Abstract
Objective
To investigate the combined effect of albumin (ALB) and globulin (GLB) on the overall survival (OS) of patients with heart failure (HF).
Design
Retrospective cohort study.
Setting
A hospital.
Participants
404 patients first diagnosed with HF.
Measurements
Serum ALB and GLB were measured within 3 days after admission. The albumin to globulin ratio (AGR) was calculated as the ALB divided by the GLB. The receiver operating characteristic curve was used to calculate the cut-off points for ALB, GLB and AGR. Patients with low ALB levels (≤35.3 g/L) and high GLB levels (>27.0 g/L) were assigned an albumin-globulin score (AGS) of 2, those with only one of the two abnormalities were assigned an AGS of 1 and those with neither of the two abnormalities were assigned an AGS of 0.
Results
The mean age of the 404 patients was 62.69±15.62, and 54.5% were male. 14 patients were lost to follow-up. 120 patients died from HF and 211 patients were readmitted to the hospital for worsening HF. Multivariate Cox regression analysis showed that higher AGR was significantly associated with favourable OS (HR, 0.61, 95% CI 0.38 to 0.98, p=0.040) but not AGS.
Conclusion
Serum levels of ALB and GLB are objective and easily measurable biomarkers which can be used in combination to predict the survival of patients with HF.
Keywords: albumin, globulin, survival, heart failure
Strengths and limitations of this study.
This is the first study investigating the prognostic value of albumin-globulin score and albumin to globulin ratio in patients with heart failure (HF).
A strength of this study is its cohort study design.
To avoid bias, only patients first diagnosed with HF were selected.
Information bias could not be avoided owing to the retrospective cohort study design.
Introduction
Heart failure (HF), with a prevalence of more than 23 million worldwide,1 2 is a global public health problem. In China, there are 4.2 million people living with HF, with 500 000 new cases diagnosed each year, and this number is expected to increase still further,3 causing enormous social and economic burden. Over the last 30 years, although great improvements have been made in drug and device therapy, the survival and the rehospitalisation rate of patients with HF often remain unsatisfactory.4 5 Hence, identification of promising prognostic factors that contribute to risk classification and clinical management of such patients could improve long-term survival.
Numerous prognostic markers of death and/or HF hospitalisation have been identified in patients with HF. In recent decades, several multivariable prognostic risk scores have been developed for different populations of patients with HF.6–8 However, their clinical applicability is limited and precise risk stratification in HF remains challenging. Simple but effective prognostic biomarker models are needed to improve the management of the HF epidemic.
The correlation between serum albumin (ALB) and globulin (GLB) with HF has recently been emphasised. ALB and GLB, the two major components of serum proteins, have been confirmed to be involved in the systemic inflammatory process. Serum ALB, one of the biochemical tests, indicates nutritional status and relates to chronic inflammation in HF.9 10 Moreover, increased levels of GLB could serve as a marker of chronic inflammation response and reflect a cumulative exposure of various proinflammatory cytokines.11 Previous studies have demonstrated that hypoalbuminaemia was associated with impaired survival in patients with HF.12 However, no study investigated the cumulative effects of ALB and GLB on patients with HF. Therefore, the purpose of the present study was to assess the effects of the albumin-globulin score (AGS) and albumin to globulin ratio (AGR) on the long-term survival of patients with HF.
Materials and methods
Participants
Between January 2010 and September 2015, 404 consecutive patients who were first diagnosed with HF at the First Affiliated Hospital of Zhengzhou University were included. The diagnosis of HF was based on a history of dyspnoea with symptomatic exercise intolerance, and signs of documentation of left ventricular enlargement or peripheral oedema or pulmonary congestion or radionuclide ventriculography or dysfunction by chest X-ray and/or echocardiography.13 Informed consent was obtained from all patients prior to participation. All methods were performed in accordance with the relevant guidelines and regulations.
The exclusion criteria were as follows: (1) acute coronary syndrome, (2) no echocardiographic structural or functional abnormalities, (3) without HF and (4) death before discharge.
Patient and public involvement
All participants were given written informed consent before they joined the study to authorise use of data generated from the medical information system. The research question and outcome measures were handed out to each patient in hardcopy. Patients were not involved in the recruitment and conduct of the study. The results will be mailed to each participant.
