Abstract
Background
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder that lacks any disease-modifying drug for the prevention and treatment. Edaravone (EDR), an approved free radical scavenger, has proven to have potential against AD by targeting multiple key pathologies including amyloid-beta (Aβ), tau phosphorylation, oxidative stress, and neuroinflammation. To enable its oral use, novel edaravone formulation (NEF) was previously developed. The aim of the present investigation was to evaluate safety and efficacy of NEF by using in vitro/in vivo disease model.
Materials and methods
In vitro therapeutic potential of NEF over EDR was studied against the cytotoxicity induced by copper metal ion, H2O2 and Aβ42 oligomer, and cellular uptake on SH-SY5Y695 amyloid-β precursor protein (APP) human neuroblastoma cell line. For in vivo safety and efficacy assessment, totally seven groups of APP/PS1 (five treatment groups, one each as a basal and sham control) and one group of C57BL/6 mice as a positive control for behavior tests were used. Three groups were orally treated for 3 months with NEF at an equivalent dose of EDR 46, 138, and 414 µmol/kg, whereas one group was supplied with each Donepezil (5.27 µM/kg) and Soluplus (amount present in NEF of 414 µmol/kg dose of EDR). Behavior tests were conducted to assess motor function (open-field), anxiety-related behavior (open-field), and cognitive function (novel objective recognition test, Y-maze, and Morris water maze). For the safety assessment, general behavior, adverse effects, and mortality were recorded during the treatment period. Moreover, biochemical, hematological, and morphological parameters were determined.
Results
Compared to EDR, NEF showed superior cellular uptake and neuroprotective effect in SH-SY5Y695 APP cell line. Furthermore, it showed nontoxicity of NEF up to 414 µM/kg dose of EDR and its potential to reverse AD-like behavior deficits of APP/PS1 mice in a dose-dependent manner.
Conclusion
Our results indicate that oral delivery of NEF holds a promise as a safe and effective therapeutic agent for AD.
Keywords: edaravone, Soluplus, dose–response relationship, APPSwe/PS1deE9 mice, learning and memory, safety assessment
Introduction
Due to modern advancement in medical technology to combat dreadful diseases and providing a high quality of life in the 21st century, the aging population is increasing.1 Alzheimer’s disease (AD), the most common cause of dementia, is a chronic neurodegenerative disease linked to the progressive cognitive deficits specifically in elder community with a sufficient severity to compromise an individual’s daily function.2 It causes huge social and economic burden such as the estimated cost of care for global patients of AD and dementia is equivalent to 1% global gross domestic product.3 Currently, 47 million global people live with dementia, and this figure is projected to rise to 74 million by the year 2030.4 Even after a century of its discovery, effective therapy is still not available which could prevent, cure, and even stop its progression.5 Drug development for AD has also become a holy grail as 99.6% failure rate is encountered in clinical trials.6 The complexity of the disease is the main reason for several failures in clinical trials due to the involvement of multiple pathways in the development and progression of the disease such as amyloid-beta (Aβ) plaques, phosphorylated tau neurofibrillary tangles, oxidative stress, neuroinflammation, mitochondrial dysfunctions, glial activation, synaptic dysfunction, and neurotransmission-related mechanisms.2,7,8 Most mechanisms form vicious cycles to accelerate the disease progression. Thus, targeting multiple key pathways of AD pathogenesis is the key to the successful therapeutic outcome.2,9
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one, MCI-186, EDR) is a free radical scavenger, developed by Mitsubishi Tanabe Pharma Corporation (Osaka, Japan). It has been approved for the treatment of acute cerebral infarction in Japan and amyotrophic lateral sclerosis (ALS) in the USA and Japan.10,11 The protective effect of EDR against AD-like insults in neuroblastoma N2a cells was reported by attenuating cytochrome c release, suppressing the activation of caspase-3 and decreasing the Bax/Bcl-2 ratio.12 Also, its inhibitory effect on amyloid-β precursor protein (APP) processing and enhancing nonamyloidogenic pathway for the regulation of Aβ production by using human “Swedish” APP mutation APP695 (SY5Y-APP695swe) was shown by downregulating beta-secretase (BACE1) in a dose-dependent manner.13 Also, its efficacy in streptozotocin-induced and Aβ-induced rat models have been demonstrated by improvement against the cognitive dysfunction.14–16 Moreover, its therapeutic potential in double transgenic (Tg) APPSwe/PS1deE9 (APP/PS1) mice after 3 months of treatment was revealed by targeting multiple pathologies such as Aβ, oxidative stress, tau hyperphosphorylation, synaptic dysfunction, neuronal loss, neuroinflammation, and glial activation and improving the behavioral deficits.2 The dose-dependent efficacy assessment of EDR is yet to be explored as it showed a dose-dependent effect against cerebral arachidonate cascade,17 cardioembolic stroke,18 and neonatal hypoxic-ischemic encephalopathy.19
Among the various routes of drug delivery, the oral route is accepted as the most favored by the patients as well as clinicians. The poor oral bioavailability (BA) due to poor aqueous solubility, stability, dissolution, and permeability has been reported for EDR. It was considered as one of the potential reasons for the failure of a number of potential drugs including curcumin in clinical trials.20,21 Thus, there is a need for the appropriate oral formulation to conduct the clinical trials for its safety and efficacy study at a large scale for the development of EDR as a drug for AD-like chronic diseases. Several efforts have been reported for the improvement of its oral BA; however, these investigational findings have not yielded significant success for effectively translating their use for clinical purpose.11,22–24 The novel edaravone formulation (NEF) was developed in part I of this study, by using the strategy called self-nanomicellizing solid dispersion which is the combination of solid dispersion and nanomicelles strategies. There was a significant dose-dependent enhancement of oral BA of 10.2, 16.1, and 14.8-fold compared to EDR suspension for 46, 138, and 414 µM/kg doses of EDR. The dramatic improvement of solubility, stability, dissolution, intestinal permeability, and inhibitory effect on glucuronidation contributed to the exceptional outcome. Previously, several nanoformulations of drugs showed significantly better efficacy than the crude drugs for AD probably by improving oral BA.25,26 Therefore, we assessed the efficacy of NEF in AD mouse model in the second part of the study in the present paper.
AD is a progressive and irreversible brain disease in which a steady decline in cognitive, behavioral, and physical abilities is the key clinical manifestation. Memory loss is one of the main clinical manifestations of AD; hence, the efficacy of any potential intervention candidates could be judged based on its ability to prevent or restore learning and memory ability.27 Furthermore, progressive cognitive impairment is always accomplished with the progression of the pathogenesis of AD such as Aβ accumulation, downstream pathological events such as oxidative stress, tau hyperphosphorylation, glial activation, neuroinflammation, neuronal loss, and synaptic dysfunction.28 The potential therapeutic candidates that significantly inhibit the downstream pathologies could improve the behavioral deficits of APP/PS1 mice.2 Thus, the improvement in behavioral deficits was considered as a marker for the efficacy of NEF and evaluated by well-accepted behavioral tests such as open-field, novel objective recognition test (NORT), Y-maze, and Morris water maze (MWM) tests.2,29 The information regarding the toxicity of EDR via oral administration and Soluplus® (SOL)-based preparation are very limited, thus it is necessary to evaluate the safety of NEF to provide guidance for clinical applications.
The work reported here aimed to evaluate the safety and efficacy of NEF in vitro with SH-SY5Y695 APP human neuroblastoma cell line, followed by in vivo using transgenic AD mouse models in a dose-dependent manner for long-term exposure (3 months) via the oral route.
Materials and methods
Materials
EDR made by Aldrich Chemical Co with 99% purity was bought from Aladdin Industrial Corporation (Shanghai, China). SOL was gifted from BASF Australia Ltd (Victoria, Australia). DMEM, fetal bovine serum (FBS), penicillin, streptomycin, and L-glutamine were purchased from Life Technology (Victoria, Australia). Acetic acid was obtained from Chem Supply (South Australia, Australia). High-performance liquid chromatography-grade ethanol and methanol were purchased from Merck (Victoria, Australia). High purity (Milli-Q) water from Millipore Ultra-Pure Water System (Millipore, North Ryde, NSW, Australia) was used throughout the study. All other reagents were of analytical grade.
In vitro neuroprotection assay
MTT assay
Human neuroblastoma cell line SH-SY5YAPP695 was used to evaluate the efficacy of EDR and NEF against cytotoxicity induced by CuSO4, H2O2, and Aβ42 following the protocol reported in our previous study.22 Briefly, SH-SY5YAPP695 cells were seeded in a 96-well plate by using DMEM containing FBS (10%), L-glutamine (2 mM), penicillin (50 IU/mL), and streptomycin (50 IU/mL) in a humidified incubator accompanied with 95% air and 5% CO2 at 37°C. The cells were treated with CuSO4 (0.5 µM,) H2O2 (50 µM), and A-β 42 (1 µM) to induce cytotoxicity. The dosage of EDR and NEF (3 µM and equivalent dose to 3 µM of EDR) was decided based on our previous study.2,22 After incubation time (19 h), MTT reagent (25 µL) was added and incubated at 37°C for an hour. After discarding MTT reagent, dimethyl sulfoxide (200 µL) was used to solubilize the intracellular formazan crystals, and the color intensity was measured at 570 nm by using multi-well scanning spectrophotometer (WALLAC 1420; PerkinElmer Inc, Waltham, MA, USA).
