Abstract
Background
Low-intensity pulsed ultrasound (LIPUS) has been used in both basic research and clinical settings for its therapeutic potential in promoting tissue healing. Clinical data has shown that LIPUS can accelerate fresh fracture healing. However, the treatment for aging osteoporosis and non-union is still unclear. In addition, the mechanism of ultrasound promoted bone healing has remained unknown.
Objective
It is proposed that noninvasive ultrasound treatment can enhance local fluid flow within the tissue to initiate remodeling and regeneration. The goal of this study was to evaluate the effects of dynamic ultrasound in promoting cellular mechanotransduction within bioengineered organic scaffolds to trigger osteogenesis and mineralization.
Methods
The experiment was designed in two-fold: to evaluate the role of LIPUS on osteoblastic-like (MC3T3) cell proliferation and mineralization in response to acoustic waves, using biomechanical rate-dependent signals in a bioreactor; and, to evaluate the new scaffold experimentation techniques, in order to generate a potential implantable biomaterial for orthopedic tissue regeneration and repair.
Results
LIPUS treatment on MC3T3 cells yielded enhanced cellular mineralization (**p < 0.001) in 3-D scaffolding, but reduced the total cell numbers (*p < 0.05), using Alizarin Red staining and cell counting analyses, respectively, in comparison to the control.
Conclusion
This study suggests that LIPUS, if applied at proper frequency and duty cycle, can promote cell mineralization within the 3-D organic scaffold under in vitro setting. The translational component of this experiment seeks to draw a parallel to the potential pre-treatment of scaffolds for implantation before orthopedic surgery, which could prove to greatly benefit the patient in accelerating fracture healing and tissue regeneration.
The Translational Potential of this Article
LIPUS stimulation was critical in contributing to the mechanical signaling transductions that activated bone enhancement parameters in MC3T3 cells regulated by bioreactor, and thus has potential to change how we pretreat scaffolds for orthopedic surgery and noninvasively accelerate healing in the future, e.g., in an extreme condition such as long-term space mission.
Keywords: Bioreactor, Bone tissue engineering and regeneration, Low-intensity pulsed ultrasound, Scaffold
Introduction
Osteoporosis affects millions of people worldwide by increasing fracture risk and diminishing the quality of life [1]. This systemic skeletal disease results in low bone mass and global weakening of the bone tissues through bone cell imbalances [2]. This leads to increased fracture risk in areas rich in trabecular bone, such as the wrists, hips and vertebrae [3]. Medications that regulate osteoblast and osteoclast balance have a history of success in treating patients with osteoporosis [4]. Other treatments also work by correcting hormone imbalances, improper loads of cell signalling and hyperactivation of cell activity [5]. Although these therapies have shown marked success, they require the administration of a drug or supplement; these therapies have well-established adverse side effects and are unable to specifically target bone sites that are at an increased risk for failure [6]. Furthermore, these treatments may also reflect understudied changes in the bone–hypothalamic–pituitary axis, osteocytes signalling and osteoclast secretions [7]. This experiment focuses on the osteoblast to tackle the imbalance of activity seen in patients with osteoporosis.
Relatively recently, ultrasound technology has been used for noninvasive experimentation; a modified form of it has been used to test our hypothesis [8]. Ultrasound is an acoustic wave or travelling mechanical energy that has a frequency between 20 kHz and 200 MHz [9]. Low-intensity pulsed ultrasound (LIPUS) is a specific frequency of ultrasound, most commonly studied at 1.5 MHz, that has the ability to improve fresh fracture healing, supported by both clinical evidence and basic research studies [10], [11]. LIPUS manipulates mechanotransduction receptors on bone cells to activate downstream effectors that signal anabolic growth cascades [12]. LIPUS reinforces a concept embedded in the foundation of microgravity experiments. Past studies have indicated that individuals exposed to conditions of microgravity for long periods of time suffer profound bone loss [13]. Gravity is a constant source of loading linked to mechanotransduction [14]. When gravity is removed for long periods of time, e.g., astronauts during long-term space mission, the constant stimulus of mechanical loading on those receptors is lost, eventually resulting in significant decreases in bone density [15], [16]. Therefore, the converse of this situation, applying extra forces (such as exercise and LIPUS), may be able to regulate the mechanotransduction pathways positively and increase bone formation parameters [17].