Clinical and laboratory parameters
Patients’ baseline characteristics, including demographic parameters, comorbidities, medications and laboratory variables, were retrospectively reviewed and collected from the electronic medical records by two researchers. Fasting venous blood samples were collected from all patients within 3 days after admission and were immediately sent for analysis. The serum levels of ALB, GLB and other variables were assayed using an automatic biochemical analyser (Hitachi 7600, Japan). The receiver operating characteristic curve was used to calculate the cut-off points for ALB, GLB and AGR. Patients with low ALB levels (≤35.3 g/L) and high GLB levels (>27.0 g/L) were assigned an AGS of 2, those with only one of the two abnormalities were assigned an AGS of 1 and those with neither of the two abnormalities were assigned an AGS of 0. AGS=1–2 was defined as high, AGS=0 was defined as low, AGR>1.48 was defined as high and AGR≤1.48 was defined as low. Moreover, AGR was divided into three equal tertiles.
Follow-up
All patients were followed up every 3 months for the first 2 years, every 6 months in the third and every 1 year afterwards. The primary endpoint was death due to a cardiovascular event (myocardial infarction, progressive HF, stroke, other vascular causes or sudden cardiac death), while the secondary endpoint was progressive HF requiring rehospitalisation. Follow-up was performed until death of the patient or until July 2016, which was the cut-off date for this study.
Statistical analysis
t-Test was used to examine the difference in age. Rank-sum test was used to measure the difference in non-normally continuous variables (ALB, B-type natriuretic peptide (BNP), interventricular septum thickness (IVST), posterior wall thickness, left ventricular end-diastolic volume (LVDv), left ventricular end-systolic volume (LVSv), left ventricular ejection fraction (LVEF) and left atrial dimension (LAD)). The χ2 test was used to assess the difference in categorical variables (including gender, hypertension, diabetes mellitus, chronic kidney disease (CKD), history of HF, idiopathic dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, hypertensive heart disease, atrial fibrillation (AF) and medications). The Kaplan-Meier method with log-rank test was used to estimate survival curves. Univariable Cox regression analysis was used to identify variables associated with overall survival. Variables with a p<0.05 on univariable analysis were further assessed with a multivariable Cox regression model. SPSS V.21 software was used for statistical analysis. The level of significance was established as a two-sided p value of 0.05.
Results
The baseline characteristics of patients are presented in table 1. A total of 404 patients with HF, with a mean age of 62.70±15.62 years old, were included in the analysis. Among them, 260 patients (64.25%) were classified as high AGS, while 189 patients (46.78%) were classified as higher AGR. Patients with lower AGS were more often among individuals without CKD, with idiopathic DCM, and were using beta-blocker and ACE inhibitor (ACE-I). They also had lower BNP, higher LVSv, higher LVEF and lower LAD. Patients with higher AGR were more often among individuals without diabetes mellitus, without coronary heart disease, with DCM, and were using beta-blocker and ACE-I. They also had lower BNP.
Table 1.
