell cultures. For example, SC cells in culture express vascular endothelial cadherin, α6 integrin and fibulin 2, but these are not expressed by TM cells (Perkumas and Stamer, 2012). Other markers expressed by contaminating cells include desmin and keratin (Fuchshofer et al., 2006).
11. Future directions
While much progress has been made since the first description methods to isolate and culture human TM cells by Polansky and colleagues in 1979 (Polansky et al., 1979), we still have not identified a differential marker protein expressed uniquely by TM cells. Given the distinctive responsibilities and location of TM cells, it would be surprising that one does not exist. To date, only one biomarker has been identified to distinguish the two TM cell populations: JCT cells express alpha-B-crystallin whereas corneoscleral TM cells do not, likely due to the fact that cells in the JCT are under constant tension in vivo (Siegner et al., 1996). Interestingly, even while not under tension the JCT cells retained a strong expression of alpha-B-crystallin (with weak expression by corneoscleral TM cells) (Fuchshofer et al., 2006; Welge-Lussen et al., 1999). Future studies should focus on identifying additional biomarkers of TM cells, possibly from all 3 anatomical regions. In addition to providing a valuable tool to positively identify TM cells from possible contaminants, a biomarker may also provide a means to directly target gene alterations specifically in the TM (transgenic/knockout mice, virus targeting, etc.). Biomarkers are also essential for those who plan on devising differentiation protocols for embryonic, induced-pluripotent and mesenchymal stem cells into TM cells for transplantation.
There is still some debate as to whether corneoscleral TM and JCT cells are the same cell “type” in different biological environments, or whether they are two different cell types (Coroneo et al., 1991; Flugel et al., 1991; Ge et al., 2016). In situ, the cells adopt different morphologies: corneoscleral TM cells have a round to oval shape, and a large cell body with some overlapping processes, while cells in the JCT region have a more elongated, spindle-shaped or stellate morphology with many overlapping processes. However, it is unlikely that the morphologies described in situ are completely maintained in the artificial cell culture environments described herein. In cultures using cells isolated from each region, both cell populations expressed many of the same basement membrane and extracellular matrix genes and responded similarly to TGFβ2 or dexamethasone treatment (Fuchshofer et al., 2006; Welge-Lussen et al., 1999). Thus, it remains uncertain whether the two populations are the same or different. To help TM cells maintain their identity in culture (and aid in their positive identification), it may be useful to identify aqueous humor “factors” that impact TM expression pattern and biological function. This has the potential to facilitate creation of a TM-specific growth media.
Lastly, there is a specialized population of TM stem cells primarily found in the anterior, non-filtering region of the TM (also known as the TM insert region) which is located beneath Schwalbe’s line and inserts into the cornea (Acott et al., 1989; Braunger et al., 2014; Kelley et al., 2009; Whikehart et al., 2005). However, some investigators have isolated stem cells from the entire TM (Du et al., 2012; Gonzalez et al., 2006). The stem cells are likely to account for no more than 1–2% of the entire TM cell number. A recent publication has identified expression ofdifferences between mesenchymal stem cells and TM cells (Snider et al., 2017), but further studies are required to characterize these endogenous TM stem cells.
Supplementary Material
Acknowledgments
The authors thank Griff Samples, Thelma De Souza, Aerie Pharmaceuticals, Bausch and Lomb, Alcon/Novartis, Glaukos and the Western Glaucoma Foundation for continued support of the trabecular meshwork study club; enabling 16 annual scientific gatherings of researchers dedicated to better understanding trabecular meshwork biology and facilitating the assembly of this position paper.
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethyl sulfoxide
- FBS
Fetal bovine serum
- EDTA
Ethylenediaminetetraacetic acid
- FGF
Fibroblast growth factor
- HIV
Human immunodeficiency virus
- IRB
Institutional review board
- IL
Interleukin
- JCT
Juxtacanalicular tissue
- MGP
Matrix gla protein
- PBS
Phosphate-buffered saline
- SC
Schlemm’s canal
- TM
Trabecular meshwork
- TNF
Tumor necrosis factor
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.exer.2018.03.001.