This experiment will study those pathways by concentrating on the three components of the tissue engineering triad, which includes the cell, the growth-stimulating signals (provided physically by the bioreactor) and the scaffold [18]. Most commonly, a scaffold is a microscopic structure that abets the development of tissues in a given 3-D cellular space [19]. A scaffold that develops seeded cell does this by fulfilling the in vivo environment, which, in nonpathological conditions, supplies vascularisation, cell attachment points and optimised migration [20]. This experiment decellularised the extracellular matrix of bovine trabecular bone to produce a macroscopic scaffold.
LIPUS is known to enhance mesenchymal stem cell recruitment and differentiation through a variety of cellular signalling transductions [21], [22]. There is evidence that LIPUS can induce cavitation and acoustic streaming in interstitial fluid and mechanical vibrations in the extracellular matrix [23]. This yields shear stresses and strains to osteoblasts and deformation to cell membranes, which can then activate mitogen-activated protein kinase (MAPK), angiotensin I (ATI ) mechanoreceptors, integrin, mechanosensitive calcium channels, G-proteins, insulin-like growth factors (IGF) and a variety of other downstream affecters [24], [25].
Another component of our experiment is the bioreactor, which simulates the biological environment of the sample, a bovine trabecular bone scaffold, for our experiment. The bioreactor we used supported cell life by nourishing it with media at a constant rate [26]. This is analogous to how blood from capillary beds supply human cells with oxygen and nutrients [27]. We use the three components of the tissue engineering triad here by treating the cells seed in the scaffold, inside the bioreactor, with LIPUS probes.
The effect of LIPUS as a noninvasive modality for tissue regeneration and cellular proliferation in a tissue engineering setting has not been investigated. Therefore, the primary objective of this study was to test the hypothesis that LIPUS treatment on scaffolds embedded with MC3T3 cells in a bioreactor setting enhances both cell proliferation and bone formation parameters, namely matrix mineralisation. We found our hypothesis to be true regarding LIPUS treatment on MC3T3-yielded enhanced cellular mineralisation (**p < 0.001) but false regarding its effect on the total cell numbers, which was reduced (*p < 0.05). The results section further elaborates on the LIPUS- versus control-treated groups' cellular proliferation rate in the scaffold and cell mineralisation as a function of calcium deposition. Results of this experiment also include the preliminary data on cell counts over 2 weeks as an assessment of the scaffolds and cells for experimentation.
Materials and methods
Scaffolds and cells
Six trabecular scaffolds were isolated from the proximal head of bovine femur (purchased from United States Department of Agriculture (USDA)-approved local market). Bovine trabecular bone was used because of its high porosity, a critical factor in promoting cell seeding, vascularisation and waste removal [28]. The culture media used for this experiment was a mixture of 89% alpha-MEM (minimum essential medium, Gibco, Life Technologies, Grand Island, NY, USA), 10% foetal bovine serum (Gibco, Life Technologies) and 1% Penicillin-Streptomycin (Gibco, Life Technologies). MC3T3, mouse osteoblastic precursor cell line (Sigma Inc., USA), was cultured in 11 mL of this media at a passage of 23 and 13 (in separate experiments) to 85% confluency. The plates were then incubated under standard conditions (95% humidity, 5% CO2 and 37°C) for 24 h to secure firm attachment.
Bioreactor and LIPUS
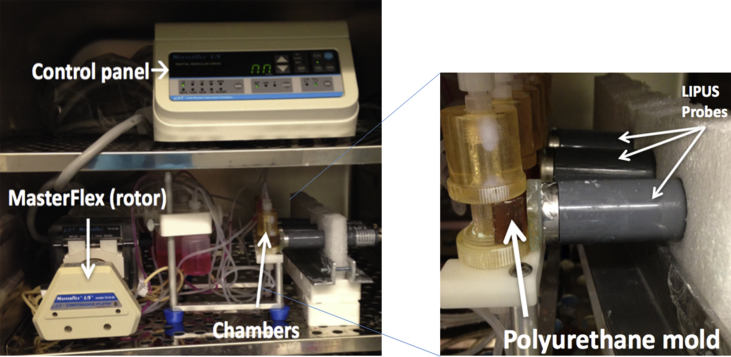
TGT's OsteoGen bioreactor (Instron TGT, MA), a device that uses a system of cartridges, tubing and chambers to simulate fluid perforation by controlling the flow rate of media, was used to perfuse the seeded scaffolds with cell culturing media. We used a rate of 0.1 mL/min as that rate permits a balance between sufficient vascularisation and seeding efficacy [29]. The Sonicator 740 (Mettler Electronics Corporation, CA, USA) with gel-coupled plane wave US applicator (ME7410) was used to apply ultrasound stimulation to the bioreactor chambers (Fig. 1).
