Summary
Dye-coupling in an in vitro preparation of the supporting cells of the guinea-pig organ of Corti was evaluated by use of the fluorescent dyes, Lucifer Yellow, fluorescein and 6 carboxyfluorescein. Despite the presence of good electrical coupling in Hensen cells (coupling ratios >0.6) the spread of Lucifer yellow was inconsistent. Hensen cells are very susceptible to photoinactivation, i.e., cell injury upon illumination of intracellular dye; and this in conjunction with Lucifer Yellow’s charge and K +-induced precipitability may account for its variability of spread. Fluorescein and 6 carboxyfluorescein, on the other hand, spread more readily and to a greater extent than Lucifer Yellow, often spreading to cell types other than those of Hensen. Dye spread is rapid, occurring within a few minutes. These results suggest that molecules of metabolic importance also may be shared by the supporting cells of the organ of Corti.
Keywords: Organ of Corti, Supporting cells, Dye coupling, Gap junctions, Photoinactivation, Guinea pig
The supporting cells of the mammalian organ of Corti are joined together by gap junctions (Jahnke 1975; Gulley and Reese 1976; Iurato et al. 1976). In a previous study, electrical coupling among these cells was shown to be present, although it was thought to be poor (Santos-Sacchi and Dallos 1983). At that time, dye spreading studies with the fluorescent dye Lucifer Yellow did not reveal dye-coupling in the supporting cells, except on very rare occasions. In recent studies on electrical coupling of Hensen cells with more precise electrophysiological techniques, coupling has been shown to be very good in vitro (Santos-Sacchi 1984, 1985). This result prompted a reinvestigation of dye coupling in the supporting cells of the mammalian inner ear.
Methods
Guinea pigs were anesthetized with pentobarbital and killed by decapitation. The cochleas were quickly removed, and the apical and third turn was microdissected in one piece after removing the spiral ligament and stria vascularis. The preparation was transferred to a perfusion chamber. Alternately, the whole temporal bone was placed in a perfusion chamber on a Zeiss ACM microscope, and the bony capsule around the two most apical turns chipped away. The stria vascularis and spiral ligament were removed. Both chambers were maintained near 37° C with Peltier devices (Bailey Instruments, N.J.). Medium 199 (with Hanka salts [1.26 mM CaCl2, 1.7 uM Fe(No3)3, 5.36 mM KCl, 0.44 mM KH2PO4, 0.81 mM MgSO4, 137 mM NaCl, 4.16 mM NaHCO3, 0.33 mM Na2HPO4]; pH 7.2–7.4, GIBCO, NY) was perfused at a rate of 0.8 to 1.5 ml/min. Electrodes were pulled on a Narishige puller.
Coupling measurements were made with high input impedance devices (WPI KS-700, Dagan 8100-1) capable of constant current injection. Coupling was assessed by injecting negative current pulses of varying magnitudes into one cell and noting the voltage drop in the same and an adjacent cell. Under visual control, Hensen cells were impaled with electrodes. Double-barreled electrodes or theta glass electrodes were used to separately inject current (I1) and record voltage drops (V1) in one cell, while a neighboring cell was impaled with a single-barreled voltage recording electrode (V2).
Coupling responses and membrane potentials were recorded on a Gould strip chart recorder. Individual coupling responses were digitally stored within a Data 6000 wave-form analyzer (Data Precision, MA) and saved to floppy disk. Coupling ratio is defined as the voltage drop in cell 2 divided by the voltage drop in cell 1 in response to current injection in cell 1 (V2/V1) (Bennett 1966).
Dye injections were made iontophoretically into individual supporting cells through single barreled electrodes. One to five percent solutions of Lucifer Yellow CH (Sigma), 6 carboxy fluorescein (Calbiochem) and fluorescein (sodium salt, Sigma) in H2O were used. Epifluorescence observations were made with Zeiss filters (G436, FT510, LP520), with a 50 watt high pressure mercury illumination system. A Zeiss F10/0.25 objective lens was used. Photographs were taken with Polaroid type 667 black and white film. Dye spread was also evaluated under reduced epi-illumination with neutral density filters. For these studies video analyses were made with an ISIT video camera (Dage-MTI) and high resolution monitor (Ikagami). Photographs of video images were made with a Kodak Instagraphic CRT camera.
