Abstract
Anaplastic astrocytoma (AA) is a diffusely infiltrating, malignant, astrocytic, primary brain tumor. AA is currently defined by histology although future classification schemes will include molecular alterations. AA can be separated into subgroups, which share similar molecular profiles, age at diagnosis and median survival, based on 1p/19q co-deletion status and IDH mutation status. AA with co-deletion of chromosomes 1p and 19q and IDH mutation have the best prognosis. AA with IDH mutation and no 1p/19q co-deletion have intermediate prognosis and AA with wild-type IDH have the worst prognosis and share many molecular alterations with glioblastoma. Treatment of noncodeleted AA based on preliminary results from the CATNON clinical trial consists of maximal safe resection followed by radiotherapy with post-radiotherapy temozolomide (TMZ) chemotherapy. The role of concurrent TMZ and whether IDH1 subgroups benefit from TMZ is currently being evaluated in the recently completed randomized, prospective Phase III clinical trial, CATNON.
KEYWORDS : anaplastic astrocytoma, malignant glioma, WHO grade III glioma
Practice points.
AA is malignant astrocytic tumor most commonly seen in comparatively young patients (median age 41 years).
Molecular pathology has further subdivided AA into multiple molecular subtypes based upon interrogation of 1p/19q codeletion, IDH1, ATRX and p53 mutations.
The main molecular categories of AA are IDH1 mutated and IDH1 wild type which have profoundly differing survival.
Based upon two recently completed clinical trials, CATNON and RTOG 9813, initial treatment of AA entails post-radiotherapy TMZ or a nitrosourea.
Uncertain currently is whether the IDH1 mutation alters these recommendations and in particular is there benefit in treating IDH1 wild type AA with post-radiotherapy chemotherapy.
Salvage therapy for recurrent AA similar to GBM utilizes re-resection, re-radiation and cytotoxic chemotherapy in the appropriate clinical context notwithstanding only modest efficacy.
The role for immunotherapy (vaccine or immune checkpoint inhibitors) as well as small molecule inhibitors of IDH1 are yet to be realized and are under active investigation.
Background
Anaplastic astrocytoma (AA) is a diffusely infiltrating, malignant, astrocytic, primary brain tumor with a median age of onset of 41 years [1]. Presently, AA is defined by the histologic characteristics of nuclear atypia, increased cellularity, significant proliferative activity as manifested by mitoses and lacking either endothelial proliferation or necrosis, the two pathologic hallmarks of glioblastoma [1]. Approximately a quarter of AA arise as a de novo tumor, whereas it is estimated that three quarters are a consequence of transformation from a lower-grade astrocytoma [2]. AA constitutes 4% of all malignant CNS tumors and 10% of all gliomas [3]. The survival of patients with AA varies depending upon molecular pathology. With conventional treatment, median overall survival (mOS) and 5-year survival rates are 3 years and 28%, respectively [3,4]. Exposure to ionizing radiation and rare genetic syndromes such as neurofibromatosis type 1 and 2, tuberous sclerosis and the Li-Fraumeni syndrome are the only established risk factors.
Patients with AA most often present with focal or generalized neurologic symptoms. The specific focal symptom depends upon the anatomic localization of the tumor and may include weakness, sensory loss, visual impairment, language dysfunction and gait disorder. Generalized symptoms include personality changes, seizures and headaches. Compared to low grade astrocytoma, seizure at presentation is less common: 83 versus 46% [5].
Imaging
MRI with administration of gadolinium contrast is the optimal imaging modality for diagnosis and management of AA. MRI assists in establishing a differential diagnosis, guides biopsy or resection, is fundamental for treatment (i.e., radiotherapy) planning, monitoring response to treatment and in part determines disease progression. MRI reveals AA to be an ill-defined, T1-weighted hypointense and T2-weighted hyperintense mass with surrounding vasogenic edema. Nodular areas of enhancement are usually observed, although nearly one third of AA display no contrast enhancement [2]. Contrast enhancement usually implies the presence of a higher grade component notwithstanding if a biopsy demonstrates a low-grade tumor as sampling error may occur. Advanced imaging such as diffusion-weighted MRI imaging, MR spectroscopy, MR perfusion and amino acid positron emission tomography imaging may further assist in the diagnosis and management of AA [2,6–10].
Pathology
• Histology
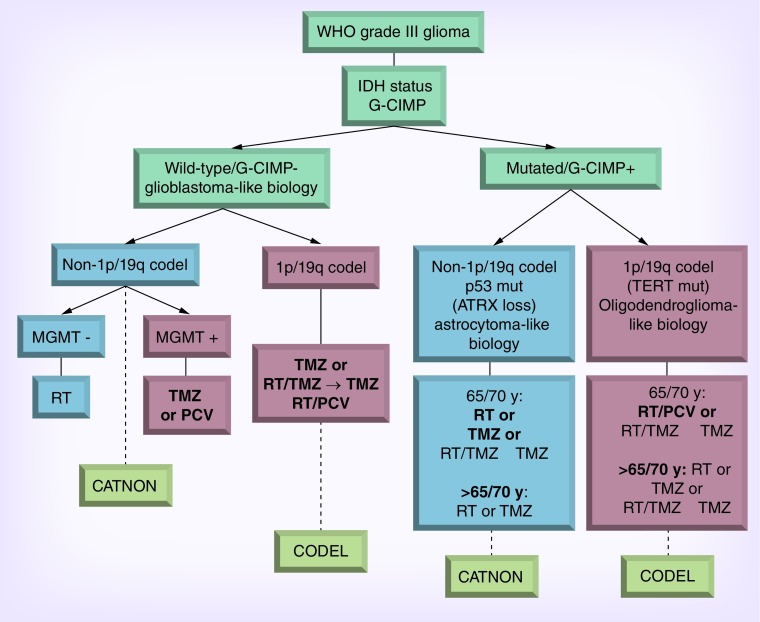
AA frequently reveals heterogeneous histology consisting of areas of low- and high-grade tumor that is thought to reflect progression from a lower grade tumor precursor. The diagnosis of AA is sometimes determined from biopsy in which sampling error may occur. The WHO classification system is the most widely used instrument for grading glial tumors. AA, defined by WHO as a grade III anaplastic glioma, is characterized by mitotic activity, increased cellularity, presence of glial makers (e.g., GFAP), absence of neuronal markers and nuclear atypia. Necrosis or microvascularization by contrast absent and rather define glioblastoma [1]. The MIB-1 labeling index in AA is usually 5–10% but may overlap with both low grade astrocytoma and glioblastoma (GB) and may show considerable variation within a given tumor [11,12]. Notably the histologic classification and grading of infiltrating low grade and anaplastic gliomas has poor reproducibility among pathologists and often poorly predicts clinical outcome [13,14]. Increasingly, clinicians are utilizing the molecular classification of gliomas to guide clinical decision-making (Figure 1) [15–18]. Perhaps this is no more evident than in anaplastic oligoastrocytoma wherein this anachronistic term is now resolvable by molecular pathology into either anaplastic oligodendroglioma or anaplastic astrocytoma (see below).