Figure 1.
Bioreactor setup in incubator and probes. The control panel was installed into the hood for technical reasons. The Masterflex rotor could then be operated using the control panel to circulate media at the specified flow rate of 0.1 mL/min to the OsteoGen chambers. This setup has the LIPUS console outside of the incubator. This allowed control LIPUS stimulation without opening the incubator, thus minimizing environmental perturbations. Probes were coupled to the chambers through a polyurethane mold and gel to remove airspace and seal coupling. To ensure the consistence of the treatment, we have set the ultrasound exposure in the far field, which operated by an acoustic lens (∼7 mm), acoustic coupling gel (∼1–2 mm) and the thickness of the bioreactor chamber (∼3 mm). Within the ultrasound far field zone, the energy is consistent and is controlled at approximately 30 mW/cm2.
LIPUS = low-intensity pulsed ultrasound.
Time course
The first set of samples was tested five months before experimentation with the bioreactor and LIPUS. This set was used to determine the efficacy of bovine trabecular bone as a scaffold and MC3T3 as seeding material. After obtaining results for this part of the experiment, we cultured the cells again and made new scaffolds for both the first experiment and the second experimentation. One month of preparation was required to allow appropriate incubation time of the cells in the scaffold before experimentation with the bioreactor and LIPUS.
Treatment regimen
In experiments with MC3T3, the control group (scaffolds 1–3) received no ultrasound treatment. Reher et al’s study on mouse calvaria bone in tissue culture found that transducer output energy, using a spatial-averaged and temporal-averaged intensity, of 100 mW/cm2 can provide approximately 30 mW/cm2 acoustic energy in tissue with acoustic coupling distance, which can provide significant bone formation stimulation [30]. Therefore, scaffolds 4–6 received a sinusoidal ultrasonic pulse at 1 MHz with a repetition frequency of 100 Hz and at an intensity of 30 mW/cm2, with 20% duty cycle for 20 min each day for 5 days total. After the first experiment was completed, the scaffolds were stored to be reused for future experiments.
Count and quantification
Cell counting and Alizarin Red staining were used to determine if LIPUS treatment promoted effective tissue engineering. The cells were counted with the Scepter 2.0 Handheld Automated Cell Counter (Millipore Corporation, MA, USA). After cells (from first experiment with MC3T3) were counted, they were stained with 40 mM Alizarin Red staining solution (pH 4.2) and imaged using an Axiovert 2000M Inverted Microscope (Axiocam MRC; Carl Zeiss Inc., Thornwood, NY). After imaging, the stain was removed from the cells using 10% cetylpyridinium chloride (CPC) and 10 mM sodium phosphate solution for 20 min. This eluted stain was then measured at 592 nm in the Bio-Tek EL800 spectrophotometer (Winooski, VT, USA). Quantification was achieved through an Alizarin Red standard curve in 10% CPC and normalised to the total number of cells [21].
Statistical analysis
GraphPad QuickCalcs (GraphPad Software, La Jolla, CA, USA) was used to perform all unpaired t tests. The student t test was then used to define all values of significance. A p-value < 0.05 was considered significant. Averaged data were presented with error bars equal to the averaged data's standard deviation [21].
Results
Assessment of scaffold and MC3T3 cells
Preliminary data demonstrated good MC3T3 seeding in bovine trabecular bone and longitudinal growth over a 2-week period. Previous studies have indicated that decellularised bovine trabecular bone can support bone cells; therefore, this part of the experiment was more important for determining if cell seeding and culturing were possible in an incubator before using a bioreactor [31]. Furthermore, we estimated that successful testing of both LIPUS and the bioreactor with MC3T3 cells would require a minimum of 10,000 cells to seed in the bovine trabecular bone scaffold. Our preliminary tests yielded an average of 10% seeding success rate as a percentage of total cells in the dish. This count proved to be more than sufficient in every single scaffold, which seeded from 13,000- 86,400 cells (Table 1). This part of the experiment was also a longitudinal study to test approximately when the scaffolds would have the most optimal confluency. Plates 1 and 2 were counted after 3 days. Plates 3 and 4 were counted after 7 days. Plates 5 and 6 were counted after 14 days (Table 1). Results from 3 to 7 days demonstrated the best confluency. The cell count seemed to decrease around 14 days but not to numbers below a tolerable confluency for an experiment with the bioreactor.