In vitro cellular uptake study
The cellular uptake of EDR and NEF in SH-SY5YAPP695 cells was studied as per the protocol reported in our previous publication.22 After seeding cells with density 2×105 cells/mL in a 6-well plate, cells were treated with EDR (3 mM) and NEF containing equivalent concentrations of EDR (3 mM) for 0.5 and 2 h. Later, the cells were washed three times with cold PBS, followed by lysing by using radioimmunoprecipitation assay buffer. Liquid chromatography-mass spectrometry was used to quantify EDR in order to determine the cellular uptake at 0.5 and 2 h.
Behavioral tests
Suitability of drinking water to supply NEF
The behavior of animals could be adversely affected due to daily gavage or injection for 3 months. Supplying with drinking water was considered as the best way to perform long-term safety and efficacy study. The acidic water (pH of 2.5–3.0) is provided to the animals to prevent the spread of bacterial disease.30,31 Thus, stability study was conducted to investigate the suitability of drinking water to supply NEF. To determine the stability of NEF, 300 mL of the formulation at the concentration equivalent to 0.27 (46 µM/kg), 0.82 (138 µM/kg), and 2.48 mM (414 µM/kg) of EDR using NEF was prepared and filled in the water bottles. The dosage was determined by the measurement of the volume of water consumed by the mice prior to drug delivery. These bottles were stored at room temperature in the animal house facility. The samples were collected at predetermined time intervals and loaded in high-performance liquid chromatography for further analysis after dilution and filtration.
Animals
APP/PS1 transgenic mice with C57BL/6 background was procured from Jackson Laboratory, Inc (West Grove, PA, USA). The animal ethics committee of the University of South Australia approved animal ethics for breeding procedures and the experiments under South Australian Animal Welfare Act 1985. The breeding was done in the Reid animal facility of the University of South Australia. The standard conditions were maintained, which include standard light/dark cycles of 12 h each, the temperature of 22°C±1°C, and humidity of 52%±2%. All the animals were assessed for their health, general behavior, and appearance to confirm the suitability for the whole experiment.
Based on our previous publications, SD was determined within the 20% of means. Thus, sample size (n=8–12) was calculated for the detection of the difference of 20% between groups at beta level of 80% and alpha at 5%.2,29 The APP/PS1 (at the age of 14 months) was randomly divided into seven groups mentioned in Table 1. The group of 17-month-old wild-type (WT) C57BL/6 mice supplied with normal drinking water was utilized as a positive control for the behavioral tests. The required amount of SOL and NEF were dissolved in drinking water, filtered, and supplied to animals as per the requirement. For donepezil (DNP), Aricept tablet (Pfizer, Inc, New York, NY, USA) was crushed using mortar and pestle and mixed with the required quantity of drinking water, filtered, and supplied to the animals. Open-field, NORT, Y-maze, and MWM tests were conducted to investigate the effect of 3 months of treatment of NEF on change in the behavioral phenotype of APP/PS1 compared to the control groups. All behavioral tests were carried out in isolated behavior room in the Reid animal facility. To acclimate to the environment, animals were moved to the behavior room 1 h before initiating the test. ANY-maze video tracking software (Stoelting Co., Wood Dale, IL, USA) and a digital camera were used to record the animal behaviors as per the previous reports.2,29,32 The persons who did behavioral tests were blind to the group identities, and all data were analyzed according to precoded labels. The results were revealed after the data analysis was completed.
Table 1.
Experimental design for behavioral tests with mouse models of AD
| Groups | Treatment | Dose | APP/PS1 mice
|
||
|---|---|---|---|---|---|
| Identity | Age (months) | Sample size (n) | |||
| Normal control | Normal drinking water | – | 17 mo WT Ctrl | 17 | 8 |
| Basal control | Normal drinking water | – | 14 mo Tg Ctrl | 14 | 11 |
| Sham control | Normal drinking water | – | 17 mo Tg Ctrl | 17 | 12 |
| BC | SOL | – | 17 mo Tg BC | 17 | 10 |
| NEF treated | NEF LD | 46 µM/kg | 17 mo Tg NEF LD | 17 | 11 |
| NEF treated | NEF MD | 138 µM/kg | 17 mo Tg NEF MD | 17 | 12 |
| NEF treated | NEF HD | 414 µM/kg | 17 mo Tg NEF HD | 17 | 9 |
| DNP | DNP | 5.27 µM/kg | 17 mo Tg DNP | 17 | 9 |
Abbreviations: AD, Alzheimer’s disease; LD, low dose; MD, medium dose; HD, high dose; Tg, transgenic mice; DNP, donepezil; NEF, novel edaravone formulation; BC, blank control; WT Ctrl, wild-type control; SOL, Soluplus.
Measurement of water and food consumption
To determine the appropriate dosing, average water and food intake of the animals were recorded. The animals were divided into different groups, and average water and food consumption was documented up to 14 days before and after the addition of NEF to confirm the appropriate dosing. The initial amount and the amount left at the end of the day were recorded to get the amount consumed over a period.
Open-field test
The white square open arena with an area of 40×40×40 cm was used for the test. Each animal was placed gently at the center of the arena, letting them move freely for the first 5 min. Each test lasts for about 10 min, and the arena was cleaned by using 70% ethanol and water after each trial. In this test, a number of various conventional and ethological parameters were recorded during each session including 1) spontaneous locomotor activity assessed by the entire distance covered and the number of rearing and 2) anxious behavior determined by the % time spent in the central arena, the number of grooming, defecation, and urination.32
Novel objective recognition test
NORT test assesses the mice’s ability to identify the novel object in the environment. The experiment was carried out in the same arena used in open-field test. The test was conducted in the three different phases that include 1) habituation; 2) familiarization; and 3) test phase. During habituation phase, each mouse was allowed to explore the whole arena without any objects. The mouse was then removed from the arena and put back into the holding cage. In the familiarization phase, the mouse was kept for some time in the open arena consisting of two identical objects (a+a). In the final testing phase, one of the identical objects was replaced with the novel object and the mouse was then kept in the white open arena composed of one identical and one novel object (a+b). In the second and third phases, the objects were kept in the opposite direction and 15 cm away from each other. Here, the time spent close to the familiar object versus the time spent close to the novel object on days 2 and 3 were recorded to generate the recognition and discrimination index.33 The ratio of time spent exploring one object over the total time spent in exploring both the objects was calculated as a recognition index. The equation [DI=(TN−TF)/(TN+TF)], where time spent to explore the novel object (TN) and familiar object (TF) was used to calculate discrimination index.
Y-maze test
Y-maze spontaneous alteration test was carried out to assess the willingness of mice to discover new environment. An immediate spatial working memory was evaluated by using a Y-shaped maze composed of three opaque arms at the angle of 120° from each other as per the previous study.2 The mouse was kept at the center of the opaque block and allowed to walk freely during 5-min session. The movement of each mouse on the opaque block was visually recorded. Alteration means successive entry to each arm when performed. The % alteration was calculated based on the actual alterations to possible alterations. For the novel arm exploration, the mouse was kept on any of the two arms and allowed to walk through the opaque block during the 5 min of the first session. In the second session, the mouse was allowed to explore all three arms and to discover the novel arm. The total number of entries in the novel arm and the time spent on the novel arm were recorded.
MWM test
A circular arena of 120 cm diameter and 45 cm height was used to carry out the MWM test as per the previous reports.2,32 The test apparatus was filled up to the height of 30 cm with water having a temperature of 23°C–25°C. The water was made cloudy using a nontoxic white dye prior to initiating the test. The MWM test pool was theoretically divided into four different parts. In one of the four quadrants, a white platform of 7.5 cm diameter was placed, and others were marked in accordance with the testing quadrant with left, right, and opposite. The mice were allowed to freely swim in the pool for ~1 min so as to adjust themselves in the testing environment (habituation phase). Here, the mice were trained in two phases known as visible and hidden phases. In the visible phase, the platform was 0.5 cm above the water level, whereas in the hidden phase, the platform was below 0.5 cm water level. Here, the training to find out a visible platform was given for 1 day (60 s/trial, 3 trials/d), and for the hidden platform, the training was given for 4 days (60 s/trial, 3 trials/d). The animals were allowed to swim from each quadrant except the quadrant closer to the platform during each trial for up to 60 s. They were led to the platform if they could not find it by themselves within 60 s and allowed to be there for 30 s. Moreover, they were allowed to be on the platform even if they find it in 60 s. The probe test (single trial of 60 s) without a platform for each mouse was performed on the last day of the study. The escape latency curve, swimming speed, and path length (habituation phase) in platform trials, while a number of annulus crossing and time spent in target quadrant compared to all other quadrants were recorded.
