Abstract
Tumor-treating fields (TTFields) is a novel antimitotic treatment modality for patients with glioblastoma. To assess response to TTFields, a newly diagnosed patient with glioblastoma underwent diffusion, perfusion and 3D echo-planar spectroscopic imaging prior to initiation of TTFields plus temozolamide (baseline) and at 1- and 2-month follow-up periods. Increased mean diffusivity along with decreased fractional anisotropy and maximum relative cerebral blood volume were noted at 2 months relative to baseline suggesting inhibition of tumor growth and angiogenesis. Additionally, a reduction in choline/creatine was also noted during this period. These preliminary data indicate the potential of physiologic and metabolic MRI in assessing early treatment response to TTFields in combination with temozolamide.
KEYWORDS : diffusion tensor imaging, echo-planar spectroscopic imaging, glioblastoma, perfusion-weighted imaging, therapeutic response, tumor-treating fields
Practice points.
Tumor-treating fields (TTFields) is a novel US FDA approved antimitotic treatment modality for patients with glioblastoma.
TTFields deliver low intensity, alternating electric energy at an intermediate frequency of 200 kHz as a loco-regional intervention inhibiting cell division and neoplastic cell death with minimal effect on the normal quiescent cells.
Physiological imaging techniques such as diffusion tensor imaging, and dynamic susceptibility contrast-perfusion weighted imaging and proton MR spectroscopy have the potential to evaluate treatment response in patients with gliomas.
A 51-year-old woman with left thalamic glioblastoma was enrolled in the Optune TTFields trial, and underwent serial diffusion tensor imaging, perfusion weighted imaging and whole brain spectroscopic MRI on a 3T MRI scanner prior to TTFields and at 1 and 2 months postinitiation of TTFields.
A trend toward increasing mean diffusivity and decreasing in fractional anisotropy, relative cerebral blood volume and choline/creatine was noted at 2 months relative to baseline suggesting the potential of physiologic and metabolic MRI in assessing early treatment response to TTFields in combination with maintenance TMZ chemotherapy.
Background
Glioblastoma (GBM) is the most common form of malignant brain tumor, affecting approximately 10,000 adults annually in the USA. It is also one of the most aggressive and lethal primary brain tumor, with only a third of patients surviving more than 1 year after diagnosis, and a median survival is only 12–15 months despite maximal aggressive and multimodal therapy [1,2]. Standard therapeutic approaches provide modest improvement in progression-free and overall survival, necessitating the investigation of novel therapies [3].
Recently, the US FDA approved the use of alternating electric fields, also known as tumor-treating fields (TTFields), for the treatment of newly diagnosed GBM. TTFields deliver low intensity, alternating electric energy at an intermediate frequency of 200 kHz as a loco-regional intervention inhibiting cell division and causing neoplastic cell death with minimal effect on the normal quiescent cells [4,5]. In a recent clinical trial [6], patients treated with TTFields and temozolamide (TMZ) survived an average of 3 months longer than those treated with TMZ alone.
Conventional MRI is usually not reliable for assessment of the treatment response due to a lack of specificity [7]. However, physiological imaging techniques such as diffusion tensor imaging (DTI) [8], and dynamic susceptibility contrast (DSC) perfusion weighted imaging (PWI) [9,10] and proton MR spectroscopy (1H MRS ) [11,12] have shown great potential in evaluating treatment response to different therapeutic regimens in patients with gliomas. Until now, no study has investigated the treatment response to TTFields in gliomas using these advanced MRI techniques. We report our initial experience in evaluating treatment response to TTFields in combination with low-dose TMZ in a newly diagnosed GBM patient using DTI, DSC-PWI and 3D-echo-planar spectroscopic imaging (EPSI).
Presentation of case initial diagnosis/assessment
A 51-year-old previously healthy woman presented with 1 week of left-sided numbness and fatigue. MRI of the brain revealed a 3.3 cm necrotic mass in the left thalamus, abutting the left aspect of the third ventricle. It was deemed unresectable due to its deep and eloquent location and a stereotactic biopsy was performed to ascertain tissue diagnosis. Pathology was consistent with a GBM, WHO Grade IV. Due to the small size of the biopsy tissue, it was not possible to perform immunohistochemistry to determine the genetic profile of the neoplasm.