Table 1.
Test for longitudinal growth. Six scaffolds were tested over a 2-week period to see when MC3T3 cells reached an optimal confluency in the bovine trabecular bone scaffold. Results from 3 days to 7 days were optimal and seemed to decrease around 14 days but not to values that would be considered below a tolerable confluency for an experiment with the bioreactor.
| Plate number | 1 (3 day) | 2 (3 day) | 3 (7 day) | 4 (7 day) | 5 (14 day) | 6 (14 day) |
|---|---|---|---|---|---|---|
| Plate count (× 105) | 4.267 | 4.940 | 5.627 | 4.615 | 4.247 | 4.443 |
| Scaffold count (× 105) | 0.4184 | 0.8640 | 0.6000 | 0.6460 | 0.2266 | 0.1382 |
Cell count and analysis
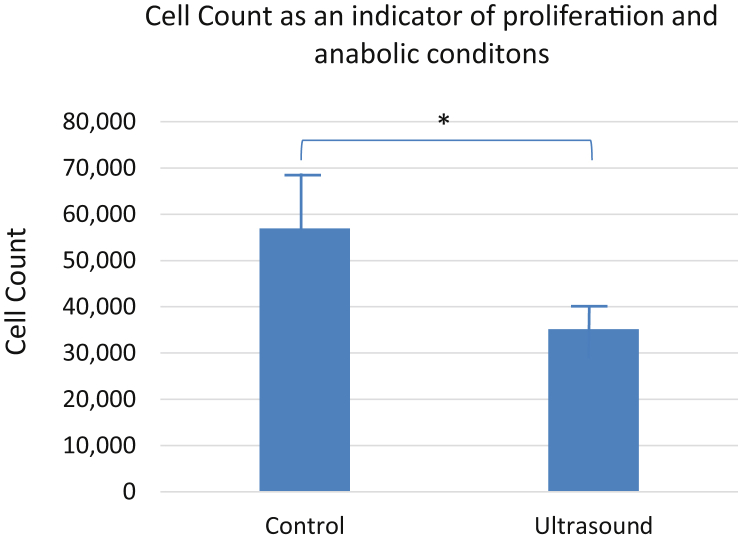
To evaluate the anabolic nature of LIPUS treatment on MC3T3 cells in bioreactor conditions, we measured cell proliferation in each scaffold after experimentation. The results initially showed a surprising decrease in cell count when the chambers were treated with LIPUS. This was seen consistently throughout the two sets of triplicates, with scaffold 2 yielding numbers around the average LIPUS experimental group count. The control MC3T3 groups on average had a higher count of cells than the average of the LIPUS experimental group (Fig. 2).
Figure 2.
LIPUS decreases the cell count after 5 days of stimulation. The average number of cells counted after control (5.70 × 104) and LIPUS treatment (3.52 × 104) on the scaffolds in conditions of bioreactor (*p < 0.05) demonstrates results in favour of the null hypothesis regarding the effect of LIPUS treatment on cell proliferation.