Assessment of long-term repeated dose toxicity
Body weight
Once the treatment was initiated, the weight of each mouse was documented on daily basis for up to 2 weeks and then on weekly basis.
Water and food consumption
Food and water consumption (g/d/10 g body weight) were recorded during the whole treatment period on weekly basis.
General behavior and mortality
All the mice were monitored for any abnormal behavior and symptoms every day such as mortality or morbidity, dull/ruffled coat, hunched posture, reluctant to move, dehydration by skin tent, diarrhea/abnormal bloating, changes in skin and fur, eyes and mucous membranes, and behavior patterns.34 The clinical record sheet for each group was maintained for everyday and was recorded up to 3 months. The individual clinical record sheet was used for mice with abnormal behavior or symptoms for close monitoring. The weight loss of >10% was used as a standard for humane killing under veterinary advice.
Hematological parameters
To investigate the different hematological parameters, the blood samples were collected in the EDTAK2-coated vials at the end the experiment. A fully automated five-part differential hematology analyzer (Sysmex XN550, Sysmex Europe GmbH, Norderstedt, Germany) was used for the analysis of hematological parameters such as white blood cells, red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration, platelets (PLT), mean platelet volume, platelet hematocrit, platelet distribution width, neutrophils, lymphocytes, monocytes, eosinophils, and basophils.35
Coagulation parameters
Different coagulation parameters including prothrombin time (PT) and activated partial thromboplastin time (APTT) were measured manually by using blood plasma. For PT estimation, the Innovin reagent was preheated at 37°C by using a water bath. 100 µL of plasma was taken in the glass test tube. In the glass test tube with plasma, 200 µL of the previously heated Innivon reagent was added, and the time for the fibrin strand formation was recorded. In case of APTT determination, APTT reagent and 0.025 M calcium chloride were preheated at 37°C. 100 µL of plasma sample was mixed with 100 µL of APTT reagent in the glass test tube. The resultant mixture was then incubated for 180 s at 37°C. In the above mixture, 100 µL of the calcium chloride was added and the time to start the fibrin strand formation (first appearance of the fibrin) was documented at 37°C. Analysis of each sample was performed in triplicate.
Serum biochemistry
An automatic biochemistry meter (SELECRTA-E; Vital Scientific, Van Rensselaerweg, AV Spankeren, the Netherlands) was used to measure the different biochemical parameters such as alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, amylase, lipase, gamma-glutamyl transferase, sodium, bicarbonate, anion gap, potassium, calcium, sodium:potassium ratio, chloride, inorganic phosphorus, calcium:phosphorus ratio, albumin, total protein, globulin, total bilirubin, creatinine, urea nitrogen, glucose, and total cholesterol.
Organ weight
Each animal was euthanized by carbon dioxide inhalation and perfused intracardially with cold PBS (pH 7.4) after completing the experiment. Different organs including brain, stomach, small intestine (SI), large intestine (LI), liver, kidneys, spleen, and heart were collected and weighed individually.
Histopathology
The histopathological examination was performed on all the collected tissues including brain, stomach, SI, LI, liver, kidneys, spleen, heart, and lungs. The tissues were fixed in 10% formalin and subsequently processed in Fully Enclosed Tissue Processor (Leica ASP300, Leica Microsystems Pty Ltd, Mt Waverley, VIC, Australia) with a routine schedule of treatments of 70% ethanol, 90% ethanol, absolute ethanol, xylene, and paraffin wax (Table S1). The tissues were embedded in the paraffin (Leica EG 1160) prior to taking standard sections via Microm Microtome and histology water bath (Leica HI1210). The sections were then stained with H&E dye and observed under the microscope (Olympus CX 41; Olympus Corporation, Tokyo, Japan).36
Statistical analysis
Mean and SD or mean and standard error of the mean was used to express all values. The statistical analysis of data was conducted by using GraphPad Prism 6 (GraphPad Software, Inc, La Jolla, CA, USA) for normal distribution first by using Shapiro–Wilk test followed by Student’s t-test for two groups, one way and two-way analysis of variance for multiple groups. P-values <0.05 were considered significant.
Results and discussion
In vitro neuroprotection assay
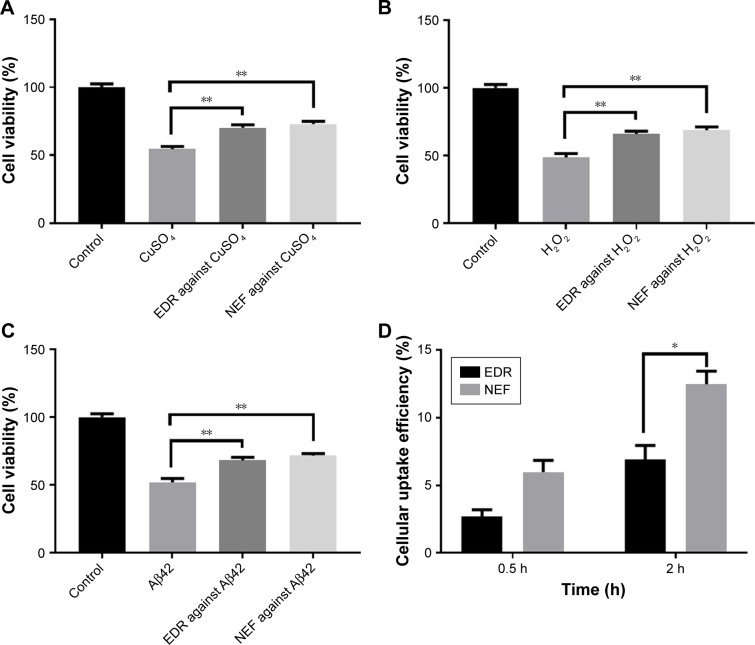
Therapeutic efficacy of EDR and NEF was investigated by using human neuroblastoma cell line which is most widely accepted to test the neuroprotection in vitro.22 In AD pathogenesis, the viability of neuron is significantly affected with cytotoxicity induced by hydrogen peroxide, copper metal ions, and Aβ42.2,22 The statistically significant neuroprotection action was observed in the treatment group EDR and NEF compared to nontreated group against cytotoxicity by copper metal ions (Figure 1A; Table S2), H2O2 (Figure 1B; Table S3), and Aβ42 (Figure 1C; Table S4). Moreover, NEF showed better neuroprotection compared to EDR; however, it was nonsignificant statistically. The results of cellular uptake demonstrated that NEF was internalized more efficiently compared to crude EDR at 0.5 and 2 h (Figure 1D; Table S5). The results of significant enhancement in cellular uptake and neuroprotective effect of NEF demonstrated that NEF may be useful as a potential anti-Alzheimer drug and considered for further evaluation in vivo.
Figure 1.
In vitro safety and efficacy of NEF compared with EDR in SH-SY5Y695 cell line.
Notes: Effect of EDR and NEF on cell viability in the presence of CuSO4 (A), H2O2 (B), and Aβ42 (C). In vitro cellular uptake efficiency of NEF and EDR in SH-SY5Y695 cell line after incubating for 0.5 and 2 h (D) (mean±SE, n=3). *P<0.05 and **P<0.01. One and two-way ANOVA and Sidak’s multiple comparisons test. Data for EDR were adapted from the earlier report.22
Abbreviations: NEF, novel edaravone formulation; EDR, edaravone; ANOVA, analysis of variance.
Behavior tests
The purpose of the study was to determine the therapeutic potential of NEF against AD, and for that, suitable animal model, age, duration of treatment, and evaluation tests were the key requirements. The ideal animal model should closely mimic the symptoms and neuropathology of AD disease. APP/PS1 mouse model was created by expressing variants of chimeric human APP and a mutant human presenilin 1.37 It is well accepted to investigate the therapeutic potential candidates against AD because it showed key characters such as cognitive dysfunction as a result of pathological changes manifested by senile plaques, neuroinflammation, and neuronal loss. The potential therapeutic candidates such as EDR,2 docosahexaenoic acid,38 Puerarin,39 and p75NTR ectodomain protein29 ameliorated the cognitive dysfunction in APP/PS1 mice by improving AD-like pathology. Therefore, the efficacy of NEF against AD was evaluated based on its potential to improve learning and memory in AD mice. In addition, the age-dependent cognitive decline was observed with APP/PS1 mouse models; hence, selection of suitable age is critical. The 14 months of age for APP/PS1 mice as a starting point because of the fully developed AD-like pathology and behavioral deficits similar to the clinical AD was observed in the previous studies.28 Moreover, due to the chronic nature of AD, 3 months of the treatment period was selected as appropriate for efficacy evaluation from the previous report.2 As an improvement of learning and memory of APP/PS1 mice used as a marker in evaluating efficacy against AD, a number of behavior tests were selected to assess the motor function (open field), anxiety-related behavior (open field), and cognitive function (NORT, Y-maze, and MWM). APP/PS1 mice showed AD-like behavioral deficits in the open-field, NORT, Y-maze, and MWM tests.2,29,40 A group of C57BL/6 WT mice was used as a normal aging control as APP/PS1 mice were genetically modified mice with the background of C57BL/6. One group of APP/PS1 mice aged 14 months was considered as a basal control, which had undergone for the behavioral tests at the starting point of treatments. Other groups of mice were used as a negative control without any treatment, blank formulation control (without EDR), and DNP-positive control group (for details of grouping designs, see Table 1). All behavioral tests were conducted at 17 months similar to NEF treatment groups, except the basal control group which was done at 14 months. The data of control groups such as 17 mo WT Ctrl, 14 mo Tg Ctrl, 17 mo Tg Ctrl, 17 mo Tg BC, and 17 mo Tg DNP are used in other similar projects to reduce the utilization of an overall number of animals as per the animal ethics guideline.