Figure 1. . Molecular stratification of anaplastic gliomas.
PCV: Procarbazine, CCNU (lomustine), vincristine; RT: Radiotherapy; TMZ: Temozolomide.
• Molecular
There is no single molecular marker that defines AA, although mutations in TP53 and ATRX are most frequent (>70%) [19], and differentiate AA from oligodendroglial tumors which are defined by codeletion of chromosomes 1p and 19q. Mutation of the ATRX gene is identified by immunohistochemistrty and is mutually exclusive with 1p/19q codeletion and TERT promoter mutations (discussed below). Mutations of the ATRX gene result in a truncated protein and abrogated protein expression. ATRX is a multiprotein complex important in incorporating histones H3.3 into telmeric regions of chromosomes and are one mechanism of telomere maintenance. Mutations of the isocitrate dehydrogenase enzymes (IDH1 and IDH2) play a critical role in the pathogenesis of most AA and secondary GB [20–23]. The IDH enzymes catalyze the metabolic conversion of isocitrate to α-ketoglutarate, a key metabolite of the Krebs cycle [21]. The IDH enzymes utilize NAD+ as a cofactor in generating α-ketoglutarate and NADPH in a reversible reaction. The overwhelming majority (95%) of IDH mutations in gliomas, affect IDH1 and in particular, the IDH1 R132H genotype (see below) [23]. The IDH1 mutations target specific arginine residues resulting in a novel gain-of-function phenotype whereby the mutant enzymes produce high levels of what is ordinarily a minor metabolic product R(-)-2-hydroxyglutarate and NADPH [20,22–25]. Unclear at present is the role of 2-hydroxyglutarate in glioma development. It is uncertain whether therapy targeting IDH mutations will be therapeutically beneficial, a hypothesis currently being evaluated in early phase clinical trials using small molecule inhibitors of IDH1 [26]. The presence of the IDH mutation in AA is a positive prognostic factor independent of age and MGMT promoter methylation status [27–29].
The Cancer Genome Atlas (TCGA) GB and diffuse lower-grade glioma projects have resulted in an enormous amount of data concerning genomic alterations in gliomas [17,20,22,30]. The initial TCGA data focused primarily on primary (de novo) GB and the small fraction of secondary glioblastoma. AA is believed to share a common lineage and molecular pathology with secondary GB [31,32]. Utilizing gene expression profiling, Phillips et al. identified three main subgroups of high-grade glioma (proneural, proliferative and mesenchymal) defined by distinct molecular pathology [33]. Essentially all AA and good prognosis GB that included all secondary GB were classified as proneural. The proneural subtype had the best prognosis among all GB and the expression profile overexpressed genes associated with normal brain processes and neurogenesis rather than gene expression indicative of cell proliferation and angiogenesis typically found in the other two GB subtypes. Notwithstanding this categorization, the relevance of these molecular subtypes to treatment is not yet determined.
More recently, TCGA Research Network performed a genome-wide analyses of 239 grade II and grade III gliomas from adults, incorporating exome sequence, DNA copy number, DNA methylation, messenger RNA expression, miRNA expression and targeted protein expression [17]. The sequencing revealed frequent mutations in IDH1, TP53, ATRX, CIC, FUBP1, NOTCH1 and the TERT promoter. In an unsupervised analyses, the authors compiled clusters of tumors with shared molecular profiles generated on multiple platforms and proceeded to integrate these in a higher level of analysis termed a ‘cluster of clusters’ analysis [34]. This resulted in three principle disease subgroups on the basis of IDH mutation and 1p/19q codeletion, two molecular alterations thought to occur early in glial oncogenesis. These subgroups and their frequently associated genetic alterations are: #1 IDH mutation and 1p/19q deletion (telomerase reverse transcriptase promoter [TERTp], CIC and FUBP1 mutated); #2 IDH mutation and no 1/19q codeletion (TP53 and ATRX mutated); and #3 IDH wild-type (EGFR, NF1 and PTEN mutated, amplification of EGFR or focal deletion of CDKN2A). The prognostic significance of these subgroups was more accurate than that produced by histology. All AA clustered into groups #2 and #3. The age at diagnosis and median survival for groups #2 and #3 were 38 years, 6.3 years and 50 years, 1.7 years, respectively.
Similarly, Suzuki et al. delineated the genetic alterations and affected pathways in WHO grade II and grade III gliomas by combining two large sets of throughput sequencing data (a Japanese data set and the TCGA data set) [35]. Their analysis revealed that WHO grade II and grade III gliomas could be classified into three subtypes which they designated types I–III: type I (38–42%, mOS 8 years) IDH1 mutation and 1p19q codeleted (associated with CIMP [CpG island methylation phenotype] positive, TERTp, CIC, FUBP1, NOTCH and PI3KCA mutated); type II (37%, mOS 6 years) IDH mutation and TP53 mutation (associated with CIMP positive and ATRX mutated); type 3 (25%, mOS 2 years) IDH1 wild-type (associated with EGFR amplification, PDGFRA mutated, 10p deletion and 7p gain). Further WHO grade III tumors in the type I or type II subtype showed overall survival comparable to that of corresponding oligodendroglioma and astrocytoma WHO grade II tumors. In terms of clinical outcome, pattern of mutations, DNA methylation and gene expression, type III glioma was most similar to GB.
Another study defined five glioma molecular groups based on the use of three molecular alterations (1p/19q codeletion, IDH mutation and TERTp mutation) that are thought to occur early during glioma formation, prevalent in glioma, or are strongly associated with overall survival [18]. These five subgroups (and their frequently associated genetic alterations) included: #1: triple-positive (31% overall, frequently associated with CIC and FUBP1 mutations); #2: IDH and TERTp mutation (5% overall, associated with TP53 and ATRX mutations); #3: IDH mutation only (47% overall, associated with TP53 and ATRX mutations); #4: triple-negative (7% overall, associated with EGFR, NF1 and PTEN mutations); and #5: TERT mutation only (10% overall, associated with EGFR, EGFRviii, NF1, PTEN, RB1 and PIK3CA or PIK3R1 mutations, amplification of EGFR or focal deletion of CDKN2A). Histologically defined AA clustered to subgroups #2–5. These five subgroups of glioma had characteristic distributions of age at diagnosis, clinical behavior, acquired genetic alterations and associated germline variants. The molecular subgroups more accurately predict prognosis than histology, similar to that seen in the TCGA study.
Epigenetic silencing of the O6-methyl-guanyl-methyl-transferase (MGMT) DNA repair enzyme gene by promoter methylation is associated with longer survival for GB patients and particularly those treated with alkylating chemotherapy [36–38]. Much less is certain regarding the prognostic value of MGMT methylation in AA. One retrospective study demonstrated MGMT promoter methylation in 54.7% (35 of 64) AA patients [39]. In this study, the median survival of anaplastic gliomas with a methylated promoter showed a trend to longer survival, although not statistically significant (9.7 vs 6.1 years). The study concluded that MGMT methylation was neither prognostic nor predictive although study was confounders included the fact that only one third of patients were treated with TMZ after malignant progression and another quarter did not receive any upfront chemotherapy.