Correlation between AGS, AGR and clinicopathological parameters in 404 patients with chronic HF
| Variables | AGS (high=260, low=144) | AGR (high=189, low=215) | ||||
| High (1–2) | Low (0) | P values | High (>1.48) | Low (≤1.48) | P values | |
| Age (years) | 63.82±15.63 | 60.67±15.45 | 0.051 | 59.46±15.34 | 65.41±15.34 | <0.001 |
| Gender | ||||||
| Male | 133 (51.2%) | 87 (60.4%) | 0.073 | 111 (58.7%) | 109 (51.2%) | 0.129 |
| Albumin (g/dL) | 37.8 (26.8–47.1) | 36.0 (24.2–45.1) | 0.026 | 36.3 (26.1–46.3) | 38.2 (25.7–47.5) | 0.017 |
| Hypertension | 107 (41.2%) | 59 (41.0%) | 0.972 | 78 (41.3%) | 88 (41.3%) | 0.993 |
| Diabetes mellitus | 80 (30.8%) | 32 (22.2%) | 0.066 | 42 (22.2%) | 70 (32.9%) | 0.018 |
| CKD | 32 (12.3%) | 7 (4.9%) | 0.015 | 16 (8.5%) | 23 (10.8%) | 0.430 |
| History of HF | 185 (71.2%) | 110 (76.4%) | 0.256 | 145 (76.7%) | 148 (69.5%) | 0.103 |
| VHD | 35 (13.5%) | 18 (12.5%) | 0.784 | 24 (12.7%) | 29 (13.6%) | 0.786 |
| CHD | 118 (45.4%) | 68 (47.2%) | 0.723 | 75 (39.7%) | 110 (51.6%) | 0.016 |
| DCM | 39 (15.0%) | 33 (22.9%) | 0.046 | 47 (24.9%) | 25 (11.7%) | 0.001 |
| HCM | 7 (2.7%) | 2 (1.4%) | 0.395 | 5 (2.6%) | 4 (1.9%) | 0.604 |
| HHD | 5 (1.9%) | 5 (3.5%) | 0.337 | 5 (2.6%) | 5 (2.3%) | 0.848 |
| AF | 81 (31.2%) | 40 (27.8%) | 0.478 | 60 (31.7%) | 60 (28.2%) | 0.434 |
| BNP (pg/mL) | 3948.5 (500.7–18 807.6) | 2643.0 (409.6–12 089.1) | <0.001 | 2881.0 (532.4–20 725.8) | 4155.5 (445.2–17 685.2) | <0.001 |
| Echocardiography findings | ||||||
| IVST (mm) | 10.0 (7.8–13.0) | 9.7 (8.0–13.4) | 0.027 | 10.0 (8.0–14.0) | 10.0 (7.7–13.0) | 0.813 |
| PWT (mm) | 9.0 (8.0–12.0) | 9.0 (7.2–12.9) | 0.172 | 9.0 (2.2–12.8) | 9.0 (8.0–11.0) | 0.423 |
| LVDv (mL) | 152.0 (71.6–347.6) | 170.0 (72.6–346.0) | 0.014 | 150.5 (72.0–365.7) | 170.0 (67.8–343.2) | 0.023 |
| LVSv (mL) | 75.5 (27.0–225.6) | 98.0 (27.0–232.1) | 0.007 | 95.0 (26.4–230.0) | 74.5 (27.0–231.1) | 0.019 |
| LVEF (%) | 48.0 (28.0–67.5) | 45.0 (27.0–64.0) | 0.031 | 45.0 (27.0–65.0) | 48.0 (28.0–67.0) | 0.086 |
| LAD (mm) | 40.0 (29.0–58.4) | 42.0 (28.3–63.7) | 0.047 | 42.0 (29.4–62.2) | 39.0 (29.0–60.6) | 0.032 |
| Medications, n (%) | ||||||
| Beta-blocker | 152 (58.5%) | 99 (68.8%) | 0.041 | 130 (68.8%) | 120 (56.3%) | 0.010 |
| CCB | 57 (21.9%) | 31 (21.5%) | 0.927 | 43 (22.8%) | 45 (21.1%) | 0.694 |
| Statins | 120 (46.2%) | 70 (48.6%) | 0.636 | 92 (48.7%) | 97 (45.5%) | 0.529 |
| ARB | 83 (31.9%) | 41 (28.5%) | 0.471 | 63 (33.3%) | 61 (28.6%) | 0.309 |
| ACE-I | 74 (28.5%) | 69 (47.9%) | <0.001 | 83 (43.9%) | 59 (27.7%) | 0.001 |
ACE-I, ACE inhibitor; AF, atrial fibrillation; AGR, albumin to globulin ratio; AGS, albumin-globulin score; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; CCB, calcium-channel blocker; CHD, coronary heart disease; CKD, chronic kidney disease; DCM, idiopathic dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; HF, heart failure; HHD, hypertensive heart disease; IVST, interventricular septum thickness; LAD, left atrial dimension; LVDv, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVSv, left ventricular end-systolic volume; PWT, posterior wall thickness; VHD, valvular heart disease.