Treatment/management
Patient was started on concurrent chemotherapy with TMZ (140 mg/m2/day) and radiation therapy in January 2015, until her platelet count dropped to 35,000/µl requiring a single platelet transfusion. TMZ was discontinued but she was able to complete radiation therapy by the beginning of February 2015 with a total dose of 6000 cGy. Her platelet count recovered and she enrolled in the Optune TTFields trial on 27 July 2015, which was prescribed by a certified neuroradiologist (SM). The interval between the end of standard chemoradiation therapy and initiation of TTFields was 5 months. At the time of writing this case report, the patient was on low dose (20–80 mg/m2/day) of maintenance TMZ. The patient started TTFields therapy (intensity ˜1 V/cm and frequency ˜200 kHz), with an average daily compliance of 87.165%. At this time her KPS is 100, and she is clinically stable and has returned to work full time.
MRI
The patient underwent serial MRI scans including a baseline (prior to TTFields) and two follow-ups (1 and 2 months postinitiation of TTFields). MRI was performed on a 3 T MRI scanner, (Tim Trio; Siemens, Erlangen, Germany) using a 12-channel phased array head coil. The imaging protocol included an axial 3D T1-weighted magnetization-prepared rapid acquisition of gradient-echo (MPRAGE) images with a repetition time (TR)/echo time (TE)/inversion time (TI)=1760/3.1/950 ms; matrix size = 192 × 256; and 1 mm section thickness; and fluid attenuated inversion recovery (FLAIR) images (TR/TE/TI = 9420/384/2500 ms; matrix size = 192 × 256; and 3 mm section thickness).
DTI data were acquired using 30 noncollinear/noncoplanar directions with a single-shot spin-echo, echo-planar read-out sequence with parallel imaging by using generalized auto-calibrating partially parallel acquisition (GRAPPA) and acceleration factor of 2. Sequence parameters were as follows: TR/TE = 5000/86 ms, number of acquisitions = 3, field of view (FOV) = 22 × 22 cm2, matrix size = 128 × 128, slice thickness = 3 mm, b = 0, 1000 s/mm2, slices = 40 covering the whole brain. The diffusion-weighted images were co-registered to the nondiffusion weighted (b = 0 s/mm2) images to minimize eddy-current and/or subject motion induced artifacts [13]. The corrected raw images were combined to compute mean diffusivity (MD) and fractional anisotropy (FA) maps using in-house software (IDL, ITT Visual Information Solutions, CO, USA).
For DSC-PWI, a bolus of gadobenate dimeglumine (MultiHance; Bracco Imaging, Milano, Italy) was injected as the preloading dose of 0.07 mmol/kg to reduce the effect of contrast leakage on cerebral blood (volume) CBV measurements. A DSC T2*-weighted gradient-echo, echo-planar imaging (GRE-EPI) sequence was obtained after approximately 3.5 min of preloading dose during which FLAIR sequence was acquired. DSC-PWI was obtained during the second 0.07 mmol/kg bolus of intravenous contrast agent. The injection rate was 5 ml/s and was immediately followed by a bolus injection of saline (total of 20 ml at the same rate). DSC-PWI parameters included: TR/TE = 2000/45 ms; FOV = 22 × 22 cm2; in-plane resolution = 1.72 × 1.72 × 3 mm3; bandwidth (BW) = 1346 Hz/pixel; flip angle = 90°; echo planar imaging (EPI) factor = 128; echo spacing = 0.83; 20 slices covering the tumor region; acquisition time 1 min 38 s. Forty-five sequential measurements were acquired for each section, with a temporal resolution of 2.1 s. A slightly longer TR of 2s was used to acquire the DSC-PWI data with 3 mm thick, 20 axial sections to encompass the brain and the entire volume of the neoplasm with a temporal resolution of 2.1 s. Postcontrast T1-weighted MPRAGE images were acquired after completion of DSC-PWI. Leakage corrected cerebral blood volume (CBV) maps were constructed using Nordic ICE program (nordicICE, Nordic Imaging Lab, Bergen, Norway).
