Abstract
We describe a 62-year-old of Egyptian origin who presented with sudden, severe and symptomatic anemia requiring hospitalization shortly after beginning concurrent radiation and temozolomide for his newly diagnosed glioblastoma. He had also recently been started on steroids, anticonvulsants and Pneumocystis jirovecii prophylaxis. He was ultimately diagnosed with G6PD deficiency with an acute hemolytic anemia precipitated by dapsone. Screening for G6PD deficiency should be considered in high-risk patient populations where P. jirovecii prophylaxis is planned.
KEYWORDS : dapsone, glioblastoma multiforme, glucose-6-phosphate dehydrogenase deficiency (G6PD), hemolytic anemia
Practice points.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzymatic disorder in red blood cells and can result in acute hemolytic anemia after exposure to certain drugs including those routinely used for Pneumocystis jirovecii prophylaxis (dapsone, trimethoprim-sulfamethoxazole).
If G6PD deficiency is suspected, patients should be worked up for hemolytic anemia, and any potentially offending drug should be discontinued.
Patients of African, Mediterranean or Middle Eastern origin should be screened for G6PD deficiency prior to initiating P. jirovecii prophylaxis.
Case study
A 62-year-old male of Egyptian origin presented with progressive left upper extremity weakness, headache, altered taste sensation and facial twitching. His neurological examination was significant for left hemiparesis, and neuroimaging demonstrated numerous contrast enhancing masses in the right frontal and parietal lobes with extensive vasogenic edema and midline shift. He underwent subtotal resection of his frontal lesion, which was found to be an MGMT-unmethylated glioblastoma. He had an uneventful postoperative recovery and after evaluation by radiation and medical oncology, he was recommended to start standard concurrent radiation and temozolomide [1]. He was noted to be mildly thrombocytopenic at baseline and thus dapsone was chosen for Pneumocystis jirovecii pneumonia (PJP) prophylaxis rather than standard trimethoprim-sulfamethoxazole. He was seen in the emergency room during his second week of treatment complaining of a worsening hemiparesis as well as severe fatigue and was noted to have tachycardia, tachypnea and mild hypoxia in room air. He underwent an emergency computed tomography angiogram, which was negative for pulmonary embolism. Prior to the initiation of therapy, his hemoglobin was 15.1 g/dl (normal range: 12–15 g/dl) and hematocrit was 43.5% (normal range: 36–46%). However, in the emergency room, his hemoglobin was 7.8 g/dl and his hematocrit was 23.8%. His other laboratory evaluations included a negative stool guaiac test, an elevated lactate dehydrogenase 682 U/l (normal range: 120–220 U/l), normal direct bilirubin 0.4 mg/dl (normal range 0–0.4 mg/dl) and low haptoglobin <6 mg/dl (normal range: 36–195 mg/dl). He was transfused two units of packed red cells with improvement in his hemoglobin and hematocrit, and improvement in all symptoms except for his hemiparesis. On questioning after admission to the hospital, his family remembered that he once had a reaction after eating fava beans over 50 years before as a child. Due to concerns of G6PD deficiency, dapsone was discontinued. One month later, his G6PD level was measured at 0.8 U/g Hb (normal range: 4.6–13.5 U/g Hb). His haptoglobin level and lactate dehydrogenase were also normalized. He was commenced on atovaquone for PJP prophylaxis and was able to successfully complete his course of concomitant temozolomide and radiation treatment without further incident.