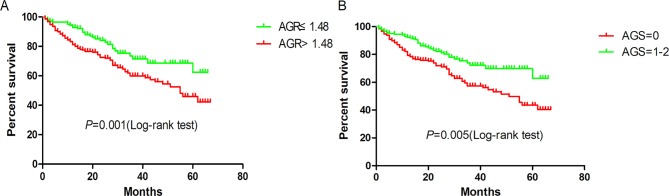
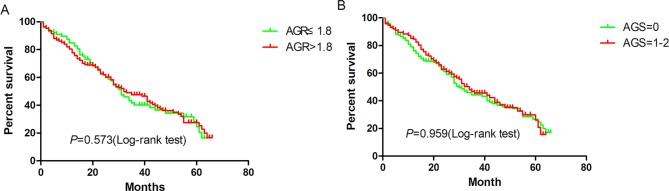
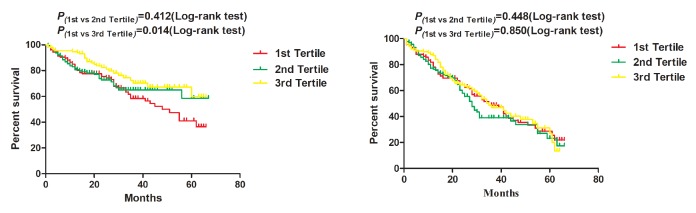
By July 2016, 404 patients had been followed up. Of these patients, 120 died, 211 were readmitted to the hospital and 14 were lost to follow-up. The follow-up rate was 96.5%. The mean and median survival times were 47.23 months and 62.00 months, respectively. Figures 1 and 2 show the Kaplan-Meier curves of the overall survival and hospital-free survival according to AGR (>1.48 vs ≤1.48) and AGS (0 vs 1–2), while figure 3 shows the Kaplan-Meier curves of overall survival and hospital-free survival according to the AGR tertiles.
Figure 1.
Kaplan-Meier survival curves according to (A) albumin to globulin ratio (AGR) and (B) albumin-globulin score (AGS).
Figure 2.
Kaplan-Meier hospital-free curves according to (A) albumin to globulin ratio (AGR) and (B) albumin-globulin score (AGS).
Figure 3.
Kaplan-Meier (A) survival and (B) hospital-free curves according to albumin to globulin ratio tertiles.
As shown in table 2, univariate analysis showed that higher AGR and lower AGS were significantly associated with favourable overall survival: 0.54 (95% CI 0.37 to 0.80) and 0.56 (95% CI 0.37 to 0.84), respectively. Other significant prognostic variables identified by univariate analysis were age (per year), CKD (yes vs no), BNP (per 100 pg/mL), LVDv (per mL), LVSv (per mL) and LAD (per mm). On multivariate analysis, AGR remained an independent predictor for overall survival (HR, 0.61, 95% CI 0.38 to 0.98), but not AGS (HR, 0.81, 95% CI 0.41 to 1.57).
Table 2.
Univariate and multivariate analyses of overall survival
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95% CI) | P values | HR (95% CI) | P values | |
| Age (per year) | 1.02 (1.01 to 1.03) | 0.011 | 1.03 (1.01 to 1.05) | 0.006 |
| Gender (female vs male) | 0.94 (0.78 to 1.13) | 0.501 | ||
| Hypertension (yes vs no) | 0.95 (0.66 to 1.37) | 0.774 | ||
| Diabetes mellitus (yes vs no) | 1.27 (0.87 to 1.87) | 0.221 | ||
| CKD (yes vs no) | 2.37 (1.43 to 3.94) | 0.001 | 2.13 (1.11 to 4.09) | 0.023 |
| History of HF (yes vs no) | 1.27 (0.85 to 1.90) | 0.244 | ||
| VHD (yes vs no) | 1.50 (0.95 to 2.37) | 0.079 | ||
| CHD (yes vs no) | 0.96 (0.67 to 1.37) | 0.808 | ||
| DCM (yes vs no) | 0.63 (0.37 to 1.11) | 0.114 | ||
| HCM (yes vs no) | 0.70 (0.17 to 2.81) | 0.610 | ||
| HHD (yes vs no) | 0.64 (0.16 to 2.60) | 0.536 | ||
| AF (yes vs no) | 1.26 (0.87 to 1.84) | 0.221 | ||
| BNP (per 100 pg/mL) | 1.01 (1.00 to 1.02) | <0.001 | 1.01 (1.00 to 1.02) | 0.034 |
| LVEF (per 1%) | 1.00 (0.98 to 1.01) | 0.623 | ||
| IVST (per mm) | 1.10 (0.99 to 1.21) | 0.066 | ||
| PWT (per mm) | 1.09 (0.99 to 1.19) | 0.076 | ||
| LVDv (per mL) | 1.01 (1.00 to 1.02) | 0.028 | 1.00 (0.99 to 1.01) | 0.393 |
| LVSv (per mL) | 1.01 (1.00 to 1.02) | 0.033 | 1.01 (1.00 to 1.02) | 0.006 |
| LAD (per mm) | 1.02 (1.01 to 1.04) | 0.001 | 1.03 (1.01 to 1.05) | 0.001 |
| AGR (>1.48 vs ≤1.48) | 0.54 (0.37 to 0.80) | 0.001 | 0.61 (0.38 to 0.98) | 0.040 |
| AGR (reference: first tertile) | ||||
| AGR second tertile | 0.84 (0.55 to 1.28) | 0.412 | 0.82 (0.54 to 1.25) | 0.350 |
| AGR third tertile | 0.56 (0.36 to 0.89) | 0.014 | 0.62 (0.39 to 0.98) | 0.040 |
| AGS (0 vs 1–2) | 0.56 (0.37 to 0.84) | 0.005 | 0.81 (0.41 to 1.57) | 0.525 |