In the NOA-o4 trial MGMT promoter methylation status was analyzed in 202 evaluable anaplastic gliomas treated on a randomized Phase III trial [27]. MGMT promoter methylation was determined to be present in 50% (48 of 96) of AA patients. MGMT promoter methylation was associated with improved progression-free survival (PFS) regardless of treatment allocation (alkylating chemotherapy agents or RT alone). The authors concluded that MGMT promoter hypermethylation in AA is prognostic for good outcome in patients treated with RT or predictive for response to RT itself. The authors further hypothesized that MGMT methylation is reflective of a hypermethylation phenotype (the glioma-CpG island methylation phenotype) in which a general defect in regulation of DNA methylation is observed leading to epigenetic inactivation of multiple genes, including genes linked to radiotherapy resistance.
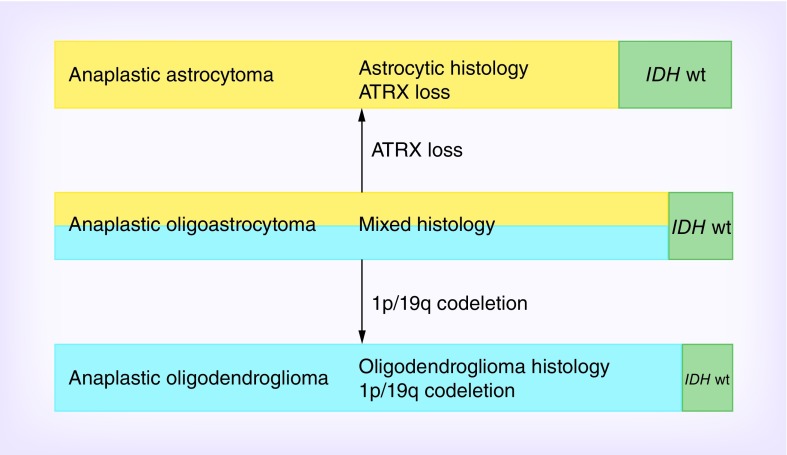
Consequently, the accumulating data have consistently demonstrated that molecular classification of gliomas is a better predictor of prognosis than histology alone, however the strongest classifier is achieved by combining both molecular and histology classifications. Patients with AA with wild-type IDH had worse prognosis than IDH-mutated GB (median survival 24 vs 36 months) [28,35]. Reuss et al. reported on a series of 1360 patients in which there was no significant difference in AA with wild-type IDH from grade II astrocytoma with wild-type IDH in median age, age distribution or overall survival, which is in contrast to survival outcomes predicted by histology only [40]. Based on molecular profiles and hallmark alterations IDH wild-type AA (and grade II astrocytoma) can be grouped into four major sets: molecular equivalent of conventional GB; GB-H3F3A mutated (GBM-H3); GB-H3-K27 (mostly infratentorial or midline location with a trend toward better survival); lower grade gliomas (examples include ganglioglioma, dysembryoplastic neuroepithelial tumor, pleomorphic xanthroastrocytoma, pilocytic astrocytoma) [41]. Thus, caution must be taken when using the diagnosis of IDH wild-type grade II astrocytoma or AA as the majority can be allocated to other tumor entities based on molecular pathology. It is likely that molecular alterations will be an important component in future revisions of the WHO criteria for gliomas as evidenced by the recent Haarlem consensus [42]. A recent study explored the role of ATRX status in the molecular classification of anaplastic glioma and its impact on survival in the biomarker cohort of the NOA-04 trial [43] (Figure 2). ATRX loss was detected in approximately 40% of AA, 25% of anaplastic oligoastrocytoma (AOA) and 10% of anaplastic oligodendroglioma (AO). The ATRX mutation was mostly restricted to IDH mutant tumors and almost mutually exclusive with 1p/19q co-deletion. The authors proposed that IDH wild-type anaplastic tumor be considered a distinct group that is most closely related to GB. They suggest grouping AA and mixed AOA without 1p/19q deletion but with ATRX loss as ‘molecular anaplastic astrocytoma’ and AO and AOA which harbor 1p/19q co-deletion should be classified as ‘molecular anaplastic oligodendroglioma’ (Figure 2).
Figure 2. . Molecular classification of anaplastic gliomas [43].
Proposed molecular classification of anaplastic gliomas based on histology and molecular markers. Length of the green bars represents the proportion of IDH wild-type tumors, whereas the yellow and blue bars represent IDH mutant tumors. Mixed AOA harboring IDH mutation is molecularly classified as either oligodendroglioma (carrying 1p/19q co-deletion) or astrocytomas (carrying ATRX loss).
Another study group has proposed an integrated diagnosis of glioma subtypes based on molecular data and histologic appearance [44]. Their study had two primary aims: first to establish a technical standard for ATRX immunohistochemistry (IHC) and second to establish an algorithm for using ATRX, IDH1-R132H IHC, 1p/19q analysis and IDH sequencing in the diagnosis of diffuse infiltrative gliomas. Based on the analysis of tumor tissue from 405 patients from three German institutions, the proposed schema is as follows: Begin by performing IHC for ATRX and IDH1-R132H expression. Tumors with loss of nuclear ATRX staining are defined as astrocytic. Tumors with nuclear ATRX expression, in other words, wild-type, are subjected to undergo 1p/19q analysis. Tumors without 1p/19q deletion are defined as astrocytic tumors regardless of IDH status. This group should undergo sequencing for rare IDH1 and IDH2 mutations because of the importance of IDH status on prognosis. Astrocytic tumors without ATRX loss, nondeleted 1p/19q and IDH wild-type are defined as glioblastoma. All tumors exhibiting 1p/19q loss are oligodendrogliomas. Oligodendrogliomas without IDH1-R132H positivity do not need to be sequenced for rare IDH mutations, since they would be expected to be detectable in all of them. This ‘integrated diagnosis’ results in definitive separation of astrocytoma from oligodendroglioma, omitting the need for the diagnoses of mixed gliomas and GB with oligodendroglial component and reduces the number of molecular tests required for unequivocal diagnosis.
Treatment
• Initial
Maximal safe surgical resection is the recommended initial surgical treatment in patients with suspected or biopsy proven anaplastic astrocytoma. Lacking a prospective, randomized study demonstrating an advantage of resection over biopsy, retrospective analyses suggest that there is improved overall survival (OS) with resective surgery [4]. Although, meta-analysis of European Organization for the Research and Treatment of Cancer (EORTC) trials suggest age and performance status dominate with extent of resection subordinate [45]. Resection additionally has the benefit of providing sufficient tissue for histologic diagnosis, reduces the tumor mass which may improve neurologic deficits and provides tissue for molecular studies. Notwithstanding image verified complete resection, surgery is not curative due to the diffuse infiltration of tumor into surrounding brain parenchyma. Advances in neuroimaging have improved the ability to resect tumor while minimizing postsurgical neurological deficits. Functional MRI and diffusion tensor MRI further permit localization of eloquent brain, white matter tracts and infiltrative tumor thereby allowing these areas to be avoided during surgery [2].