LIPUS exposure significantly increases calcification
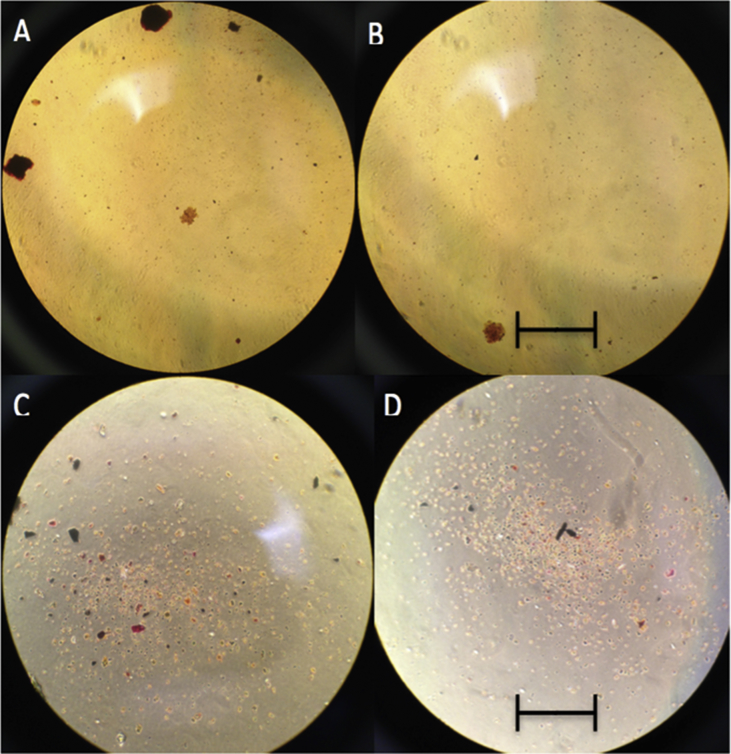
To establish the anabolic effects of LIPUS on matrix mineralisation, matrix calcification was determined using Alizarin Red staining. At 4× magnification, it was difficult to discern noticeable differences in levels of Alizarin Red when comparing the control and LIPUS groups (Fig. 3). At 10× magnification, there is a similar difficulty as both seem to have strong reddened areas. Qualitative analysis thus showed no significant difference in matrix mineralisation between the control- and LIPUS-treated samples.
Figure 3.
Alizarin Red Stain on fixed MC3T3 cells. Images A and C depict the control under 4× and 10× magnification, respectively. Images B and D depict the LIPUS-treated cells under 4× and 10× magnification, respectively. It is difficult to qualify the difference in calcific deposition by the images alone. Scale bar 4× = 5000 μm and 10× = 2000 μm.
LIPUS = low-intensity pulsed ultrasound.
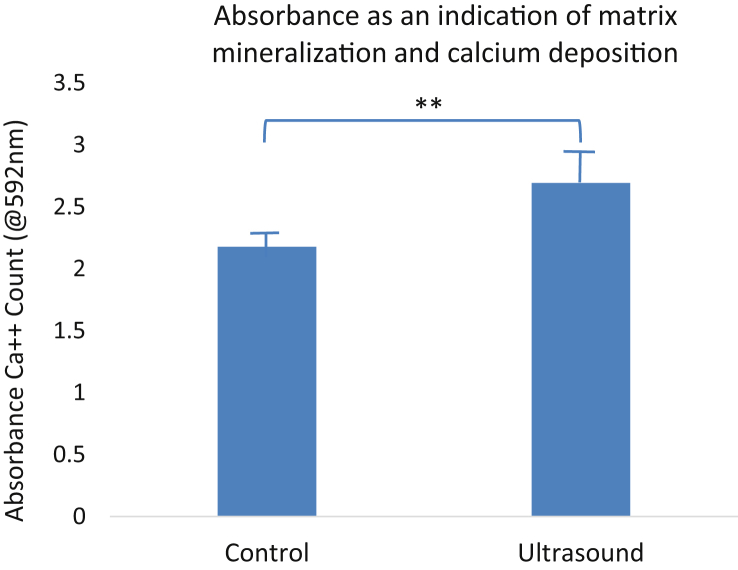
Quantitative data, however, give strong statistical evidence supporting the hypothesised anabolic effect of LIPUS on MC3T3 cells in the bioreactor. High absorbance from this stain reflects an increase in Alizarin Red stain, which measures calcium deposition. This quantification was achieved through comparison of our results with an Alizarin Red standard curve in 10% CPC and normalised to the total number of cells. 2.18 is the absorbance average of the two control groups; 2.69 is the average absorbance of the two LIPUS groups (Fig. 4). LIPUS treatment showed a significantly higher absorption than the control group (Fig. 4). These data support that LIPUS treatment increased the matrix mineralisation in MC3T3 cell cultures seeded in scaffolds under conditions of the bioreactor.
Figure 4.
LIPUS increases matrix mineralization after 5 days of stimulation. Alizarin Red quantification demonstrated a 62% increase in extracellular matrix calcific depositions measured by a significant increase in absorbance - 2.18 control vs. 2.69 ultrasound (**p < 0.001).
LIPUS = low-intensity pulsed ultrasound.