AF, atrial fibrillation; AGR, albumin to globulin ratio; AGS, albumin-globulin score; BNP, B-type natriuretic peptide; CHD, coronary heart disease; CKD, chronic kidney disease; DCM, idiopathic dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; HF, heart failure; HHD, hypertensive heart disease; IVST, interventricular septum thickness; LAD, left atrial dimension; LVDv, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVSv, left ventricular end-systolic volume; PWT, posterior wall thickness; VHD, valvular heart disease.
The univariate and multivariate analyses of rehospitalisation are presented in table 3. Univariate analysis showed that AGR and AGS had no significant effect on rehospitalisation: 0.92 (95% CI 0.70 to 1.22) and 0.99 (95% CI 0.75 to 1.32), respectively. However, age (per year), CKD (yes vs no), history of HF (yes vs no), BNP (per 100 pg/mL), IVST (per mm) and LAD (per mm) were significant prognostic variables for rehospitalisation. On multivariate analysis, age (per year), CKD (yes vs no), IVST (per mm) and LAD (per mm) were independent prognostic indicators for rehospitalisation.
Table 3.
Univariate and multivariate analyses of rehospitalisation
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95% CI) | P values | HR (95% CI) | P values | |
| Age (per year) | 1.01 (1.00 to 1.02) | 0.009 | 1.01 (1.00 to 1.02) | 0.028 |
| Gender (female vs male) | 1.01 (1.00 to 1.02) | 0.882 | ||
| Hypertension (yes vs no) | 1.00 (0.76 to 1.32) | 0.998 | ||
| Diabetes mellitus (yes vs no) | 1.25 (0.94 to 1.68) | 0.130 | ||
| CKD (yes vs no) | 1.68 (1.07 to 2.62) | 0.023 | 1.80 (1.07 to 3.03) | 0.027 |
| History of HF (yes vs no) | 1.42 (1.04 to 1.94) | 0.028 | 1.30 (0.87 to 1.95) | 0.202 |
| VHD (yes vs no) | 1.14 (0.78 to 1.68) | 0.490 | ||
| CHD (yes vs no) | 1.26 (0.96 to 1.65) | 0.096 | ||
| DCM (yes vs no) | 0.61 (0.33 to 1.12) | 0.108 | ||
| HCM (yes vs no) | 1.28 (0.60 to 2.73) | 0.523 | ||
| HHD (yes vs no) | 0.77 (0.29 to 2.08) | 0.609 | ||
| AF (yes vs no) | 1.11 (0.83 to 1.47) | 0.487 | ||
| BNP (per 100 pg/mL) | 1.01 (1.00 to 1.02) | 0.050 | 1.00 (0.99 to 1.01) | 0.967 |
| LVEF (per 1%) | 1.01 (0.99 to 1.02) | 0.187 | ||
| IVST (per mm) | 1.09 (1.01 to 1.17) | 0.023 | 1.10 (1.00 to 1.20) | 0.050 |
| PWT (per mm) | 1.04 (0.97 to 1.13) | 0.296 | ||
| LVDv (per mL) | 1.00 (0.99 to 1.01) | 0.967 | ||
| LVSv (per mL) | 1.00 (0.99 to 1.01) | 0.839 | ||
| LAD (per mm) | 1.01 (1.00 to 1.03) | 0.041 | 1.02 (1.00 to 1.03) | 0.011 |
| AGR (>1.48 vs ≤1.48) | 0.92 (0.70 to 1.22) | 0.573 | ||
| AGR (reference: first tertile) | ||||
| AGR second tertile | 1.14 (0.82 to 1.58) | 0.448 | ||
| AGR third tertile | 0.97 (0.69 to 1.36) | 0.850 | ||
| AGS (0 vs 1–2) | 0.99 (0.75 to 1.32) | 0.959 | ||
AF, atrial fibrillation; AGR, albumin to globulin ratio; AGS, albumin-globulin score; BNP, B-type natriuretic peptide; CHD, coronary heart disease; CKD, chronic kidney disease; DCM, idiopathic dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; HF, heart failure; HHD, hypertensive heart disease; IVST, interventricular septum thickness; LAD, left atrial dimension; LVDv, left ventricular end-diastolic volume; LVSv, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; PWT, posterior wall thickness; VHD, valvular heart disease.