Scan parameters for the 3D-EPSI sequence were: TR/TE = 1550/17.6 ms, spatial points = 50 × 50 × 18, FOV = 280 × 280 × 180 mm3, voxel size = 5.6 × 5.6 × 10 mm3 (0.31 cm3), excitation angle = 73°, 512 complex points, spectral BW = 616.2 Hz with radiofrequency excitation pulse centered at water resonance, number of excitations = 1. Water suppression using frequency-selective saturation pulses and inversion-recovery nulling of lipid signal was performed with TI of 198 ms. The acquisition time was 15 min including an interleaved water reference acquisition scan obtained using a gradient-echo acquisition with 20° excitation angle and a 6.3 ms TE. This water-unsuppressed image was used to perform signal normalization, eddy current correction and image co-registration. 3D-EPSI data were processed offline using the metabolic imaging and data analysis system (MIDAS) package developed by Maudsley et al. [14]. Following corrections for inhomogeneity in the B0 field, and eddy current and data were interpolated to a spatial resolution of 64 × 64 × 32 voxels. Signal intensity normalization of metabolite maps was performed using tissue water as an internal reference. The automated MIDAS tool was used to generate Cho and Cr parametric maps.
MD, FA, CBV maps and FLAIR images were co-registered to postcontrast T1-weighted images and a semi-automated routine was used to segment the contrast-enhancing region of tumor [13]. The CBV values were normalized to the contralateral normal white matter to generate relative CBV (rCBV) values. Median values of MD and FA were computed from the enhancing region at each time point. The top 90th percentile rCBV values were measured from the enhancing region and reported as maximum rCBV (rCBVmax). To compute absolute values of Cho, Cr and metabolite ratio of choline/creatine (Cho/Cr), a total of 8 voxels (effective size of voxel = 1 cm3 after interpolation) from similar contrast-enhancing regions at both time points were used. A custom MATLAB-based image reconstruction and visualization platform was used to project Cho/Cr ratio maps