Discussion
Past studies on LIPUS have demonstrated its anabolic effect both in vivo and in vitro [32]. Stimulating the mechanoreceptors of bone cells, both osteoclasts and osteoblasts, has proven to be just as important as maintaining biochemical/hormonal signal transduction homoeostasis [33]. This concept led us to the expectation that ultrasound should enhance cell proliferation and mineralisation. The statistically significant results of the Alizarin Red stain agreed with our hypothesis regarding the anabolic actions of LIPUS treatment. Although qualitative analysis from the picture could not help us truly differentiate which plate had calcium deposits, it was suggested from quantitative analysis that the LIPUS treatment increased matrix mineralisation in the MC3T3 cells under conditions of the bioreactor.
The discrepancy between cell proliferation and matrix mineralisation can be due to a range of factors. The additional shear stress and deformation of the cell membranes may not have been well favoured in a bioreactor environment, where the cells are already experiencing such deformation from a fluid flow shear stress. This could potentially explain why fewer cells were able to survive or proliferate. There must be some limit to cell membrane deformation that the MC3T3 can survive before it starts to become harmful. However, for those that were able to survive, the additional acoustic streaming in the interstitial fluid of the MC3T3 cells should explain the increase in matrix mineralisation as LIPUS increases activation of downstream activators [21]. Furthermore, from previous 2-D osteoblast-like cell studies, ultrasound exposure showed maintenance or increased cell differentiation and enhanced mineralisation [34], [35]. Although this 3-D cell culture has shown significance with LIPUS in promoting cell mineralisation, the cell counts was reduced. Perhaps, MC3T3 is most likely comparable with the 2-D cell dish environment but operates at different optimised differentiation duration in the 3-D scaffold environment. Future studies would be needed to explore the duration or time-dependent differentiation in such 3-D scaffold conditions. Further research should also concentrate on defining a set upper limit to the cell membrane deformation that a preosteoblastic cell can survive. Furthermore, the sources of deformation should vary in stimulus form as LIPUS- and bioreactor-induced fluid flow shear stress differs. A limitation of this study was the inability to perform multiple tests on the scaffolds with seeded MC3T3 cells to see if unoptimised LIPUS parameters could be responsible for the decreased cell count. We understand that repeated experimentation could further elucidate how matrix mineralisation could increase in the presence of decreased cell count; however, technical difficulties prevent such repetition.
The primary osteogenic assessment was using cell counts and Alizarin Red. Previous studies from our laboratory on ultrasound-regulated stem cell differentiation and mineralisation have shown that ultrasound has the potential to promote both osteogenesis and mineralisation on human mesenchymal stem cells (hMSC), in which analyses of collagen, alkaline phosphatase (ALP), osterix (OSX), receptor activator of nuclear factor kappa-Β ligand (RANKL), runt-related transcription factor 2 (RUNX2), and osteoprotegerin (OPG) have shown significance in cell viability and mineralisation (assessed by Alizarin Red) [18]. While this work was the first attempt to extend the study from 2-D cell culture into 3-D cell motility in the scaffold, the analyses were mainly focused on mineralisation and motility. In the future, we will extend the analyses to include extensive broader assessment.
Conclusion
The objective of this experiment was to study the effects of LIPUS on MC3T3 cells in bovine trabecular bone scaffolds under conditions of dynamic flow bioreactor. It was discovered that LIPUS decreased cell proliferation but significantly increased extracellular matrix mineralisation. Future studies should investigate the role of LIPUS in bioreactor environments to better understand how dynamic fluid flow interacts with LIPUS treatment. From the gathered information, we conclude that LIPUS stimulation was critical in contributing to the mechanical signalling transductions that activated bone enhancement parameters and thus has essential potential to create a change in how we pretreat scaffolds for orthopaedic surgery in the future.
Conflict of Interest
The authors certify that they have no affiliations with or involvement in any organisation/entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Acknowledgment/Funding
This work is supported by the National Institute of Health (AR52379, AR61821) and the National Space Biomedical Research Institute through NASA Cooperative Agreement NCC 9–58. The authors would like to thank Suphannee Pongkitwitoon for her superior advice and training regarding technical methods. The authors would also like to thank Drs. Liangjun Lin and Jesse Muir, and Ms. Alyssa Tuthill for their excellent technical supports.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jot.2018.02.002.