Daily injection or gavage for 3 months could affect the behaviors of mice and impact on the outcome as well as a welfare issue. Therefore, mixing of NEF with drinking water was selected to allow voluntary oral administration of NEF, Blank formulation and DNP. In animal house facility, acidic drinking water was used to avoid the spreading of bacterial infections among the animals in a cage.30,31 Thus, evaluating the stability of NEF in acidic drinking water was performed to determine its suitability as an administration technique and frequency of changing the water bottle. The frequency of changing water bottle in our animal house facility was weekly as per the standard; hence, stability study was conducted for 7 days. Less than 1% decrease in EDR content with all three doses of NEF was detected in 1 week, which confirmed the suitability of mixing NEF with acidic drinking water as a way of administration. The concentration of NEF, SOL, and DNP in acidic drinking water was calculated for appropriate dosing as per the data of average water consumption and weight of the mice mentioned in Table 2. The amount of water intake was used to calculate the dose for the preparation of drinking water mixed with the different doses of NEF, blank formulation, and DNP.
Table 2.
Measurement of food and water consumption and body weight of Tg mice (mean±SD, n=5)
| Parameter | Time | APP/PS1 mice
|
||
|---|---|---|---|---|
| 17 mo Tg Ctrl | 17 mo Tg BC | 17 mo Tg NEF HD | ||
| Food intake (g/d/10 g body weight) | Before addition (14 months)* | 1.34±0.19 | 1.36±0.26 | 1.38±0.25 |
| After addition (14 months)+ | – | 1.33±0.22 | 1.33±0.31 | |
| At 17 months | 1.22±0.31 | 1.24±0.22 | 1.19±0.25 | |
| Water intake (g/d/10 g body weight) | Before addition (14 months)* | 1.42±0.42 | 1.41±0.32 | 1.43±0.32 |
| After addition (14 months)+ | – | 1.48±0.24 | 1.52±0.34 | |
| At 17 months | 1.25±0.20 | 1.27±0.28 | 1.23±0.27 | |
| Body weight (g) | Before addition (14 months)* | 33.13±3.24 | 33.9±4.82 | 32.81±2.59 |
| After addition (14 months)+ | – | 34.6±3.45 | 34.32±2.99 | |
| At 17 months | 34.15±3.13 | 34.24±3.17 | 34.58±3.12 | |
Notes:
Before addition of treatment such as blank formulation (17 mo Tg BC) and NEF (17 mo Tg NEF HD).
After addition of treatment such as blank formulation (17 mo Tg BC) and NEF (17 mo Tg NEF HD).
Abbreviations: Tg, transgenic mice; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; NEF, novel edaravone formulation.
Open-field test
The locomotor and exploration activity (measuring total distance traveled and the number of rearing) and anxiety behaviors (% time spent in the central zone and number of rearing, grooming, urination, and defecation) of all APP/PS1 mice were evaluated by open-field test.29 There was a significant decrease in locomotor and exploration activity as well as high level of anxiety observed at 14 and 17 months of age of APP/PS1 mice without any treatment and with blank formulation treatment compared to WT control mice. The treatment groups with NEF displayed dose-dependent increase in the locomotor activity, which revealed a statistically significant increase in total distance traveled (Figure 2A; Table S6) and number of rearing (Figure 2B; Table S6). Moreover, mice treated with NEF with middle dose (MD) and high dose (HD) but not low dose (LD) spent significantly longer time in the central zone (Figure 2C; Table S6), which indicated the lowering of anxiety-type behavior. Also, NEF-treated mice showed decreased but not statistically significant number of grooming (Figure 2D; Table S6), urination (Figure 2E; Table S6), and defecation (Figure 2F; Table S6). Besides, the DNP-treated mice showed similar results to the mice treated with NEF LD, but NEF MD- and HD-treated mice revealed better performance than DNP-treated mice. There was no statistically significant difference in locomotor and exploration activities and anxiety behaviors of NEF-treated mice with standard WT mice, suggesting a potential of NEF treatment on the reversal of the behavioral deficits to the normal level. It is worthy to note that the NEF groups with all dosages performed better than the basal control group at 14 months on the total travel distance, number of rearing, and the time spent in the central zone in the open-field test (Figure 2A–C), suggesting that the NEF treatment can reverse the mood status of aged AD mice to a much younger age.
Figure 2.
Results of open-field test.
Notes: Total distance traveled (A), number of rearing (B), time spent in central zone (C), number of grooming (D), number of urination (E), and number of defecation (F). *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 (one-way ANOVA, Tukey’s test) (mean±SEM).
Abbreviations: NEF, novel edaravone formulation; 17 mo WT Ctrl, 17-month-old normal wild-type control mice; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 14 mo Tg Ctrl, 14-month-old transgenic mice as a basal control; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF LD, 17-month-old transgenic mice treated with low dose of NEF; 17 mo Tg NEF MD, 17-month-old transgenic mice treated with medium dose of NEF; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; 17 mo Tg DNP, 17-month-old transgenic mice treated with high dose of donepezil; SEM, standard error of the mean; ANOVA, analysis of variance.
Novel objective recognition test
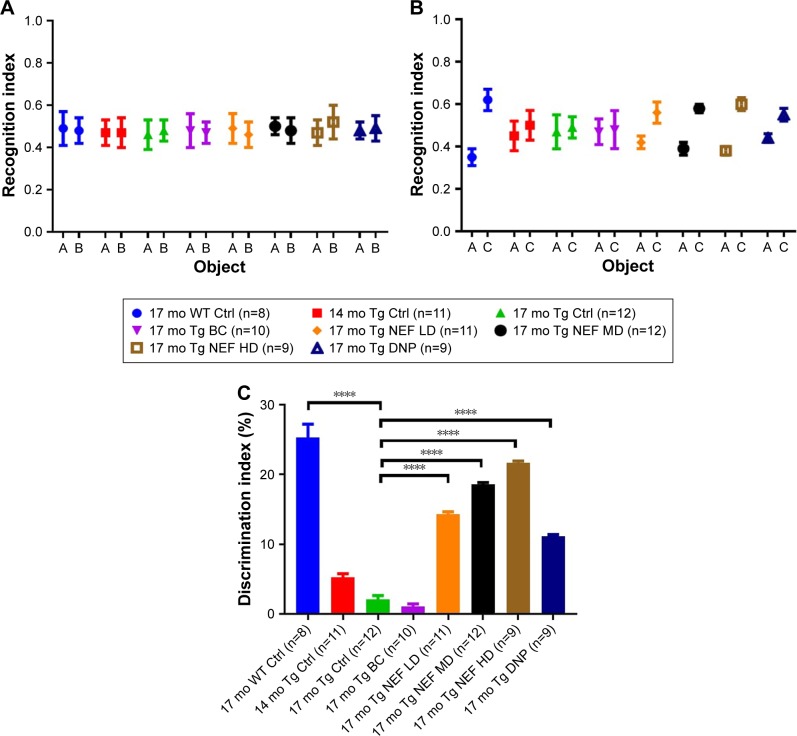
The NORT reflects the learning and memory of mice based on their natural tendency of exploring novel object instead of familiar one after exposed to a novel environment.41 The poor performance of APP/PS1 in NORT made it an ideal study to examine the potential of intervention therapy for AD.42 All APP/PS1 and WT mice showed statistically insignificant difference in recognition index in the phase of familiarization (Figure 3A; Table S7). In the test phase, the 17-month-old APP/PS1 mice without any treatment and with blank formulation treatment showed no tendency to explore the novel object, while NEF-treated mice presented significantly higher and dose-dependent increase in the discrimination index (Figure 3B and C; Tables S7 and S8). Moreover, NEF-treated mice with all doses displayed significantly better performance in a dose-dependent manner compared to the basal control at 14 months. DNP-treated mice reflected the improvement of AD-related deficits to the much younger aged mice and better than untreated control groups. The performance of NEF-treated mice evaluated based on discrimination index in test phase was comparable to WT normal mice at 17 months and better than the positive control DNP group. These results suggest that NEF treatment at all dosages can reverse the cognitive decline to the much younger age of AD mice and to the level near WT normal aging mice.
Figure 3.
Results of NORT.