Following surgery, RT is standard treatment for malignant gliomas wherein the benefit has been established in multiple prospective clinical studies [46–53]. Nonetheless RT is not curative as higher doses though able to sterilize tumor, damage the normal brain. Additionally, the use of radiotherapy boost in addition to standard RT has failed to show a survival benefit for newly diagnosed malignant glioma in a randomized trial [54].
Typically, RT is administered to the contrast enhancing tumor, the T2-weighted or FLAIR peritumoral surround and an additional 1–2 cm margin [55]. The planned tumor volume receives approximately 50 Gray (Gy) and the contrast enhancing volume receives an additional 10 Gy; all administered in 1.8–2.0 Gy fractions per day, 5 fractions per week, for a total of 60 Gy. Elderly patients or patients with poor performance status often have difficulty tolerating this therapy and preferably receive alternative therapy as described below.
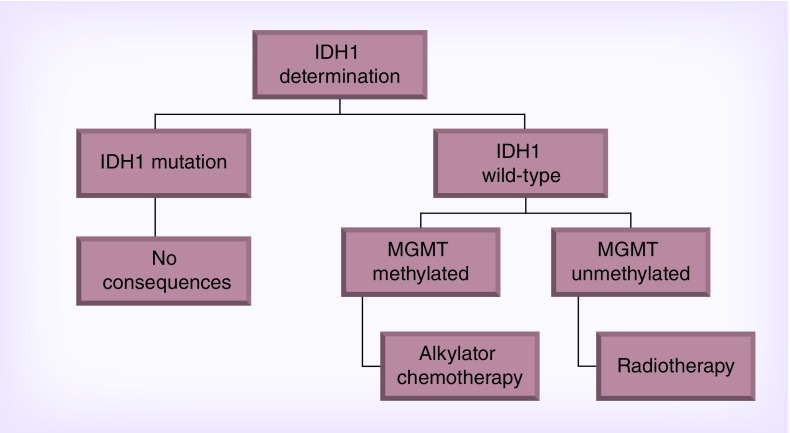
The NOA-04 study demonstrated that initial chemotherapy (TMZ or PCV) with deferred RT is equivalent to RT only [27]. The NOA-04 study was a prospective trial that randomized patients with anaplastic gliomas to: conventional RT, procarbazine, lomustine and vincristine (PCV) or temozolomide (TMZ) as initial therapy. There was no difference in PFS between initial RT versus chemotherapy, suggesting that chemotherapy is a biologically effective treatment in anaplastic gliomas. The study also showed that IDH1 mutation is a prognostic factor with a positive impact that is superior to that of 1p/19q co-deletion or MGMT promoter methylation. Furthermore, in patients with IDH1 wild-type anaplastic gliomas, MGMT determination assists in treatment allocation as per the NOA-04 study wherein tumors that are unmethylated are best treated with RT only (Figure 3).
Figure 3. . NOA-04 [27].
There is increasing evidence that all diffuse gliomas, regardless of histologic grade, may benefit from the combination of chemotherapy after or with RT. Three prospective, randomized clinical trials have demonstrated an OS benefit with the use of RT with adjuvant PCV for patients with anaplastic oligodendroglioma (RTOG 9402 and EORTC 26955) and high risk low grade glioma (RTOG 9802) (Table 1) [56–58]. Similarly, RT with concurrent TMZ and adjuvant TMZ was shown to be beneficial over RT alone for GB in a prospective, randomized clinical trial (EORTC/National Cancer Institute, Canada [NCIC} TMZ/RT study) [59]. Cairncross suggests that the survival benefit of the addition of PCV chemotherapy to RT in anaplastic oligodendroglial tumors was not limited to 1p/19q codeleted tumors but to those with IDH and ATRX mutations as well [60] (Table 2). As discussed in preceding sections, from a molecular/biologic perspective, a glioma characterized histologically as AA may share molecular features with 1p/19q intact anaplastic oligodendroglial tumors, IDH mutant low grade astrocytoma and IDH wild-type GB. Thus it could be argued that the results from RTOG 9402, EORTC 26955, RTOG 9802 and EORTC/NCIC TMZ/RT studies can be extrapolated to all diffuse gliomas. That is, treatment sensitivity is related to the molecular background of a tumor and not to histologic grading.
Table 1. . Anaplastic oligodendroglial tumors: upfront treatment.
| Chromosomal status | Median overall survival | Median progression-free survival | ||
|---|---|---|---|---|
| RT/PCV | RT | RT/PCV | RT | |
| Combined 1p19q loss | ||||
| EORTC 26951 | NR | 111.8 months | 156.8 months | 49.9 months |
| RTOG 9402 | 176.4 months | 87.6 months | 100.8 months | 34.8 months |
| Noncodeleted | ||||
| EORTC 26951 | 25 months | 21 months | 14.8 months | 8.7 months |
| RTOG 9402 | 31.2 months | 32.4 months | 14.4 months | 12 months |
EORTC: European Organization for Research and Treatment of Cancer; NR: Not reported; PCV: Procarbazine, lomustine (CCNU), vincristine; RT: Radiotherapy; RTOG: Radiation therapy Oncology Group.
Table 2. . RTOG 9402: molecular subtyping and response to RT versus RT + PCV.
| Molecular signature | Frequency (%) | Median overall survival (years) | |
|---|---|---|---|
| Radiotherapy | Radiotherapy + PCV | ||
| IDH1 mutation | 74 | 5.7 | 9.4 |
| IDH wild-type | 26 | 1.3 | 1.8 |
| 1p19q codeletion + IDH1 mutation (90% codeleted tumors w/IDH1 mutation; 78% AO) | 42 | 6.8 | 14.7 |
| Nondeleted + IDH1 mutation (64% AOA; 72% ATRX mutated) | 32 | 3.3 | 5.5 |
| + ATRX mutation | 2.7 | 11.0 | |
| + ATRX wild-type | 3.5 | 4.4 | |
| Nondeleted + IDH1 wild-type | 21 | 1.0 | 1.3 |
| 1p19q codeletion + IDH1 wild-type | 5 | ||
Anaplastic oligodendroglial tumors = AO and AOA.
AO: Anaplastic oligodendroglioma; AOA: Anaplastic oligoastrocytoma; PCV: Procarbazine, lomustine (CCNU), vincristine.
The utility of TMZ in 1p/19q intact anaplastic gliomas is the subject of the recently completed CATNON trial (Phase III trial of Concurrent and Adjuvant Temozolomide (TMZ) Chemotherapy in 1p/19q nondeleted Anaplastic Glioma) [61]. An interim analysis by an independent Data Monitoring Committee reported superiority of the addition of adjuvant TMZ (for 12 cycles) following RT versus RT alone. A second interim analysis is planned regarding the added value of TMZ given concurrently with radiotherapy. In the near future, it is anticipated CATNON will begin to address best treatment of varying AA genotypes as the current molecular pathology classification was integrated into the trial design.