Author contributions
SSM and YXQ conceived and designed the experiments. SSM performed the experiments, analysed the data, contributed reagents/materials/analysis tools, and wrote the article. YXQ edited and reviewed the article.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rendina E., Hembree K.D., Davis M.R., Marlow D., Clarke S.L., Halloran B.P. Dried plum's unique capacity to reverse bone loss and alter bone metabolism in postmenopausal osteoporosis model. PLoS One. 2013;8(3):e60569. doi: 10.1371/journal.pone.0060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faienza M.F., Ventura A., Marzano F., Cavallo L. Postmenopausal osteoporosis: the role of immune system cells. Clin Dev Immunol. 2013;2013:575936. doi: 10.1155/2013/575936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandao C.M., Machado G.P., Acurcio Fde A. Pharmacoeconomic analysis of strategies to treat postmenopausal osteoporosis: a systematic review. Rev Bras Reumatol. 2012;52(6):924–937. [PubMed] [Google Scholar]
- 4.Briot K., Cortet B., Thomas T., Audran M., Blain H., Breuil V. 2012 update of French guidelines for the pharmacological treatment of postmenopausal osteoporosis. Joint Bone Spine. 2012;79(3):304–313. doi: 10.1016/j.jbspin.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Josse R., Khan A., Ngui D., Shapiro M. Denosumab, a new pharmacotherapy option for postmenopausal osteoporosis. Curr Med Res Opin. 2013;29(3):205–216. doi: 10.1185/03007995.2013.763779. [DOI] [PubMed] [Google Scholar]
- 6.Ferreri S.L., Talish R., Trandafir T., Qin Y.X. Mitigation of bone loss with ultrasound induced dynamic mechanical signals in an OVX induced rat model of osteopenia. Bone. 2011;48(5):1095–1102. doi: 10.1016/j.bone.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colaianni G., Cuscito C., Colucci S. FSH and TSH in the regulation of bone mass: the pituitary/immune/bone axis. Clin Dev Immunol. 2013;2013:382698. doi: 10.1155/2013/382698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihara Y., Ueki K., Sotobori M., Marukawa K., Moroi A. Bone regeneration by statin and low-intensity pulsed ultrasound (LIPUS) in rabbit nasal bone. J Cranio-Maxillo-Fac Surg. 2014;42(3):185–193. doi: 10.1016/j.jcms.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Abramowicz J.S., Kremkau F.W., Merz E. [Obstetrical ultrasound: can the fetus hear the wave and feel the heat?] Ultraschall Med. 2012;33(3):215–217. doi: 10.1055/s-0032-1312759. [DOI] [PubMed] [Google Scholar]
- 10.Pounder N.M., Harrison A.J. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48(4):330–338. doi: 10.1016/j.ultras.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N.C.S., Leung K.S., Cheung W.H. Ultrasound as a stimulus for musculoskeletal disorders. Journal of Orthopaedic Translation. 2017;9:52–59. doi: 10.1016/j.jot.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watabe H., Furuhama T., Tani-Ishii N., Mikuni-Takagaki Y. Mechanotransduction activates alpha(5)beta(1) integrin and PI3K/Akt signaling pathways in mandibular osteoblasts. Exp Cell Res. 2011;317(18):2642–2649. doi: 10.1016/j.yexcr.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Blaber E.A., Dvorochkin N., Lee C., Alwood J.S., Yousuf R., Pianetta P. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS One. 2013;8(4):e61372. doi: 10.1371/journal.pone.0061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vorselen D., Roos W.H., MacKintosh F.C., Wuite G.J., van Loon J.J. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014;28(2):536–547. doi: 10.1096/fj.13-236356. [DOI] [PubMed] [Google Scholar]
- 15.Smith S.M., Zwart S.R., Heer M., Hudson E.K., Shackelford L., Morgan J.L. Men and women in space: bone loss and kidney stone risk after long-duration spaceflight. J Bone Miner Res. 2014;29(7):1639–1645. doi: 10.1002/jbmr.2185. [DOI] [PubMed] [Google Scholar]
- 16.Uddin S.M., Qin Y.X. Dynamic acoustic radiation force retains bone structural and mechanical integrity in a functional disuse osteopenia model. Bone. 2015;75:8–17. doi: 10.1016/j.bone.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czarkowska-Paczek B., Wesolowska K., Przybylski J. Physical exercise prevents osteoporosis. Przegl Lek. 2011;68(2):103–106. [PubMed] [Google Scholar]
