Abstract
Objectives
To assess Italian medical oncologists’ opinion on the implications of conflict of interest (COI) on medical education, care and research, and to evaluate their direct financial relationships.
Design
National cross-sectional survey conducted between March and April 2017 among Italian oncologists.
Setting
Online survey sponsored by the Italian College of Medical Oncology Chiefs through its website.
Participants
Italian oncologists who filled out an anonymous questionnaire including 19 items and individual and working characteristics.
Main outcome measure
The proportion of medical oncologists perceiving COI as an outstanding issue and those receiving direct payments from industry.
Results
There were 321 respondents, representing 13% of Italian tenured medical oncologists. Overall, 62% declared direct payments from the pharmaceutical industry in the last 3 years. Sixty-eight per cent felt the majority of Italian oncologists have a COI with industry, but 59% suppose this is not greater than that of other specialties. Eighty-two per cent consider that most oncology education is supported by industry. More than 75% believe that current allocation of industry budget on marketing and promotion rather than research and development is unfair, but 75% consider it appropriate to receive travel and lodging hospitality from industry. A median net profit margin of €5000 per patient enrolled in an industry trial was considered appropriate for the employee institution. Sixty per cent agree to receive a personal fee for patients enrolled in industry trials, but 79% state this should be reported in the informed consent. Over 90% believe that scientific societies should publish a financial report of industry support. Finally, 79% disagree to being a coauthor of an article written by a medical writer when no substantial scientific contribution is made.
Conclusions
Among Italian oncologists COI is perceived as an important issue influencing costs, education, care and science. A more rigorous policy on COI should be implemented.
Keywords: conflict of interest, survey oncologist, physician industry relationship, cancer drug prices, ghost writing
Strengths and limitations of this study.
This is the first national survey performed by Italian oncologists and one of the few prompted by medical oncologists regarding conflict of interest and physician–industry relationships in Europe.
The sample size of 321 is quite large, as it represents 13% of the 2260 tenured Italian certified medical oncologists from the 319 units of the country, making the results of the survey well founded.
Another strength of the questionnaire is its anonymous form, which favoured the disclosure of financial relationships with industry and an open attitude by respondents.
The limitations of this study include the non-random selection of the respondents and the greater representation of chiefs of staff compared with the overall population of medical oncologists.
Introduction
A conflict of interest (COI) exists when professional judgement concerning a primary interest such as patient welfare or the validity of research may be influenced by a secondary interest such as financial gain or career advancement. Financial relationships between industry and physicians and/or researchers are common, and may be direct, consisting of stock options, advisory fees, honoraria, speaking fees, travel and lodging expenses, or indirect, such as research support to researchers’ institutions. COI increasingly affects every aspect of medicine, including care, education, research integrity, patient trust, guideline formulation, regulatory approval and scientific prominence.1–7
Collaboration between industry and clinicians and/or researchers creates challenges and opportunities. While these relationships may contribute to advancement in the field, there is a need to better understand the negative consequences of COI and how best to report and manage it. Systematic reviews have found that pharmaceutical industry-sponsored studies are more often favourable to the sponsor’s product compared with studies having other sources of sponsorship.4 5 Public opinion on physician–pharmaceutical industry interactions differs depending on the context and specific country healthcare models,8 9 but some studies suggest a significant level of concern regarding interaction involving direct financial benefit to physicians.9 10
In medical oncology, financial relationships have increased through the years and have influenced clinical research, scientific prominence and visibility.11 12 The issue is particularly important given the increasing volume of investments made by the pharmaceutical industry in cancer treatment.13 In this price increase strategy,14 pharmaceutical companies tend to spend much more for marketing and promotional activities than for research and development.15 16 Evaluation of the clinical benefits that oncology drugs offer as a function of their cost has become complex, and for some clinical indications, health benefits are diminishing over time.17 Moreover, these benefits do not always follow the criteria of innovation18 and provide increasing financial toxicity to patients.19 There is concern that the substantial increase in drug prices may hamper both universal and private healthcare systems’ sustainability in many countries,14 20–22 while this is also of concern to top managers of pharmaceutical industries.23
The debate on COI has received attention in the USA since the introduction of the Physician Payments Sunshine Act (PPSA), which requires healthcare product manufacturers to report payments of more than $10 to physicians to the federal government. Together with transparency, the PPSA may increase medical professionalism, but it has received mixed opinions among physicians and experts in the field of COI.24 25 Conversely, little is known about the opinion of medical doctors in universal health systems such as those in Europe. A recent survey conducted in Italy showed that industry sponsorship of medical conferences is common, while the presence of a structured regulatory system is not. Disclosure of industry funding to medical societies was very limited.26
To ascertain the Italian situation, we assessed the opinion of Italian medical oncologists on the different aspects and implications of COI in a national survey.