RTOG 9813 randomized newly diagnosed AA patients to receive RT with adjuvant TMZ versus adjuvant nitrosoureas (BCNU or CCNU) [62]. TMZ was much better tolerated than nitrosoureas chemotherapy in that no patients stopped therapy because of the toxicity in the TMZ arm as compared with 27.6% in the nitrosoureas arm. The study showed equivalence in OS (3.9 vs 3.8 years) between TMZ versus nitrosoureas despite the high frequency of discontinuation in the nitrosoureas arm. Notably RTOG 9813 demonstrated the prognostic benefit of the IDH1 mutation when comparing OS to AA with IDH1 wild-type (7.9 vs 2.8 years). Whether this statement can be applied to adjuvant PCV is uncertain however there are plentiful examples in oncology where combination chemotherapy is superior to single agent chemotherapy. However, there was no superiority in OS for initial treatment of AA with PCV versus single agent TMZ in the German NOA-04 trial recognizing however that upfront RT was not used in either treatment arm and rather utilized at first recurrence.
The European Association of Neuro-Oncology (EANO) recommends maximal safe surgical resection followed by RT alone or alkylating chemotherapy (temozolomide or PCV) alone for the treatment of newly diagnosed AA/oligoastrocytoma lacking 1p/19q co-deletion [55]. Two randomized, prospective clinical trials, CATNON and CODEL, will better define the optimum treatment for anaplastic gliomas without and with 1p/19q deletion respectively [55]. The CATNON trial (EORTC 26053, NCT00626990) examined RT only to the addition of TMZ to RT administered concurrently or adjuvant post-RT, or both, for patients with anaplastic gliomas without the 1p/19q codeletion [55]. As mentioned above early results suggest superior outcome in patients treated with RT followed by post-RT TMZ. The CODEL trial ((NCT00887146) is examining RT followed by PCV versus TMZ concurrent with RT followed by adjuvant TMZ versus TMZ alone [55]. Early results from CODEL suggest single agent TMZ is inferior to initial RT with either TMZ or PCV.
• Initial treatment of elderly poor performance patients
The treatment of elderly and poor performance AA patients deserves special discussion. There is a near consensus that elderly (age >70 years) and poor performance patients have more difficulty tolerating standard RT than younger patients. Additionally elderly and poor performance patients most often manifest an unfavorable molecular signature which in part reflects poor response to treatment. Recommended treatment options for elderly patients may include RT or RT/TMZ (for fit, otherwise healthy elderly patients), accelerated hypofractionated RT (20–40 Gy in 5–15 fractions), and primary TMZ chemotherapy with deferred RT. The NOA-08 l trial randomized 373 patients, age >65 years, with AA or GB to standard RT (54–60 Gy) versus dose dense TMZ [63,64]. Outcome was similar in both treatment arms suggesting that TMZ alone, particularly in those with methylated MGMT promoter gene, is an option for newly diagnosed elderly or poor performance AA patients. A similar conclusion was reached in the Nordic trial which compared standard RT to hypofractionated RT to TMZ only [65]. Whether combined hypofractionated RT + TMZ is superior to hypofractionated RT only in elderly patient with GB will be determined in the recently completed NCIC/EORTC trial [66,67].
• Treatment at recurrence/progression
In AA that progress following initial therapy, there is no standard treatment and options are limited (Tables 3–5). Re-resection can be useful in selected patients particularly those that are symptomatic from tumor mass, patients with relatively preserved performance and with tumors located in noneloquent brain. Although surgery may improve function, the survival benefit has not been substantiated. Obtaining tissue by surgery may be advantageous if progression to GB is demonstrated as this may substantially increase the availability of investigative clinical trial options for which the patient may be eligible.
Table 3. . Recurrent anaplastic astrocytoma trials.
| Study (year) | Trial design | Patient number | Overall response rate (%) | Median progression-free survival (months) | 6-month progression-free survival (%) | Median overall survival (months) | Ref. |
|---|---|---|---|---|---|---|---|
| Wong et al. (1999) | Phase II (total of 8) | 150 | 14 | 3.25 | 31 | 7.5 | [68] |
| Lamborn et al. (2008) | Phase II (total of 12) | 159 | 7 | 6 | 28 | 8 | [69] |
| Yung et al. (1999) | Randomized Phase III | 162 (AA/AOA) | 35 | 5.4 | 46 | 13.6 | [70] |
| Bevacizumab trials | Retrospective and prospective (total of 8) | 258 (65% AG) | 24–64 | 2.9–7.0 | 21–60 | 11–16 | [71,72] |
| Chamberlain (2015) | Retrospective | 35 | 5.7 | 4.5 | 40 | 9.5 | [73] |
AA: Anaplastic astrocytoma; AOA: Anaplastic oligodendroglioma; AG: Anaplastic glioma.
Table 4. . First-line salvage chemotherapy: recurrent grade II and III gliomas.
| Histology (study and year) | Patient number | Drugs | Response (%) | PFS-6 (%) | PFS-12 (%) | mOS (months) | Ref. |
|---|---|---|---|---|---|---|---|
| All Gr2 (Taal 2011) | 70 | TMZ | 47 | 63 | – | 13 | [74] |
| AA/AOA (Yung 1999) | 65 | TMZ | 43 | 50 | – | 11.5 | [75] |
| AO/AOA (Brandes 2006) | 67 | TMZ | 46 | – | 50 | 31 | [76] |
| AO/AOA (van den Bent 2003) | 38 | TMZ | 54 | 71 | 40 | – | [77] |
| AO/AOA (van den Bent 1998) | 52 | PCV | 63 | – | 50 | 20 | [78] |
| AOA (Brandes 2004) | 37 | PCV | 59 | 72 | 52 | 30.7 | [79] |
AA: Anaplastic astrocytoma; AOA: Anaplastic oligoastrocytoma; AO: Anaplastic oligodendroglioma; mOS: Median overall survival; TMZ: Temozolomide.
Table 5. . Treatment of recurrent anaplastic astrocytoma.
| Study (year) | Trial | # | Outcome | Ref. | ||
|---|---|---|---|---|---|---|
| Response (%) | PFS-6 (%) | mOS (weeks) | ||||
| Wong (1999) | Composite | 150 | 14 | 31 | 47 | [80] |
| Yung (1999) | TMZ | 162 | 35 | 44 | 54 | [81] |
| Brem (1995) | Gliadel | 28 | NS | 64 | 31 | [82] |
| Yung (1996) | cRA | 28 | 11 | NS | 34 | [83] |
| Jaeckle (2003) | cRA+TMZ | 28 | 15 | 46 | 47 | [84] |
| Levin (1992) | DFMO | 44 | 9 | NS | 62 | [85] |
| Levin (1992) | TPCH | 38 | 34 | NS | 50 | [86] |
| Chamberlain (1999) | Taxol | 24 | 13 | NS | 72 | [87] |
| Chamberlain (2007) | Cytoxan | 40 | 22.5 | 40 | 28 | [88] |
| Chamberlain(2008) | CPT-11 | 40 | 23 | 40 | 28 | [89,90,91] |
| Vredenbergh(2007) | CPT-11+Avastin | 9 | 67 | 56 | NS | [92] |
CPT-11: Irinotecan; cRA: Cis-retinoic acid; DFMO: Difluoromethyl ornithine; TMZ: Temozolomide; TPCH: 6-thioguanine, procarbazine, CCNU (lomustine), hydroxyurea.