in 3D and overlay segmented regions of interests corresponding to voxels that exceed a threshold value for Cho/Cr ratio. The product of largest biperpendicular diameters of the neoplasm were determined on the axial postcontrast T1 weighted images at each time point. Additionally, tumor volumes involving contrast enhancing and central necrotic regions were measured. Percent changes of each parameter between baseline and follow-up time points were calculated as (N – baseline)/baseline × 100.
Outcome/results
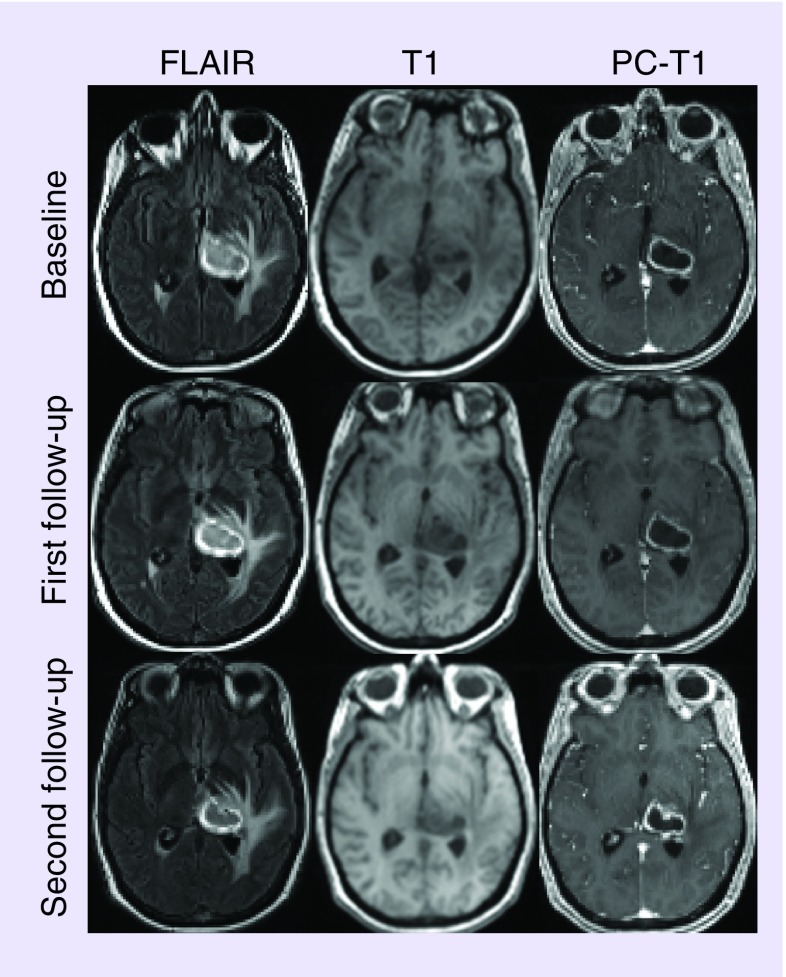
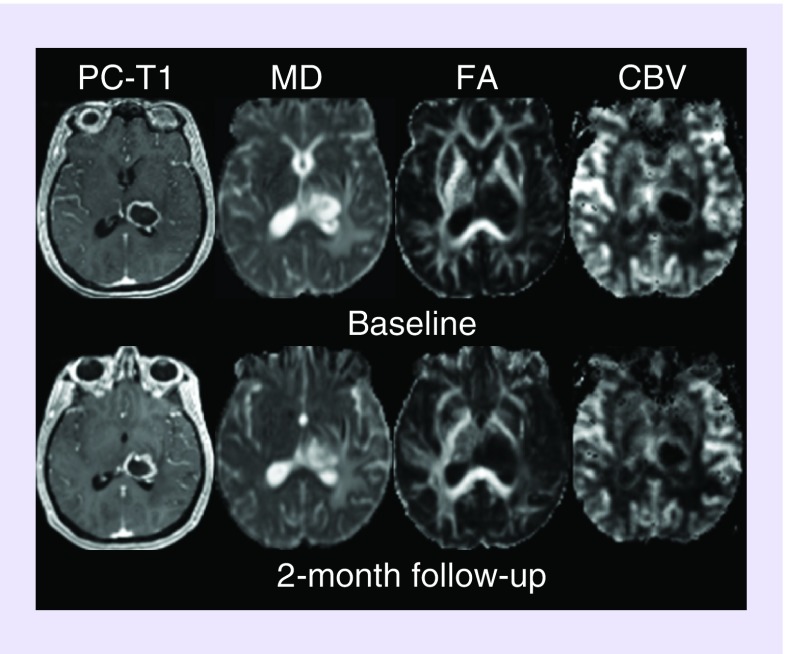
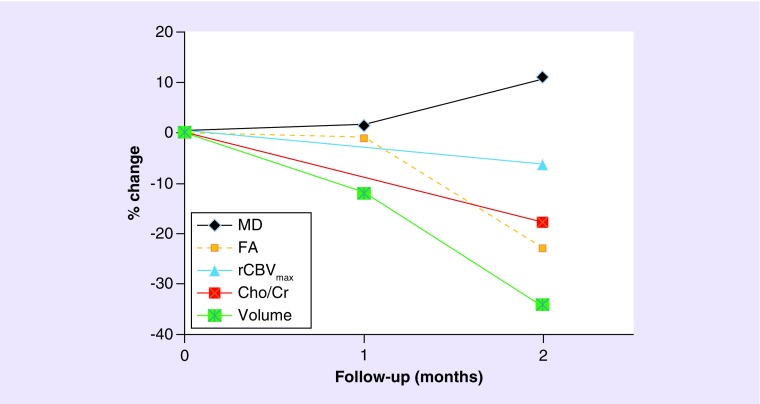
FLAIR, pre- and post-contrast T1 weighted images (Figure 1), demonstrate steady declines in tumor volume as observed at first (˜12%) and second (˜34%) follow-up periods relative to baseline. An approximately 7.6 and 29% decrease in the product of largest biperpendicular diameters was noted at first and second follow-up periods respectively, compared with baseline, according to the RANO criteria. A summary of tumor measurements, volume, DTI, DSC-PWI and EPSI findings at different timepoints are presented in Table 1. Representative images and parametric maps from baseline and two follow-up scans are presented in Figure 2. At 1-month follow-up, MD (1.41% increase) and FA (0.92% decrease) values were relatively stable relative to baseline. Poor quality of CBV maps secondary to contrast extravasation and corrupted raw EPSI data precluded us from computing CBV and Cho levels at 1 month (first) follow-up. Compared with baseline, a larger increase in MD (10.9%) and decreases in FA (22.9%), and rCBVmax (6.21%) were observed at 2-month (second) follow-up. We also observed a 17.8% decrease in Cho/Cr from the enhancing region at the second follow-up compared with baseline. The total number of voxels that exceed the threshold value of 0.55 were 50 at baseline and 34 at second follow-up (Figure 3). Percent changes in volume, MD, FA, rCBVmax and Cho/Cr from baseline to post-TTFields at 1- and 2-month follow-up periods are shown in Figure 4.