References
- Acott TS, Samples JR, Bradley JM, Bacon DR, Bylsma SS, Van Buskirk EM. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am J Ophthalmol. 1989;107:1–6. doi: 10.1016/0002-9394(89)90805-2. [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714–727. [PubMed] [Google Scholar]
- Anderssohn AM, Cox K, O’Malley K, Dees S, Hosseini M, Boren L, Wagner A, Bradley JM, Kelley MJ, Acott TS. Molecular chaperone function for myocilin. Invest Ophthalmol Vis Sci. 2011;52:7548–7555. doi: 10.1167/iovs.11-7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley CG, Yue BY, Hendricks RL. Murine trabecular meshwork cells in tissue culture. Curr Eye Res. 1991;10:1015–1030. doi: 10.3109/02713689109020340. [DOI] [PubMed] [Google Scholar]
- Bermudez JY, Webber HC, Brown B, Braun TA, Clark AF, Mao W. A comparison of gene expression profiles between glucocorticoid responder and non-responder bovine trabecular meshwork cells using RNA sequencing. PLoS One. 2017;12:e0169671. doi: 10.1371/journal.pone.0169671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44:5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- Braunger BM, Ademoglu B, Koschade SE, Fuchshofer R, Gabelt BT, Kiland JA, Hennes-Beann EA, Brunner KG, Kaufman PL, Tamm ER. Identification of adult stem cells in Schwalbe’s line region of the primate eye. Invest Ophthalmol Vis Sci. 2014;55:7499–7507. doi: 10.1167/iovs.14-14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AF, Miggans ST, Wilson K, Browder S, McCartney MD. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J Glaucoma. 1995;4:183–188. [PubMed] [Google Scholar]
- Coroneo MT, Korbmacher C, Flugel C, Stiemer B, Lutjen-Drecoll E, Wiederholt M. Electrical and morphological evidence for heterogeneous populations of cultured bovine trabecular meshwork cells. Exp Eye Res. 1991;52:375–388. doi: 10.1016/0014-4835(91)90032-a. [DOI] [PubMed] [Google Scholar]
- Danias J, Gerometta R, Ge Y, Ren L, Panagis L, Mittag TW, Candia OA, Podos SM. Gene expression changes in steroid-induced IOP elevation in bovine trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:8636–8645. doi: 10.1167/iovs.11-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Roh DS, Mann MM, Funderburgh ML, Funderburgh JL, Schuman JS. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Invest Ophthalmol Vis Sci. 2012;53:1566–1575. doi: 10.1167/iovs.11-9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci. 2000;41:4163–4168. [PubMed] [Google Scholar]
- Fautsch MP, Howell KG, Vrabel AM, Charlesworth MC, Muddiman DC, Johnson DH. Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Invest Ophthalmol Vis Sci. 2005;46:2848–2856. doi: 10.1167/iovs.05-0101. [DOI] [PubMed] [Google Scholar]
- Filla MS, Liu X, Nguyen TD, Polansky JR, Brandt CR, Kaufman PL, Peters DM. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002;43:151–161. [PubMed] [Google Scholar]
- Flugel C, Tamm E, Lutjen-Drecoll E. Different cell populations in bovine trabecular meshwork: an ultrastructural and immunocytochemical study. Exp Eye Res. 1991;52:681–690. doi: 10.1016/0014-4835(91)90020-f. [DOI] [PubMed] [Google Scholar]
- Freddo TF. A contemporary concept of the blood-aqueous barrier. Prog Retin Eye Res. 2013;32:181–195. doi: 10.1016/j.preteyeres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E, Birke M. Biochemical and morphological analysis of basement membrane component expression in corneoscleral and cribriform human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:794–801. doi: 10.1167/iovs.05-0292. [DOI] [PubMed] [Google Scholar]
- Ge P, Navarro ID, Kessler MM, Bernier SG, Perl NR, Sarno R, Masferrer J, Hannig G, Stamer WD. The soluble guanylate cyclase stimulator IWP-953 increases conventional outflow facility in mouse eyes. Invest Ophthalmol Vis Sci. 2016;57:1317–1326. doi: 10.1167/iovs.15-18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microsc Res Tech. 1996;33:336–367. doi: 10.1002/(SICI)1097-0029(19960301)33:4<336::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Epstein DL, Luna C, Liton PB. Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp Eye Res. 2006;82:959–967. doi: 10.1016/j.exer.