Discussion
G6PD deficiency is the most common enzymatic disorder of red cells and affects >400 million people worldwide [2]. It is an X-linked disorder that affects primarily males of African, Mediterranean and Middle Eastern ancestry [2]. It was first described in 1955 when it was noted that African–American soldiers treated prophylactically with the antimalarial primaquine were more likely to develop jaundice and anemia than their Caucasian counterparts [3]. These reactions were subsequently found to correlate with reduced G6PD enzymatic activity in the red cells of affected individuals [4]. A wide spectrum of enzymatic deficiency has been described ranging from class I (severe, <10% of normal) to class IV (no enzyme deficiency or hemolysis). Patients of Middle Eastern descent, such as the one presented in this manuscript, commonly harbor the Mediterranean G6PD variant, which can result in severe hemolysis [5]. Under normal conditions, spontaneous hemolysis is uncommon, although patients with mild-moderate deficient levels of G6PD will undergo hemolysis in the presence of medications, which provide an oxidative stress to these susceptible red blood cells (Figure 1). A listing of these medications is provided in Table 1.
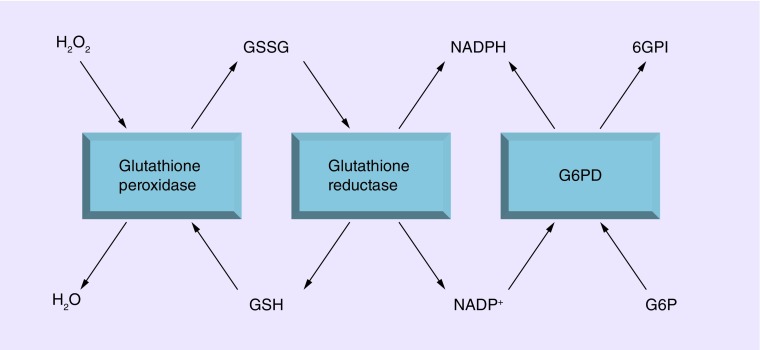
Figure 1. . Role of G6PD in managing oxidative stress.
Glutathione peroxidase protects cells from oxidative damage by reducing reactive oxygen species, such as H2O2 to H2O. It does this by reducing glutathione disulfide to monomeric glutathione. Glutathione reductase then completes the cycle by reducing oxidized glutathione using NADPH as the reducing agent. In normal erythrocytes, G6P dehydrogenase activity continuously regenerates reducing energy supplies by maintaining the level of NADPH when it converts G6P to 6PGI. In G6PD-deficient cells, glutathione levels are rapidly exhausted when subjected to oxidative challenge, thereby resulting in excessive levels of H2O2 which results in hemolysis.
6PGI: Glucose-6-phosphate-isomerase; G6P: Glucose-6-phosphate; G6PD: Glucose-6-phosphate dehydrogenase; H2O: Water; H2O2: Hydrogen peroxide.
Table 1. . List of medications relevant to oncologists that may precipitate hemolysis in G6PD deficient individuals.
| Category of drug | Definite risk | Possible risk |
|---|---|---|
| Antimalarials | Dapsone | |
| Primaquine | ||
| Anti-infectives | Quinolones (ciprofloxacin, levofloxacin) | |
| Nitrofurantoin | ||
| Cotrimoxazole | ||
| Miscellaneous | Methylene blue | |
| Rasburicase | ||
| Analgesic/antipyretic | Acetaminophen | |
| Aspirin | ||
PJP is a serious and potentially life-threatening infection that most commonly affects severely immunocompromised individuals such as patients with HIV. It has also been reported in patients with hematologic malignancies and transplant recipients who are also profoundly immunosuppressed [6,7]. PJP has also been reported in patients with primary brain tumors [8]. This was originally thought to be solely related to the use of dexamethasone in this patient population to control peritumoral brain edema. Dexamethasone is known to result in reactivation of latent PJP infection due to its immunosuppressant effects [8]. More recent studies have demonstrated that severe (grade III and IV) and sustained treatment-related lymphopenia occurs in 40% of patients with newly diagnosed glioblastoma receiving standard radiation and temozolomide [9]. It now appears that radiation therapy to a partial brain field may be responsible for the remarkable and sustained fall in CD4 counts that these patients develop [10]. Prior to the routine use of PJP prophylaxis during systemic treatment of CNS tumors, over 50% of patients contracting this opportunistic infection died of progressive interstitial pneumonia [11]. PJP prophylaxis has been shown to result in a ninefold reduction in PJP development in the HIV population [12] and has also been shown to dramatically reduce PJP-related mortality in non-HIV patients. As a result, published guidelines recommend the use of PJP prophylaxis for patients with cancer [13], where the risk of contracting this infection is >3.5% [14]. In general, this prophylaxis is of most benefit to patients with a CD4 count below 200/µl.