Figure 1. . A 51-year-old patient with newly diagnosed glioblastoma treated with tumor-treating fields plus temozolamide.
Axial FLAIR images at three time points demonstrate a well-defined hyperintense mass centered in the left thalamus with surrounding vasogenic edema. This mass appears as hypointense on the corresponding T1-weighted images. A heterogeneously enhancing lesion with hypointense central necrotic core and peritumoral edema is visible on the postcontrast T1-weighted images.
Table 1. . Biperpendicular diameters, tumor volume, mean diffusivity, fractional anisotropy, maximum relative cerebral blood volume and choline/creatine values from the patient at different time points.
| Time | Diameters (mm) | Volume (cm3) | MD (×10-3 mm2/s) | FA | rCBVmax | Cho/Cr |
|---|---|---|---|---|---|---|
| Baseline | 27.7 × 32.5 | 15.5 | 1.49 | 0.125 | 1.76 | 0.457 |
| First follow-up | 25.6 × 32.5 | 13.6 | 1.51 | 0.124 | NA | NA |
| Second follow-up | 24.9 × 25.8 | 10.2 | 1.65 | 0.097 | 1.41 | 0.375 |
Cho/Cr: Choline/creatine; FA: Fractional anisotropy; MD: Mean diffusivity; NA: Not available; rCBVmax: Maximum relative cerebral blood volume.
Figure 2. . Axial co-registered contrast-enhanced T1-weighted image, and corresponding mean diffusivity, fractional anisotropy, cerebral blood volume and choline/creatine maps are shown at baseline and at a 2-month follow-up period.
CBV: Cerebral blood volume; FA: Fractional anisotropy; MD: Mean diffusivity.
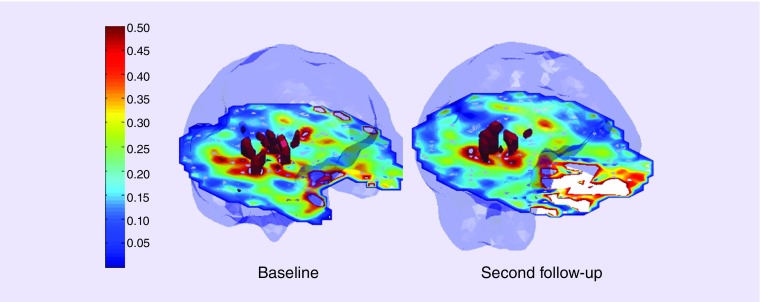
Figure 3. . Choline/creatine maps show red volumes correspond to voxels that exceed a threshold value for choline/creatine ratio of 0.55 at baseline and at 2 months follow-up periods.
The total number of voxels that exceed the threshold value of 0.55 were 50 at baseline and were 34 at second follow-up suggesting reduced levels of choline/creatine at follow-up relative to baseline.
Figure 4. . Percentage changes in parameters from baseline to 1- and 2-month follow-up periods from a patient with glioblastoma treated with tumor-treating fields plus temozolamide.