Results
Electrical coupling among the supporting cells is good; adjacent cells usually have coupling ratios around 0.6, under favorable culture conditions (Fig. 1a). Despite the fact that Hensen cells are well coupled electrically, spread of the injected dye Lucifer Yellow occurs inconsistently. Observations have been made in which dye spread did not occur with Lucifer Yellow, although simultaneous measurements indicated the existence of electrical coupling between the injected Hensen cell and adjacent cells.
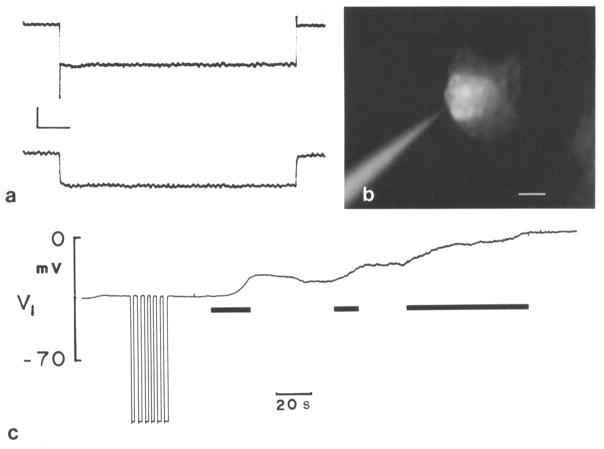
Fig. 1.
a Two adjacent Hensen cells impaled under visual observation with 3M KCl glass electrodes. In one cell current pulses (− 10 nA) were injected down one barrel of a theta glass pipet and the voltage drop was measured with the other barrel. Coupling responses were measured in the other cell with a single barreled electrode. One event was digitally stored and displayed. Calculated coupling ratio 0.8. Vertical scale: 4 mV. Horizontal scale: 0.2 sec. b Hensen cell injected with Lucifer Yellow without epi-illumination 45 sec prior to fluorescence observation as indicated in the trace from this cell (Fig. 1c). Dye spread had occurred. Scale: 15 μm. c Hensen cell impaled with single barreled electrode containing Lucifer Yellow. A stable membrane potential was recorded for a few min whereupon dye was injected into cell with negative current pulses (−1 nA; negative deflections). Membrane potential remained stable after dye injection until blue light illumination was turned on (first black bar). Consequently cell depolarized but stabilized after light was turned off. Photoinactivation process continued upon reinstatement of epi-illumination (second black bar). Finally, light source was left on until total depolarizarion had occurred (third black bar)
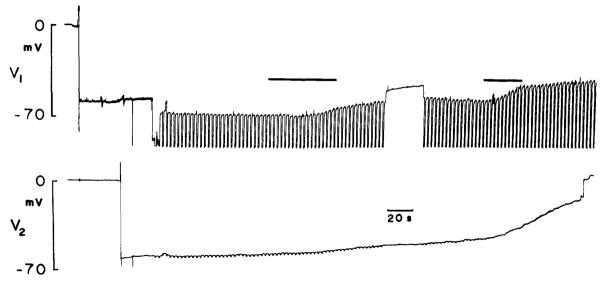
It was noted that frequently cell membrane potentials drop upon injection of Lucifer Yellow. In fact, the Hensen cells are very susceptible to photoinactivation. Photoinactivation (Miller and Selverston 1979; Cohan et al. 1983) denotes the selective killing of cells or parts of cells by irradiating intracellularly injected dyes. Apparently, during absorption of light, heat is generated which deleteriously affects cells, causing drops in membrane potential and electrical activity. Membrane potentials of supporting cells are normally very stable; however, blue light irradiation causes an immediate and coincident drop in membrane potential of dye filled cells (Fig. 1c). Membrane potential declines when the light is turned on and stablizes when the light is turned off. Usually, spread of Lucifer Yellow did not occur during simultaneous epifluorescent observation. However, sometimes dye spread was observable when cells were injected prior to fluorescence observation (Fig. 1b). Spread of Lucifer Yellow, when it occurs, is typically rapid (within one to a few min) and is limited to no more than several neighboring cells. In addition to reducing membrane potentials, blue light exposure causes coupling responses to decrease in coupled Hensen cells (Fig. 2).
Fig. 2.