Methods
The Italian College of Medical Oncology Chiefs (CIPOMO) set up an online national cross-sectional survey of its members. CIPOMO accounts for 184 chiefs of hospital oncology divisions/departments. Questionnaires were not sent directly to CIPOMO members. We used a passive approach to avoid intrusive claims, given the sensitivity of the topic, so the denominators are unknown. The survey was posted on the CIPOMO website for 6 weeks, and three reminder emails were sent to the regional delegates of CIPOMO to advertise the survey and to involve collaborators. Medical oncologists working in research institutions and university hospitals do not belong to CIPOMO, but those willing to participate who were informed by word of mouth were not excluded from the survey.
The questionnaire was authored by three members of CIPOMO and was based on outstanding issues in the oncology community and reviewed by eight members of the CIPOMO board of directors. After approval, the questionnaire was written using the ‘Surveymonkey’ platform (www.surveymonkey.com) and presented online from 1 March to 15 April 2017. CIPOMO members were reminded to complete the survey through three repeated email messages. Completion of the survey was anonymous, although baseline information (country area, age, sex, duration of oncology experience, type of institution and position) was requested before proceeding.
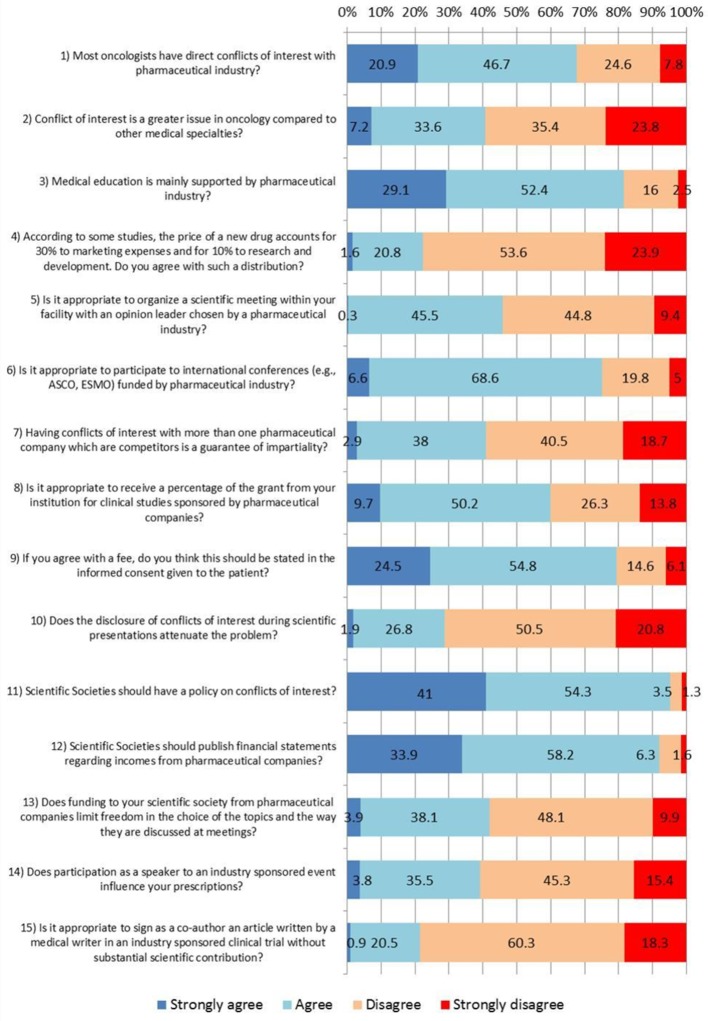
The survey was composed of 19 questions investigating feelings, opinions and experience of the respondents on different aspects of COI (figure 1 and the text). These include the following areas: the influence of COI on medical oncology and drug pricing; influence of the drug industry on continuing medical education (CME); the percentage of direct payments from industry; the acceptability of travel and lodging coverage by industry and per-patient fee for clinical trials and its disclosure in the informed consent; the payment amount of per-patient fee to the institution for a trial; the role of disclosure as a deterrent of COI; the influence of COI on scientific societies; the influence of COI on drug prescriptions; and opinion on ghost writing in scientific articles. The main outcome measure was the proportion of medical oncologists perceiving COI as an outstanding issue and those receiving direct payments from industry.
Figure 1.
Questions and answers evaluated with a 4-point Likert scale on conflict of interest (%). ASCO, American Society of Clinical Oncology; ESMO, European Society of Medical Oncology.