Reirradiation has been found to be safe and may have palliative benefit based upon two single institution retrospective studies. However both studies suffer from being retrospective with likely treatment selection bias [93,94].
Standard dose TMZ demonstrated activity in patients with recurrent AA treated initially with surgery and RT only in a multicenter, Phase II study [70]. The study demonstrated 6-month PFS (PFS-6) of 46%, objective radiologic response rate of 35% and OS of 13.6 months. Continuous dose-dense TMZ (50 mg/m2/day) is another treatment option for recurrent AA [95]. The hypothesis is that daily (metronomic) dose dense TMZ overcomes drug resistance by reducing intra-tumoral MGMT activity and additionally provides an antiangiogenic effect by limiting endothelial cell recovery, inhibiting the activity of circulating endothelial precursors and upregulating thrombospondin. In the study by Perry, PFS-6, 1-year OS and radiographic response rates were 35.7, 60.7 and 15.4%, respectively. It is, however, uncertain whether metronomic TMZ is more effective than rechallenge with standard dose TMZ. Although another failed to demonstrate a benefit for rechallenging GB patients at first recurrence with dose-intense TMZ (75–100 mg/m2 on a 21/28 day schedule) [96]. PFS-6 and radiographic response rates were 11 and 13%, respectively.
Bevacizumab has been evaluated in recurrent AA in two prospective, Phase II studies and six retrospective studies. The initial Phase II study used the combination of bevacizumab and irinotecan in patients with recurrent AA (n = 25/33) and anaplastic oligodendroglioma (n = 8/33) [71]. The PFS-6, 6-month OS, and radiologic response rate were 55, 79 and 61%, respectively, suggesting that bevacizumab may have similar activity to that seen in glioblastoma. The second single arm Phase II study used bevacizumab only in patients with recurrent malignant glioma (68% were AA) [72]. Median OS, median PFS, PFS-6 and radiographic response rate were 12 months, 2.93 months, 20.9% and 43%, respectively. Notwithstanding the modest PFS-6 patients experienced a significant clinical benefit as suggested by patients on steroids at study onset were able to substantially decrease the dose and in nearly half of patients neurologic symptoms improved with treatment.
The use of lomustine for TMZ refractory recurrent AA was explored in a retrospective study of 35 patients [73]. All patients had prior surgery, RT, and TMZ. Lomustine was utilized at first recurrence in seven and second recurrence in 28. Median PFS, 6-month PFS, 12-month PFS and median OS after onset of lomustine were 4.5 months, 40%, 11.4% and 2.7 years.
Conclusion
The treatment of AA continues to evolve with the increasing recognition of molecular subgroups as illustrated by the two large categories of non-codeleted IDH1 mutated and IDH1 wild type AA. Whether treatment should differ for these two molecular subtypes of AA is not yet clear notwithstanding significant differences in outcome [62]. Initial treatment of AA following maximum safe surgery entails involved field radiotherapy followed by 12 cycles of post-radiotherapy TMZ based on early results of the CATNON trial [61]. Unclear is whether TMZ given in conjunction with RT adds further benefit, an analysis yet to be completed by the CATNON investigators. Alternatively and based on the RTOG 9813 study, either lomustine (CCNU) or carmustine (BCNU) may be substituted for TMZ and appears to confer similar survival benefits [62]. There are no adjuvant therapies aside from TMZ or nitrosoureas (CCNU or BCNU) that have demonstrated a survival benefit in newly diagnosed AA, a significant unmet need not dissimilar to the up-front treatment of GBM. Whether immunotherapy (e.g. vaccines or check point inhibitors) will provide another adjunct treatment strategy is dependent in large part on success of these therapies in on-going trials in GBM. IDH1 small molecule inhibitors, a rationale targeted therapy for the majority of patients with IDH1 mutant AA awaits on-going clinical trials. Much as in GBM, salvage therapy for recurrent AA utilizes re-resection, re-radiation and cytotoxic chemotherapies in an appropriate context with only modest efficacy. A novel randomized trial in recurrent AA (sponsor Orbus Pharmaceutical) is comparing lomustine with or without the oral agent DFMO in patients having progressed on TMZ. New approaches for both the up-front and salvage treatment of AA remain a significant need in neuro-oncology.
Future perspective
Notwithstanding initial promise, trials investigating agents that target the molecular pathology of malignant gliomas such as EGFR, mTOR, PI3K and VEGFR have been overwhelmingly disappointing [97]. Identifying subsets of AA with oncogene addiction by gene expression profiling may permit improved efficacy of available targeted therapies however oncogene dependent tumors are likely to be quite rare. In addition, the use of correlative studies, that utilize pre- and post-treatment surgical specimens to determine if the target of interest is present in the tumor and affects the targeted signal pathway, is encouraged.
Since IDH mutations are highly prevalent in AA and enzymatic defects are attractive candidates for targeted therapy, IDH-related therapy may have a novel role in patients with AA and IDH mutation. However, the oncogenic mechanism associated with IDH mutations is not yet determined. As previously discussed, clinical trials utilizing small molecule inhibitors of the IDH1 mutant protein and vaccine immunotherapy against the IDH1 mutant protein are planned.
Finally, preclinical studies have revealed promising results for various immunotherapeutic treatment strategies of malignant gliomas, particularly GB [64]. These immunotherapy treatment options include vaccines, cell-based approaches and immune checkpoint blockade. Clinical studies to evaluate these therapies are underway. Important considerations going forward include optimizing response assessment and identifying correlative biomarkers to predict therapeutic benefit [64].