Trends toward decreased tumor volume, rCBVmax, Cho/Cr and FA along with an increased MD were observed at follow-up relative to baseline indicating tumor growth arrest.
Cho/Cr: Choline/creatine; FA: Fractional anisotropy; MD: Mean diffusivity; rCBVmax: Maximum relative cerebral blood volume.
Discussion
In this report, we present our initial experience using physiological and metabolic imaging in evaluating treatment response to TTFields in combination with low-dose maintenance TMZ in a patient with newly diagnosed GBM. TTFields have been under active investigation with rapid ascent from the lab to clinical trials leading to FDA approval for recurrent GBM on 8 April 2011 and for newly diagnosed GBM on 5 October 2015.
The electrical fields in this therapy are delivered using four insulated transducer arrays composed of biocompatible ceramic discs (nine discs per array) that are applied to the shaved scalp of the GBM patient. The position and size of the arrays are adjusted depending upon the head size, tumor dimensions and location. There is relatively low toxicity with this method with the most common complaint of skin irritation [15]. There appears to be a time-dependent treatment effect with efficacy observed with compliance of wearing the treatment mask over 18 h per day (75%) [6].
The precise mechanism of action of TTFields is not completely understood. However, delivery of alternating electric fields from a portable external device probably disrupts cell division, most notably by interfering with cellular microtubule organization during metaphase of mitosis, resulting in disordered cell division and/or immunogenic cell death as observed in multiple cancer cell lines and animal tumor models [16,17]. We believe that a synergestic effect of TTFields and TMZ chemotherapy might have inhibited the growth of tumor cells in our case.
In comparison to the 1-month time point, we observed larger changes in MD and FA at 2-month period following treatment with TTFields in our patient. Previous studies have reported increased MD and reduced FA from the tumor [18,19] as well as the normal brain parenchyma [20,21] in patients treated with chemo-radiation therapy. However, the interpretation of changes in MD following radiation therapy and adjuvant chemotherapy is complex because of co-localization of treatment-induced gliosis, necrosis and edema [22]. Recently, Wang et al. [23] reported higher MD and significantly lower FA in post-treatment GBM patients with pseudo-progression (PsP) compared with those with true-progression (TP), suggesting that elevated MD and reduced FA are associated with positive treatment response. Our DTI results are in agreement with these studies and imply that DTI can assess therapeutic response to TTFields. We believe that cellular growth inhibition and associated cell death by 2 months might have accounted for the large increase in MD observed in our patient. It has been widely reported that organized microstructures secondary to closely packed proliferating tumor cells in gliomas result in high FA [19,24]. A 23% reduction in FA in the current case may be due to reduced cell density and incoherent orientation of neoplastic cells.
Rich capillary network secondary to angiogenesis is a common feature of GBMs, responsible for high rCBV [25]. Several studies [9,10] have reported reduced rCBV in gliomas following radiotherapy and anti-angiogenetic therapy. Fibrinoid necrosis, endothelial injury and occlusion of blood vessels have been proposed as potential reasons for decreased rCBV levels in treated GBMs [26]. In agreement with these studies, reduced rCBVmax were also noted in the present case suggesting reduced vascularity and tissue perfusion within the tumor bed. A previous study reported substantial decrease in the levels of CD34 (an immunohistochemical marker of microvessel density) and downregulation of VEGFs (proangiogenetic factors) in murine melanomas exposed to intermediate frequency alternating electric field compared with control group [27]. While, it is not clear how a combination of TTFields and TMZ chemotherapy modulates tumor vasculature of gliomas, it may be speculated that inhibited angiogenesis might have caused decreased perfusion in our case.
Several prior 1H MRS studies [11,12] have reported decreased levels of Cho as a surrogate marker of positive treatment response in patients with brain tumors. In accordance with these previous studies, we also observed decreased levels of absolute Cho and Cho/Cr in our case at 2-month period following treatment. It is well documented that Cho content correlates with cell density and with indices of cellular proliferation [28]. We believe that these reductions in Cho levels in our case were most likely a direct consequence of combined anti-proliferative effect of TTFields and TMZ on cellular metabolism of gliomas.