Respondents were requested to quantify in a 4-point Likert scale the extent to which they agreed with the proposed questions or statements. In the analysis, 17 answers were grouped to facilitate understanding of results (ie, ‘strongly agree’ plus ‘agree’ vs ‘strongly disagree’ plus ‘disagree’). One item on net profit margin led to an answer as a continuous variable, whereas another item on direct payment was dichotomised (yes, no).
Statistical analysis
Answers were collected by the online platform and transformed in a datasheet for analysis. Descriptive statistics (number, percentage) were used to show both the respondents’ characteristics and the general results. Moreover, an exploratory analysis of subgroups was performed considering the following explicative variables: geographical area (north, centre, south), sex (male, female), age (<45, 45–59, ≥60 years), place of work (hospital, university, research institute, other), nature of institution (public, private), job position (assistant chief, chief, other), years of oncology experience (<15, ≥15) and direct payment from industry in the last 3 years (no, yes). All answers to the questionnaire items were in turn used as dependent variables. Due to the explorative purpose of the analysis, no adjustment for Bonferroni’s inequality was made. Given the cross-sectional nature of the study, where the respondents were not randomly chosen, bidirectional χ2 tests assuming an alpha=0.05 as the significance level were calculated to provide a measure of the strength of association and not with inferential purposes. A sample size of at least 220 respondents was considered adequate as it represents 10% of the total medical oncologist population in Italy.
Patient and public involvement
The issues of increasing healthcare costs and of a trustful relationship between patients and physicians were the main reasons of the survey and were highlighted in the Introduction section. Neither patients nor the public were involved in this study. The findings of the survey will be disseminated through a press release and media coverage. A position paper on COI by CIPOMO is under preparation.
Results
There were 321 respondents, from all 20 Italian regions, representing 13% of the 2260 tenured Italian certified medical oncologists from the 319 oncology units of the country, according to the White Book of the Italian Association of Medical Oncology.27 The characteristics of the respondents are summarised in table 1. They reflect the main characteristics of the Italian population of oncologists, with the majority of them employed in northern Italy, having equal sex distribution, a third being aged 45 years or younger, and working predominantly in public hospitals. However, there was a greater proportion of chiefs of staff because of the nature of the study sponsor.
Table 1.
Main characteristics of the respondents
| n (%) | |
| Geographical area | |
| North | 161 (50.2) |
| Centre | 108 (33.6) |
| South | 52 (16.2) |
| Age in years | |
| <45 | 103 (32.1) |
| 45–59 | 133 (41.4) |
| ≥60 | 85 (26.5) |
| Sex* | |
| Male | 170 (53.3) |
| Female | 149 (46.7) |
| Place of work | |
| Hospital | 283 (88.2) |
| University | 20 (6.2) |
| Research institute | 11 (3.4) |
| Other | 7 (2.2) |
| Nature of institution | |
| Public | 296 (92.2) |
| Private | 25 (7.8) |
| Job position | |
| Assistant chief | 190 (59.2) |
| Chief | 98 (30.5) |
| Other | 33 (10.3) |
| Years of experience | |
| <15 | 88 (27.4) |
| ≥15 | 233 (72.6) |
| Direct payment from industries in the last 3 years* | |
| No | 120 (37.6) |
| Yes | 199 (62.4) |
*Two oncologists did not answer the question.
The questionnaire and the answers concerning COI are described in figure 1. Over two-thirds (68%) believe the majority of Italian oncologists have a COI with industry. A subgroup analysis indicates a greater proportion of them among women, younger physicians, assistant chiefs and those who did not receive payments from industry in the last 3 years (p<0.05; table 2). However, 59% assume COI in oncology is no greater than in other medical specialties.
Table 2.
Subgroup analysis on question 1: do you believe most oncologists have direct conflict of interests with pharmaceutical companies?