Notes: Recognition index in the phase of familiarization (A) and the test phase (B), discrimination index in the test phase (C). ****P<0.0001 (one-way ANOVA, Tukey’s test) (mean±SEM).
Abbreviations: NEF, novel edaravone formulation; NORT, novel objective recognition test; 17 mo WT Ctrl, 17-month-old normal wild-type control mice; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 14 mo Tg Ctrl, 14-month-old transgenic mice as a basal control; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF LD, 17-month-old transgenic mice treated with low dose of NEF; 17 mo Tg NEF MD, 17-month-old transgenic mice treated with medium dose of NEF; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; 17 mo Tg DNP, 17-month-old transgenic mice treated with high dose of donepezil; SEM, standard error of the mean; ANOVA, analysis of variance.
Y-maze test
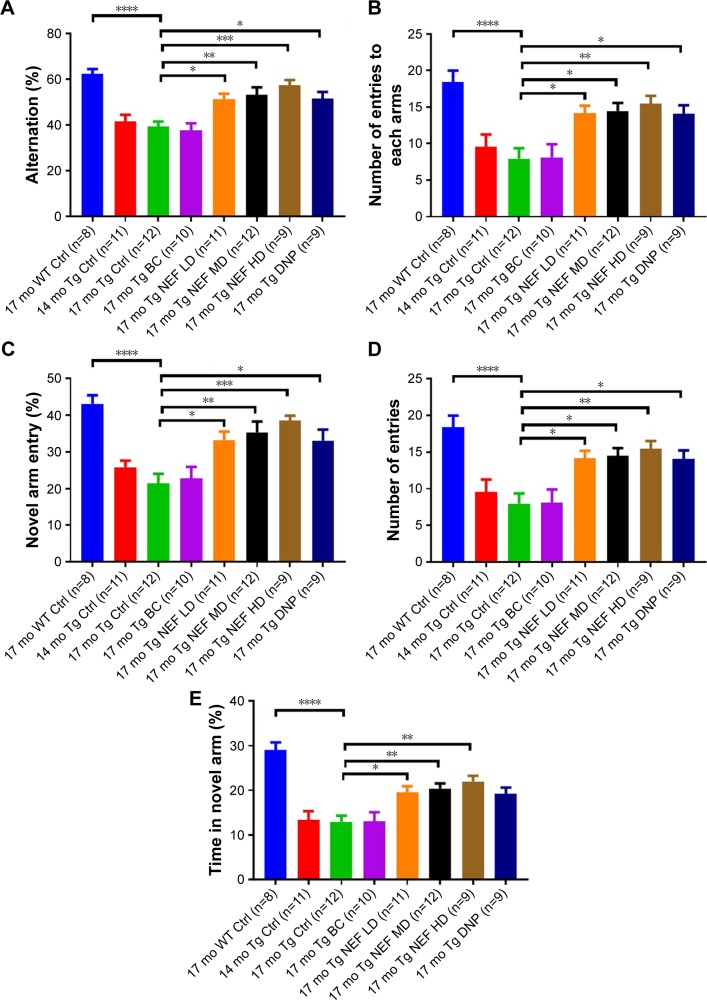
Mouse has a natural tendency to explore a novel environment, but AD-like cognitive deficits could affect its ability to discriminate between familiar and novel environment.43 Y-maze test was conducted in two phases including spontaneous alternation and novel arm exploration tests.2,29 The performance of APP/PS1 mice without any treatment and treated with blank formulation was poor in both the phases compared with WT control, which indicated AD-like behavioral deficits such as impairment in remembering the already explored arm in spontaneous alternation and the previously blocked arm in novel arm exploration test. The NEF-treated mice showed the significantly greater number of alteration (Figure 4A; Table S9) and entry to each arm (Figure 4B; Table S9) in a dose-dependent manner compared to control mice without any treatment or treated with the SOL vehicle. Moreover, significantly higher number of entry (Figure 4C and D; Table S9) and time spent (Figure 4E; Table S9) in the novel arm in comparison with other arms were witnessed in NEF-treated mice compared with the mice without any treatment or treated with the vehicle. All NEF-treated mice performed better than the basal control mice at 14 months of age, indicating that the NEF treatment can reverse the cognitive status to a much younger age than 14 months, although these basal control younger mice performed slightly better than 17-month-old untreated controls but had no statistical difference. Also, NEF-treated mice showed better results but statistically insignificant compared with mice treated with DNP in both the phases. Also, most importantly, the performance of NEF-treated mice in both the phases was not statistically different from that of WT normal aging control, indicating amendment by NEF at all dosages in AD-like cognitive functions to the normal mice with same age.
Figure 4.
The behavioral performances of Tg mice in Y-maze test.
Notes: % Alternation (A) and numbers of entries in each arm (B) and in the spontaneous alternation test, % of total entry in novel arm compared with other arms (C), total number of entry to the novel arm (D), and time (%) (E) in the novel arm test. *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 (one-way ANOVA, Tukey’s test) (mean±SEM).
Abbreviations: NEF, novel edaravone formulation; 17 mo WT Ctrl, 17-month-old normal wild-type control mice; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 14 mo Tg Ctrl, 14-month-old transgenic mice as a basal control; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF LD, 17-month-old transgenic mice treated with low dose of NEF; 17 mo Tg NEF MD, 17-month-old transgenic mice treated with medium dose of NEF; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; 17 mo Tg DNP, 17-month-old transgenic mice treated with high dose of donepezil; SEM, standard error of the mean; ANOVA, analysis of variance.
MWM test
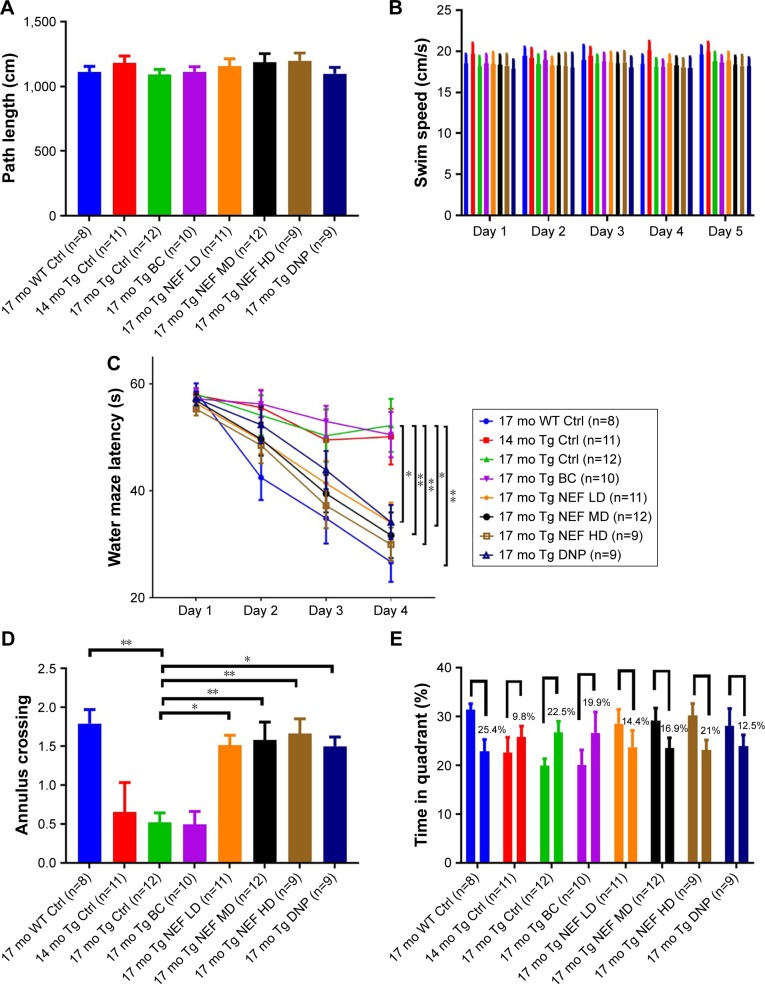
The MWM test is one of the most sensitive tests which represents the AD-like deficits as it examines the function of the hippocampus, the most affected region of the brain in AD. The determination of hippocampal spatial memory deficits was performed by motivating mice to escape from water by finding the platform after placing into the water tank.2,29 The test was performed in four phases including habituation, visible platform, hidden platform, and probe test. The performance of all APP/PS1 and WT mice in first and second phases was evaluated by measuring the path length (Figure 5A; Table S10), swimming speed (Figure 5B; Table S11), and escape latency time, which was not significantly different among groups, which indicated that all groups had similar motor and visual capabilities. The learning and memory of all mice were assessed in the third phase by comparing escape latency time (Figure 5C; Table S12) and the fourth phase by measuring a number of annulus crossing (Figure 5D; Table S13) and time spent in target quadrant compared with all other quadrants (Figure 5E; Table S14) of MWM. The mice without treatment or treated with the blank formulation in APP/PS1 mice at 14 or 17 months displayed significantly impaired learning compared with WT mice. NEF-treated mice performed significantly better in a dose-dependent manner showing a reduction in the escape latency time in the third phase and a higher number of annulus crossing, and longer time spent in the target quadrant in the fourth phase, compared with those of untreated or vehicle-treated mice in both the phases. Moreover, these mice treated with MD and HD of NEF exhibited better performance than the DNP group. Again, the most important finding is that all mice treated with NEF improved their capacity of learning and memory to the extent comparable to the level of WT, as no statistical difference was seen between the WT normal aging control and NEF-treated groups.