Revised criteria for assessment of treatment response have been proposed by the RANO Working Group [29]. According to this method that has served as the current benchmark for tumor assessment, evaluation of therapy response is based on the change in contrast-enhancing lesion size via 2D perpendicular diameter of the enhancing region. Our patient demonstrated approximately 29% decline in the product of the perpendicular diameters suggesting ‘stable disease’ according to the RANO criteria. There was approximately a 34% decrease in neoplasm volume with moderate variations in the physiological and metabolic parameters as observed at the second follow-up indicating an overall favorable response to TTFields in combination with TMZ. We believe that multiparametric approach utilizing the unique strengths of these advanced imaging techniques as performed in the present case may provide a comprehensive assessment of treatment response in these patients. A larger cohort of patients with newly diagnosed GBM is required to further elucidate the efficacy of TTFields in combination with TMZ.
In addition to response assessment, the neuroradiologist may also function as the certified prescriber of this novel therapy perhaps adding another dimension to ‘interventional’ neuroradiology. In the future, these devices will likely become smaller, lighter, more convenient for use and increasingly tailored for the tumor size. The indications for use in other brain and nonbrain malignancies will likely continue to expand, and the incorporation into combination therapies will continue to be optimized.
Conclusion & future perspective
This case demonstrates the potential of advanced physiologic and metabolic MRI in more accurate assessment of treatment response to TTFields in combination with TMZ indicating that TTFields should be considered as an adjunct modality in the treatment of GBM. However, these early findings need to be corroborated in a larger patient cohort.
Acknowledgements
The authors would also like to thank the University of Pennsylvania radiology research team, Lisa Desiderio, Katelyn O'Reilly and MRI technicians, for their valuable contributions to the project.
Footnotes
Financial & competing interests disclosure
The study was funded in part by a grant from Novocure Ltd., Haifa, Israel. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Young RM, Jamshidi A, Davis G, Sherman JH. Current trends in the surgical management and treatment of adult glioblastoma. Ann. Transl Med. 2015;3(9):121. doi: 10.3978/j.issn.2305-5839.2015.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong ET, Lok E, Swanson KD. An evidence-based review of alternating electric fields therapy for malignant gliomas. Curr. Treat Options Oncol. 2015;16(8):40. doi: 10.1007/s11864-015-0353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Efficacy of tumor-treating fields (TTFields) in the treatment of malignant gliomas can be increased by combining it with other anticancer treatment modalities.
- 5.Pless M, Weinberg U. Tumor treating fields: concept, evidence and future. Expert Opin. Investig. Drugs. 2011;20(8):1099–1106. doi: 10.1517/13543784.2011.583236. [DOI] [PubMed] [Google Scholar]; •• TTFields destroy mitotic cells via apoptosis, thereby inhibiting tumor growth without effecting the nondividing quiescent cells.
- 6.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]; •• Adding TTFields to maintenance temozolomide chemotherapy after standard chemoradiation therapy significantly prolongs progression-free and overall survival of patients with glioblastomas.
- 7.Jackson EF, Barboriak DP, Bidaut LM, Meyer CR. Magnetic resonance assessment of response to therapy: tumor change measurement, truth data and error sources. Transl Oncol. 2009;2(4):211–215. doi: 10.1593/tlo.09241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraswathy S, Crawford FW, Lamborn KR, et al. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J. Neurooncol. 2009;91(1):69–81. doi: 10.1007/s11060-008-9685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmainda KM, Prah M, Connelly J, et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro. Oncol. 2014;16(6):880–888. doi: 10.1093/neuonc/not216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aquino D, Di Stefano AL, Scotti A, et al. Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to bevacizumab and irinotecan. PLoS ONE. 2014;9(3):e90535. doi: 10.1371/journal.pone.0090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon JY, Kovanlikaya I, Boockvar JA, et al. Metabolic response of glioblastoma to superselective intraarterial cerebral infusion of bevacizumab: a proton MR spectroscopic imaging study. Am. J. Neuroradiol. 2012;33(11):2095–2102. doi: 10.3174/ajnr.A3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muruganandham M, Clerkin PP, Smith BJ, et al. 3-Dimensional magnetic resonance spectroscopic imaging at 3 Tesla for early response assessment of glioblastoma patients during external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014;90(1):181–189. doi: 10.1016/j.ijrobp.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Kim S, Chawla S, et al. Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage. 2009;44(3):653–660. doi: 10.1016/j.neuroimage.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maudsley AA, Darkazanli A, Alger JR, et al. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006;19(4):492–503. doi: 10.1002/nbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Develoment of 3D echo-planar spectroscopic imaging sequence along with sophisticated postprocessing tool enables mapping of metabolite distributions from different tissue compartments encompassing the entire volume of the neoplasm without partial volume effects.