Neighboring Hensen cells impaled with single barreled electrodes, one containing KCl, the other Lucifer Yellow. Current pulses (− 5 nA) injected into Lucifer electrode and coupling responses measured in the other cell. Voltage drop across Lucifer electrode unbalanceable and thus off scale; however, since input resistance of Hensen cells is about 0.4 megaohms initial calculated coupling ratio is 0.7. Dye spred occurred in this example. Upon illumination of cells with blue light (first black bar), membrane potentials begin to fall. Subsequently, coupling response in other cell decreases. Further exposure to blue light depolarizes cells further (second black bar)
Unlike Lucifer Yellow, fluorescein spreads more consistently to neighboring cells (Fig. 3a–c), and is often observed during epi-illumination, although photoinactivation can occur with this dye. As with Lucifer Yellow, the spread of dye is almost immediate. However, a greater number of cells are usually stained, and supporting cells other than Hensen cells are involved. These other cells include Deiters cells and possibly outer pillar cells. Fluorescein is somewhat membrane permeable; yet, the spread does not appear to be due to non-junctional membrane passage from one cell to another because 1) several attempts to observe dye passage through plasma membranes by directly iontophoreseing fluorescein onto the external aspect of Hensen cell membranes failed to reveal cytoplasmic uptake; 2) extended soaking (up to 20 min) of Hensen cells with external solutions of fluorescein revealed much poorer staining of Hensen cell cytoplasm after washout than that of cells previously stained by intracellular spread of the dye; 3) on a few occasions fluorescein injection did not result in spread of the dye to adjacent cells, probably indicating that these cells were uncoupled; and 4) extracellular spaces between stained Hensen cells showed less fluorescence than intracellular spaces.
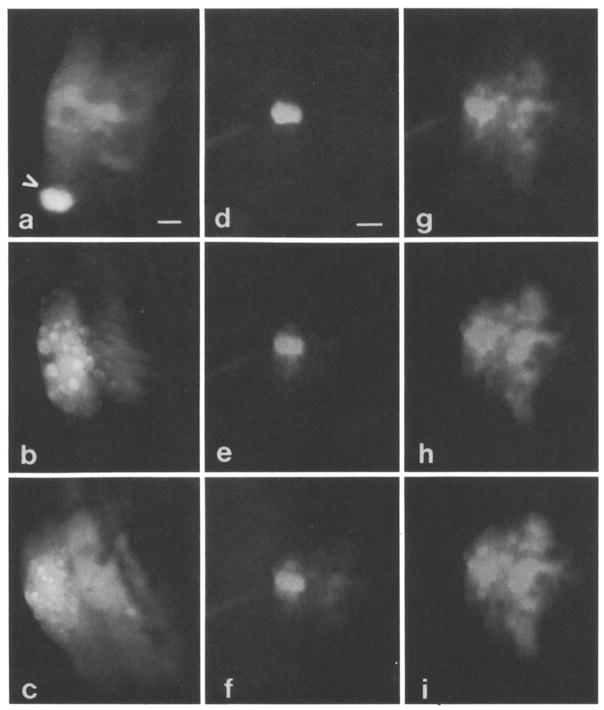
Fig. 3.
a Organ of Corti spirals around bony modiolus through which eighth nerve courses. Hensen cells are most distal to modiolus which is located to right in all photographs. Arrow depicts Hensen cell injected with Lucifer Yellow. No dye spread occurred. Subsequently, fluorescein was injected about four cells away, and dye did spread to adjacent Hensen cells as well as other supporting cells closer to modiolus. Scale: 15 μm. b Fluorescein injected into single Hensen cell, spread of dye occurred rapidly to adjacent Hensen cells and to presumed processes of Deiters cells. Bright spherical structures in Hensen cells are lipid droplets. Scale: same as in Fig. 3a. c Fluorescein observed to spread from Hensen cells to other supporting cell types of organ of Corti. All injections depicted thus far made during epi-illumination. Scale: same as in Fig. 3a. d–i Series of photographs depicting time course of spread of 6 carboxyfluorescein through supporting cells. Epi-illumination present during whole process; however, neutral density filters were used to reduce intensity. Spread of dye video taped with low light level Dage MTI camera and individual photographs taken of monitor screen, d 1 min after start of injection. Barely detectable spread in immediately adjacent Hensen cells. Scale: 15 μm for this and subsequent photographs. e 1 min 30 sec. Dye now seen clearly in spirally adjacent Hensen cells and in cells nearer modiolus, f 1 min 40 sec. Cell staining more clearly seen near modiolus, g 2 min. Spread of dye continues spirally, h 2 min 30 sec. Intensity of dye in adjacent cells approaching that of injected cell. i 3 min. Dye more uniformly distributed in group of supporting cells of different types.