As over 40% of patients with newly diagnosed glioblastoma will develop CD4 counts of <200/µl and as the mortality rate for patients with solid tumors who develop PJP is >50%, these patients should be either routinely offered PJP prophylaxis or their total lymphocyte and CD4 counts are followed closely and PJP prophylaxis initiated if total lymphocyte counts are below 500/µl or CD4 counts are below 200/µl. For patients following the Stupp protocol [1], we recommend monitoring counts weekly during concomitant temozolomide/radiation therapy and monthly during the period when patients receive temozolomide monotherapy. Trimethoprim-sulfamethoxazole (TMZ-SMX) is the preferred agent used in PJP prophylaxis, and common side effects include fever, rash, myelosuppression, hepatitis and headache. Alternative agents include aerosolized pentamidine, atovaquone and dapsone. There are some data which suggest that these alternatives are not equivalent to TMZ-SMX in the allogeneic bone marrow transplant population, although such data do not exist in the brain tumor population [15,16]. Pentamidine and atovaquone have been shown to be equivalent in patients with HIV who are intolerant of TMZ-SMX [17]. Hematological toxicities are uncommon with atovaquone making it an attractive alternative during cytotoxic chemotherapy. Pentamidine is available in nebulized form which is only required to be taken once monthly.
Dapsone (4-4′-diaminodiphenylsulfone) is generally well tolerated but has the potential for serious, although rare, hematologic complications including methemoglobinema, agranulocytosis, aplastic anemia and hemolytic anemia [18]. Dapsone induces hemolysis by generating free radicals from its metabolite, hydroxylamine, resulting in the depletion of glutathione stores in erythrocytes (Figure 1) [19]. While dapsone can induce hemolysis in those with normal G6PD function [20], its effects are significantly more pronounced in those with G6PD deficiency. Patients with G6PD deficiency who receive dapsone may experience jaundice, anemia and methemoglobinemia [21]. A peripheral blood smear obtained during active hemolysis may show the presence of a bite cells or Heinz bodies.
Our patient had recently received high doses of dexamethasone for management of peritumoral and surgically induced brain edema and presented with a sudden symptomatic anemia prompting concerns for gastrointestinal bleeding. Once this and other causes of anemia were excluded and his distant history of a fava bean reaction was uncovered, he received red cell transfusions and the dapsone was discontinued. Methylene blue was not administered as this could increase the oxidative stress on red cells [22] and the patient was not treated with ascorbic acid which may be of some benefit [23]. As is evident from Table 1 and other sources, this patient would have been at significant risk for the same complication with trimethoprim-sulfamethoxazole, several other antibiotics commonly used in cancer patients, anthracyclines and antipyretics [24,25].
His G6PD deficiency was confirmed about 1 month after his presentation to allow time for his native red cells to be evaluated. There are many different assay methods that are inexpensive, accurate and provide rapid results. [26]. In our patient, G6PD activity was measured using a validated quantitative spectrophotometric assay (normal range: 7–10 IU/g Hb) that costs $68. Screening for unrecognized G6PD is routinely performed in selected populations prior to commencing antimalarial treatment. For example, the US military mandates G6PD testing before deployment to regions where malaria is endemic. G6PD data from 63,302 military personnel collected between 2004 and 2005 were retrospectively analyzed with self-reported ethnicity [27]. G6PD deficiency was noted in 2.5% of males (most with class III variants) and 1.6% of females (most with class IV variants). The highest proportions of military personnel with G6PD deficiency were African–American males (12.2%), Asian males (4.3%) and African–American females (4.1%). Although these data involve healthy subjects, it nonetheless provide an important insight into the prevalence of G6PD deficiency in the population.