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D, Ill C. Extracellular matrix and control of proliferation of vascular endothelial cells. J Clin Invest. 1980;65:1351–1364. doi: 10.1172/JCI109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J, Tran V, Bhattacharya SK, Bianchi L. Ionic currents of human trabecular meshwork cells from control and glaucoma subjects. J Membr Biol. 2013;246:167–175. doi: 10.1007/s00232-012-9517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson I, Lee WR, Abraham S, Howes RC. Associations between the cells of the walls of Schlemm’s canal. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;208:33–47. doi: 10.1007/BF00406980. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Weinstein BI, Schwartz J, Ritch R, Gordon GG, Southren AL. Human trabecular meshwork cells in culture: morphology and extracellular matrix components. Invest Ophthalmol Vis Sci. 1987;28:1655–1660. [PubMed] [Google Scholar]
- Hosaka F, Rodriguez-Vazquez JF, Abe H, Murakami G, Fujimiya M, Ohguro H. Qualitative changes in fetal trabecular meshwork fibers at the human iridocorneal angle. Anat Cell Biol. 2013;46:49–56. doi: 10.5115/acb.2013.46.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Shefield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Keller KE, Acott TS. The juxtacanalicular region of ocular trabecular meshwork: a tissue with a unique extracellular matrix and specialized function. J Ocul Biol. 2013;1:3. [PMC free article] [PubMed] [Google Scholar]
- Kelley MJ, Rose AY, Keller KE, Hessle H, Samples JR, Acott TS. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res. 2009;88:747–751. doi: 10.1016/j.exer.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Lee OT, Minasi P, Wong J. Isolation, culture, and characterization of human fetal trabecular meshwork cells. Curr Eye Res. 2007;32:43–50. doi: 10.1080/02713680601107058. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Mao W, Liu Y, Wordinger RJ, Clark AF. A magnetic bead-based method for mouse trabecular meshwork cell isolation. Invest Ophthalmol Vis Sci. 2013a;54:3600–3606. doi: 10.1167/iovs.13-12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao WM, Liu Y, Wordinger RJ, Clark AF. A magnetic bead-based method for mouse trabecular meshwork cell isolation. Invest Ophthalmol Vis Sci. 2013b;54:3600–3606. doi: 10.1167/iovs.13-12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, Steptoe RJ. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J Anat. 1991;178:65–77. [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Raghunathan VK, Chang YR, Murphy CJ, Russell P. The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget. 2015;6:15362–15374. doi: 10.18632/oncotarget.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Wood JA, Walker NJ, Raghunathan VK, Borjesson DL, Murphy CJ, Russell P. Human trabecular meshwork cells exhibit several characteristics of, but are distinct from, adipose-derived mesenchymal stem cells. J Ocul Pharmacol Therapeut. 2014;30:254–266. doi: 10.1089/jop.2013.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994;13:51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- Perkumas KM, Stamer WD. Protein markers and differentiation in culture for Schlemm’s canal endothelial cells. Exp Eye Res. 2012;96:82–87. doi: 10.1016/j.exer.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Zimmerman CC. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye (Lond) 2000;14(Pt 3B):503–514. doi: 10.1038/eye.2000.137. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Weinreb R, Alvarado JA. Studies on human trabecular cells propagated in vitro. Vis Res. 1981;21:155–160. doi: 10.1016/0042-6989(81)90151-6. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979;18:1043–1049. [PubMed] [Google Scholar]