The dye 6 carboxyfluorescein, which is less membrane permeant than fluorescein, shows similar dye spread patterns and also spreads more consistently than Lucifer Yellow. Nevertheless, there were cases where the dye did not spread. The kinetics of dye movement were interesting for 6 carboxyfluorescein and fluorescein. Often, during the first min or two following injection of dye into a particular cell, spread was absent or was limited to closely neighboring cells. Subsequently, a sudden and rapid movement of the dye into a more extensive group of cells occurred. This is demonstrated in Fig. 3d–e, which illustrates the time course of dye spread for an injection of 6 carboxyfluorescein into a Hensen cell. After one min, spread of dye is barely detectable in the two immediately adjacent cells. At 1.5 min, the dye has begun to spread rapidly and by 3 min has spread to a large portion of the organ of Corti, including areas occupied by supporting cells other than those of Hensen, i.e., Deiters and pillar cells.
Discussion
Lucifer Yellow is a highly fluorescent molecule which has been used to demonstrate dye coupling in many cell systems (Stewart 1981; Kaneko and Stewart 1984). Presumably, electrically coupled cells permit the spread of dye through gap junctions. Yet, there is a growing number of observations of electrically coupled cells which do not demonstrate dye coupling or demonstrate dye coupling inconsistently by use of Lucifer Yellow (Takato and Goldring 1979; Audesirk et al. 1982; Kater and Hadley 1982).
In our previous study of dye coupling in the in vitro organ of Corti, fluorescence observations were made during injections of Lucifer Yellow and spread of the dye was observed only occasionally (Santos-Sacchi and Dallos 1983). Possibly the cells were killed by photoirradiation during observations. It would appear that Lucifer Yellow does not spread as easily to coupled cells as does fluorescein and 6 carboxyfluorescein. Perhaps the difference in molecular weights (LY 457, F 330) is responsible for this disparity. Yet, Flagg-Newton et al. (1979) have shown that the molecular cut-off for mammalian junctional spread is around 1000 Daltons. Furthermore, molecular models of the two dyes do not reveal sufficient size differences to account for dye spread differences, so that other factors, e.g., charge and precipitability, may be influential (D.C. Spray, personal communication). Lucifer Yellow readily precipitates as the potassium salt, and thus may do so intracellularly. Fluorescein is somewhat membrane permeable, and care must be taken in dye spread studies to exclude possible spread across non-junctional membranes. Several facts suggest that dye spread is through junctions, most notably the speed of dye spread and the observation that some flourescein injections did not result in spread. 6 carboxyfluorescein spreads as well and because of its charge it is likely that the route of passage is through the aqueous channels of gap junctions.
It is clear that the presence of electrical or ionic coupling cannot necessarily indicate that dye coupling exists. This has been directly demonstrated by Kettenmann and Orkand (1983), who showed that in electrically and dye coupled cells, dye coupling can be abolished without uncoupling ionic communication between the cells. It is probably true, however, that dye coupling indicates the presence of electrical coupling. Thus the observation that fluorescent dyes spread from Hensen cells to other supporting cells, including Deiter and pillar cells, indicates that electrical communication occurs among supporting cells of different types. This may have important consequences for the organ of Corti.
The supporting cells of the organ of Corti provide structural support for the sensory hair cells. Other roles that they may play are speculative at present, yet the presence of electrical and dye coupling between them may provide clues. For example, metabolic cooperation between the supporting cells may be required for normal cochlear function under conditions of high sound stimulation. Perhaps metabolites diffuse intracellularly through the supporting cells from areas of high metabolic activity, e.g., from the lateral wall of the cochlear duct. In addition, the supporting syncytium may provide a K+ sink as is thought to occur in astrocytes of the CNS (Somjen 1979). Potassium levels may be kept low in those areas of the organ (e.g., spaces of Nuel) where its presence would interfere with electrical activity. This may be specially important considering the recently identified outer hair cell contracture associated with potassium induced hair cell depolarization (Brownell et al. 1985; Zenner et al. 1985). Finally, the possibility exists that supporting cells may influence cochlear mechanics. The fact that Hensen cells are dye coupled and can be photoinactivated (killed) may permit an evaluation of the role supporting cells play in cochlear mechanics. That is, selective destruction of dye-injected supporting cells by photoirradiation may alter electrical responses to sound.