Universal screening for G6PD deficiency should be strongly considered in selected patients with newly diagnosed glioblastoma before they begin PJP prophylaxis. These patients are also at risk to receive other antibiotics and antipyretics or investigational or standard chemotherapeutic agents that could also precipitate an acute hemolytic anemia [28]. The patients at highest risk include men with African–American or Asian ethnicity or those from other regions with endemic malaria. Currently, almost 30% of Marylanders are African–American, 8% are Hispanic, 6% are Asian and 3% are described as multiracial [29]. As the population of the USA becomes increasingly diverse, the number of patients at risk will continue to increase for this treatment-related complication.
Conclusion
Patients receiving immunosuppressive anti-cancer therapies, such as temozolomide and radiation therapy for high grade gliomas, require prophylaxis against PJP. This case highlights the consequences of a patient with an undiagnosed G6PD deficiency who developed hemolytic anemia after taking dapsone for PJP prophylaxis. We propose that selected patients due to receive PJP prophylaxis should be screened for G6PD deficiency.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Mason PJ, Bautista JM, Gilsanz F. G6PD deficiency: the genotype–phenotype association. Blood Rev. 2007;21(5):267–283. doi: 10.1016/j.blre.2007.05.002. [DOI] [PubMed] [Google Scholar]; •• Excellent review of the pathophysiology, clinical features and genetics of G6PD deficiency.
- 3.Dern RJ, Beutler E, Alving AS. The hemolytic effect of primaquine. V. Primaquine sensitivity as a manifestation of a multiple drug sensitivity. J. Lab. Clin. Med. 1955;45(1):30–39. [PubMed] [Google Scholar]
- 4.Alving AS, Carson PE, Flanagan CL, Ickes CE. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124(3220):484–485. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- 5.Cappellini MD, Martinez Di Montemuros F, De Bellis G, Debernardi S, Dotti C, Fiorelli G. Multiple G6PD mutations are associated with a clinical and biochemical phenotype similar to that of G6PD Mediterranean. Blood. 1996;87(9):3953–3958. [PubMed] [Google Scholar]
- 6.Sepkowitz KA, Brown AE, Telzak EE, Gottlieb S, Armstrong D. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA. 1992;267(6):832–837. [PubMed] [Google Scholar]
- 7.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin. Proc. 1996;71(1):5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Schiff D. Pneumocystis pneumonia in brain tumor patients: risk factors and clinical features. J. Neurooncol. 1996;27(3):235–240. doi: 10.1007/BF00165480. [DOI] [PubMed] [Google Scholar]
- 9.Campian JL, Ye X, Gladstone DE, et al. Pre-radiation lymphocyte harvesting and post-radiation reinfusion in patients with newly diagnosed high grade gliomas. J. Neurooncol. 2015;124(2):307–316. doi: 10.1007/s11060-015-1841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(2):140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahindra AK, Grossman SA. Pneumocystis carinii pneumonia in HIV negative patients with primary brain tumors. J. Neurooncol. 2003;63(3):263–270. doi: 10.1023/a:1024217527650. [DOI] [PubMed] [Google Scholar]; • Highlights the need for Pneumocystis jirovecii pneumonia prophylaxis in immunocompromised populations including those receiving chemotherapy/steroids for CNS malignancies.
