Abstract
Objective
The objective of this study was to describe a case of severe lamotrigine toxicosis in a dog, which was successfully treated using minimal medical interventions.
Case summary
A 7-month-old male, intact, Labrador mix was evaluated because of acute onset of vomiting, rigidity, and dull mentation after ingesting lamotrigine tablets. The estimated oral dose that had been ingested was 278 mg/kg (611.6 mg/lb). Physical examination was unremarkable other than abnormalities noted in the cardiovascular and neurological systems. Neurological examination revealed dull mentation, vertical nystagmus, four-legged extensor limb rigidity, and alligator rolling. Cardiovascular examination revealed pale pink mucous membranes and multifocal ventricular tachycardia. Intravenous (IV) fluids were started at three times maintenance (180 mL/kg/day). Methocarbamol (100 mg/kg [220 mg/lb], rectally) and lidocaine (2 mg/kg [4.4 mg/lb, IV]) were administered. Twenty-four and seventy-two hours after presentation, the dog was clinically normal with no ventricular tachycardia being noted.
Conclusion
Lamotrigine (6-[2,3-dichlorophenyl]-1,2,4-triazine-3,5-diamine) is an anticonvulsant medication used in humans, which inhibits voltage-gated sodium channels. The clinical success of this case suggests that administration of only methocarbamol for the neurologic effects and lidocaine for the arrhythmias, as well as supportive IV fluid therapy, could be a successful treatment strategy for dogs, even with severe lamotrigine toxicosis.
Keywords: arrhythmia, toxicity, multifocal ventricular tachycardia, poison
Video abstract
Case report
A 7-month-old male, intact, 18 kg (39.6 lb) Labrador mix presented for acute vomiting, rigidity, and dull mentation. The owners had not been home for 14 h prior. When they returned, they found the dog laterally recumbent, rigid, but conscious, with vomit, and a chewed lamotrigine (GlaxoSmithKline plc, Lamotrigine [Lamictal XR Extended Release], Brentford, London, UK) bottle nearby. The dog ingested approximately fifty 100 mg tablets. The estimated oral dose ingested was 278 mg/kg (611.6 mg/lb). There were no other animals in the household. The dog had been healthy with no previous illnesses.
On presentation, the dog had a rectal temperature of 39.4°C (103.0°F). On physical examination, the dog had good body condition. He was tachypneic at 60 breaths per minute with clear lung fields. The mouth was rigidly closed. Abdominal palpation was soft with no intra-abdominal masses. Rectal examination revealed normal, brown stool. Neurological examination revealed a dull mentation, vertical nystagmus, severe alligator rolling with extensor rigidity in all four limbs. Cardiac examination revealed a heart rate of 160 beats per minute, and he had pale pink mucous membranes with a capillary refill time of 2 s. An intermittently irregular heart rhythm was noted.
Diagnostic testing upon presentation included an electrocardiogram and blood pressure measurement. This identified the arrhythmia as multifocal ventricular tachycardia (Figure 1). The blood pressure was found to be 104 mmHg. An intravenous (IV) catheter was placed, and a balanced electrolyte solution (Plasmalyte A; Baxter International Inc, Deerfield, IL, USA) was started at three times maintenance (180 mL/kg/day). Because of the patient’s abnormal mentation and neurologic status on presentation, oral medication could not be administered and also an IV catheter could not be placed safely initially. Methocarbamol (Auxilium Pharmaceuticals, Inc, Chesterbrook, PA, USA) was administered (100 mg/kg [220 mg/lb], rectally) mixed with 3 mL of saline. A lidocaine (Lidocaine hydrochloride; Amphastar Pharmaceuticals, Inc, Rancho Cucamonga, CA, USA; 2 mg/kg [4.4 mg/lb], IV) bolus was administered IV soon thereafter converting electrocardiogram to a normal sinus rhythm.
Figure 1.

Presenting electrocardiogram.
Notes: Six-lead electrocardiogram recording of a dog that was evaluated because of concern for ingestion of ~278 mg/kg (611.6 mg/lb) of lamotrigine. He was subsequently found to be neurologic with gastrointestinal signs. Note the multifocal ventricular tachycardia with a mean heart rate of 125 beats per minute. Paper speed =25 mm/s; 1 cm = 1 mV.