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mechtler L. Neuroimaging in neuro-oncology. Neurol. Clin. 2009;27(1):171–201. doi: 10.1016/j.ncl.2008.09.015. ix. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;4(16 Suppl.):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prados MD, Gutin PH, Phillips TL, et al. Highly anaplastic astrocytoma: a review of 357 patients treated between 1977 and 1989. Int. J. Radiat. Oncol. Biol. Phys. 1992;23(1):3–8. doi: 10.1016/0360-3016(92)90537-r. [DOI] [PubMed] [Google Scholar]
- 5.Pace A, Bove L, Innocenti P, et al. Epilepsy and gliomas: incidence and treatment in 119 patients. J. Exp. Clin. Cancer Res. 1998;17(4):479–482. [PubMed] [Google Scholar]
- 6.Astrakas LG, Zurakowski D, Tzika AA, et al. Noninvasive magnetic resonance spectroscopic imaging biomarkers to predict the clinical grade of pediatric brain tumors. Clin. Cancer Res. 2004;10(24):8220–8228. doi: 10.1158/1078-0432.CCR-04-0603. [DOI] [PubMed] [Google Scholar]
- 7.Henson JW, Gaviani P, Gonzalez RG. MRI in treatment of adult gliomas. Lancet Oncol. 2005;6(3):167–175. doi: 10.1016/S1470-2045(05)01767-5. [DOI] [PubMed] [Google Scholar]
- 8.Hutterer M, Nowosielski M, Putzer D, et al. [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15(3):341–351. doi: 10.1093/neuonc/nos300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen NL, Graute V, Armbruster L, et al. MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur. J. Nucl. Med. Mol. Imaging. 2012;39(6):1021–1029. doi: 10.1007/s00259-012-2109-9. [DOI] [PubMed] [Google Scholar]
- 10.Pafundi DH, Laack NN, Youland RS, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15(8):1058–1067. doi: 10.1093/neuonc/not002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coons SW, Johnson PC. Regional heterogeneity in the proliferative activity of human gliomas as measured by the Ki-67 labeling index. J. Neuropathol. Exp. Neurol. 1993;52(6):609–618. doi: 10.1097/00005072-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Karamitopoulou E, Perentes E, Diamantis I, Maraziotis T. Ki-67 immunoreactivity in human central nervous system tumors: a study with MIB 1 monoclonal antibody on archival material. Acta Neuropathol. 1994;87(1):47–54. doi: 10.1007/BF00386253. [DOI] [PubMed] [Google Scholar]
- 13.Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79(7):1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol. 2010;120(3):297–304. doi: 10.1007/s00401-010-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theeler BJ, Yung WK, Fuller GN, De Groot JF. Moving toward molecular classification of diffuse gliomas in adults. Neurology. 2012;79(18):1917–1926. doi: 10.1212/WNL.0b013e318271f7cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weller M, Stupp R, Hegi ME, et al. Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol. 2012;4(14 Suppl.):iv100–iv108. doi: 10.1093/neuonc/nos206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network. Brat DJ, Verhaak RG, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 2015;372(26):2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N. Engl. J. Med. 2015;372(26):2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe K, Sato K, Biernat W, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin. Cancer Res. 1997;3(4):523–530. [PubMed] [Google Scholar]
- 20.Brennan C. Genomic profiles of glioma. Curr. Neurol. Neurosci. Rep. 2011;11(3):291–297. doi: 10.1007/s11910-011-0198-7. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R, Webb-Myers R, Flanagan S, Buckland ME. Isocitrate dehydrogenase mutations in diffuse gliomas: clinical and aetiological implications. J. Clin. Pathol. 2011;64(10):835–844. doi: 10.1136/jclinpath-2011-200227. [DOI] [PubMed] [Google Scholar]
- 22.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleeker FE, Atai NA, Lamba S, et al. The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119(4):487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340(6132):626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized Phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J. Clin. Oncol. 2009;27(35):5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta. Neuropathol. 2010;120(6):707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 29.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin. Cancer Res. 2010;16(5):1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 32.Jaeckle KA, Decker PA, Ballman KV, et al. Transformation of low grade glioma and correlation with outcome: an NCCTG database analysis. J. Neurooncol. 2011;104(1):253–259. doi: 10.1007/s11060-010-0476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Ellison DW. Multiple molecular data sets and the classification of adult diffuse gliomas. N. Engl. J. Med. 2015;372(26):2555–2557. doi: 10.1056/NEJMe1506813. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 2015;47(5):458–468. doi: 10.1038/ng.3273. [DOI] [PubMed] [Google Scholar]
- 36.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 2000;343(19):1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized Phase III clinical trial. J. Clin. Oncol. 2013;31(32):4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 39.Juratli TA, Kirsch M, Geiger K, et al. The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J. Neurooncol. 2012;110(3):325–333. doi: 10.1007/s11060-012-0977-2. [DOI] [PubMed] [Google Scholar]
- 40.Reuss DE, Mamatjan Y, Schrimpf D, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta. Neuropathol. 2015;129(6):867–873. doi: 10.1007/s00401-015-1438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuss DE, Kratz A, Sahm F, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta. Neuropathol. 2015;130(3):407–417. doi: 10.1007/s00401-015-1454-8. [DOI] [PubMed] [Google Scholar]
- 42.Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology – Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–435. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiestler B, Capper D, Holland-Letz T, et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta. Neuropathol. 2013;126(3):443–451. doi: 10.1007/s00401-013-1156-z. [DOI] [PubMed] [Google Scholar]
- 44.Reuss DE, Sahm F, Schrimpf D, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta. Neuropathol. 2015;129(1):133–146. doi: 10.1007/s00401-014-1370-3. [DOI] [PubMed] [Google Scholar]
- 45.van den Bent M, Hildebrand J, Brandes AA, et al. O38. Impact of extent of resection and outcome to adjuvant chemotherapy: a meta-analysis of three EORTC studies. Neuro Oncol. 2008;10(6):1061–1149. [Google Scholar]
- 46.Andersen AP. Postoperative irradiation of glioblastomas. Results in a randomized series. Acta. Radiol. Oncol. Radiat. Phys. Biol. 1978;17(6):475–484. doi: 10.3109/02841867809128178. [DOI] [PubMed] [Google Scholar]
- 47.Douglas BG, Worth AJ. Superfractionation in glioblastoma multiforme – results of a Phase II study. Int. J. Radiat. Oncol. Biol. Phys. 1982;8(10):1787–1794. doi: 10.1016/0360-3016(82)90303-0. [DOI] [PubMed] [Google Scholar]
- 48.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- 49.Salazar OM, Rubin P, Feldstein ML, Pizzutiello R. High dose radiation therapy in the treatment of malignant gliomas: final report. Int. J. Radiat. Oncol. Biol. Phys. 1979;5(10):1733–1740. doi: 10.1016/0360-3016(79)90554-6. [DOI] [PubMed] [Google Scholar]
- 50.Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J. Neurosurg. 1989;71(1):1–9. doi: 10.3171/jns.1989.71.1.0001. [DOI] [PubMed] [Google Scholar]
- 51.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J. Neurosurg. 1978;49(3):333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 52.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1979;5(10):1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 53.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 1989;16(6):1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 54.Tsao MN, Mehta MP, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 2005;63(1):47–55. doi: 10.1016/j.ijrobp.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 55.Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 56.Buckner JC, Pugh SL, Shaw EG, et al. Phase III study of radiation therapy with or without procarbazine, CCNU, and vincrisitine (PCV) in low-grade glioma: RTOG 9802 with Alliance, ECOG, and SWOG. J. Clin. Oncol. 2014;32:5s. suppl. abstr 2000. [Google Scholar]