- 15.Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur. J. Cancer. 2012;48(14):2192–2202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]; •• TTFields is a relatively new therapeutic modality in cancer treatment. NovoTTF-100A is a portable device that delivers low-intensity, intermediate frequency alternating electric fields via noninvasive transducer arrays.
- 16.Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288–3295. doi: 10.1158/0008-5472.can-04-0083. [DOI] [PubMed] [Google Scholar]
- 17.Giladi M, Weinberg U, Schneiderman RS, et al. Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo . Semin. Oncol. 2014;41(Suppl. 6):S35–S41. doi: 10.1053/j.seminoncol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, van Zijl PCM, Laterra J, et al. Unique patterns of diffusion directionality in rat brain tumors revealed by high-resolution diffusion tensor MRI. Magn. Reson. Med. 2007;58:454–462. doi: 10.1002/mrm.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beppu T, Inoue T, Shibata Y, et al. Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neur. 2005;63:56–61. doi: 10.1016/j.surneu.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Chawla S, Wang S, Kim S, et al. Radiation injury to the normal brain measured by 3D-echo-planar spectroscopic imaging and diffusion tensor imaging: initial experience. J. Neuroimaging. 2015;25(1):97–104. doi: 10.1111/jon.12070. [DOI] [PubMed] [Google Scholar]
- 21.Kitahara S, Nakasu S, Murata K, et al. Evaluation of treatment-induced cerebral white matter injury by using diffusion-tensor MR imaging: initial experience. Am. J. Neuroradiol. 2005;26(9):2200–2206. [PMC free article] [PubMed] [Google Scholar]
- 22.Tomura N, Narita K, Izumi J-I, et al. Diffusion changes in a tumor and peritumoral tissue after stereotactic irradiation for brain tumors: possible prediction of treatment response. J. Comput. Assist. Tomogr. 2006;30(3):496–500. doi: 10.1097/00004728-200605000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Martinez-Lage M, Sakai Y, et al. Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. Am. J. Neuroradiol. 2016;37(1):28–36. doi: 10.3174/ajnr.A4474. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Elevated mean diffusivity and reduced fractional anisotropy are associated with favorable treatment response in patients with glioblastomas exhibiting enhancing lesions within 6 months after completion of chemoradiation therapy.
- 24.Kinoshita M, Hashimoto N, Goto T, et al. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage. 2008;43(1):29–35. doi: 10.1016/j.neuroimage.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Kim JH, Kim YM, et al. Perfusion MR imaging in gliomas: comparison with histologic tumor grade. Korean J. Radiol. 2001;2:1–7. doi: 10.3348/kjr.2001.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu XJ, Duan CF, Fu WW, et al. Correlation between magnetic resonance perfusion weighted imaging of radiation brain injury and pathology. Genet. Mol. Res. 2015;14(4):16317–16324. doi: 10.4238/2015.December.8.23. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Liu R, Liu J, Tang J. Growth inhibition of malignant melanoma by intermediate frequency alternating electric fields, and the underlying mechanisms. J. Int. Med. Res. 2012;40:85–94. doi: 10.1177/147323001204000109. [DOI] [PubMed] [Google Scholar]
- 28.Miller BL, Chang L, Booth R, et al. In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci. 1996;58:1929–1935. doi: 10.1016/0024-3205(96)00182-8. [DOI] [PubMed] [Google Scholar]
- 29.Wen PY, MacDonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]