The dog was hospitalized for supportive care and monitoring. After the single methocarbamol and lidocaine doses, three times maintenance IV fluid therapy was continued; however, no further methocarbamol doses or lidocaine boluses were administered. Two hours after presentation, the patient was able to stand and ambulate with mild ataxia. A mild head tremor lastinĝ45 s was recorded at that time, which resolved on its own. Three hours after presentation, another electrocardiogram was taken, which revealed a normal heart rate, 136 beats per minute, with occasional ventricular premature complexes. Blood gas was evaluated to be normal (Table 1). Low–normal potassium was noted at 3.7 meq/L (reference range 3.6–4.9 meq/L). The dog began to eat within the first 4 h of presentation and had no further vomiting.
Table 1.
Blood gas testing on day 1 and 2
| Values | Day 1 | Day 2 | Reference values |
|---|---|---|---|
| pH | 7.41 | 7.41 | 7.36–7.47 |
| PCO2 | 28.6 | 27.4 | 30.0–50.0 mmHg |
| PO2 | 43.8 | 45.0 | 30.0–60.0 mmHg |
| HCT | 42 | 40 | 40%–50% |
| SO2% | 80.5 | 81.6 | 100% |
| Na+ | 146.6 | 141.9 | 145.0–155.0 meq/L |
| K+ | 3.7 | 4.6 | 3.6–4.9 meq/L |
| Cl− | 113.0 | 111.2 | 110.0–120.0 meq/L |
| Ca++ | 1.36 | 1.37 | 1.15–1.35 mmol/L |
| Mg++ | 0.5 | 0.4 | 0.1–1.5 mg/dL |
| Glucose | 94 | 97 | 80–110 mg/dL |
| Lactate | 0.9 | 1.7 | 0.5–2.5 mmol/L |
| BUN | 12 | 10 | 5–30 mg/dL |
| Creatinine | 1.1 | 1.3 | 0.7–1.8 mg/dL |
| TCO2 | 19.5 | 18.3 | 21–25 mmol/L |
| HCO3− | 18.7 | 17.5 | 18–26 mmol/L |
Notes: Abbreviated blood gas panel results of the dog ingesting ~278 mg/kg (611.6 mg/lb) of lamotrigine. Blood gas panels taken on the first and second day of hospitalization.
Abbreviations: BUN, blood urea nitrogen; HCT, hematocrit.
Three times maintenance IV fluids were administered until that evening when they were decreased to two times maintenance (120 mL/kg/day). The following day, another blood gas was taken, which revealed that all values remained within the normal limits (Table 1). An electrocardiogram was also repeated and found to have normal rate and rhythm (Figure 2). By 48 h after presentation, fluids were decreased to 60 mL/kg/day, and the patient was discharged soon thereafter.
Figure 2.
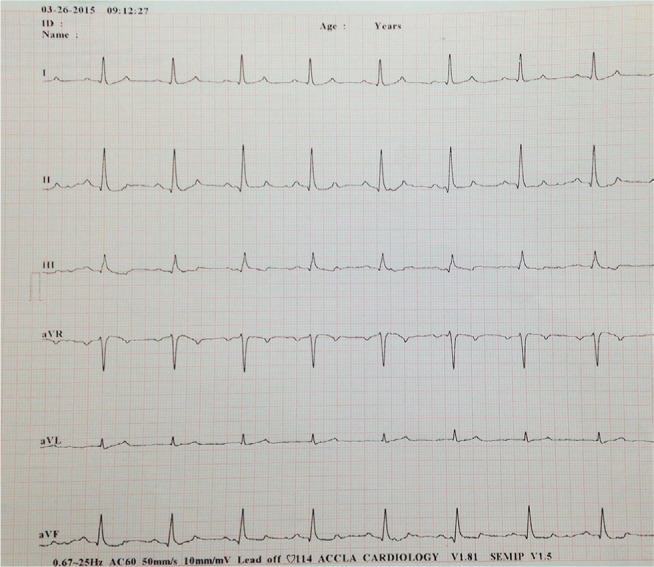
Electrocardiogram taken on the second day of hospitalization.
Notes: Six-lead electrocardiogram recording obtained 24 h after the dog ingested ~278 mg/kg (611.6 mg/lb) of lamotrigine and was treated with intravenous fluids, lidocaine, and methocarbamol. At this time, there is a normal sinus rhythm with a mean heart rate of 114 beats per minute. Paper speed =50 mm/s; 1 cm = 1 mV.