| Disagree | Agree | P values* | |||
| n | % | n | % | ||
| Country area | |||||
| North | 112 | 69.6 | 49 | 30.4 | <0.440 |
| Centre | 68 | 63.0 | 40 | 37.0 | |
| South | 37 | 71.2 | 15 | 28.8 | |
| Sex | <0.001 | ||||
| Female | 84 | 56.4 | 65 | 43.6 | |
| Male | 131 | 77.1 | 39 | 22.9 | |
| Age | <0.057 | ||||
| <45 | 61 | 59.2 | 42 | 40.8 | |
| 45–59 | 92 | 69.2 | 41 | 30.8 | |
| ≥60 | 64 | 75.3 | 21 | 24.7 | |
| Workplace | |||||
| Research institute | 9 | 81.8 | 2 | 18.2 | <0.583 |
| Hospital | 189 | 66.8 | 94 | 33.2 | |
| University | 15 | 75.0 | 5 | 25.0 | |
| Other | 4 | 57.1 | 3 | 42.9 | |
| Type of structure | <0.350 | ||||
| Private | 19 | 76.0 | 6 | 24.0 | |
| Public | 198 | 66.9 | 98 | 33.1 | |
| Job position | <0.021 | ||||
| Assistant chief | 119 | 62.6 | 71 | 37.4 | |
| Chief | 77 | 78.6 | 21 | 21.4 | |
| Other | 21 | 63.6 | 12 | 36.4 | |
| Years of experience | <0.023 | ||||
| <15 | 51 | 58.0 | 37 | 42.0 | |
| ≥15 | 166 | 71.2 | 67 | 28.8 | |
| Direct payments from industry | <0.029 | ||||
| No | 72 | 60.0 | 48 | 40.0 | |
| Yes | 143 | 71.9 | 56 | 28.1 | |
*Referred to bidirectional χ2 test.
Overall, 62% declared general payments from the pharmaceutical industry in the last 3 years, with a significantly greater proportion among those living in southern Italy, men, oncologists working in research institutes and chiefs of staff (p<0.05; table 3).
Table 3.
Subgroup analysis on the question ‘Have you received any payment to speak at educational meetings sponsored by a pharmaceutical company in the last 3 years?’
| No | Yes | P values* | |||
| n | % | n | % | ||
| Country area | <0.002 | ||||
| North | 57 | 35.4 | 104 | 64.6 | |
| Centre | 52 | 49.1 | 54 | 50.9 | |
| South | 11 | 21.1 | 41 | 78.9 | |
| Sex | |||||
| Female | 69 | 46.6 | 79 | 53.4 | <0.002 |
| Male | 51 | 30.0 | 119 | 70.0 | |
| Age | <0.715 | ||||
| <45 | 41 | 39.8 | 62 | 60.2 | |
| 45–59 | 50 | 38.2 | 81 | 61.8 | |
| ≥60 | 29 | 34.1 | 56 | 65.8 | |
| Workplace | <0.003 | ||||
| Research institute | 0 | 0.0 | 11 | 100.0 | |
| Hospital | 106 | 37.6 | 176 | 62.4 | |
| University | 8 | 42.1 | 11 | 57.9 | |
| Other | 6 | 85.7 | 1 | 14.3 | |
| Type of structure | <0.493 | ||||
| Private | 11 | 44.0 | 14 | 56.0 | |
| Public | 109 | 37.1 | 185 | 62.9 | |
| Job position | <0.016 | ||||
| Assistant chief | 72 | 38.3 | 116 | 61.7 | |
| Chief | 29 | 29.6 | 69 | 70.4 | |
| Other | 19 | 57.6 | 14 | 42.4 | |
| Years of experience | <0.314 | ||||
| <15 | 37 | 42.0 | 51 | 58.0 | |
| ≥15 | 83 | 35.9 | 148 | 64.1 | |
*Referred to bidirectional χ2 test.
Eighty-one per cent believe that most oncology education is supported by industry, with a greater proportion among older physicians and chiefs of staff (p<0.05), while over 70% think their CME should be supported by their institution or public sources, and only less than 10% and 20% think it should be paid for by themselves and the industry, respectively (table 4). The vast majority stated their first CME tool is scientific journals (89%), but 14% use pharmaceutical representatives as their main CME source.
Table 4.
Role of public entities and private industry in continuing medical education (CME) support
| Questions | Important or very important score 4+5, n (%) |
| 1. Which method do you primarily use for your CME? You can select multiple choices and attribute different scores from ‘not at all important’ (1) to ‘very important’ (5). | |
| Medical websites. | 185 (60.8) |
| Scientific journals. | 278 (89.1) |
| CME courses. | 181 (59.5) |
| Conferences. | 211 (67.4) |
| Pharmaceutical representatives. | 42 (13.7) |
| Books. | 62 (20.9) |
| 2. Who should pay for your CME? You can select multiple choices and attribute different scores from ‘not at all important’ (1) to ‘very important’ (5). | |
| Myself. | 27 (9.3) |
| Hospital. | 256 (83.1) |
| Public institutions. | 211 (70.3) |
| Pharmaceutical companies. | 51 (17.3) |
| Research foundations. | 140 (48.1) |
However, 54% of the medical oncologists consider it inappropriate to organise a scientific meeting within his/her facility with an opinion leader chosen by a pharmaceutical company, especially in the north and among the chiefs of staff (p<0.05).