Figure 5.
The performances of Tg mice in MWM test.
Notes: Path length (cm) to the platform (A), swim speed (cm/s) during platform trials and probe test (B), escape latency (s) during platform trials and distance travelled on each day of training (C), number of annulus crossings in probe trial (D), comparison of time spent in target zone (Q3) (first bar) where platform was located compared with the average of all other zones (o.a.) (second bar) (E). *P<0.05 and **P<0.01 (two-way or one-way ANOVA, Tukey’s test) (mean±SEM).
Abbreviations: NEF, novel edaravone formulation; MWM, Morris water maze; 17 mo WT Ctrl, 17-month-old normal wild-type control mice; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 14 mo Tg Ctrl, 14-month-old transgenic mice as a basal control; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF LD, 17-month-old transgenic mice treated with low dose of NEF; 17 mo Tg NEF MD, 17-month-old transgenic mice treated with medium dose of NEF; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; 17 mo Tg DNP, 17-month-old transgenic mice treated with high dose of donepezil; SEM, standard error of the mean; ANOVA, analysis of variance.
Overall, the performance of untreated or blank formulation treated mice at 17 months showed AD-like behavioral deficits in open-field, NORT, Y-maze, and MWM behavior tests compared with WT control. The basal control group of mice presented slightly better but insignificant than 17-month-old mice, while blank formulation treatment did not show any improvement compared with 17-month-old untreated mice. DNP treatment rescues the cognitive deficits of APP/PS1 mice in all behavioral tests compared with 17-month-old untreated mice but only able to match the performance of NEF LD treatment. The NEF MD- and HD-treated mice revealed exceptional learning and memory ability which was better than DNP-treated, basal control and quite similar to the WT control mice in all behavior tests. The expressed NEF treatment could not just prevent the deterioration of cognitive functions but also made a reversal of AD-like behavioral deficits in APP/PS1 mice similar to the much younger mice or same age normal mice.
Previously, EDR ameliorates the AD-like memory deficits in APP/PS1 mice by reducing Aβ deposition and alleviates oxidative stress and attenuates the downstream pathologies including tau hyperphosphorylation, glial activation, neuroinflammation, neuronal loss, and synaptic dysfunction.2 Moreover, it also showed improved streptozotocin-induced cognitive damage by reducing oxidative stress and hyperphosphorylation of tau.15 In addition, EDR amended spatial learning and memory deficits in Aβ-induced neurotoxicity in rats by lowering 4-hydroxynonenal level, acetylcholinesterase and choline acetyl transferase activities.14 NEF is a more bioavailable form (>10-fold compared with EDR suspension) of EDR which could not only achieve a higher concentration in systemic circulation but also in the brain as 50%–65% of the relative ratio of plasma to the cerebral spinal fluid of EDR was reported previously.44 The dose-dependent therapeutic effect of EDR against AD in in vitro and in vivo studies against ALS has been already revealed.2,13,45 Therefore, due to the enhancement of oral BA of EDR, NEF could more effectively target multiple pathways of AD pathogenesis and thus rescue the cognitive deficits of a mouse model of AD.
Assessment of long-term repeated dose toxicity
As a result of superior pharmacological properties of EDR against AD and the exceptional performance of NEF, a more bioavailable form or EDR in a behavioral study conducted on APP/PS1 mice, NEF could be developed as a therapeutic agent for AD. The available information on the toxicity of SOL-based preparation is very limited. Thus, as a part of safety assessment, the objective of this study was to evaluate the long-term toxicity study of NEF when supplied for consecutively 3 months. It could provide guidance for clinical applications of NEF not only for AD but various neurologic and nonneurologic diseases related to organs such as heart, lung, intestine, liver, pancreas, kidney, bladder, and testis.46 The data of 17 mo Tg Ctrl and 17 mo Tg BC control groups are used in other similar projects based on SOL-based preparations to reduce the use of an overall number of animals as per the animal ethics guideline.
No death or clinical signs were noticed related to the treatment in any groups including blank formulation and NEF with all doses. Also, the insignificant difference was observed in body weight, water, and food consumption in NEF-treated groups compared with the nontreated group except the first week (Table 2). During the first week after initiating the treatment, an increase in water intake and weight gain but a decrease in food intake was noted in both blank formulation, and NEF-treated groups than in untreated groups. The preferable taste of SOL might be the possible reason to explain the higher water intake, which led to a weight gain, and a slight and transient decrease in food intake may not affect the body weight. After 1 week, the water and food intake and weight of the mice remained consistent throughout the study.
Hematological parameter
The data of hematological parameters of NEF-treated mice showed a slight but not significant decrease in most of the parameters except MCH, PLT, monocyte, and basophil counts compared with nontreated mice group (Table 3). The SOL treatment did not cause any statistically significant increase and decrease in the values of various hematological parameters, compared with nontreated mouse groups. The biological significance of the results was further assessed from the study of organ weight and histology of the spleen.
Table 3.
Analysis of hematological and coagulation parameters of Tg mice (mean±SD, n=5)
| Parameters | APP/PS1 mice
|
||
|---|---|---|---|
| 17 mo Tg Ctrl | 17 mo Tg BC | 17 mo Tg NEF HD | |
| Hematological parameters | |||
| RBC (×1012/L) | 9.52±0.39 | 9.38±0.51 | 9.12±0.47 |
| WBC (×109/L) | 7.17±3.28 | 7.56±3.94 | 6.95±3.08 |
| HGB (g/dL) | 13.64±0.45 | 13.95±0.61 | 13.28±0.62 |
| HCT (%) | 48.92±1.43 | 47.67±1.92 | 46.18±2.42 |
| MCV (fL) | 49.81±1.36 | 48.53±1.89 | 49.17±2.05 |
| MCH (pg) | 14.68±0.82 | 15.28±0.61 | 14.80±0.49 |
| MCHC (g/dL) | 29.53±1.16 | 28.95±1.99 | 29.18±1.82 |
| PLT (×109/L) | 496.81±282.34 | 521.94±196.05 | 529.27±297.26 |
| MPV (fL) | 6.95±0.14 | 6.57±0.27 | 6.58±0.31 |
| PCT (%) | 0.23±0.16 | 0.19±0.11 | 0.22±0.18 |
| PDW (fL) | 8.3±1.62 | 8.29±1.72 | 7.96±2.44 |
| Neutrophils (%) | 14.35±4.53 | 12.24±5.25 | 13.45±4.98 |
| Lymphocytes (%) | 81.82±5.74 | 77.56±5.23 | 76.95±6.29 |
| Monocytes (%) | 4.61±2.16 | 5.25±2.49 | 4.99±2.46 |
| Eosinophils (%) | 1.86±1.65 | 1.52±1.16 | 1.79±1.04 |
| Basophils (%) | 0.04±0.05 | 0.05±0.05 | 0.05±0.03 |
| Coagulation parameters | |||
| PT (s) | 10.12±0.62 | 10.25±1.12 | 12.21±2.48 |
| APTT (s) | 26.67±8.97 | 28.45±8.12 | 26.19±9.29 |
Abbreviations: APP/PS1, APPSwe/PS1deE9; Tg, transgenic mice; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; WBC, white blood cells; RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelets; MPV, mean platelet volume; PCT, platelet hematocrit; PDW, platelet distribution width; PT, prothrombin time; APTT, activated partial thromboplastin time; NEF, novel edaravone formulation.
Coagulation parameters
There was no statistically significant increase or decrease in the value of PT and APTT detected in the case of the NEF-treated mice and the SOL vehicle-treated mice, compared with nontreated mice (Table 3).
Blood electrolytic parameters
The results indicated that there was no statistically significant increase or decrease in the values of blood electrolytic parameters between the mice treated with SOL or NEF and those control mice without any treatment (Table 4).
Table 4.