The dog was presented for recheck 72 h after original presentation. He was presented with a rectal temperature of 39.6°C (103.2°F). On physical examination, the dog was found to be in good body condition. He was panting at 60 breaths per minute. His heart rate was 125 beats per minute, and he had pink mucous membranes with a capillary refill time of 2 s. Neurological examination revealed normal mentation, normal spinal reflexes, and placing reflexes. Cardiac examination revealed normal sinus rhythm. No further arrhythmias were noted on electrocardiogram.
Discussion
To the authors’ knowledge, this is the first published case report of a massive lamotrigine overdose in a dog which was able to be treated with minimal intervention. Lamotrigine (6-[2,3-dichlorophenyl]-1,2,4-triazine-3,5-diamine) is a phenyltriazine anticonvulsant medication, which is used in humans to treat bipolar disorders and seizures.1 Unlike other antiepileptic drugs, lamotrigine reduces presynaptic excitatory amino acid (glutamate and aspartate) release rather than post synaptic release.1 It inhibits voltage-gated sodium channels, but it also inhibits voltage-gated calcium channels and is a weak 5-hydroxytryptamine (5HT3) antagonist.2
The current documented clinical signs from the American Society for the Prevention of Cruelty to Animals poison control database for dogs and cats with lamotrigine toxicosis include: vomiting in 46% of cases, ataxia in 25%, lethargy in 25%, with ~14% having tachycardia and/or seizures, and 11% having tremors or arrhythmias. Hypersalivation was noted in 8%, bradycardia was documented in 6% of patients, and 4% of patients had hypokalemia.3 The patient discussed in this case exhibited gastrointestinal, cardiac, and neurological abnormalities. The exact mechanism of action of lamotrigine in causing vomiting has not yet been elucidated. Arrhythmias in dogs have been postulated to occur because of the metabolism of lamotrigine in the canine liver to the 2-N-methyl metabolite.1 Lamotrigine is catalyzed to this metabolite by canine species-specific methyltransferase.4 This is in contrast to the glucuronic acid conjugation forming the inactive 2-N-glucuronide conjugate in humans.4 In canines, the 2-N-methyl metabolite has been shown to cause prolongation of P-R interval, widening of QRS complexes, and complete atrioventricular conduction block.5 An intermittent ventricular tachycardia was noted in the patient presented here. This is suspected to be caused by lamotrigine’s inhibition of the SCN5A sodium channel as noted in one human study.6
Although the potassium level was low–normal on presentation in this case, lamotrigine is also known to cause hypokalemia because of an inhibitory effect it has on A-type potassium current in hippocampal neuron-derived H19-7 cells.7
The estimated dose ingested in this case was 278 mg/kg (611.6 mg/lb). Although an LD50 of 205 mg/kg (451 mg/lb) has been noted in rats,8 based on the American Society for the Prevention of Cruelty to Animals database, in small animals at 3.4 mg/kg (7.5 mg/lb) dosage lethargy can be seen, at >20 mg/kg (44 mg/lb) cardiac signs begin, and at >40 mg/kg (88 mg/lb) this dosage can be life threatening.5 Clinical signs commonly occur within the first 4 h of ingestion, but can be delayed by 12 h with the extended release products. This range of clinical signs coincides with the elimination half-life being ~25 h in human subjects, but can extend to 50 h if there is renal dysfunction.9 Elimination is 94% in the urine, with a small portion being eliminated in the feces.1
Treatment of lamotrigine toxicosis includes decontamination through emesis. Apomorphine can be administered IV at 0.03 mg/kg (0.66 mg/lb).10 Emesis can be induced within 1 h of exposure to immediate release product and within 2–3 h if extended release. There is no evidence that charcoal can aid in decontamination, but its use should be considered to try to help reduce systemic absorption. IV fluid diuresis has been documented in some human cases and was used in this case as the main decontamination route.11,12 Patients with lamotrigine toxicosis should be monitored closely with an electrocardiogram. If ventricular arrhythmias are present, a lidocaine bolus at 2 mg/kg (4.4 mg/lb) to effect, as seen in this case, may be administered IV followed by a continuous rate infusion of 25–75 µg/kg/min (55–165 µg/lb/min).10 If bradyarrhythmias are present, such as atrioventricular block, continuous rate infusion of atropine or isoproterenol may be administered IV. Vomiting should be controlled with anti-emetics. Methocarbamol should be given at 55–220 mg/kg (121–484 mg/lb) IV if possible.10 Although not required clinically in this case, other anti-seizure medications such as diazepam, levetiracetam, or phenobarbital may be used. Phenobarbital may be used if the patient is not responding to initial treatments. Potassium levels should be monitored, and the patient should be supplemented with potassium if the patient is hypokalemic. Although there are no veterinary reports, there have been human reports of intralipids used for decontamination because of the lipophilic nature of lamotrigine.13,14 Intralipid therapy could be considered as a possible treatment option in severely affected veterinary patients with lamotrigine toxicosis. The clinician should weigh the benefits of intralipids prior to the administration as the therapeutic concentration of some anesthetics, such as lidocaine, have been noted to be decreased with intralipid therapy.15
Conclusion
The present study seems to be the first to provide a case documentation of a successful, simple treatment strategy for a severe lamotrigine toxicosis in a dog. The dog was successfully treated using only lidocaine, methocarbamol, and IV fluid therapy. He responded rapidly despite the high dose of medication being ingested. Further studies are needed to determine the prognosis for this case and confirm appropriate treatment strategies.