- 57.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 59.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 60.Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J. Clin. Oncol. 2014;32(8):783–790. doi: 10.1200/JCO.2013.49.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van den Bent MJ. Practice changing mature results of RTOG study 9802: another positive PCV trial makes adjuvant chemotherapy part of standard of care in low-grade glioma. Neuro Oncol. 2014;16(12):1570–1574. doi: 10.1093/neuonc/nou297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang SM, Zhang P, Cairncross JG, et al. Results of NRG oncology/RTOG 9813: a Phase III randomized study of radiation therapy (RT) and temozolomide (TMZ) versus RT and nitrosurea(NU) therapy for anaplastic astrocytoma. J. Clin. Oncol. 2015;33(Suppl.) Abstract 2002. [Google Scholar]
- 63.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 64.Reardon DA, Freeman G, Wu C, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16(11):1441–1458. doi: 10.1093/neuonc/nou212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malmstrom A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 66.Perry JR, O'Callaghan CJ, Ding K, et al. A phase III randomized controlled trial of short-course radiotherapy with or without consomitant and adjuvant temozolomide in elderly patients with glioblastoma. Neuro Oncol. 2014;16(Suppl. 3):iii46. [Google Scholar]
- 67.Roa W, Kepka L, Kumar N, et al. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2015;33(35):4145–4150. doi: 10.1200/JCO.2015.62.6606. [DOI] [PubMed] [Google Scholar]
- 68.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factor in recurrent glioma patients enrolled onto phase II clinical trials. J. Clin. Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 69.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J. Clin. Oncol. 1999;17(9):2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 71.Desjardins A, Reardon DA, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin. Cancer Res. 2008;14(21):7068–7073. doi: 10.1158/1078-0432.CCR-08-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreisl TN, Zhang W, Odia Y, et al. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011;13(10):1143–1150. doi: 10.1093/neuonc/nor091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chamberlain MC. Salvage therapy with lomustine for temozolomide refractory recurrent anaplastic astrocytoma: a retrospective study. J. Neurooncol. 2015;122(2):329–338. doi: 10.1007/s11060-014-1714-9. [DOI] [PubMed] [Google Scholar]
- 74.Taal W, van der Rijt CD, Sillevis Smitt PA, et al. [Favourable result for temozolomide in recurrenthigh-grade glioma] Ned Tijdschr Geneeskd. 2005;149(25):1393–1399. [PubMed] [Google Scholar]
- 75.Yung WK, Kyritsis AP, Gleason MJ, Levin VA. Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoicacid. Clin. Cancer Res. 1996;2(12):1931–1935. [PubMed] [Google Scholar]
- 76.BrandesAA, Tosoni A, Cavallo G, et al. Correlations between O6-methylguanine DNA methyltransferase promotermethylation status, 1p and 19q deletions, and response to temozolomide inanaplastic and recurrent oligodendroglioma: a prospective GICNO study. J. Clin. Oncol. 2006;24(29):4746–4753. doi: 10.1200/JCO.2006.06.3891. [DOI] [PubMed] [Google Scholar]
- 77.van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group):assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 78.van den Bent MJ, Schellens JH, Vecht CJ, et al. Phase II study on cisplatin and ifosfamide in recurrent high gradegliomas J. Eur. J. Cancer. 1998;34(10):1570–1574. doi: 10.1016/s0959-8049(98)00138-5. [DOI] [PubMed] [Google Scholar]
- 79.BrandesAA, Tosoni A, Basso U, et al. Second-line chemotherapy with irinotecan plus carmustine in glioblastomarecurrent or progressive after first-line temozolomide chemotherapy: a Phase IIstudy of the Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) J. Clin. Oncol. 2004;22(23):4779–4786. doi: 10.1200/JCO.2004.06.181. [DOI] [PubMed] [Google Scholar]
- 80.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolledonto Phase II clinical trials. J. Clin. Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 81.YungWK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplasticastrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J. Clin. Oncol. 1999;17(9):2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 82.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperativecontrolled delivery by biodegradable polymers of chemotherapy for recurrentgliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008-12–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 83.Yung WK, Kyritsis AP, Gleason MJ, Levin VA. Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoicacid. Clin. Cancer Res. 1996;2(12):1931–1935. [PubMed] [Google Scholar]
- 84.JaeckleKA, Hess KR, Yung WK, et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for thetreatment of recurrent and progressive malignant glioma: a North American BrainTumor Consortium study. J. Clin. Oncol. 2003;21(12):2305–2311. doi: 10.1200/JCO.2003.12.097. [DOI] [PubMed] [Google Scholar]
- 85.Levin VA, Prados MD. Treatment of recurrent gliomas and metastatic brain tumors with apolydrug protocol designed to combat nitrosourea resistance. J. Clin. Oncol. 1992;10(5):766–767. doi: 10.1200/JCO.1992.10.5.766. [DOI] [PubMed] [Google Scholar]
- 86.LevinVA, Prados MD, Yung WK, Gleason MJ, Ictech S, Malec M. Treatment of recurrent gliomas with eflornithine. J. Natl Cancer Inst. 1992;84(18):1432–1437. doi: 10.1093/jnci/84.18.1432. [DOI] [PubMed] [Google Scholar]
- 87.ChamberlainMC, Kormanik P. Salvage chemotherapy with taxol for recurrentanaplastic astrocytomas. J. Neurooncol. 1999;43(1):71–78. doi: 10.1023/a:1006277631745. [DOI] [PubMed] [Google Scholar]
- 88.Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapywith cyclophosphamide for recurrent temozolomide-refractory anaplastic astrocytoma. Cancer. 2006;106(1):172–179. doi: 10.1002/cncr.21582. [DOI] [PubMed] [Google Scholar]
- 89.Chamberlain MC, Glantz MJ. CPT-11 for recurrent temozolomide-refractory 1p19q co-deleted anaplasticoligodendroglioma. J. Neurooncol. 2008;89(2):231–238. doi: 10.1007/s11060-008-9613-6. [DOI] [PubMed] [Google Scholar]
- 90.Chamberlain MC, Wei-Tsao DD, Blumenthal DT, Glantz MJ. Salvage chemotherapy with CPT-11 for recurrent temozolomide-refractoryanaplastic astrocytoma. Cancer. Year;112(9):2038–2045. doi: 10.1002/cncr.23404. [DOI] [PubMed] [Google Scholar]
- 91.Chamberlain MC. Bevacizumab plus irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2008;26(6):1012-3–1013. doi: 10.1200/JCO.2007.15.1605. author reply 1013. [DOI] [PubMed] [Google Scholar]
- 92.VredenburghJJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 93.Adkison JB, Tomé W, Seo S, et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(3):835–841. doi: 10.1016/j.ijrobp.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 94.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J. Clin. Oncol. 2010;28(18):3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perry JR, Bélanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J. Clin. Oncol. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 96.Norden AD, Lesser GJ, Drappatz J, et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013;15(7):930–935. doi: 10.1093/neuonc/not040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quant EC, Drappatz J, Wen PY, Norden AD. Recurrent high-grade glioma. Curr. Treat. Options Neurol. 2010;12(4):321–333. doi: 10.1007/s11940-010-0078-5. [DOI] [PubMed] [Google Scholar]