Acknowledgments
This work was supported by an RCDA from NINCDS and grants from the Deafness Research Foundation, the Foundation of the UMDNJ, and the NIH (NS 21380-01). I thank Barbara Fate for technical assistance
References
- Audesirk G, Audesirk T, Bowsher P. Variability and frequent failure of lucifer yellow to pass between two electrically coupled neurons in lymnaea stagnalis. J Neurobiol. 1982;13:369–375. doi: 10.1002/neu.480130407. [DOI] [PubMed] [Google Scholar]
- Bennett MVL. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966;137:509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Cohan CS, Hadley RD, Kater SB. Zap axotomy: localized fluorescent excitation of single dye-filled neurons induces growth by selective axotomy. Brain Res. 1983;270:93–101. doi: 10.1016/0006-8993(83)90794-1. [DOI] [PubMed] [Google Scholar]
- Flagg-Newton J, Simpson I, Loewenstein W. Permeability of the cell-to-cell membrane channels in mammalian cell junction. Science. 1979;205:404–407. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- Gulley RS, Reese TS. Intercellular junctions in the reticular lamina of the organ of Corti. J Neurocytol. 1976;5:479–507. doi: 10.1007/BF01181652. [DOI] [PubMed] [Google Scholar]
- Iurato S, Franke K, Luciano L, Wermbter G, Pannese E, Reale E. Intercellular junctions in the organ of Corti as revealed by freeze fracturing. Acta Otolaryngol. 1976;82:57–69. doi: 10.3109/00016487609120863. [DOI] [PubMed] [Google Scholar]
- Jahnke K. The fine structure of freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol [Suppl] 1975:336. [PubMed] [Google Scholar]
- Kaneko A, Stuart AE. Coupling between horizontal cells in the carp retina revealed by diffuison of lucifer yellow. Neurosci Lett. 1984;47:1–7. doi: 10.1016/0304-3940(84)90377-x. [DOI] [PubMed] [Google Scholar]
- Kater SB, Hadley RD. Intracellular staining combined with video fluorescence microscopy for viewing living identified neurons. In: Liss AR, editor. Cytochemical methods in neuroanatomy. New York: 1982. pp. 441–459. [Google Scholar]
- Kettenmann H, Orkand RK. Intracellular SITS injection dye uncouples mammalian oligodendrocytes in culture. Neurosci Lett. 1983;39:21–26. doi: 10.1016/0304-3940(83)90159-3. [DOI] [PubMed] [Google Scholar]
- Miller JP, Selverston AI. Rapid killing of single neurons by irradiation of intracellularly injected dye. Science. 1979;206:702–704. doi: 10.1126/science.386514. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. A re-evaluation of cell coupling in the organ of Corti. Hear Res. 1984;14:203–204. doi: 10.1016/0378-5955(84)90019-4. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. The effects of cytoplasmic acidification upon electrical coupling in the organ of Corti. Hear Res. 1985;19:207–215. doi: 10.1016/0378-5955(85)90140-6. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Dallos P. Intercellular communication in the supporting cells of the organ of Corti. Hear Res. 1983;9:317–326. doi: 10.1016/0378-5955(83)90034-5. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Extracellular potassium in the mammalian central nervous system. Ann Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- Stewart WW. Lucifer dyes – highly fluorescent dyes for biological tracing. Nature. 1981;292:17–21. doi: 10.1038/292017a0. [DOI] [PubMed] [Google Scholar]
- Takato M, Goldring S. Intracellular marking with lucifer yellow CH and horseradish peroxidase of cells electrophysiologically characterized as glia in the cerebral cortex of the cat. J Comp Neurol. 1979;186:173–188. doi: 10.1002/cne.901860205. [DOI] [PubMed] [Google Scholar]
- Zenner HP, Zimmermann U, Schmitt U. Reversible contraction of isolated mammalian cochlear hair cells. Hear Res. 1985;18:127–133. doi: 10.1016/0378-5955(85)90004-8. [DOI] [PubMed] [Google Scholar]