About 77% believe that the greater allocation of budget placed by industry on marketing and promotion relative to research and development is inappropriate, with a greater proportion of supporters among younger physicians and non-chiefs of staff (p<0.05), but 75% of all respondents consider it appropriate to receive travel and lodging hospitality from industry to attend international meetings, with a significantly greater proportion of supporters among those receiving direct industry payments (p<0.05).
A median net profit margin of €5000 (mean±SD=€9888±€10 414) per patient enrolled in a trial was considered an appropriate amount for the investigator’s institution, although the distribution had a long tail towards higher values.
Sixty per cent would agree to receive a personal fee for each patient enrolled in an industry-sponsored trial, with a greater proportion among those who received payments from industry (p<0.05), but 79% state this should be reported in the patient’s informed consent.
Nearly 60% think that disclosing a COI with different companies that are competitors is not a guarantee of impartiality, and 71% believe that COI disclosure does not attenuate the risk of scientific bias. However, 48% of those working in private institutions vs 27% of those working in public institutions believe that COI disclosure attenuates the problem (p<0.05).
Over 90% believe that scientific societies should have a COI policy and that a detailed report of the financial support by the industry should be published annually. A total of 58% believe that industry support does not influence topic selection in meetings, and 61% believe that giving an invited speech by industry does not influence their drug prescription. However, a higher proportion of male and older physicians feel that prescription is influenced by direct industry payments (p<0.05).
Finally, 79% consider it unfair to be a coauthor of an article written by a medical writer for an industry-sponsored trial when no substantial scientific contribution is made. However, 25% of those receiving industry payments believe this is appropriate vs 15% of those who did not (p<0.05).
Discussion
With the introduction of the open PPSA and the increasing costs of healthcare, the debate on financial COI has received a great deal of attention in the USA.1 24 25 28 Particularly in Europe, however, a direct perspective by the medical community on this matter is still unclear.
The main findings from this anonymous questionnaire indicate that two-thirds of Italian medical oncologists believe that COI is a relevant issue, with a higher perception among women, young physicians, assistant chiefs of staff and those not receiving industry payments in the last 3 years. Although nearly 60% suppose this is not a greater issue in oncology than in other medical specialties, this does not mitigate the potential impact of the problem. Second, 62% of the sample declared direct payments from the pharmaceutical industry in the last 3 years, with a greater frequency in southern Italy, research hospitals, chiefs of staff and male physicians.
Over 80% confirm that most oncology education and training is financially supported by industry, with a greater proportion of followers among older physicians and chiefs. Subgroup analyses also show there is a greater awareness of COI as a problem among women and young doctors, who are also among those categories receiving fewer payments from industry. While it is difficult to establish a causal relationship between increased awareness and lower frequency of payments (the younger and female physicians groups might have a more idealistic attitude), the gender disparity in industry relationships is a well-known phenomenon. In recent American analyses, only a quarter of physicians receiving payments were female, who on average also received less money per person than men.29 In our study, 70% of male vs 53% of female physicians received direct payments from industry for speaking fees in the last 3 years. This percentage is in line with that reported by a recent survey through the open payment act in the USA, where 63% of oncologists received a general payment in 2014.30 Oncologists were also more likely to receive a general payment and to hold ownership interest compared with non-oncologists.30
Another important source of funding from industry is research. Interestingly, while 60% of physicians would agree to receive a percentage fee for every patient enrolled in an industry-sponsored trial, nearly 80% are favourable to disclose it in the patient’s informed consent. This is a significant inclination towards transparency among our professional community that has not yet been translated in regulatory acts by the current legislation regulating clinical trials. This is also important because physician payment for study participation in clinical trials is a potential COI that can adversely affect patient trust.10 31
The median net margin for the employee institution that was considered balanced for each patient enrolled in an industry trial was €5000, which appears significantly lower than the current level of industry per-patient fee, where the gross fee may now easily exceed €30 000. The vast majority of respondents is also contrary to the current escalating trend to spend more for marketing and promotion than for research and development by industry, a notion which is rarely openly declared by industry.15 16 These considerations suggest that the surveyed sample is aware that the current trend to increasing costs also has a negative impact on quality of care once the drug is licensed. In the USA, patients with cancer carry rising burdens of healthcare-related out-of-pocket expenses, and a growing number of patients are considered underinsured. To save money, a large proportion of these patients take less or nothing of the prescribed medications, a phenomenon known as financial toxicity, which has also been described in the context of the Italian healthcare system.19 32
Nearly 80% consider it unfair to be a coauthor of an article written by a medical writer for an industry-sponsored trial when no substantial scientific contribution has been made. This is in contrast to the present trend of most industry-sponsored trials to be reported by medical writers, often in concomitance with presentation at premier international meetings.11 The legal and ethical consequences of ghost writing, including the risk of plagiarism and loss of professionalism and genuine intellectual contribution to the advancement of science, are a subject of intense debate.33 34
Over 70% of the oncologists think their CME should be supported by their institution or public sources and less than 10% by personal resources. The vast majority stated their first CME tool is scientific journals, but nearly 15% use industry sales representatives as the main CME method. These findings are in line with the public landscape of our national health system medical doctors, where CME is considered a right that should be covered by public resources and not a duty to be at least partially covered by physician resources. Three-quarters of Italian oncologists would agree to be financially supported by industry for travel and lodging at international meetings, another important source of industry expenditures. It is possible that this form of financial support is perceived as less conflicting and as the only way to attend important meetings given the scarcity of public or private no-profit funding.