Analysis of biochemical parameters of Tg mice (mean±SD, n=5)
| Parameters | APP/PS1 mice
|
||
|---|---|---|---|
| 17 mo Tg Ctrl | 17 mo Tg BC | 17 mo Tg NEF HD | |
| Sodium (mmol/L) | 147.66±2.94 | 143.06±2.98 | 147.38±2.58 |
| Bicarbonate (mmol/L) | 27.16±5.79 | 27.9±5.02 | 28.31±5.48 |
| Anion gap (mmol/L) | 22.78±4.70 | 22.91±5.25 | 23.46±4.68 |
| Potassium (mmol/L) | 11.06±1.52 | 12.94±1.03 | 11.00±1.26 |
| Calcium (mmol/L) | 2.56±0.19 | 2.84±0.18 | 2.58±0.22 |
| Sodium:potassium ratio | 13.6±1.86 | 13.93±1.92 | 13.75±1.39 |
| Chloride (mmol/L) | 107.71±2.52 | 109.52±2.91 | 107.73±2.94 |
| Inorganic phosphorus (mmol/L) | 3.08±0.77 | 3.56±0.99 | 3.04±0.84 |
| Calcium:phosphorus ratio | 0.83±0.19 | 0.78±0.14 | 0.81±0.18 |
| Albumin (g/L) | 34.93±2.68 | 31.16±3.57 | 33.45±2.48 |
| Total protein (g/L) | 56.69±3.59 | 54.58±4.56 | 57.62±3.57 |
| Globulin (g/L) | 24.8±2.67 | 23.4±2.96 | 22.53±2.48 |
| Total bilirubin (µmol/L) | 0.43±0.05 | 0.67±0.07 | 0.46±0.04 |
| Creatinine (µmol/L) | 10.84±2.86 | 12.22±4.76 | 14.56±3.59 |
| Urea (mmol/L) | 7.67±2.12 | 9.2±3.45 | 7.24±2.94 |
| Glucose (mmol/L) | 15.96±3.15 | 15.06±3.78 | 15.64±3.26 |
| Cholesterol (mmol/L) | 2.56±0.59 | 2.51±0.56 | 2.64±0.61 |
| AST (U/L) | 305.07±200.04 | 326.94±179.35 | 256.62±127.48 |
| GGT (U/L) | 0 | 0 | 0 |
| Alkaline phosphatase (U/L) | 106.73±31.70 | 106.44±28.02 | 115.88±32.14 |
| Lipase (U/L) | 98.86±39.77 | 89.28±35.14 | 105.46±45.20 |
| ALT (U/L) | 97.01±21.15 | 99.64±18.04 | 103.54±23.28 |
| Amylase (U/L) | 2,181.95±339.34 | 2,295.26±290.25 | 1,944.36±424.28 |
Abbreviations: APP/PS1, APPSwe/PS1deE9; Tg, transgenic mice; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; NEF, novel edaravone formulation.
Biochemical parameters
The analysis of serum biochemistry was performed to assess the impact of the treatment of NEF and SOL on the standard function of liver, kidneys, heart, liver, and digestive system of mice (Table 4). At the end of the treatment period, there was a slight increase in values of creatinine, total bilirubin, and urea in NEF- and SOL-treated groups compared with the untreated controls, but no statistically significant difference. There was no statistically significant difference in the rest of liver enzymes and metabolites between treated and control mice. These results indicate that the treatment of SOL did not show any significant alterations in the serum biochemistry compared with nontreated groups. The biological significance of the results was further evaluated based on the study of organ weight and histology study.
Organ weight
The results of weights of all the key organs of the mice treated with blank formulations and NEF and without any treatment are presented in Table 5. The NEF-treated mice showed a slight increase in weight of all organs but without statistical significance. The average weight of NEF-treated mice was higher than the nontreated mice that could explain slight more weights of all the key organs. There was no change in the weight of organs in SOL-treated mice compared with untreated mice.
Table 5.
Analysis of organ weights of Tg mice (mean±SD, n=5)
| Organ (g) | APP/PS1 mice
|
||
|---|---|---|---|
| 17 mo Tg Ctrl | 17 mo Tg BC | 17 mo Tg NEF HD | |
| Brain | 0.48±0.08 | 0.49±0.07 | 0.49±0.08 |
| Liver | 1.86±0.48 | 1.84±0.42 | 1.89±0.44 |
| Heart | 0.22±0.06 | 0.21±0.08 | 0.24±0.05 |
| Lung | 0.52±0.12 | 0.55±0.14 | 0.55±0.10 |
| Spleen | 0.13±0.03 | 0.12±0.04 | 0.16±0.03 |
| Kidney | 0.25±0.04 | 0.23±0.03 | 0.27±0.04 |
Abbreviations: APP/PS1, APPSwe/PS1deE9; Tg, transgenic mice; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; NEF, novel edaravone formulation.
Histopathology
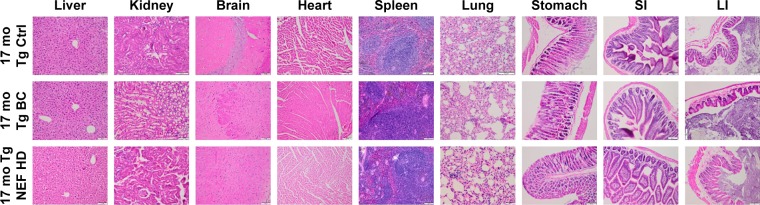
Histopathological examinations of the all the vital organs including liver, kidney, brain, heart, spleen, lung, stomach, SI, and LI did not reveal any treatment-related changes in NEF- or SOL-treated mouse group compared with nontreated mice group (Figure 6). Therefore, these results are consistent with no statistically significant change in the results observed in hematological, coagulation, blood electrolytic, and biochemical parameters after the treatment of NEF or SOL for 3 months. These results together indicate that 1) different dosages of EDA 46, 138, and 414 µM/kg are well tolerated by animals via oral intake for 3 months and 2) the vehicle SOL added into the formulation did not alter any organ functions and is also well tolerated in mice fed for 3 months.
Figure 6.
Photomicrographs of the sections of the tissues of Tg mice.
Abbreviations: NEF, novel edaravone formulation; 17 mo Tg Ctrl, 17-month-old transgenic sham control mice; 17 mo Tg BC, 17-month-old transgenic blank control mice; 17 mo Tg NEF HD, 17-month-old transgenic mice treated with high dose of NEF; SI, small intestine; LI, large intestine.
Previously, the potential of EDR as an intervention therapy for AD was disclosed by targeting multiple key AD pathways including Aβ, tau phosphorylation, oxidative stress, and neuroinflammation. NEF, the more bioavailable form of EDR, showed exceptional performance by improving AD-like behavioral deficits near the normal level that was better than the already approved drug product (DNP). In addition, based on the long-term repeated dose toxicity assessment results, good tolerability of NEF was proven via the oral route of administration. As the oral route of administration is the most preferred route of administration, our study demonstrated NEF as a promising and very safe therapeutic candidate in the current preclinical development.
Conclusion
For the first time, the greater bioavailable form of EDR- and SOL-based formulation, NEF, was systematically studied for safety and efficacy in in vitro and in vivo disease models. NEF showed greater cellular uptake and better neuroprotective effect compared with EDR against the cytotoxicity induced by copper metal ion, H2O2, and Aβ42 oligomer in SH-SY5Y695 cell line. In in vivo study, it showed dose-dependent rescues of the behavioral deficits of 17-month-old APP/PS1 mice from the open-field, NORT, Y-maze, and MWM tests after 3 months of exposure. Also, it appeared reversing the cognitive decline of APP/PS1 mice to the cognitive level of WT normal aging mice and performed better than DNP. Moreover, the toxicity study data revealed the absence of drug-induced toxicity by oral administration of up to 414 µM/kg of EDR in the APP/PS1 mice. Thus, NEF could be a promising candidate for the AD treatment. Our data in the present study, together with the earlier proof-of-concept study in 12-month-old APP/PS1 mice, warrants a large scale of clinical trials in mild cognitive impairment and sporadic and familial AD.
Acknowledgments
The authors would specially like to acknowledge Fujian Kangshimei Co, China for the financial support for the present research, the University President’s Scholarships from University of South Australia for AP and ZS for their doctorate study; NHMRC fellowship for XFZ; and a scholarship under state scholarship fund from China Scholarship Council for JL. Fujian Kangshimei Co, China owns the intellectual property for Chinese patent 200610149832.9. The authors would like to acknowledge H Md Morshed Alam (BASF Australia Ltd) for providing samples of SOL; Rupal Pradhan and Andrew Beck from University of South Australia for hematological, coagulation parameters, and histology studies; Rebecca Summerton and Dr Ian Beckman from Veterinary Diagnostic Laboratory, the University of Adelaide, for serum biochemistry study; and Noralyn Manucat-Tan and Chun-Sheng Ruan for behavior tests. The staff members of Reid animal house facility including Alex Whittaker, Ruth Brogan, Jayne Skinner, Alysha Servin, Jess Parken, and Becky Nitschke from University of South Australia are acknowledged for their generous support in animal work.
Footnotes
Disclosure
AP, XFZ, and SG are the named inventors of Chinese patent 200610149832.9. The authors report no other conflicts of interest in this work.