Discussion
This study demonstrated that the combination of ALB and GLB has potential predictive effects on the survival of patients with HF, and that higher AGR was significantly associated with favourable overall survival among patients with HF. To the best of our knowledge, this is the first study investigating the prognostic value of AGS and AGR in patients with HF.
The prediction of HF prognosis is a cornerstone of HF management. Accurately predicting prognosis can be of benefit to patients with HF. Patients with a poor prognosis might benefit more from aggressive treatment and a closer follow-up.14 There exist previous risk models for patients with HF,15 16 which adopted a systems biology approach; incorporating information from demographic, biomarker, genomic, proteomic and the initial response to therapy might create a more effective prediction model and hopefully aid in understanding HF prognosis. However, these models are complex in clinical application and only provide moderate accuracy prediction in survival and rehospitalisation in patients with HF.17 Hence, designing a simple survival model based on routine blood biochemical indexes for clinicians is helpful for better identification of patients at high risk for HF.
The role of serum ALB is complex. Serum ALB is synthesised in the liver and plays multiple physiological roles, including maintenance of pH and normal microvascular permeability and mediation of coagulation, and has antioxidant properties.18 The decrease in plasma ALB concentration may be due to malnutrition and cachexia (decreased ALB intake),19 diffuse inflammation (increased ALB consumption),20 renal impairment (increased urinary ALB loss),21 plasma volume expansion (dilutional hypoalbuminaemia) and hepatic dysfunction (decreased ALB synthesis).22 23 Serum ALB is used to access protein malnutrition without calorie malnutrition which did not affect the anthropometric measurements (eg, weight).24 Malnutrition was found to be associated with worsening of symptoms and poor prognosis. Multiple European studies showed malnourished patients with HF are weaker and experience fatigue earlier.25 26 HF with hypoalbuminaemia, the indicator of malnutrition, was found to be associated with higher New York Heart Association functional class, elevated serum blood urea nitrogen and C reactive protein (CRP).24 It should be noted that, although hypoalbuminaemia might reflect poor nutritional status, ALB reduction in chronic inflammation is frequent.11
Similar to the low ALB, high non-albumin protein was a predictor of mortality in patients with HF. We postulate that the high serum non-albumin proteins status is a marker of inflammation in patients with HF. Chronic inflammation is known to increase acute-phase proteins (eg, CRP, serum amyloid A, complement C3, fibrinogen and ceruloplasmin), which constitute part of the calculated GLB,11 and the increased level of GLB serves as a marker of chronic inflammation and reflects cumulative exposure to various proinflammatory cytokines such as interleukin (IL), particularly IL-6 and IL-1β, and tumour necrosis factor-α, which stimulates the production of acute-phase proteins.11 Chronic inflammation is a critical contributor to HF occurrence, development and survival, and is also related to the risk of recurrence among patients with HF.27 Hence, we propose that low AGR and high AGS measure the extent of such activities related to chronic inflammation, which influences mortality.