Interestingly, over 70% believe that COI disclosure during presentations does not attenuate the risk of scientific bias. A recent study35 also showed that disclosure can be incomplete by using the term ‘unpaid consultant’, whereby many doctors fail to identify research funding, conference fees, travel expenses or other benefits. However, approximately 60% believe that industry support does not influence topic selection at meetings and that giving invited speeches does not influence personal drug prescription.
Another important issue raised by our survey is the call for a higher level of transparency by scientific societies, including annual detailed reporting of industry payments. Prior studies have shown that disclosure of COI among Italian scientific societies does not attenuate the problem but in fact seems to be a justification to increase financial relationships.26
The consequences of financial COI on patient perception have been the subject of recent studies.8 10 36 In an American Society of Clinical Oncology (ASCO) survey of COI policies, the majority of non-physicians and patient advocates felt that full disclosure of COI by physicians was expected and could be a factor in patients’ decisions regarding therapy.37
Altogether, the answers to the survey clearly show that the direct economic relationship between clinicians and industry is deeply rooted in current practice. Money from industry regularly flows as the result of declared marketing investments in the context of legal pathways. The hidden question is whether a clinician who receives financial support for various activities in his profession can be impartial and objective in making clinical decisions. This is particularly true in all those clinical settings where uncertainties about the added value of new drugs make treatment choices questionable.17 18 20–22 Most recent evidence indicates that the majority of cancer drugs registered in Europe by the European Medicines Agency do not show a benefit in terms of survival or quality of life,38 indicating the necessity to raise the evidence bar before market approval.39 Moreover, in a recent analysis of 10 approved cancer drugs in the USA, the median cost of developing a drug was $648 000 000, a figure significantly lower than prior estimates. The revenue after 4 years of approval was substantial (median, $1658.4 million; range, $204.1–$22 275.0 million),40 suggesting the need for a significant reduction of expenses for marketing and promotional activities, including paying doctors for a variety of activities, to guarantee sustainable health systems. The results of our study are also consistent with the international research context on this topic,2–7 underlying the increasing importance of COI on practice41 and research.42
To our knowledge this is the first national survey performed by Italian oncologists and one of the few prompted by medical oncologists regarding their COI and physician–industry relationships. The questionnaire in an anonymous form probably favoured the disclosure of financial relationships with industry and an open attitude by respondents. The study has limitations, including the non-random selection of the respondents and the greater representation of chiefs of staff. A strength of our study, however, is the relatively large sample size, which may overcome the limitations and possibly reflect the general characteristics of medical oncologists in Italy.27
Our study indicates that among Italian oncologists, COI is perceived as an important issue influencing education, quality of care, science and costs. The overall view on COI calls for a process of rethinking of the relationship between clinicians and industry, and most importantly a courageous step towards transparency. The results seem to indicate a need for education about the effect of sponsored education on the attitudes and on the prescribing behaviour and the extent to which industry sponsorship affects clinical trial results. However, disclosure cannot be the only answer and all components of the healthcare system are called into action. Health institutions should promote and finance professional education, and industry should transparently contribute to research and increase quality of care. Most importantly, we suggest that the financial relationships between industry and clinicians should always be mediated by the employee’s institution. In the present context of increasing healthcare costs and financial toxicity, alternative ways to support education and research and strict transparency policies could contribute to increased patient trust, sustainability and equity in healthcare access. These principles are being proposed in a forthcoming policy document on COI that will be endorsed by CIPOMO, spread among all Italian oncologists and proposed to the Italian health authorities.
Supplementary Material
Acknowledgments
The authors wish to thank Katherine Brandt for reviewing the English language, and Cristina Giua and Tania Buttiron Webber for technical assistance.