References
- 1.Centers for Disease Control and Prevention Trends in aging – United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52(6):101–104. 106. [PubMed] [Google Scholar]
- 2.Jiao SS, Yao XQ, Liu YH, et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc Natl Acad Sci U S A. 2015;112(16):5225–5230. doi: 10.1073/pnas.1422998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126(4):479–497. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association Alzheimer’s & Dementia: Global Resources. 2017. [Accessed March 1, 2017]. Available from: https://alz.org/global/
- 5.Cipriani G, Dolciotti C, Picchi L, Bonuccelli U. Alzheimer and his disease: a brief history. Neurol Sci. 2011;32(2):275–279. doi: 10.1007/s10072-010-0454-7. [DOI] [PubMed] [Google Scholar]
- 6.Cummings J. Lessons learned from Alzheimer disease: clinical trials with negative outcomes. Clin Transl Sci. 2018;11(2):147–152. doi: 10.1111/cts.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sood S, Jain K, Gowthamarajan K. Intranasal therapeutic strategies for management of Alzheimer’s disease. J Drug Target. 2014;22(4):279–294. doi: 10.3109/1061186X.2013.876644. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal K, Liu F, Gong CX. Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles. Biochem Pharmacol. 2014;88(4):631–639. doi: 10.1016/j.bcp.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bu XL, Jiao SS, Lian Y, Wang YJ. Perspectives on the tertiary prevention strategy for Alzheimer’s disease. Curr Alzheimer Res. 2016;13(3):307–316. doi: 10.2174/1567205013666151215110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tokuda E, Furukawa Y. Abnormal protein oligomers for neurodegeneration. Oncotarget. 2017;8(25):39943–39944. doi: 10.18632/oncotarget.18030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh A, Kathawala K, Tan CC, Garg S, Zhou XF. Development of a novel oral delivery system of edaravone for enhancing bioavailability. Int J Pharm. 2016;515(1–2):490–500. doi: 10.1016/j.ijpharm.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Yan Y, Gong K, Ma T, et al. Protective effect of edaravone against Alzheimer’s disease-relevant insults in neuroblastoma N2a cells. Neurosci Lett. 2012;531(2):160–165. doi: 10.1016/j.neulet.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Shen YE, Wang Y, Yu GC, Liu C, Zhang ZY, Zhang LM. Effects of edaravone on amyloid-beta precursor protein processing in SY5Y-APP695 cells. Neurotox Res. 2013;24(2):139–147. doi: 10.1007/s12640-012-9370-3. [DOI] [PubMed] [Google Scholar]
- 14.He F, Cao YP, Che FY, Yang LH, Xiao SH, Liu J. Inhibitory effects of edaravone in beta-amyloid-induced neurotoxicity in rats. Biomed Res Int. 2014;2014:370368. doi: 10.1155/2014/370368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Yu G, Chi L, et al. Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Neurotoxicology. 2013;38:136–145. doi: 10.1016/j.neuro.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Yang R, Wang Q, Li F, Li J, Liu X. Edaravone injection ameliorates cognitive deficits in rat model of Alzheimer’s disease. Neurol Sci. 2015;36(11):2067–2072. doi: 10.1007/s10072-015-2314-y. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Egawa M. Effects of an antistroke agent MCl-186 on cerebral arachidonate cascade. J Pharmacol Exp Ther. 1994;271(3):1624–1629. [PubMed] [Google Scholar]
- 18.Unno Y, Katayama M, Shimizu H. Does functional outcome in acute ischaemic stroke patients correlate with the amount of free-radical scavenger treatment? A retrospective study of edaravone therapy. Clin Drug Investig. 2010;30(3):143–155. doi: 10.2165/11535500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda T, Xia YX, Kaneko M, Sameshima H, Ikenoue T. Effect of the free radical scavenger, 3-methyl-1-phenyl-2-pyrazolin-5-one (MCI-186), on hypoxia-ischemia-induced brain injury in neonatal rats. Neurosci Lett. 2002;329(1):33–36. doi: 10.1016/s0304-3940(02)00573-6. [DOI] [PubMed] [Google Scholar]
- 20.Ringman JM, Frautschy SA, Teng E, et al. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther. 2012;4(5):43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mecocci P, Polidori MC. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim Biophys Acta. 2012;1822(5):631–638. doi: 10.1016/j.bbadis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Parikh A, Kathawala K, Tan CC, Garg S, Zhou XF. Lipid-based nanosystem of edaravone: development, optimization, characterization and in vitro/in vivo evaluation. Drug Deliv. 2017;24(1):962–978. doi: 10.1080/10717544.2017.1337825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong WT, Lu YP, Tao Q, et al. Hydroxypropyl-sulfobutyl-beta-cyclodextrin improves the oral bioavailability of edaravone by modulating drug efflux pump of enterocytes. J Pharm Sci. 2014;103(2):730–742. doi: 10.1002/jps.23807. [DOI] [PubMed] [Google Scholar]
- 24.Jian Zenga YR, Zhoua C, Yua S, Chen W-H. Preparation and physicochemical characteristics of the complex of edaravone with hydroxypropyl – cyclodextrin. Carbohydr Polym. 2010;83:5. [Google Scholar]
- 25.Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15(2):324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int J Pharm. 2010;389(1–2):207–212. doi: 10.1016/j.ijpharm.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14(4):287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- 28.Arendash GW, King DL, Gordon MN, et al. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 29.Yao XQ, Jiao SS, Saadipour K, et al. p75NTR ectodomain is a physiological neuroprotective molecule against amyloid-beta toxicity in the brain of Alzheimer’s disease. Mol Psychiatry. 2015;20(11):1301–1310. doi: 10.1038/mp.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tober-Meyer BK, Bieniek HJ, Kupke IR. Studies on the hygiene of drinking water for laboratory animals. 2. Clinical and biochemical studies in rats and rabbits during long-term provision of acidified drinking water. Lab Anim. 1981;15(2):111–117. doi: 10.1258/002367781780959071. [DOI] [PubMed] [Google Scholar]
- 31.Tober-Meyer BK, Bieniek HJ. Studies on the hygiene of drinking water for laboratory animals. 1. The effect of various treatments on bacterial contamination. Lab Anim. 1981;15(2):107–110. doi: 10.1258/002367781780959026. [DOI] [PubMed] [Google Scholar]
- 32.Ruan CS, Yang CR, Li JY, Luo HY, Bobrovskaya L, Zhou XF. Mice with Sort1 deficiency display normal cognition but elevated anxiety-like behavior. Exp Neurol. 2016;281:99–108. doi: 10.1016/j.expneurol.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Gao X, Li C, et al. Impaired dendritic development and memory in Sorbs2 knock-out mice. J Neurosci. 2016;36(7):2247–2260. doi: 10.1523/JNEUROSCI.2528-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakoma B, Berke B, Eklu-Gadegbeku K, et al. Acute and sub-chronic (28 days) oral toxicity evaluation of hydroethanolic extract of Bridelia ferruginea Benth root bark in male rodent animals. Food Chem Toxicol. 2013;52:176–179. doi: 10.1016/j.fct.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Li J, Zhang W, et al. Acute and sub-chronic toxicity studies of honokiol microemulsion. Regul Toxicol Pharmacol. 2014;71(3):428–436. doi: 10.1016/j.yrtph.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Li Z, Li L, et al. Acute and sub-chronic oral toxicity profiles of the aqueous extract of Cortex Dictamni in mice and rats. J Ethnopharmacol. 2014;158(Pt A):207–215. doi: 10.1016/j.jep.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Ashe KH. Learning and memory in transgenic mice modeling Alzheimer’s disease. Learn Mem. 2001;8(6):301–308. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- 38.Perez SE, Berg BM, Moore KA, et al. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: possible gender effects. J Neurosci Res. 2010;88(5):1026–1040. doi: 10.1002/jnr.22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Xie N, Li L, Zou Y, Zhang X, Dong M. Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice. Int J Neuropsychopharmacol. 2014;17(4):635–644. doi: 10.1017/S146114571300148X. [DOI] [PubMed] [Google Scholar]
- 40.Guo HB, Cheng YF, Wu JG, et al. Donepezil improves learning and memory deficits in APP/PS1 mice by inhibition of microglial activation. Neuroscience. 2015;290:530–542. doi: 10.1016/j.neuroscience.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 41.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13(2):93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng D, Low JK, Logge W, Garner B, Karl T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1E9 mice. Psychopharmacology. 2014;231(15):3009–3017. doi: 10.1007/s00213-014-3478-5. [DOI] [PubMed] [Google Scholar]
- 43.Kim HY, Kim HV, Lee DK, Yang SH, Kim Y. Rapid and sustained cognitive recovery in APP/PS1 transgenic mice by co-administration of EPPS and donepezil. Sci Rep. 2016;6:34165. doi: 10.1038/srep34165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabrizchi R. Edaravone Mitsubishi-Tokyo. Curr Opin Investig Drugs. 2000;1(3):347–354. [PubMed] [Google Scholar]
- 45.Ito H, Wate R, Zhang J, et al. Treatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS mice. Exp Neurol. 2008;213(2):448–455. doi: 10.1016/j.expneurol.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Kikuchi K, Takeshige N, Miura N, et al. Beyond free radical scavenging: beneficial effects of edaravone (radicut) in various diseases (review) Exp Ther Med. 2012;3(1):3–8. doi: 10.3892/etm.2011.352. [DOI] [PMC free article] [PubMed] [Google Scholar]