What is more, we found that LAD was a significant independent predictor of long-term survival and rehospitalisation of patients with HF. The result is consistent with previous research findings.28 As a predictor reflecting left atrium (LA) structural remodelling, LAD relates to all key risk factors for AF, such as advancing age, male sex and higher blood pressure.29 LA enlargement, as characterised by echocardiographic LAD, is related to incident AF, HF, stroke and mortality.28 30–33 Moreover, LA enlargement can reflect atrial volume or pressure overload in valvular or ischaemic heart disease, or as a consequence of AF.34–36
Prior studies have demonstrated that low serum ALB is an independent predictor of HF long-term mortality. Liu et al 37 showed that patients with hypoalbuminaemia had a significantly lower survival rate (53% vs 84%, log-rank χ(2)=53.3, p<0.001) and a higher rate of cardiovascular death (21.8% vs 8.9%, p<0.001). Su et al 38 reported that patients with higher N-terminal pro-brain natriuretic peptide (NT-proBNP) and lower ALB than the median had the highest risk for cardiac events (HR, 2.89, CI 1.90 to 4.40). The finding in our study that AGR is an independent predictor of long-term mortality in patients with HF was consistent with previous studies.
Conclusions
In conclusion, the present study suggests that AGR is a convenient and effective tool to predict the overall survival time in patients with HF. Further larger prospective studies are required to validate this finding and to investigate other prognostic indicators in patients with HF.
Supplementary Material
Footnotes
Contributors: KL and YZ designed the study and provided critical review of the manuscript. YZ, YB and WF reviewed the literature. YZ and KL analysed the data and wrote the first draft of the manuscript. YB, WF and YZ revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The study was approved by the Ethics Research Committee of Zhengzhou University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional data are available from YZ on reasonable request.
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–46. 10.1136/hrt.2003.025270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646–59. 10.1161/CIRCRESAHA.113.300268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam CS, Teng TK, Tay WT, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J 2016;37:3141–53. 10.1093/eurheartj/ehw331 [DOI] [PubMed] [Google Scholar]
- 4. Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail 2013;15:808–17. 10.1093/eurjhf/hft050 [DOI] [PubMed] [Google Scholar]
- 5. Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009;73:1893–900. [DOI] [PubMed] [Google Scholar]
- 6. Lupón J, de Antonio M, Vila J, et al. Development of a novel heart failure risk tool: the barcelona bio-heart failure risk calculator (BCN bio-HF calculator). PLoS One 2014;9:e85466 10.1371/journal.pone.0085466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113:1424–33. 10.1161/CIRCULATIONAHA.105.584102 [DOI] [PubMed] [Google Scholar]
- 8. Mozaffarian D, Anker SD, Anand I, et al. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation 2007;116:392–8. 10.1161/CIRCULATIONAHA.106.687103 [DOI] [PubMed] [Google Scholar]
- 9. Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, et al. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am Heart J 2010;160:279–85. 10.1016/j.ahj.2010.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horwich TB, Kalantar-Zadeh K, MacLellan RW, et al. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883–9. 10.1016/j.ahj.2007.11.043 [DOI] [PubMed] [Google Scholar]
- 11. Gabay C, Kushner I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N Engl J Med Overseas Ed 1999;340:448–54. 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 12. Grodin JL, Lala A, Stevens SR, et al. Clinical Implications of Serum Albumin Levels in Acute Heart Failure: Insights From DOSE-AHF and ROSE-AHF. J Card Fail 2016;22:884–90. 10.1016/j.cardfail.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu T, Bu ZD, Li ZY, et al. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: a meta-analysis of randomized clinical trials. BMC Cancer 2015;15:322 10.1186/s12885-015-1341-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail 2014;2:429–36. 10.1016/j.jchf.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 15. Giamouzis G, Kalogeropoulos A, Georgiopoulou V, et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail 2011;17:54–75. 10.1016/j.cardfail.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 16. Betihavas V, Davidson PM, Newton PJ, et al. What are the factors in risk prediction models for rehospitalisation for adults with chronic heart failure? Aust Crit Care 2012;25:31–40. 10.1016/j.aucc.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 17. Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014;2:440–6. 10.1016/j.jchf.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 18. Roche M, Rondeau P, Singh NR, et al. The antioxidant properties of serum albumin. FEBS Lett 2008;582:1783–7. 10.1016/j.febslet.2008.04.057 [DOI] [PubMed] [Google Scholar]