Footnotes
Contributors: ADC, GN, FR: idea, planning the data set, data analysis, wrote the manuscript, final approval of the version to be published. EB, BR, VL: planning the data set, statistical analysis, wrote the manuscript, final approval of the version to be published. FA, LF, CV: revision of the manuscript for important intellectual content, final approval of the version to be published. MT, MC: revision of the manuscript for important intellectual content, standing funding, final approval of the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The Italian College of Medical Oncology Chiefs (CIPOMO) supported the online questionnaire set-up, but the content of the paper does not necessarily represent its view.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Ethics approval was not required because the research survey was considered morally acceptable and could not risk harming the study participants. Moreover, Italian legislation does not require ethics approval for research not involving patients.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are stored on the CIPOMO server and are available on request.
Collaborators: Katherine Brandt, Cristina Giua, Tania Buttiron Webber.
Contributor Information
Collaborators: Katherine Brandt, Cristina Giua, and Tania Buttiron Webber
References
- 1. Stead WW. The Complex and Multifaceted Aspects of Conflicts of Interest. JAMA 2017;317:1765–7. 10.1001/jama.2017.3435 [DOI] [PubMed] [Google Scholar]
- 2. McCarthy M. PubMed is urged to include competing interest information in abstracts. BMJ 2016;353:i2018 10.1136/bmj.i2018 [DOI] [PubMed] [Google Scholar]
- 3. McCarthy M. US doctors earn speaking and consulting fees from drug companies that sponsor their research. BMJ 2014;348:g2410 10.1136/bmj.g2410 [DOI] [PubMed] [Google Scholar]
- 4. Lundh A, Lexchin J, Mintzes B, et al. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;2:MROOOO33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahn R, Woodbridge A, Abraham A, et al. Financial ties of principal investigators and randomized controlled trial outcomes: cross sectional study. BMJ 2017;356:i6770 10.1136/bmj.i6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell AP, Basch EM, Dusetzina SB. Financial Relationships With Industry Among National Comprehensive Cancer Network Guideline Authors. JAMA Oncol 2016;2:1628–31. 10.1001/jamaoncol.2016.2710 [DOI] [PubMed] [Google Scholar]
- 7. Wise J. Still too little transparency among guideline writers and others. BMJ 2017;356:j276 10.1136/bmj.j276 [DOI] [PubMed] [Google Scholar]
- 8. Hampson LA, Agrawal M, Joffe S, et al. Patients' views on financial conflicts of interest in cancer research trials. N Engl J Med 2006;355:2330–7. 10.1056/NEJMsa064160 [DOI] [PubMed] [Google Scholar]
- 9. Holbrook A, Lexchin J, Pullenayegum E, et al. What do Canadians think about physician-pharmaceutical industry interactions? Health Policy 2013;112:255–63. 10.1016/j.healthpol.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 10. Klein E, Solomon AJ, Corboy J, et al. Physician compensation for industry-sponsored clinical trials in multiple sclerosis influences patient trust. Mult Scler Relat Disord 2016;8:4–8. 10.1016/j.msard.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 11. Moy B, Bradbury AR, Helft PR, et al. Correlation between financial relationships with commercial interests and research prominence at an oncology meeting. J Clin Oncol 2013;31:2678–84. 10.1200/JCO.2012.46.6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundh A, Barbateskovic M, Hróbjartsson A, et al. Conflicts of interest at medical journals: the influence of industry-supported randomised trials on journal impact factors and revenue - cohort study. PLoS Med 2010;7:e1000354 10.1371/journal.pmed.1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. IMS Institute for healthcare information. Global oncology trend report a review of 2015 and outlook to 2020. http://www.imshealth.com/en/thought-leadership/quintilesims-institute/reports/global-oncology-trend-report-a-review-of-2015-and-outlook-to-2020
- 14. Saltz LB. Perspectives on cost and value in cancer care. JAMA Oncol 2016;2:19–21. 10.1001/jamaoncol.2015.4191 [DOI] [PubMed] [Google Scholar]
- 15. Laurance J. Makers of anticancer drugs are "profiteering," say 100 specialists from around the world. BMJ 2013;346:f2810 10.1136/bmj.f2810 [DOI] [PubMed] [Google Scholar]
- 16. Anderson R. Pharmaceutical industry gets high on fat profits. By Business reporter, BBC News 6 November 2014. http://www.bbc.com/news/business-28212223
- 17. Cressman S, Browman GP, Hoch JS, et al. A time-trend economic analysis of cancer drug trials. Oncologist 2015;20:729–36. 10.1634/theoncologist.2014-0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol 2015;1:539–40. 10.1001/jamaoncol.2015.0373 [DOI] [PubMed] [Google Scholar]