- 18.Chan B.P., Leong K.W. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17(Suppl 4):467–479. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moshaverinia A., Chen C., Akiyama K., Xu X., Chee W.W., Schricker S.R. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res A. 2013;101(11):3285–3294. doi: 10.1002/jbm.a.34546. [DOI] [PubMed] [Google Scholar]
- 20.Mourino V., Cattalini J.P., Roether J.A., Dubey P., Roy I., Boccaccini A.R. Composite polymer-bioceramic scaffolds with drug delivery capability for bone tissue engineering. Expert Opin Drug Deliv. 2013;10(10):1353–1365. doi: 10.1517/17425247.2013.808183. [DOI] [PubMed] [Google Scholar]
- 21.Uddin S.M., Qin Y.X. Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS One. 2013;8(9):e73914. doi: 10.1371/journal.pone.0073914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uddin S.M., Richbourgh B., Ding Y., Hettinghouse A., Komatsu D.E., Qin Y.X. Chondro-protective effects of low intensity pulsed ultrasound. Osteoarthritis Cartilage. 2016;24(11):1989–1998. doi: 10.1016/j.joca.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mundi R., Petis S., Kaloty R., Shetty V., Bhandari M. Low-intensity pulsed ultrasound: fracture healing. Indian J Orthop. 2009;43(2):132–140. doi: 10.4103/0019-5413.50847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padilla F., Puts R., Vico L., Raum K. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics. 2014;54(5):1125–1145. doi: 10.1016/j.ultras.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Zia Uddin S.M., Cheng J., Lin W., Qin Y.X. Low-intensity amplitude modulated ultrasound increases osteoblastic mineralization. Cell Mol Bioeng. 2011;4(1):81–90. doi: 10.1007/s12195-010-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David V., Guignandon A., Martin A., Malaval L., Lafage-Proust M.H., Rattner A. Ex Vivo bone formation in bovine trabecular bone cultured in a dynamic 3D bioreactor is enhanced by compressive mechanical strain. Tissue Eng Part A. 2008;14(1):117–126. doi: 10.1089/ten.a.2007.0051. [DOI] [PubMed] [Google Scholar]
- 27.Botchwey E.A., Pollack S.R., Levine E.M., Laurencin C.T. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J Biomed Mater Res. 2001;55(2):242–253. doi: 10.1002/1097-4636(200105)55:2<242::aid-jbm1011>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimko D.A., Shimko V.F., Sander E.A., Dickson K.F., Nauman E.A. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J Biomed Mater Res B Appl Biomater. 2005;73(2):315–324. doi: 10.1002/jbm.b.30229. [DOI] [PubMed] [Google Scholar]
- 29.Cartmell S.H., Porter B.D., Garcia A.J., Guldberg R.E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9(6):1197–1203. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 30.Reher P., Elbeshir el N.I., Harvey W., Meghji S., Harris M. The stimulation of bone formation in vitro by therapeutic ultrasound. Ultrasound Med Biol. 1997;23(8):1251–1258. doi: 10.1016/s0301-5629(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 31.Shahabipour F., Mahdavi-Shahri N., Matin M.M., Tavassoli A., Zebarjad S.M. Scaffolds derived from cancellous bovine bone support mesenchymal stem cells' maintenance and growth. In Vitro Cell Dev Biol Anim. 2013;49(6):440–448. doi: 10.1007/s11626-013-9591-7. [DOI] [PubMed] [Google Scholar]
- 32.Mostafa N.Z., Uludag H., Dederich D.N., Doschak M.R., El-Bialy T.H. Anabolic effects of low-intensity pulsed ultrasound on human gingival fibroblasts. Arch Oral Biol. 2009;54(8):743–748. doi: 10.1016/j.archoralbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Thompson W.R., Rubin C.T., Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503(2):179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uddin S.M., Hadjiargyrou M., Cheng J., Zhang S., Hu M., Qin Y.X. Reversal of the detrimental effects of simulated microgravity on human osteoblasts by modified low intensity pulsed ultrasound. Ultrasound Med Biol. 2013;39(5):804–812. doi: 10.1016/j.ultrasmedbio.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia P., Wang X., Qu Y., Lin Q., Cheng K., Gao M. TGF-beta1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res Ther. 2017;8(1):281. doi: 10.1186/s13287-017-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.