- Polansky JR, Wood IS, Maglio MT, Alvarado JA. Trabecular meshwork cell culture in glaucoma research: evaluation of biological activity and structural properties of human trabecular cells in vitro. Ophthalmology. 1984;91:580–595. doi: 10.1016/s0161-6420(84)34241-5. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Hann CR, Cook KA, Fautsch MP. Aqueous humor rapidly stimulates myocilin secretion from human trabecular meshwork cells. Exp Eye Res. 2010;91:901–908. doi: 10.1016/j.exer.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee DJ, Tamm ER, Russell P. Donor corneoscleral buttons: a new source of trabecular meshwork for research. Exp Eye Res. 2003;77:749–756. doi: 10.1016/j.exer.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Schachtschabel OO, Matthiessen PF. In vitro studies on the trabecular meshwork of the primate eye. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;193:95–107. doi: 10.1007/BF00419354. [DOI] [PubMed] [Google Scholar]
- Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR. Measurement of outflow facility using iPerfusion. PLoS One. 2016;11:e0150694. doi: 10.1371/journal.pone.0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegner A, May CA, Welge-Lussen UW, Bloemendal H, Lutjen-Drecoll E. Alpha B-crystallin in the primate ciliary muscle and trabecular meshwork. Eur J Cell Biol. 1996;71:165–169. [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, Savinova OV, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001;1:3. doi: 10.1186/1471-213X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider EJ, Vannatta RT, Schildmeyer L, Stamer WD, Ethier CR. Characterizing differences between MSCs and TM cells: toward autologous stem cell therapies for the glaucomatous trabecular meshwork. J Tissue Eng Regen Med. 2017 doi: 10.1002/term.2488. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000;20:347–350. [PubMed] [Google Scholar]
- Stamer WD, Clark AF. The many faces of the trabecular meshwork cell. Exp Eye Res. 2017;158:112–123. doi: 10.1016/j.exer.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:1804–1812. [PubMed] [Google Scholar]
- Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992;33:2242–2250. [PubMed] [Google Scholar]
- Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88:648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Kellenberger A. Aqueous humor dynamics and trabecular meshwork. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. MIT Press; Cambridge: 2008. pp. 129–134. [Google Scholar]
- Tamm ER, Russell P, Johnson DH, Piatigorsky J. Human and monkey trabecular meshwork accumulate alpha B-crystallin in response to heat shock and oxidative stress. Invest Ophthalmol Vis Sci. 1996;37:2402–2413. [PubMed] [Google Scholar]
- Tamm ER, Russell P, Piatigorsky J. Development and characterization of an immortal and differentiated murine trabecular meshwork cell line. Invest Ophthalmol Vis Sci. 1999a;40:1392–1403. [PubMed] [Google Scholar]
- Tamm ER, Russell P, Piatigorsky J. Development of characterization of a immortal and differentiated murine trabecular meshwork cell line. Invest Ophthalmol Vis Sci. 1999b;40:1392–1403. [PubMed] [Google Scholar]
- Taniguchi F, Suzuki Y, Kurihara H, Kurihara Y, Kasai H, Shirato S, Araie M. Molecular cloning of the bovine MYOC and induction of its expression in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2000;41:2070–2075. [PubMed] [Google Scholar]
- Tripathi RC, Tripathi BJ. Human trabecular endothelium, corneal endothelium, keratocytes, and scleral fibroblasts in primary cell culture. A comparative study of growth characteristics, morphology, and phagocytic activity by light and scanning electron microscopy. Exp Eye Res. 1982;35:611–624. doi: 10.1016/s0014-4835(82)80074-2. [DOI] [PubMed] [Google Scholar]
- Welge-Lussen U, May CA, Eichhorn M, Bloemendal H, Lutjen-Drecoll E. AlphaB-crystallin in the trabecular meshwork is inducible by transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1999;40:2235–2241. [PubMed] [Google Scholar]
- Whikehart DR, Parikh CH, Vaughn AV, Mishler K, Edelhauser HF. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005;11:816–824. [PubMed] [Google Scholar]
- Wirtz MK, Samples JR, Xu H, Severson T, Acott TS. Expression profile and genome location of cDNA clones from an infant human trabecular meshwork cell library. Invest Ophthalmol Vis Sci. 2002;43:3698–3704. [PubMed] [Google Scholar]
- Xue W, Comes N, Borras T. Presence of an established calcification marker in trabecular meshwork tissue of glaucoma donors. Invest Ophthalmol Vis Sci. 2007;48:3184–3194. doi: 10.1167/iovs.06-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.