- 12.Stansell JD, Osmond DH, Charlebois E, et al. Predictors of Pneumocystis carinii pneumonia in HIV-infected persons. Pulmonary Complications of HIV Infection Study Group. Am. J. Respir. Crit. Care Med. 1997;155(1):60–66. doi: 10.1164/ajrccm.155.1.9001290. [DOI] [PubMed] [Google Scholar]
- 13.Baden LR, Bensinger W, Angarone M, et al. Prevention and treatment of cancer-related infections. J. Natl Compr. Canc. Netw. 2012;10(11):1412–1445. doi: 10.6004/jnccn.2012.0146. [DOI] [PubMed] [Google Scholar]
- 14.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin. Proc. 2007;82(9):1052–1059. doi: 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 15.Marras TK, Sanders K, Lipton JH, Messner HA, Conly J, Chan CK. Aerosolized pentamidine prophylaxis for Pneumocystis carinii pneumonia after allogeneic marrow transplantation. Transpl. Infect. Dis. 2002;4(2):66–74. doi: 10.1034/j.1399-3062.2002.t01-1-00008.x. [DOI] [PubMed] [Google Scholar]
- 16.Sangiolo D, Storer B, Nash R, et al. Toxicity and efficacy of daily dapsone as Pneumocystis jiroveci prophylaxis after hematopoietic stem cell transplantation: a case–control study. Biol. Blood Marrow Transplant. 2005;11(7):521–529. doi: 10.1016/j.bbmt.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Chan C, Montaner J, Lefebvre EA, et al. Atovaquone suspension compared with aerosolized pentamidine for prevention of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected subjects intolerant of trimethoprim or sulfonamides. J. Infect. Dis. 1999;180(2):369–376. doi: 10.1086/314893. [DOI] [PubMed] [Google Scholar]
- 18.Hughes WT. Use of dapsone in the prevention and treatment of Pneumocystis carinii pneumonia: a review. Clin. Infect. Dis. 1998;27(1):191–204. doi: 10.1086/514626. [DOI] [PubMed] [Google Scholar]
- 19.Grossman SJ, Jollow DJ. Role of dapsone hydroxylamine in dapsone-induced hemolytic anemia. J. Pharmacol. Exp. Ther. 1988;244(1):118–125. [PubMed] [Google Scholar]
- 20.Olteanu H, Harrington AM, George B, Hari PN, Bredeson C, Kroft SH. High prevalence of dapsone-induced oxidant hemolysis in North American SCT recipients without glucose-6-phosphate-dehydrogenase deficiency. Bone Marrow Transplant. 2012;47(3):399–403. doi: 10.1038/bmt.2011.83. [DOI] [PubMed] [Google Scholar]
- 21.Luzzatto LP, VE Orkin SH, Nathan DG, Ginsburg D, Look TA, Fisher DE, Lux SE. Hematology of Infancy and Childhood. Saunders; PA, USA: 2009. Glucose 6-phosphate dehydrogenase deficiency; pp. 883–907. [Google Scholar]
- 22.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann. Emerg. Med. 1999;34(5):646–656. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 23.Sonbol MB, Yadav H, Vaidya R, Rana V, Witzig TE. Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am. J. Hematol. 2013;88(2):152–154. doi: 10.1002/ajh.23182. [DOI] [PubMed] [Google Scholar]
- 24.www.g6pd.org G6PD Deficiency Association.
- 25.http://www.g6pddeficiency.org g6pd Deficiency.org.
- 26.Baird JK, Dewi M, Subekti D, Elyazar I, Satyagraha AW. Noninferiority of glucose-6-phosphate dehydrogenase deficiency diagnosis by a point-of-care rapid test vs the laboratory fluorescent spot test demonstrated by copper inhibition in normal human red blood cells. Transl Res. 2014;165(6):677–688. doi: 10.1016/j.trsl.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows the relative ease of obtaining a laboratory confirmation of G6PD deficiency.
- 27.Chinevere TD, Murray CK, Grant E, Jr., Johnson GA, Duelm F, Hospenthal DR. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil. Med. 2006;171(9):905–907. doi: 10.7205/milmed.171.9.905. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JA, Petty WJ, Harmon M, et al. Hemolytic anemia in two patients with glioblastoma multiforme: a possible interaction between vorinostat and dapsone. J. Oncol. Pharm. Pract. 2015;21(3):220–223. doi: 10.1177/1078155214524085. [DOI] [PubMed] [Google Scholar]
- 29.United States Census Bureau. 2015. http://quickfacts.census.gov/qfd/states/24000.html