- 19. Lourenço BH, Vieira LP, Macedo A, et al. Nutritional status and adequacy of energy and nutrient intakes among heart failure patients. Arq Bras Cardiol 2009;93:541–8. [DOI] [PubMed] [Google Scholar]
- 20. Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 2011;13:1161–71. 10.1093/eurjhf/hfr122 [DOI] [PubMed] [Google Scholar]
- 21. Zamora E, Lupón J, Vila J, et al. Estimated glomerular filtration rate and prognosis in heart failure: value of the Modification of Diet in Renal Disease Study-4, chronic kidney disease epidemiology collaboration, and cockroft-gault formulas. J Am Coll Cardiol 2012;59:1709–15. 10.1016/j.jacc.2011.11.066 [DOI] [PubMed] [Google Scholar]
- 22. Adlbrecht C, Kommata S, Hülsmann M, et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body’s red cell volume. Eur Heart J 2008;29:2343–50. 10.1093/eurheartj/ehn359 [DOI] [PubMed] [Google Scholar]
- 23. Herzer K, Kneiseler G, Bechmann LP, et al. Onset of heart failure determines the hepatic cell death pattern. Ann Hepatol 2011;10:174–9. [PubMed] [Google Scholar]
- 24. Amare H, Hamza L, Asefa H. Malnutrition and associated factors among heart failure patients on follow up at Jimma university specialized hospital, Ethiopia. BMC Cardiovasc Disord 2015;15:128 10.1186/s12872-015-0111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobsson A, Pihl-Lindgren E, Fridlund B. Malnutrition in patients suffering from chronic heart failure; the nurse’s care. Eur J Heart Fail 2001;3:449–56. [DOI] [PubMed] [Google Scholar]
- 26. Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med 2004;36:518–29. 10.1080/07853890410017467 [DOI] [PubMed] [Google Scholar]
- 27. Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res 2016;119:159–76. 10.1161/CIRCRESAHA.116.308030 [DOI] [PubMed] [Google Scholar]
- 28. Ristow B, Ali S, Whooley MA, et al. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study). Am J Cardiol 2008;102:70–6. 10.1016/j.amjcard.2008.02.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McManus DD, Yin X, Gladstone R, et al. Alcohol Consumption, Left Atrial Diameter, and Atrial Fibrillation. J Am Heart Assoc 2016;5:e004060 10.1161/JAHA.116.004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McManus DD, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left atrial diameter over the adult life course: Clinical correlates in the community. Circulation 2010;121:667–74. 10.1161/CIRCULATIONAHA.109.885806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vaziri SM, Larson MG, Benjamin EJ, et al. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994;89:724–30. 10.1161/01.CIR.89.2.724 [DOI] [PubMed] [Google Scholar]
- 32. Kizer JR, Bella JN, Palmieri V, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS). Am Heart J 2006;151:412–8. 10.1016/j.ahj.2005.04.031 [DOI] [PubMed] [Google Scholar]
- 33. Benjamin EJ, D’Agostino RB, Belanger AJ, et al. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation 1995;92:835–41. 10.1161/01.CIR.92.4.835 [DOI] [PubMed] [Google Scholar]
- 34. Sanfilippo AJ, Abascal VM, Sheehan M, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 1990;82:792–7. 10.1161/01.CIR.82.3.792 [DOI] [PubMed] [Google Scholar]
- 35. Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006;47:2357–63. 10.1016/j.jacc.2006.02.048 [DOI] [PubMed] [Google Scholar]
- 36. Pritchett AM, Mahoney DW, Jacobsen SJ, et al. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol 2005;45:87–92. 10.1016/j.jacc.2004.09.054 [DOI] [PubMed] [Google Scholar]
- 37. Liu M, Chan CP, Yan BP, et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2012;14:39–44. 10.1093/eurjhf/hfr154 [DOI] [PubMed] [Google Scholar]
- 38. Su W, An T, Zhou Q, et al. Serum albumin is a useful prognostic indicator and adds important information to NT-proBNP in a Chinese cohort of heart failure. Clin Biochem 2012;45:561–5. 10.1016/j.clinbiochem.2012.02.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.