- 19. Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 2013;18:381–90. 10.1634/theoncologist.2012-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kantarjian HM, Fojo T, Mathisen M, et al. Cancer drugs in the United States: Justum Pretium--the just price. J Clin Oncol 2013;31:3600–4. 10.1200/JCO.2013.49.1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfister DG. The just price of cancer drugs and the growing cost of cancer care: oncologists need to be part of the solution. J Clin Oncol 2013;31:3487–9. 10.1200/JCO.2013.50.3466 [DOI] [PubMed] [Google Scholar]
- 22. Fricker J. New NICE criteria for drug access. Lancet Oncol 2017;18:576 10.1016/S1470-2045(17)30235-8 [DOI] [PubMed] [Google Scholar]
- 23. Jimenez J. Why the approach to drug pricing has to change now. 2016. https://www.forbes.com/sites/sciencebiz/2016/11/01/why-the-approach-to-drug-pricing-has-to-change-now/#62f0330657fc
- 24. Chimonas S, DeVito NJ, Rothman DJ. Bringing transparency to medicine: exploring physicians' views and experiences of the sunshine act. Am J Bioeth 2017;17:4–18. 10.1080/15265161.2017.1313334 [DOI] [PubMed] [Google Scholar]
- 25. Lenzer J, Brownlee S. Diverting attention from financial conflicts of interest. BMJ 2015;350:h3505 10.1136/bmj.h3505 [DOI] [PubMed] [Google Scholar]
- 26. Fabbri A, Gregoraci G, Tedesco D, et al. Conflict of interest between professional medical societies and industry: a cross-sectional study of Italian medical societies' websites. BMJ Open 2016;6:e011124 10.1136/bmjopen-2016-011124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Libro bianco VI edizione. Milan, Italy: Associazione Italiana di Oncologia Medica. 2015. http://www.aiom.it/libro-bianco-2015/professionisti/documenti-scientifici/pubblicazioni/libro-bianco-2015/libro-bianco-2015/1,759,1
- 28. Rose SL, Krzyzanowska MK, Joffe S. Relationships between authorship contributions and authors' industry financial ties among oncology clinical trials. J Clin Oncol 2010;28:1316–21. 10.1200/JCO.2008.21.6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tringale KR, Marshall D, Mackey TK, et al. Types and distribution of payments from industry to physicians in 2015. JAMA 2017;317:1774–84. 10.1001/jama.2017.3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marshall DC, Moy B, Jackson ME, et al. Distribution and patterns of industry-related payments to oncologists in 2014. J Natl Cancer Inst 2016;108:djw163 10.1093/jnci/djw163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen L. Patients can’t trust doctors' advice if we hide our financial connections with drug companies. BMJ 2014;348:g167 10.1136/bmj.g167 [DOI] [PubMed] [Google Scholar]
- 32. Perrone F, Jommi C, Di Maio M, et al. The association of financial difficulties with clinical outcomes in cancer patients: secondary analysis of 16 academic prospective clinical trials conducted in Italy. Ann Oncol 2016;27:2224–9. 10.1093/annonc/mdw433 [DOI] [PubMed] [Google Scholar]
- 33. Das N, Panjabi M. Plagiarism: why is it such a big issue for medical writers? Perspect Clin Res 2011;2:67–71. 10.4103/2229-3485.80370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wislar JS, Flanagin A, Fontanarosa PB, et al. Honorary and ghost authorship in high impact biomedical journals: a cross sectional survey. BMJ 2011;343:d6128 10.1136/bmj.d6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menkes DB, Masters JD, Bröring A, et al. What does ’Unpaid Consultant' signify? A survey of euphemistic language in conflict of interest declarations. J Gen Intern Med 2018;33:139–41. 10.1007/s11606-017-4225-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong YN, Schluchter MD, Albrecht TL, et al. Financial concerns about participation in clinical trials among patients with cancer. J Clin Oncol 2016;34:479–87. 10.1200/JCO.2015.63.2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lockhart AC, Brose MS, Kim ES, et al. Physician and stakeholder perceptions of conflict of interest policies in oncology. J Clin Oncol 2013;31:1677–82. 10.1200/JCO.2012.47.5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davis C, Naci H, Gurpinar E, et al. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ 2017;359:j4530 10.1136/bmj.j4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen D. Cancer drugs: high price, uncertain value. BMJ 2017;359:j4543 10.1136/bmj.j4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prasad V, Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med 2017;177:1569 10.1001/jamainternmed.2017.3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med 2007;356:1742–50. 10.1056/NEJMsa064508 [DOI] [PubMed] [Google Scholar]
- 42. Rasmussen K, Schroll J, Gøtzsche PC, et al. Under-reporting of conflicts of interest among trialists: a cross-sectional study. J R Soc Med 2015;108:101–7. 10.1177/0141076814557878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.