Acknowledgments
Verbal consent was obtained from the clients in order to include the patient in this case report.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lamictal . Physicians’ Desk Reference. 63rd ed. Montvale, NJ: Thomson Reuters; 2009. p. 1489. [Google Scholar]
- 2.Thomas SP, Nandhra HS, Jayaraman A. Systemic review of lamotrigine augmentation of treatment resistant unipolar depression (TRD) J Ment Health. 2010;19(2):168–175. doi: 10.3109/09638230903469269. [DOI] [PubMed] [Google Scholar]
- 3.AnTox Database. Urbana, IL: ASPCA Animal Poison Control Center; 2003–2011. [Google Scholar]
- 4.Fong KL, Hwang BY. Dog liver N-methyltransferase. A drug-metabolizing enzyme. Biochem Pharmacol. 1983;32(18):2781–2786. doi: 10.1016/0006-2952(83)90092-8. [DOI] [PubMed] [Google Scholar]
- 5.Stern LA. Lamotrigine toxicosis in dogs and cats. Vet Med. 2015;110(2):45–47. [Google Scholar]
- 6.Antzelevitch C, Brugada P, Borggrefe M. Brugada syndrome: report of the second consensus conference. Circulation. 2005;111:650–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Huang C, Liu Y, et al. Inhibitory effect of lamotrigine on A-type potassium current in hippocampal neuron-derived H19-7 cells. Epilepsia. 2004;45(7):729–736. doi: 10.1111/j.0013-9580.2004.58403.x. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Occupational Safety and Health . Registry of toxic effects of chemical substances. Department of Health, Education and Welfare; Cincinnati, OH: 2001. [Accessed March 1, 2017]. (Lamotrigine RETCS number: XY5850700). Available from: https://www.cdc.gov/niosh/rtecs/rtecsac-cess.html. [Google Scholar]
- 9.Fillastre J, Taburet A, Fialaire A, et al. Pharmacokinetics of lamotrigine in patients with renal impairment: influence of haemodialysis. Drugs Exp Clin Res. 1993;19(1):25–32. [PubMed] [Google Scholar]
- 10.Plumb DC. Plumb’s Veterinary Drug Handbook. 6th ed. Ames, IA: Blackwell Publishing; 2008. [Google Scholar]
- 11.Daana M, Nevo Y, Tenenbaum A, et al. Lamotrigine overdose in a child. J Child Neurol. 2007;22(5):642–644. doi: 10.1177/0883073807302600. [DOI] [PubMed] [Google Scholar]
- 12.Venkatraman N, O’Neil D, Hall A. Life-threatening overdose with lamotrigine, citalopram, and chlorpheniramine. J Postgrad Med. 2008;54(4):316–317. doi: 10.4103/0022-3859.43516. [DOI] [PubMed] [Google Scholar]
- 13.Castanares-Zapatero D, Wittebole X, Huberlant V, et al. Lipid emulsion as rescue therapy in lamotrigine overdose. J Emerg Med. 2012;42:48–51. doi: 10.1016/j.jemermed.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 14.Dagtekin O, Marcus H, Müller C, Böttiger BW, Spöhr F. Lipid therapy for serotonin syndrome after intoxication with venlafaxine, lamotrigine and diazepam. Minerva Anesthesiol. 2011;77(1):93–95. [PubMed] [Google Scholar]
- 15.Laine J, Lokajova J, Parshintsev J, et al. Interaction of a commercial lipid dispersion and local anesthetics in human plasma: implications for drug trapping by “lipid sinks.”. Anal Bio Chem. 2010;396(7):2599–2607. doi: 10.1007/s00216-009-3435-z. [DOI] [PubMed] [Google Scholar]


